Sensitization Potential of the Major Soybean Allergen Gly m 4 and Its Cross-Reactivity with the Birch Pollen Allergen Bet v 1
Abstract
1. Introduction
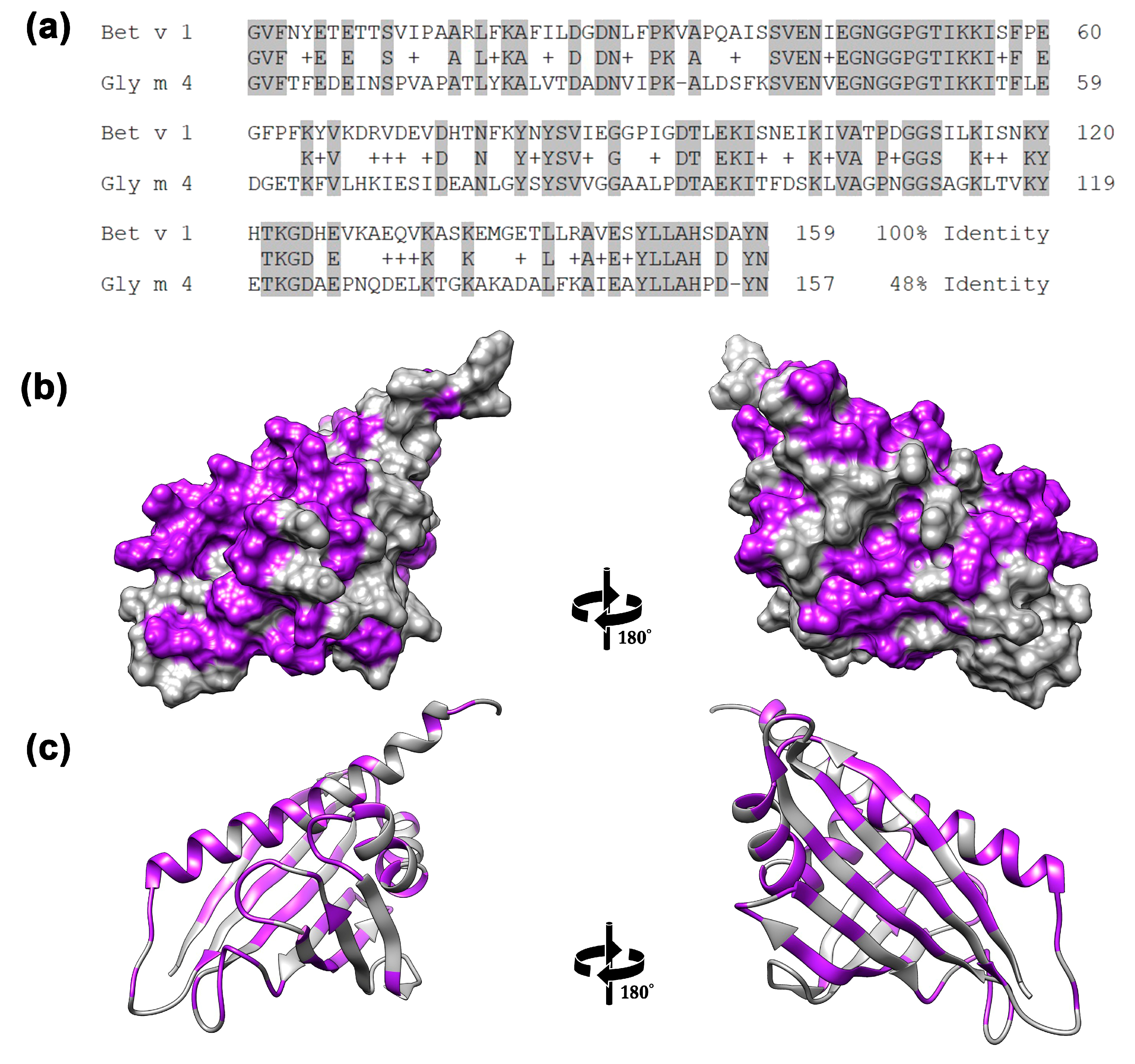
2. Results and Discussion
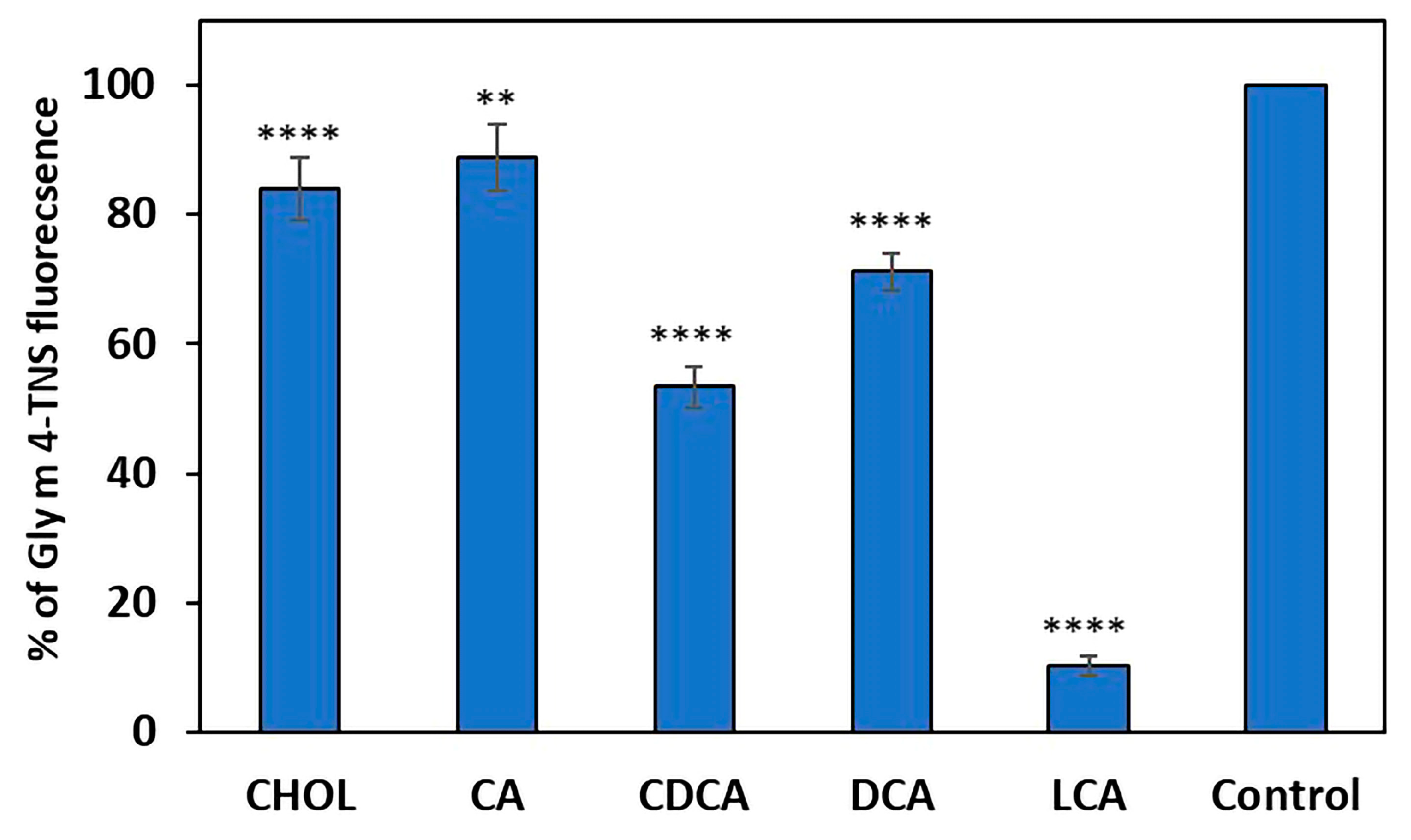
2.1. The Ability of Gly m 4 to Bind Cholesterol and Bile Acids
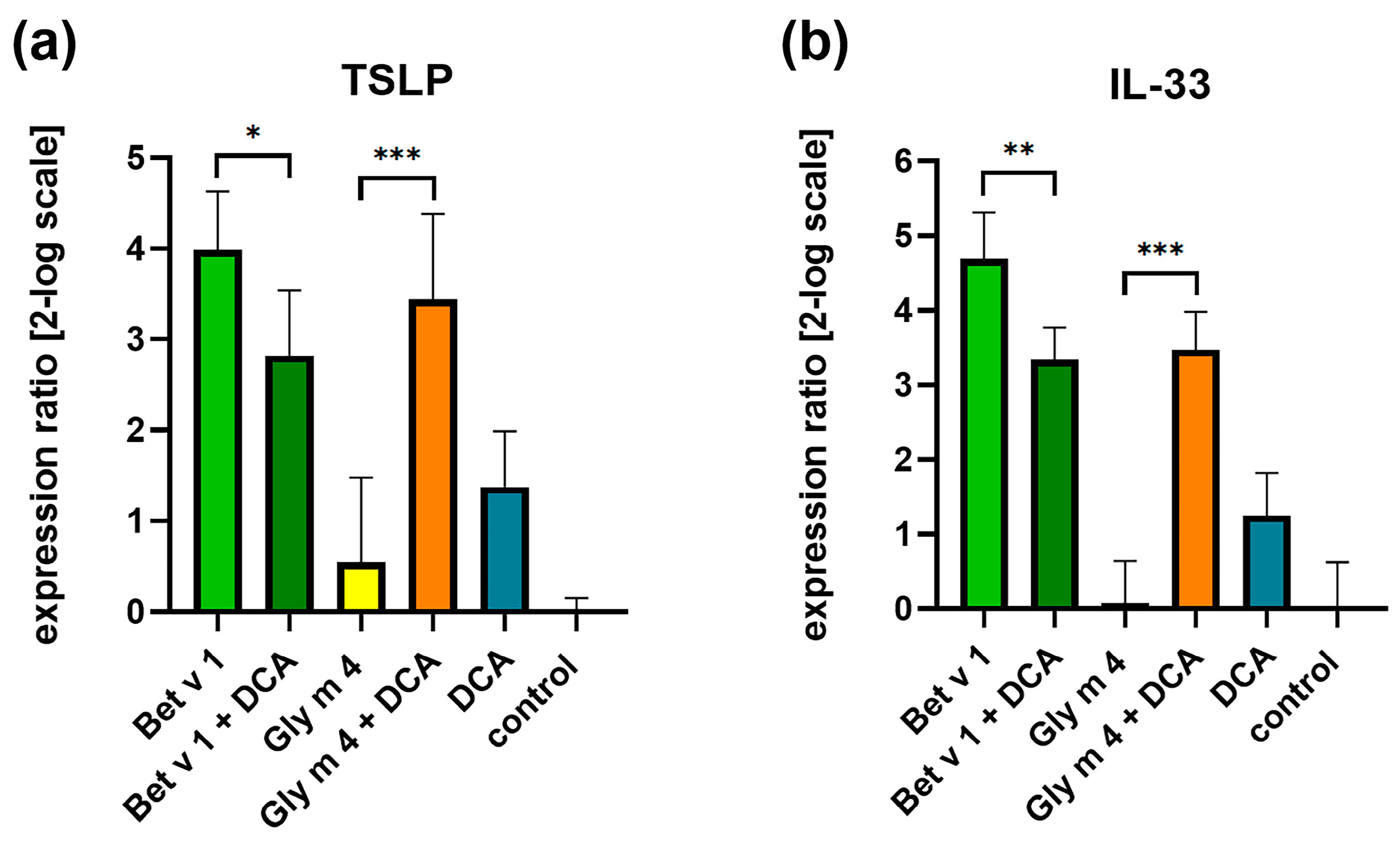
2.2. Effects of Gly m 4 on the Expression of Alarmin Genes in Epithelial Cells
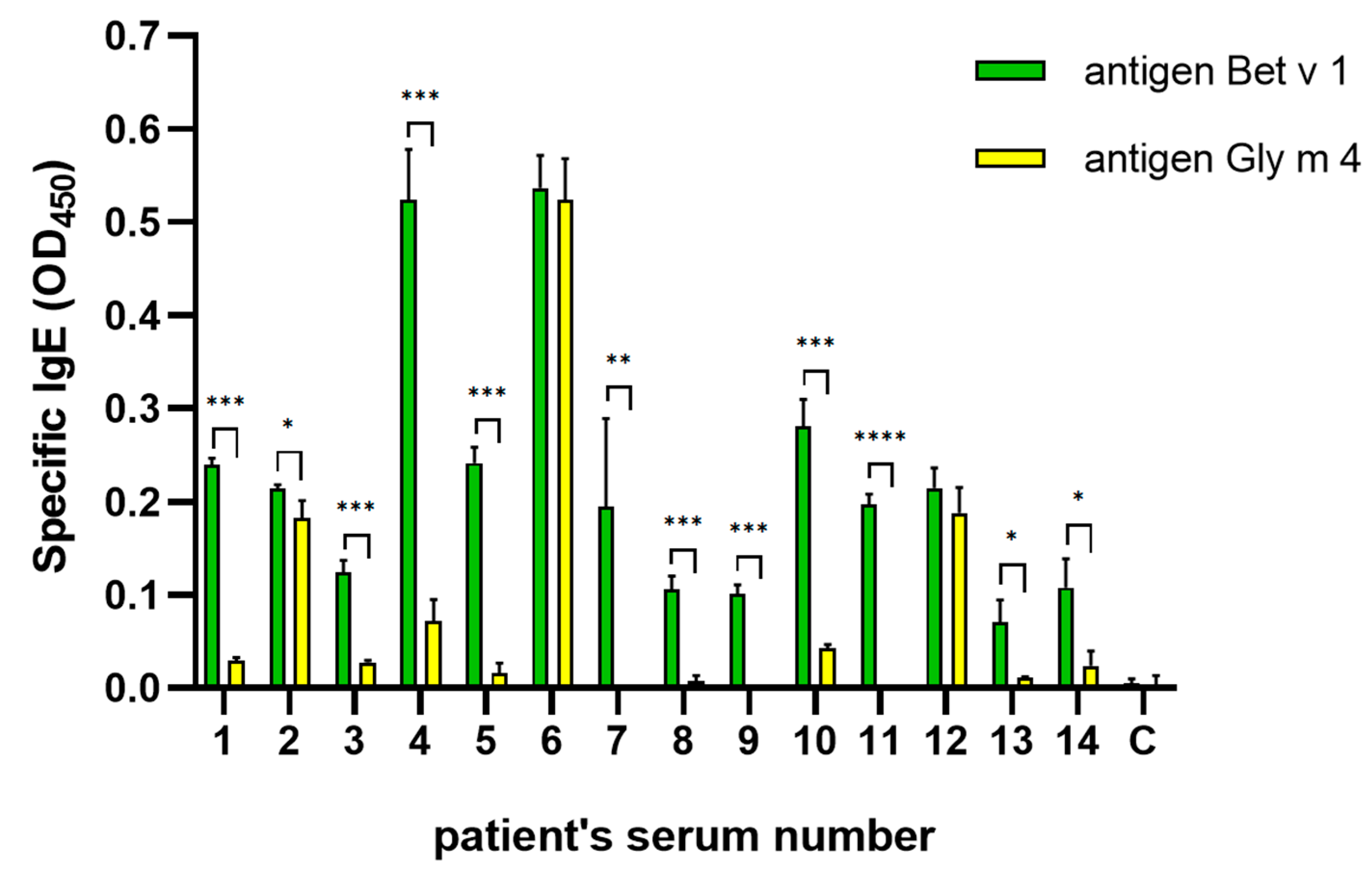
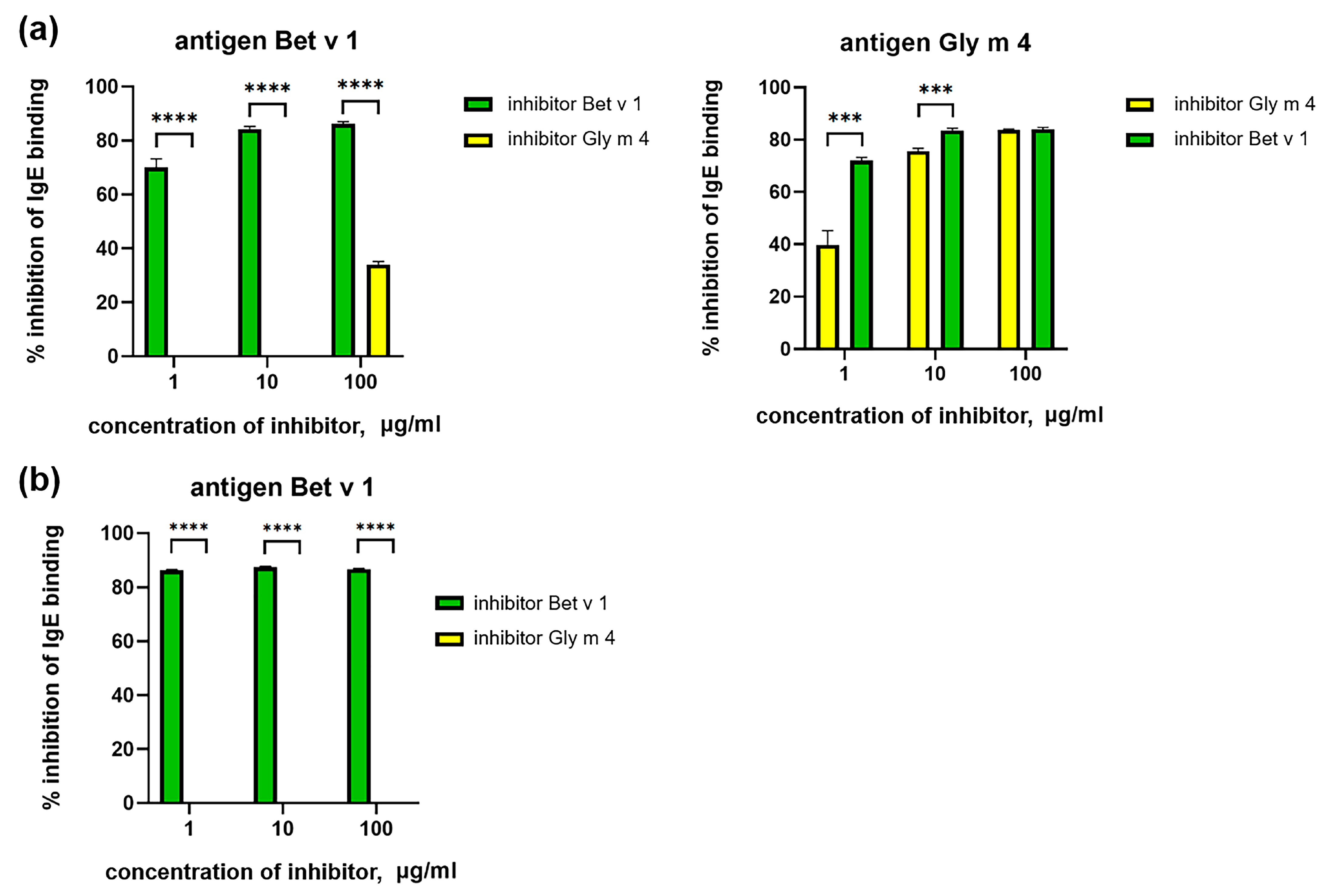
2.3. Cross-Reactivity of IgE from Sera of Allergic Patients
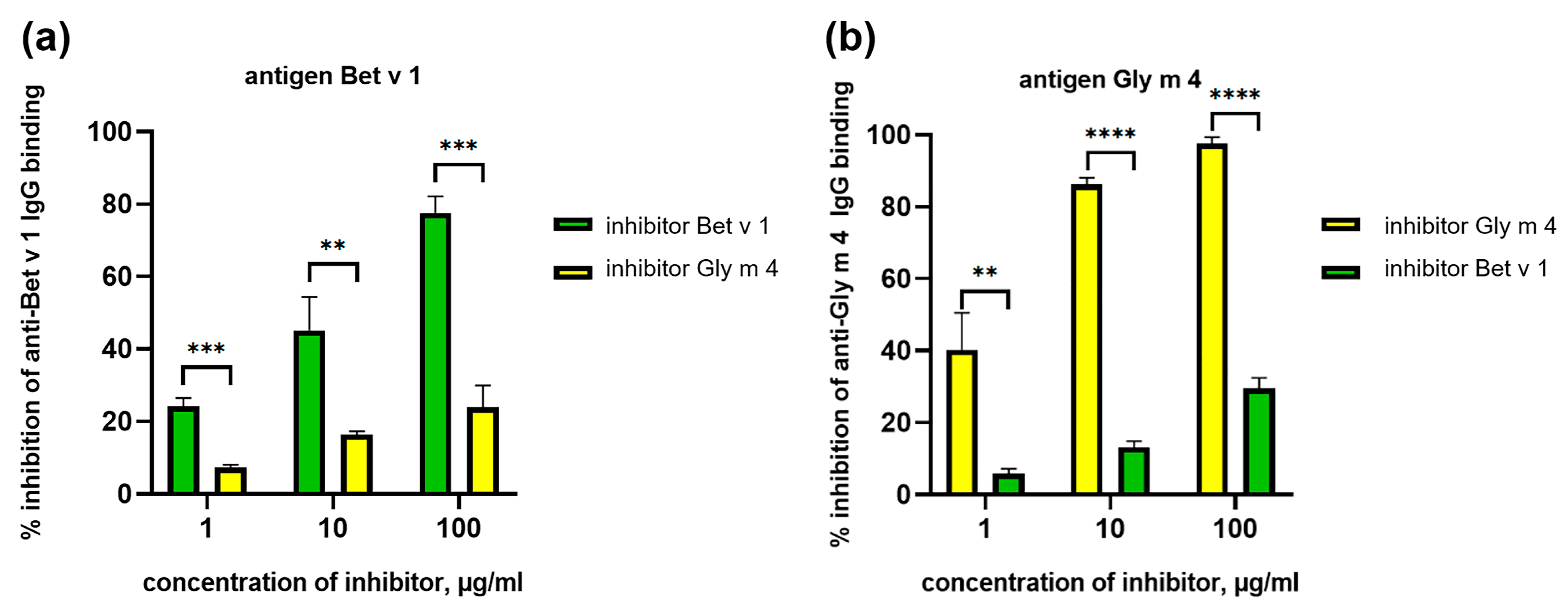
2.4. Cross-Reactivity of Animal Polyclonal Anti-Bet v 1 and Anti-Gly m 4 IgG
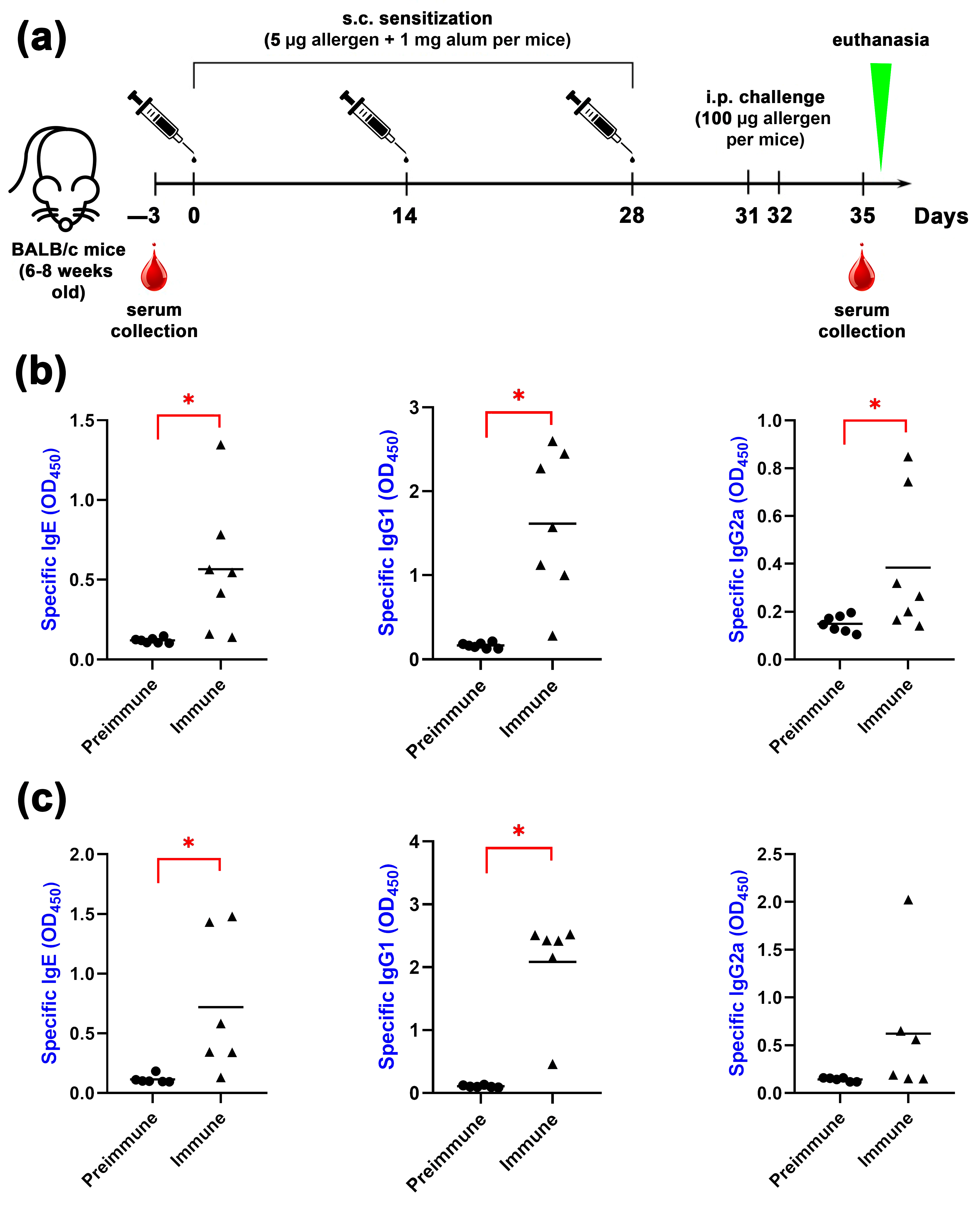
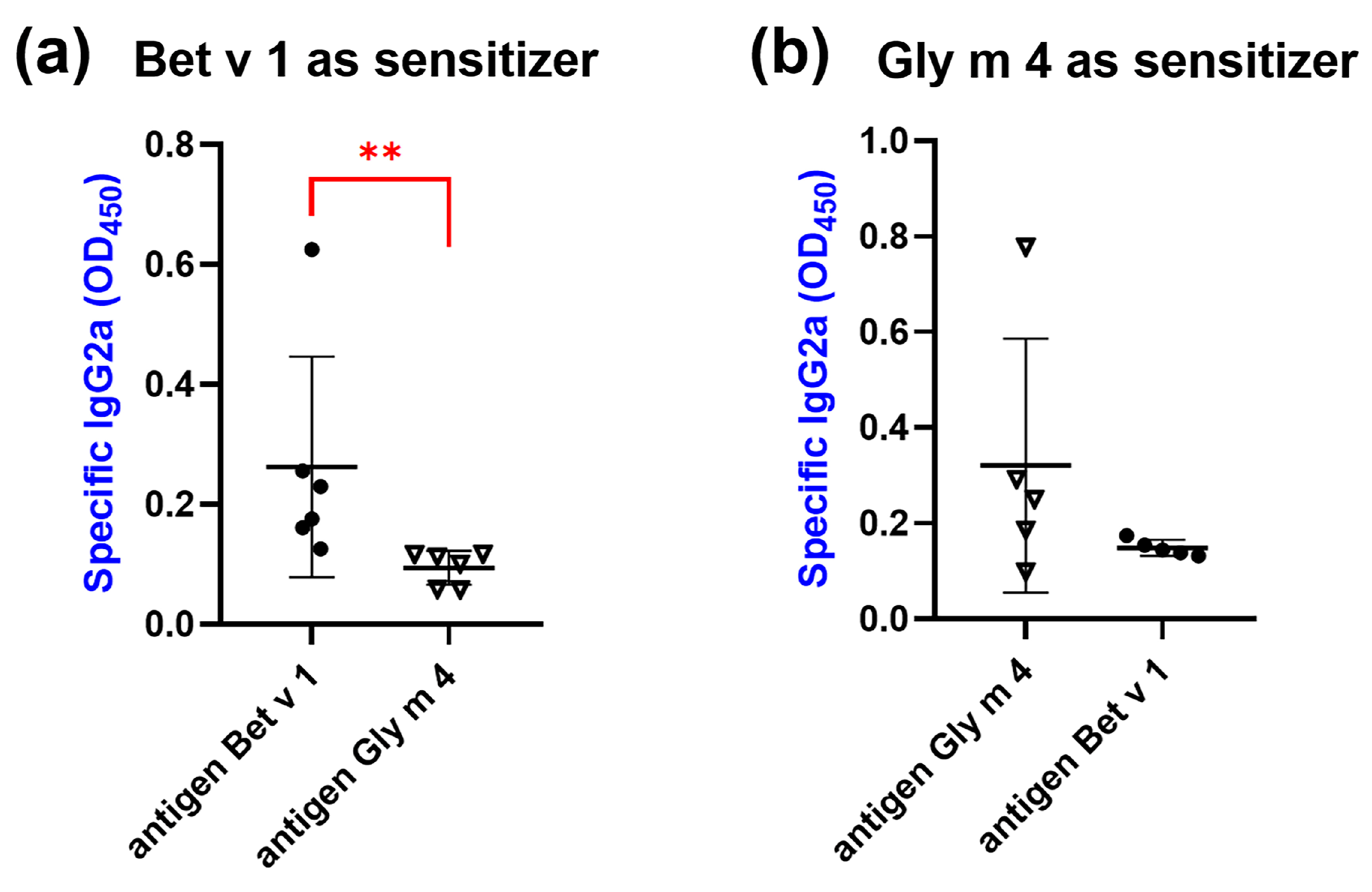
2.5. Animal Sensitization by Bet v 1 or Gly m 4
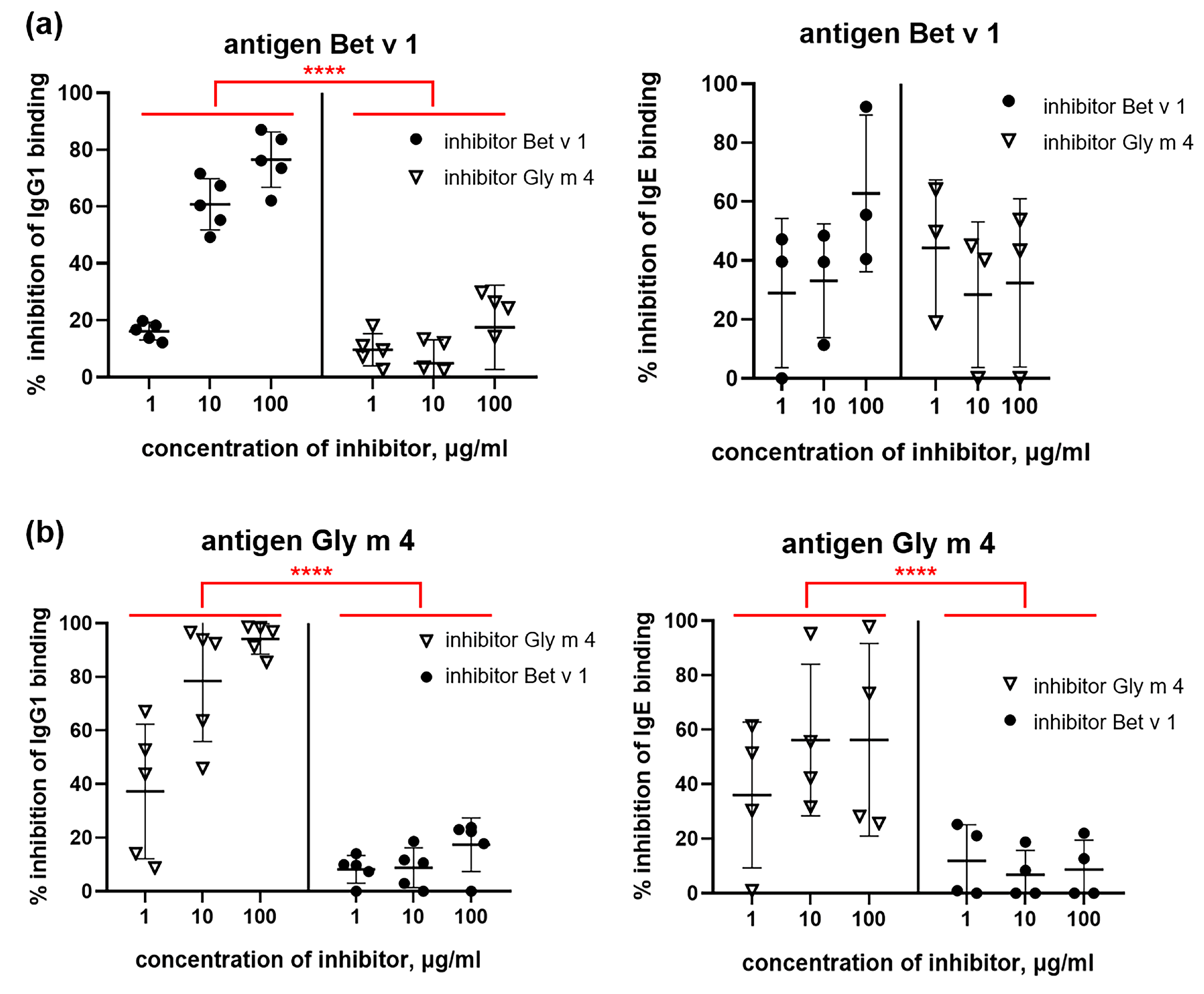
2.6. Cross-Reactivity of IgG1, IgE and IgG2a from Sera of Sensitized Mice
3. Materials and Methods
3.1. Materials
3.2. Recombinant Production of Allergens
3.3. Ligand Binding Assay
3.4. Stimulation of Caco-2 Cells
3.5. RNA Extraction
3.6. qPCR
3.7. Mice Models of Allergic Sensitization by Bet v 1 and Gly m 4
3.8. Immunoglobulin Binding Assay
3.9. ELISA Inhibition Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akdis, C.A.; Agache, I. Vision and roadmap to fight with allergies. In EAACI Global Atlas of Allergy; European Academy of Allergy and Clinical Immunology: Zurich, Switzerland, 2014; pp. 385–388. [Google Scholar] [CrossRef]
- McKenna, O.E.; Asam, C.; Araujo, G.R.; Roulias, A.; Goulart, L.R.; Ferreira, F. How relevant is panallergen sensitization in the development of allergies? Pediatr. Allergy Immunol. 2016, 27, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Plant Pathogenesis-Related Proteins PR-10 and PR-14 as Components of Innate Immunity System and Ubiquitous Allergens. Curr. Med. Chem. 2017, 24, 1772–1787. [Google Scholar] [CrossRef]
- Polak, D.; Vollmann, U.; Grilo, J.; Bogdanov, I.V.; Aglas, L.; Ovchinnikova, T.V.; Ferreira, F.; Bohle, B. Bet v 1-independent sensitization to major allergens in Fagales pollen: Evidence at the T-cell level. Allergy 2023, 78, 743–751. [Google Scholar] [CrossRef]
- Husslik, F.; Nürnberg, J.; von Loetzen, C.S.; Mews, T.; Ballmer-Weber, B.K.; Kleine-Tebbe, J.; Treudler, R.; Simon, J.; Randow, S.; Völker, E.; et al. The conformational IgE epitope profile of soya bean allergen Gly m 4. Clin. Exp. Allergy 2016, 46, 1484–1497. [Google Scholar] [CrossRef]
- Kleine-Tebbe, J.; Vogel, L.; Crowell, D.N.; Haustein, U.F.; Vieths, S. Severe oral allergy syndrome and anaphylactic reactions caused by a Bet v 1- related PR-10 protein in soybean, SAM22. J. Allergy Clin. Immunol. 2002, 110, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Berneder, M.; Bublin, M.; Hoffmann-Sommergruber, K.; Hawranek, T.; Lang, R. Allergen chip diagnosis for soy-allergic patients: Gly m 4 as a marker for severe food-allergic reactions to soy. Int. Arch. Allergy Immunol. 2013, 161, 229–233. [Google Scholar] [CrossRef]
- Grilo, J.R.; Kitzmüller, C.; Aglas, L.; Acosta, G.S.; Vollmann, U.; Ebner, C.; Horak, F.; Kinaciyan, T.; Radauer, C.; Ferreira, F.; et al. IgE-cross-blocking antibodies to Fagales following sublingual immunotherapy with recombinant Bet v 1. Allergy 2021, 76, 2555–2564. [Google Scholar] [CrossRef]
- Bohle, B.; Radakovics, A.; Lüttkopf, D.; Jahn-Schmid, B.; Vieths, S.; Ebner, C. Characterization of the T cell response to the major hazelnut allergen, Cor a 1.04: Evidence for a relevant T cell epitope not cross-reactive with homologous pollen allergens. Clin. Exp. Allergy 2005, 35, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Kinaciyan, T.; Jahn-Schmid, B.; Radakovics, A.; Zwölfer, B.; Schreiber, C.; Francis, J.N.; Ebner, C.; Bohle, B. Successful sublingual immunotherapy with birch pollen has limited effects on concomitant food allergy to apple and the immune response to the Bet v 1 homolog Mal d 1. J. Allergy Clin. Immunol. 2007, 119, 937–943. [Google Scholar] [CrossRef]
- Treudler, R.; Franke, A.; Schmiedeknecht, A.; Ballmer-Weber, B.; Worm, M.; Werfel, T.; Jappe, U.; Biedermann, T.; Schmitt, J.; Brehler, R.; et al. BASALIT trial: Double-blind placebo-controlled allergen immuno-therapy with rBet v 1-FV in birch-related soya allergy. Allergy 2017, 72, 1243–1253. [Google Scholar] [CrossRef]
- Asam, C.; Batista, A.L.; Moraes, A.H.; de Paula, V.S.; Almeida, F.C.L.; Aglas, L.; Kitzmüller, C.; Bohle, B.; Ebner, C.; Ferreira, F.; et al. Bet v 1-a Trojan horse for small ligands boosting allergic sensitization? Clin. Exp. Allergy 2014, 44, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, A. The HDM allergen orchestra and its cysteine protease maestro: Stimulators of kaleidoscopic innate immune responses. Mol. Immunol. 2023, 156, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ignatova, A.A.; Ovchinnikova, T.V. Do lipids influence gastrointestinal processing: A case study of major soybean allergen Gly m 4. Membranes 2021, 11, 754. [Google Scholar] [CrossRef] [PubMed]
- Šarenac, T.M.; Mikov, M. Bile Acid Synthesis: From Nature to the Chemical Modification and Synthesis and Their Applications as Drugs and Nutrients. Front. Pharmacol. 2018, 9, 39. [Google Scholar] [CrossRef]
- Albrecht, M.; Garn, H.; Buhl, T. Epithelial-immune cell interactions in allergic diseases. Eur. J. Immunol. 2024, 54, e2249982. [Google Scholar] [CrossRef]
- Melnikova, D.N.; Finkina, E.I.; Potapov, A.E.; Danilova, Y.D.; Toropygin, I.Y.; Matveevskaya, N.S.; Ovchinnikova, T.V.; Bogdanov, I.V. Structural and Immunological Features of PR-10 Allergens: Focusing on the Major Alder Pollen Allergen Aln g 1. Int. J. Mol. Sci. 2024, 25, 4965. [Google Scholar] [CrossRef]
- Finkina, E.I.; Bogdanov, I.V.; Ziganshin, R.H.; Strokach, N.N.; Melnikova, D.N.; Toropygin, I.Y.; Matveevskaya, N.S.; Ovchinnikova, T.V. Structural and Immunologic Properties of the Major Soybean Allergen Gly m 4 Causing Anaphylaxis. Int. J. Mol. Sci. 2022, 23, 15386. [Google Scholar] [CrossRef]
- Tordesillas, L.; Gómez-Casado, C.; Garrido-Arandia, M.; Murua-García, A.; Palacín, A.; Varela, J.; Konieczna, P.; Cuesta-Herranz, J.; Akdis, C.A.; O’Mahony, L.; et al. Transport of Pru p 3 across gastrointestinal epithelium—An essential step towards the induction of food allergy? Clin. Exp. Allergy 2013, 43, 1374–1383. [Google Scholar] [CrossRef]
- Bogdanov, I.V.; Finkina, E.I.; Melnikova, D.N.; Ziganshin, R.H.; Ovchinnikova, T.V. Investigation of sensitization potential of the soybean allergen Gly m 4 by using Caco-2/immune cells co-culture model. Nutrients 2021, 13, 2058. [Google Scholar] [CrossRef]
- Mandanas, M.V.; Barrett, N.A. Epithelial sensing in allergic disease. Curr. Opin. Immunol. 2024, 91, 102490. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Won, T.H.; Li, T.T.; Yano, H.; Digumarthi, S.; Heras, A.F.; Zhang, W.; Parkhurst, C.N.; Kashyap, S.; Jin, W.B.; et al. Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation. Nature 2022, 611, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Briceno Noriega, D.; Savelkoul, H.F.J.; Jansen, A.; Teodorowicz, M.; Ruinemans-Koerts, J. Pollen Sensitization Can Increase the Allergic Reaction to Non-Cross-Reactive Allergens in a Soy-Allergic Patient. Int. J. Environ. Res. Public Health 2023, 20, 6045. [Google Scholar] [CrossRef]
- Fukutomi, Y.; Sjölander, S.; Nakazawa, T.; Borres, M.P.; Ishii, T.; Nakayama, S.; Tanaka, A.; Taniguchi, M.; Saito, A.; Yasueda, H.; et al. Clinical relevance of IgE to recombinant Gly m 4 in the diagnosis of adult soybean allergy. J. Allergy Clin. Immunol. 2012, 129, 860–863.e3. [Google Scholar] [CrossRef]
- Schülke, S.; Albrecht, M. Mouse Models for Food Allergies: Where Do We Stand? Cells 2019, 8, 546. [Google Scholar] [CrossRef]
- Pichler, U.; Asam, C.; Weiss, R.; Isakovic, A.; Hauser, M.; Briza, P.; Ferreira, F.; Wallner, M. The fold variant BM4 is beneficial in a therapeutic Bet v 1 mouse model. Biomed. Res. Int. 2013, 2013, 832404. [Google Scholar] [CrossRef]
- Adel-Patient, K.; Créminon, C.; Bernard, H.; Clément, G.; Négroni, L.; Frobert, Y.; Grassi, J.; Wal, J.M.; Chatel, J.M. Evaluation of a high IgE-responder mouse model of allergy to bovine beta-lactoglobulin (BLG): Development of sandwich immunoassays for total and allergen-specific IgE, IgG1 and IgG2a in BLG-sensitized mice. J. Immunol. Methods 2000, 235, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Bidad, K.; Nicknam, M.H.; Farid, R. A review of allergy and allergen specific immunotherapy. Iran. J. Allergy Asthma Immunol. 2011, 10, 1–9. [Google Scholar]
- Delayre-Orthez, C.; Becker, J.; de Blay, F.; Frossard, N.; Pons, F. Exposure to endotoxins during sensitization prevents further endotoxin-induced exacerbation of airway inflammation in a mouse model of allergic asthma. Int. Arch. Allergy Immunol. 2005, 138, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Mamat, U.; Wilke, K.; Bramhill, D.; Schromm, A.B.; Lindner, B.; Kohl, T.A.; Corchero, J.L.; Villaverde, A.; Schaffer, L.; Head, S.R.; et al. Detoxifying Escherichia coli for endotoxin-free production of recombinant proteins. Microb. Cell Factories 2015, 14, 57. [Google Scholar] [CrossRef]
- Gizzarelli, F.; Corinti, S.; Barletta, B.; Iacovacci, P.; Brunetto, B.; Butteroni, C.; Afferni, C.; Onori, R.; Miraglia, M.; Panzini, G.; et al. Evaluation of allergenicity of genetically modified soybean protein extract in a murine model of oral allergen-specific sensitization. Clin. Exp. Allergy 2006, 36, 238–248. [Google Scholar] [CrossRef]
- Murakami, H.; Ogawa, T.; Takafuta, A.; Yano, E.; Zaima, N.; Moriyama, T. Identification of the 7S and 11S globulins as percutaneously sensitizing soybean allergens as demonstrated through epidermal application of crude soybean extract. Biosci. Biotechnol. Biochem. 2018, 82, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Demir, H.; Radauer, C.; Strobl, M.R.; Scheurer, S.; Kinaciyan, T.; Bohle, B. Cross-protection of AIT-induced antibodies to related allergens requires a high degree of structural identity. Allergy 2024, 80, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Bahri, R.; Eggel, A.; Sabato, V.; Tontini, C.; Elst, J. Flow cytometry-based basophil and mast cell activation tests in allergology: State of the art. J. Allergy Clin. Immunol. 2025, 155, 286–297. [Google Scholar] [CrossRef]
- Beisser, P.S.; Laurent, L.; Virelizier, J.-L.; Michelson, S. Human cytomegalovirus chemokine receptor gene US28 is transcribed in latently infected THP-1 monocytes. J. Virol. 2001, 75, 5949–5957. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.C.; Friderici, K.H. A Novel Actin mRNA Splice Variant Regulates ACTG1 Expression. PLoS Genet. 2013, 9, e1003743. [Google Scholar] [CrossRef]
- Guo, P.F.; Du, M.R.; Wu, H.X.; Lin, Y.; Jin, L.P.; Li, D.J. Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory TH2 bias in the decidua during early gestation in humans. Blood 2010, 116, 2061–2069. [Google Scholar] [CrossRef]
- Pastorelli, L.; Garg, R.R.; Hoang, S.B.; Spina, L.; Mattioli, B.; Scarpa, M.; Fiocchi, C.; Vecchi, M.; Pizarro, T.T. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc. Natl. Acad. Sci. USA 2010, 107, 8017–8022. [Google Scholar] [CrossRef]
- Untergasser, A.; Ruijter, J.M.; Benes, V.; van den Hoff, M.J.B. Web-based LinRegPCR: Application for the Visualization and Analysis of (RT)-qPCR Amplification and Melting Data. BMC Bioinform. 2021, 22, 398. [Google Scholar] [CrossRef]

| No. | Age (y.o.) | Sex (M/F) | sIgE to Birch | sIgE to Soybean | Another Plant Allergen Source | ||
|---|---|---|---|---|---|---|---|
| IU/mL | RAST Class | IU/mL | RAST Class | ||||
| 1 | 4 | M | >100 | 6 | 0.57 | 1.6 | H, O, hz, pn, wn, po, cl, ct, wt, r, ss |
| 2 | 31 | M | >100 | 6 | 0.4 | 1.1 | H, O, A, hz, pn, po, wn, cl, ct, wt, r, ss |
| 3 | 5 | M | >100 | 6 | 0.34 | 0.9 | H, O, A, hz |
| 4 | 6 | M | >100 | 6 | 0.05 | 0.1 | G, ct, po, wt, hz, pn |
| 5 | 60 | F | >100 | 6 | - | - | A, H, hz, r, pn |
| 6 | 4 | M | >100 | 6 | nd | nd | A, H |
| 7 | 3 | F | >100 | 6 | nd | nd | A, H |
| 8 | 9 | M | >100 | 6 | nd | nd | A, H, G, r |
| 9 | 4 | М | >100 | 6 | - | - | G, ct, po, wt, hz, pn |
| 10 | 10 | F | >100 | 6 | nd | nd | A, H, O, G, r |
| 11 | 6 | F | >100 | 6 | - | - | A, H, G, pn, hz, ct, wt, r |
| 12 | 6 | F | >100 | 6 | nd | nd | A, H, G, r |
| 13 | 14 | F | >100 | 6 | nd | nd | A, H, O, G |
| 14 | 3 | М | 13.44 | 3.7 | - | - | wt, hz, pn |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finkina, E.I.; Danilova, Y.D.; Melnikova, D.N.; Ovchinnikova, T.V.; Bogdanov, I.V. Sensitization Potential of the Major Soybean Allergen Gly m 4 and Its Cross-Reactivity with the Birch Pollen Allergen Bet v 1. Int. J. Mol. Sci. 2025, 26, 2932. https://doi.org/10.3390/ijms26072932
Finkina EI, Danilova YD, Melnikova DN, Ovchinnikova TV, Bogdanov IV. Sensitization Potential of the Major Soybean Allergen Gly m 4 and Its Cross-Reactivity with the Birch Pollen Allergen Bet v 1. International Journal of Molecular Sciences. 2025; 26(7):2932. https://doi.org/10.3390/ijms26072932
Chicago/Turabian StyleFinkina, Ekaterina I., Yulia D. Danilova, Daria N. Melnikova, Tatiana V. Ovchinnikova, and Ivan V. Bogdanov. 2025. "Sensitization Potential of the Major Soybean Allergen Gly m 4 and Its Cross-Reactivity with the Birch Pollen Allergen Bet v 1" International Journal of Molecular Sciences 26, no. 7: 2932. https://doi.org/10.3390/ijms26072932
APA StyleFinkina, E. I., Danilova, Y. D., Melnikova, D. N., Ovchinnikova, T. V., & Bogdanov, I. V. (2025). Sensitization Potential of the Major Soybean Allergen Gly m 4 and Its Cross-Reactivity with the Birch Pollen Allergen Bet v 1. International Journal of Molecular Sciences, 26(7), 2932. https://doi.org/10.3390/ijms26072932









