Virtual Screening and Molecular Dynamics of Cytokine–Drug Complexes for Atherosclerosis Therapy
Abstract
1. Introduction
2. Results
2.1. Structural Analysis of TNF-α, Il-1β and IFN-γ
2.2. Virtual Screening and Molecular Docking of Active Compounds
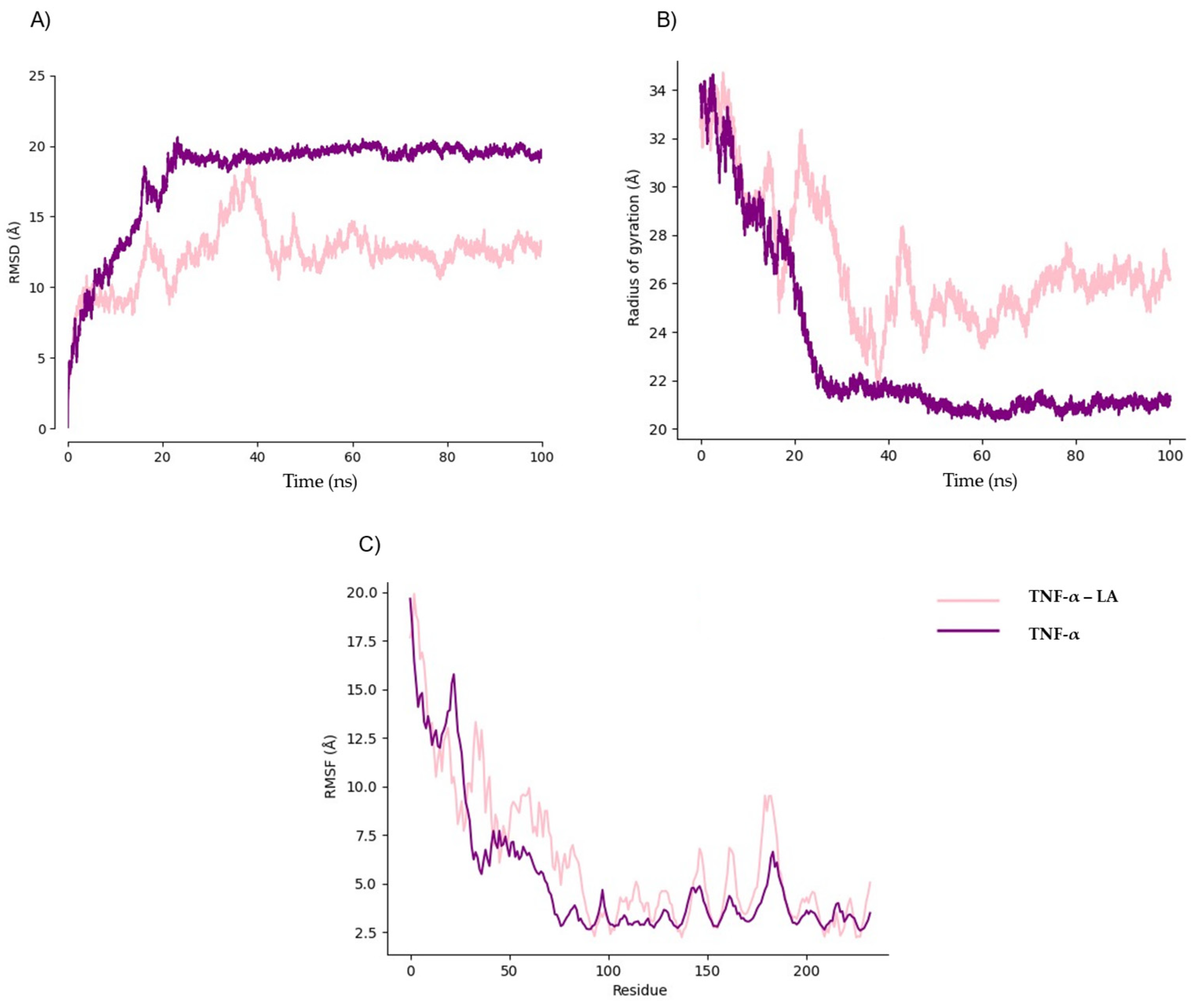
2.3. Pharmacokinetic Predicted Profiles of Selected Ligands and Molecular Dynamics Simulations
3. Discussion
4. Materials and Methods
4.1. Ligand Preparation
4.2. Protein Selection for Ligand Docking
4.3. Pharmacokinetic and Drug-Likeness Prediction
4.4. Molecular Dynamics Simulations
4.5. Protein Flexibility Predictions
4.6. Principal Component Analysis (PCA)
4.7. Dynamic Cross-Correlation Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNF-α | Tumor necrosis factor-α |
| IL-1β | Interleukin-1β |
| IFN-γ | Interferon-γ |
| mRNA | Messenger RNA |
| AI | Artificial intelligence |
| FDA | Food and Drug Administration |
| pLDDT | predicted local distance difference test |
| DHA | docosahexaenoic acid |
| ns | Nanosecond |
| MD | Molecular dynamics |
| atm | Atmospheres |
| PCA | Principal Component Analysis |
References
- Frantz, S.; Ertl, G.; Bauersachs, J. Mechanisms of Disease: Toll-like Receptors in Cardiovascular Disease. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Zayeri, F.; Salehi, M. Trend Analysis of Cardiovascular Disease Mortality, Incidence, and Mortality-to-Incidence Ratio: Results from Global Burden of Disease Study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef]
- Di Cesare, M.; McGhie, D.V.; Perel, P.; Mwangi, J.; Taylor, S.; Pervan, B.; Kabudula, C.; Narula, J.; Bixby, H.; Pineiro, D.; et al. The Heart of the World. Glob. Heart 2024, 19, 11. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Björkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent Developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Pagler, T.A.; Wang, M.; Mondal, M.; Murphy, A.J.; Westerterp, M.; Moore, K.J.; Maxfield, F.R.; Tall, A.R. Deletion of ABCA1 and ABCG1 Impairs Macrophage Migration Because of Increased Rac1 Signaling. Circ. Res. 2011, 108, 194–200. [Google Scholar] [CrossRef]
- Wang, T.; Palucci, D.; Law, K.; Yanagawa, B.; Yam, J.; Butany, J. Atherosclerosis: Pathogenesis and Pathology. Diagn. Histopathol. 2012, 18, 461–467. [Google Scholar] [CrossRef]
- Chen, X.; Deng, Z.; Feng, J.; Chang, Q.; Lu, F.; Yuan, Y. Necroptosis in Macrophage Foam Cells Promotes Fat Graft Fibrosis in Mice. Front. Cell Dev. Biol. 2021, 9, 651360. [Google Scholar] [CrossRef]
- Javadifar, A.; Rastgoo, S.; Banach, M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. Foam Cells as Therapeutic Targets in Atherosclerosis with a Focus on the Regulatory Roles of Non-Coding Rnas. Int. J. Mol. Sci. 2021, 22, 2529. [Google Scholar] [CrossRef]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF Therapy: Past, Present and Future. Int. Immunol. 2015, 27, 55–62. [Google Scholar] [CrossRef]
- Dinarello, C.A. Historical Insights into Cytokines. Eur. J. Immunol. 2007, 37, S34–S45. [Google Scholar] [CrossRef]
- Ma, J.; Luo, J.; Sun, Y.; Zhao, Z. Cytokines Associated with Immune Response in Atherosclerosis. Am. J. Transl. Res. 2022, 14, 6424. [Google Scholar] [PubMed]
- Tabas, I. Consequences and Therapeutic Implications of Macrophage Apoptosis in Atherosclerosis: The Importance of Lesion Stage and Phagocytic Efficiency. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2255–2264. [Google Scholar] [PubMed]
- Ruuls, S.R.; Sedgwick, J.D. Unlinking Tumor Necrosis Factor Biology from the Major Histocompatibility Complex: Lessons from Human Genetics and Animal Models. Am. J. Hum. Genet. 1999, 65, 294–301. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Buckley, C.D.; Isaacs, J.D. Cytokines in Rheumatoid Arthritis—Shaping the Immunological Landscape. Nat. Rev. Rheumatol. 2016, 12, 63–68. [Google Scholar] [CrossRef]
- Sartor, R.B. Mechanisms of Disease: Pathogenesis of Crohn’s Disease and Ulcerative Colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef]
- Sands, B.E.; Kaplan, G.G. The Role of TNFα in Ulcerative Colitis. J. Clin. Pharmacol. 2007, 47, 930–941. [Google Scholar] [CrossRef]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF Receptor Superfamilies. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef]
- Sack, M. Tumor Necrosis Factor in Myocardial Hypertrophy and Ischaemia—An Anti-Apoptotic Perspective. Cardiovasc. Res. 2000, 45, 688–695. [Google Scholar] [CrossRef]
- Zia, K.; Ashraf, S.; Jabeen, A.; Saeed, M.; Nur-e-Alam, M.; Ahmed, S.; Al-Rehaily, A.J.; Ul-Haq, Z. Identification of Potential TNF-α Inhibitors: From in Silico to in Vitro Studies. Sci. Rep. 2020, 10, 20974. [Google Scholar] [CrossRef]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Braddock, M.; Quinn, A. Targeting IL-1 in Inflammatory Disease: New Opportunities for Therapeutic Intervention. Nat. Rev. Drug Discov. 2004, 3, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Dewberry, R.; Holden, H.; Crossman, D.; Francis, S. Interleukin-1 Receptor Antagonist Expression in Human Endothelial Cells and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yin, Y.; Zhou, Z.; He, M.; Dai, Y. OxLDL-Induced IL-1beta Secretion Promoting Foam Cells Formation Was Mainly via CD36 Mediated ROS Production Leading to NLRP3 Inflammasome Activation. Inflamm. Res. 2014, 63, 33–43. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.E.; Ramji, D.P. Interferon Gamma: A Master Regulator of Atherosclerosis. Cytokine Growth Factor. Rev. 2009, 20, 125–135. [Google Scholar] [CrossRef]
- Whitman, S.C.; Ravisankar, P.; Elam, H.; Daugherty, A. Exogenous Interferon-γ Enhances Atherosclerosis in Apolipoprotein E−/− Mice. Am. J. Pathol. 2000, 157, 1819–1824. [Google Scholar] [CrossRef]
- Inagaki, Y.; Yamagishi, S.; Amano, S.; Okamoto, T.; Koga, K.; Makita, Z. Interferon-γ-Induced Apoptosis and Activation of THP-1 Macrophages. Life Sci. 2002, 71, 2499–2508. [Google Scholar] [CrossRef]
- Chaffey, L.; Roberti, A.; Greaves, D.R. Drug Repurposing in Cardiovascular Inflammation: Successes, Failures, and Future Opportunities. Front. Pharmacol. 2022, 13, 1046406. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- van Breugel, M.; Rosa e Silva, I.; Andreeva, A. Structural Validation and Assessment of AlphaFold2 Predictions for Centrosomal and Centriolar Proteins and Their Complexes. Commun. Biol. 2022, 5, 312. [Google Scholar] [CrossRef]
- Eck, M.J.; Sprang, S.R. The Structure of Tumor Necrosis Factor-α at 2.6 Å Resolution. J. Biol. Chem. 1989, 264, 17595–17605. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.T.P.; Le, M.D.; Trinh, H.X.; Nong, H.V. In Silico Studies for the Interaction of Tumor Necrosis Factor-Alpha (TNF-α) with Different Saponins from Vietnamese Ginseng (Panax vietnamesis). Biophys. Physicobiol 2016, 13, 173–180. [Google Scholar] [CrossRef]
- Jameei, A.; Nagarajan, D.; Sarikhani, M.; Chandra, N.; Karande, A.A. Development and Characterization of a Potent Tumor Necrosis Factor-Alpha-Blocking Agent. Monoclon. Antib. Immunodiagn. Immunother. 2019, 38, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Wróblewski, K.; Kmiecik, S. Integrating AlphaFold PLDDT Scores into CABS-Flex for Enhanced Protein Flexibility Simulations. Comput. Struct. Biotechnol. J. 2024, 23, 4350–4356. [Google Scholar] [CrossRef]
- Dufour, A.; Bellac, C.L.; Eckhard, U.; Solis, N.; Klein, T.; Kappelhoff, R.; Fortelny, N.; Jobin, P.; Rozmus, J.; Mark, J.; et al. C-Terminal Truncation of IFN-γ Inhibits Proinflammatory Macrophage Responses and Is Deficient in Autoimmune Disease. Nat. Commun. 2018, 9, 2416. [Google Scholar] [CrossRef]
- Dinarello, C. Interleukin-1 and Interleukin-1 Antagonism. Blood 1991, 77, 1627–1652. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhang, T.; Zhang, S.; Tang, Q.; Yan, Y.; Feng, H. Integrated Bioinformatics and Validation Reveal IL1B and Its Related Molecules as Potential Biomarkers in Chronic Spontaneous Urticaria. Front. Immunol. 2022, 13, 850993. [Google Scholar] [CrossRef]
- Hailey, K.L.; Li, S.; Andersen, M.D.; Roy, M.; Woods, V.L.; Jennings, P.A. Pro-Interleukin (IL)-1β Shares a Core Region of Stability as Compared with Mature IL-1β While Maintaining a Distinctly Different Configurational Landscape: A Comparative Hydrogen/Deuterium Exchange Mass Spectrometry Study. J. Biol. Chem. 2009, 284, 26137–26148. [Google Scholar] [CrossRef]
- Torres, P.H.M.; Sodero, A.C.R.; Jofily, P.; Silva-Jr, F.P. Key Topics in Molecular Docking for Drug Design. Int. J. Mol. Sci. 2019, 20, 4574. [Google Scholar] [CrossRef]
- Ramji, D.P. Polyunsaturated Fatty Acids and Atherosclerosis: Insights from Pre-Clinical Studies. Eur. J. Lipid Sci. Technol. 2019, 121, 1800029. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain ω-3 Fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Perez-Martinez, P.; Garcia-Rios, A.; Delgado-Lista, J.; Perez-Jimenez, F.; Lopez-Miranda, J. Mediterranean Diet Rich in Olive Oil and Obesity, Metabolic Syndrome and Diabetes Mellitus. Curr. Pharm. Des. 2011, 17, 769–777. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Hollander, D.; Laung Chow, S.; Dadufalza, V.D. Intestinal Absorption of Free Oleic Acid in the Unanesthetized Rat: Evidence for a Saturable Component? Can. J. Physiol. Pharmacol. 1984, 62, 1136–1140. [Google Scholar] [CrossRef]
- Yao, H.T.; Chang, Y.W.; Lan, S.J.; Chen, C.T.; Hsu, J.T.A.; Yeh, T.K. The Inhibitory Effect of Polyunsaturated Fatty Acids on Human CYP Enzymes. Life Sci. 2006, 79, 2432–2440. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid Mediators in the Resolution of Inflammation. Cold Spring Harb. Perspect. Biol. 2015, 7, a016311. [Google Scholar] [CrossRef]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health Effects of Dietary Phospholipids. Lipids Health Dis. 2012, 11, 1–16. [Google Scholar] [CrossRef]
- Guixà-González, R.; Javanainen, M.; Gómez-Soler, M.; Cordobilla, B.; Domingo, J.C.; Sanz, F.; Pastor, M.; Ciruela, F.; Martinez-Seara, H.; Selent, J. Membrane Omega-3 Fatty Acids Modulate the Oligomerisation Kinetics of Adenosine A2A and Dopamine D2 Receptors. Sci. Rep. 2016, 6, 19839. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Zoubdane, N.; Heshmati, J.; Alami, M.; Berrougui, H.; Khalil, A. High-Density Lipoprotein Metabolism and Function in Cardiovascular Diseases: What about Aging and Diet Effects? Nutrients 2024, 16, 653. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.L.; Brook, R.D.; Rubenfire, M.; Eagle, K.A. Cardiovascular Impact of Nutritional Supplementation With Omega-3 Fatty Acids. J. Am. Coll. Cardiol. 2021, 77, 593–608. [Google Scholar] [CrossRef]
- Li, J.; Guasch-Ferré, M.; Li, Y.; Hu, F.B. Dietary Intake and Biomarkers of Linoleic Acid and Mortality: Systematic Review and Meta-Analysis of Prospective Cohort Studies. Am. J. Clin. Nutr. 2020, 112, 150–167. [Google Scholar] [CrossRef]
- Rodriguez, D.; Lavie, C.J.; Elagizi, A.; Milani, R.V. Update on Omega-3 Polyunsaturated Fatty Acids on Cardiovascular Health. Nutrients 2022, 14, 5146. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.S.; Martin, N.; Bridges, C.; Brainard, J.S.; Wang, X.; Brown, T.J.; Hanson, S.; Jimoh, O.F.; Ajabnoor, S.M.; Deane, K.H.O.; et al. Polyunsaturated Fatty Acids for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2018, 7, 41. [Google Scholar] [CrossRef]
- Walter, M.R.; Nagabhushan, T.L. Crystal Structure of Interleukin 10 Reveals an Interferon.Gamma.-like Fold. Biochemistry 1995, 34, 12118–12125. [Google Scholar] [CrossRef]
- Vandenbroeck, K.; Alloza, I.; Brehmer, D.; Billiau, A.; Proost, P.; McFerran, N.; Rüdiger, S.; Walker, B. The Conserved Helix C Region in the Superfamily of Interferon-γ/Interleukin-10-Related Cytokines Corresponds to a High-Affinity Binding Site for the HSP70 Chaperone DnaK. J. Biol. Chem. 2002, 277, 25668–25676. [Google Scholar] [CrossRef]
- Sanchez, H.P.; Tatarenko, K.; Nigen, M.; Pavlov, G.; Imberty, A.; Lortat-Jacob, H.; De La Torre, J.G.; Ebel, C. Organization of Human Interferon γ-Heparin Complexes from Solution Properties and Hydrodynamics. Biochemistry 2006, 45, 13227–13238. [Google Scholar] [CrossRef]
- Reinisch, W.; De Villiers, W.; Bene, L.; Simon, L.; Rácz, I.; Katz, S.; Altorjay, I.; Feagan, B.; Riff, D.; Bernstein, C.N.; et al. Fontolizumab in Moderate to Severe Crohn’s Disease: A Phase 2, Randomized, Double-Blind, Placebo-Controlled, Multiple-Dose Study. Inflamm. Bowel Dis. 2010, 16, 233–242. [Google Scholar] [CrossRef]
- Harden, J.L.; Johnson-Huang, L.M.; Chamian, M.F.; Lee, E.; Pearce, T.; Leonardi, C.L.; Haider, A.; Lowes, M.A.; Krueger, J.G. Humanized Anti-IFN-γ (HuZAF) in the Treatment of Psoriasis. J. Allergy Clin. Immunol. 2015, 135, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Hitoshi, K.; Kozo, H.; Shingu, T.; Yoshio, K.; Ohtani, H.; Okura, Y.; Tanaka, K.; Yasunobu, Y.; Nomura, K.; Kajiyama, G. Opposite Effects on Cholesterol Metabolism and Their Mechanisms Induced by Dietary Oleic Acid and Palmitic Acid in Hamsters. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1995, 1258, 251–256. [Google Scholar] [CrossRef]
- Sayols-Baixeras, S.; Irvin, M.R.; Elosua, R.; Arnett, D.K.; Aslibekyan, S.W. Epigenetics of Lipid Phenotypes. Curr. Cardiovasc. Risk Rep. 2016, 10, 31. [Google Scholar] [CrossRef]
- Turunen, M.P.; Aavik, E.; Ylä-Herttuala, S. Epigenetics and Atherosclerosis. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Lund, G.; Andersson, L.; Lauria, M.; Lindholm, M.; Fraga, M.F.; Villar-Garea, A.; Ballestar, E.; Esteller, M.; Zaina, S. DNA Methylation Polymorphisms Precede Any Histological Sign of Atherosclerosis in Mice Lacking Apolipoprotein E. J. Biol. Chem. 2004, 279, 29147–29154. [Google Scholar] [CrossRef]
- Silva-Martínez, G.A.; Rodríguez-Ríos, D.; Alvarado-Caudillo, Y.; Vaquero, A.; Esteller, M.; Carmona, F.J.; Moran, S.; Nielsen, F.C.; Wickström-Lindholm, M.; Wrobel, K.; et al. Arachidonic and Oleic Acid Exert Distinct Effects on the DNA Methylome. Epigenetics 2016, 11, 321–334. [Google Scholar] [CrossRef]
- Sąhin, A.; Derin, M.E.; Albayrak, F.; Karakaş, B.; Karagöz, Y. Assessment of Effectiveness of Anakinra and Canakinumab in Patients with Colchicine-Resistant/Unresponsive Familial Mediterranean Fever. Adv. Rheumatol. 2020, 60, 12. [Google Scholar] [CrossRef]
- Frostegård, J. Immunity, Atherosclerosis and Cardiovascular Disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Denis Alexander, H.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef]
- Balta, I.; Stef, L.; Pet, I.; Iancu, T.; Stef, D.; Corcionivoschi, N. Essential Fatty Acids as Biomedicines in Cardiac Health. Biomedicines 2021, 9, 1466. [Google Scholar] [CrossRef]
- Das, B.; Sarkar, C.; Rawat, V.S.; Kalita, D.; Deka, S.; Agnihotri, A. Promise of the Nlrp3 Inflammasome Inhibitors in in Vivo Disease Models. Molecules 2021, 26, 4996. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, N.; Pillinger, M.H.; Simon, L.S.; Lipsky, P.E. Interleukin-1β Inhibitors for the Management of Acute Gout Flares: A Systematic Literature Review. Arthritis Res. Ther. 2023, 25, 128. [Google Scholar] [CrossRef]
- Rivalta, I.; Lisi, G.P.; Snoeberger, N.S.; Manley, G.; Loria, J.P.; Batista, V.S. Allosteric Communication Disrupted by a Small Molecule Binding to the Imidazole Glycerol Phosphate Synthase Protein-Protein Interface. Biochemistry 2016, 55, 6484–6494. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Glassman, C.R.; Garcia, K.C. Emerging Principles of Cytokine Pharmacology and Therapeutics. Nat. Rev. Drug Discov. 2023, 22, 21–37. [Google Scholar] [CrossRef]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE). Chemical Computing Group ULC. 2025. Available online: https://www.chemcomp.com/en/Research-Citing_MOE.htm (accessed on 20 March 2025).
- Knox, C.; Wilson, M.; Klinger, C.M.; Franklin, M.; Oler, E.; Wilson, A.; Pon, A.; Cox, J.; Chin, N.E.; Strawbridge, S.A.; et al. DrugBank 6.0: The DrugBank Knowledgebase for 2024. Nucleic Acids Res 2024, 52, D1265–D1275. [Google Scholar] [CrossRef] [PubMed]
- Mariani, V.; Biasini, M.; Barbato, A.; Schwede, T. LDDT: A Local Superposition-Free Score for Comparing Protein Structures and Models Using Distance Difference Tests. Bioinformatics 2013, 29, 2722–2728. [Google Scholar] [CrossRef]
- Guo, H.-B.; Perminov, A.; Bekele, S.; Kedziora, G.; Farajollahi, S.; Varaljay, V.; Hinkle, K.; Molinero, V.; Meister, K.; Hung, C.; et al. AlphaFold2 Models Indicate That Protein Sequence Determines Both Structure and Dynamics. Sci. Rep. 2022, 12, 10696. [Google Scholar] [CrossRef]
- Arantes, P.R.; Polêto, M.D.; Pedebos, C.; Ligabue-Braun, R. Making It Rain: Cloud-Based Molecular Simulations for Everyone. J. Chem. Inf. Model. 2021, 61, 4852–4856. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Vander Meersche, Y.; Cretin, G.; de Brevern, A.G.; Gelly, J.-C.; Galochkina, T. MEDUSA: Prediction of Protein Flexibility from Sequence. J. Mol. Biol. 2021, 433, 166882. [Google Scholar] [CrossRef] [PubMed]
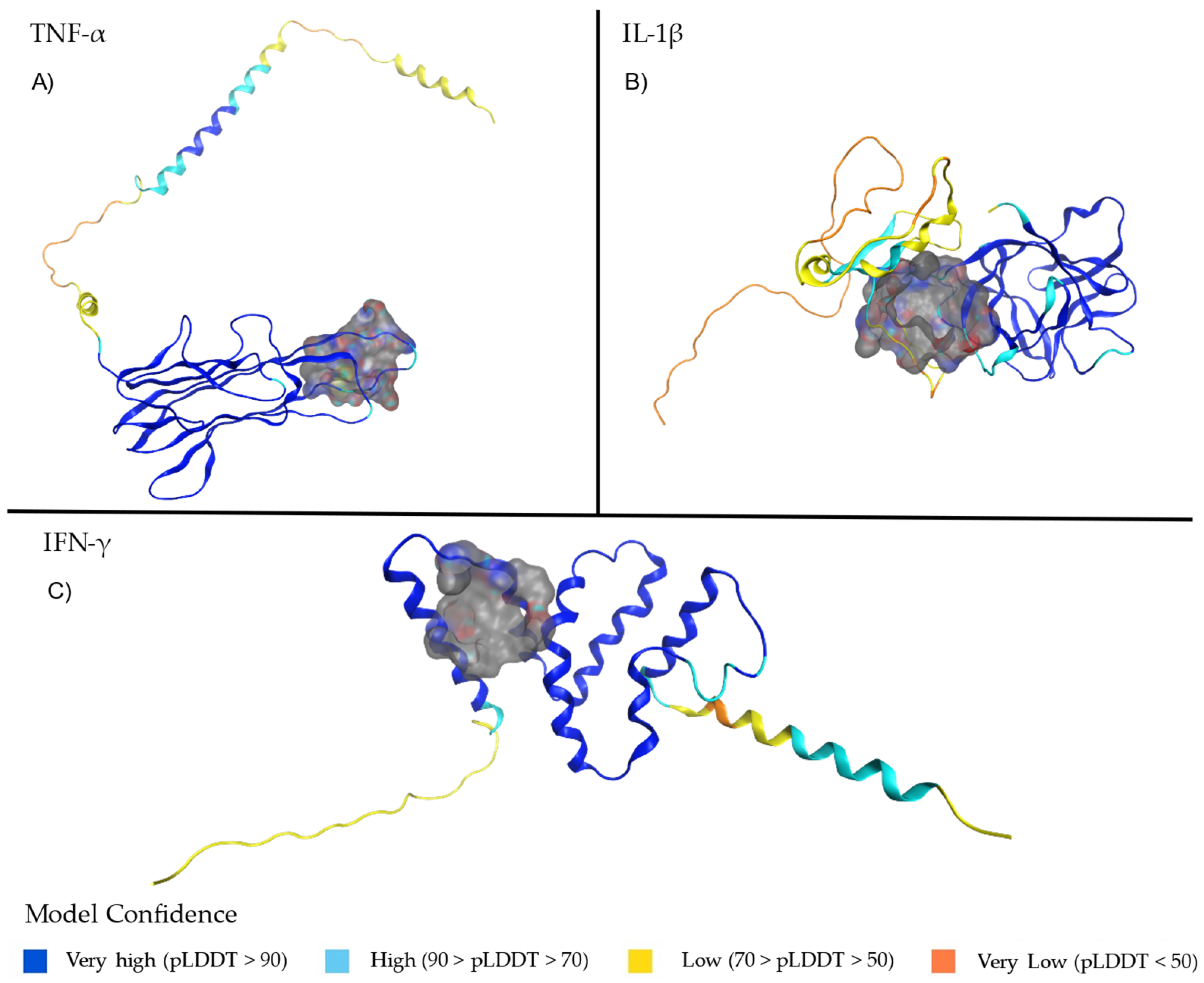
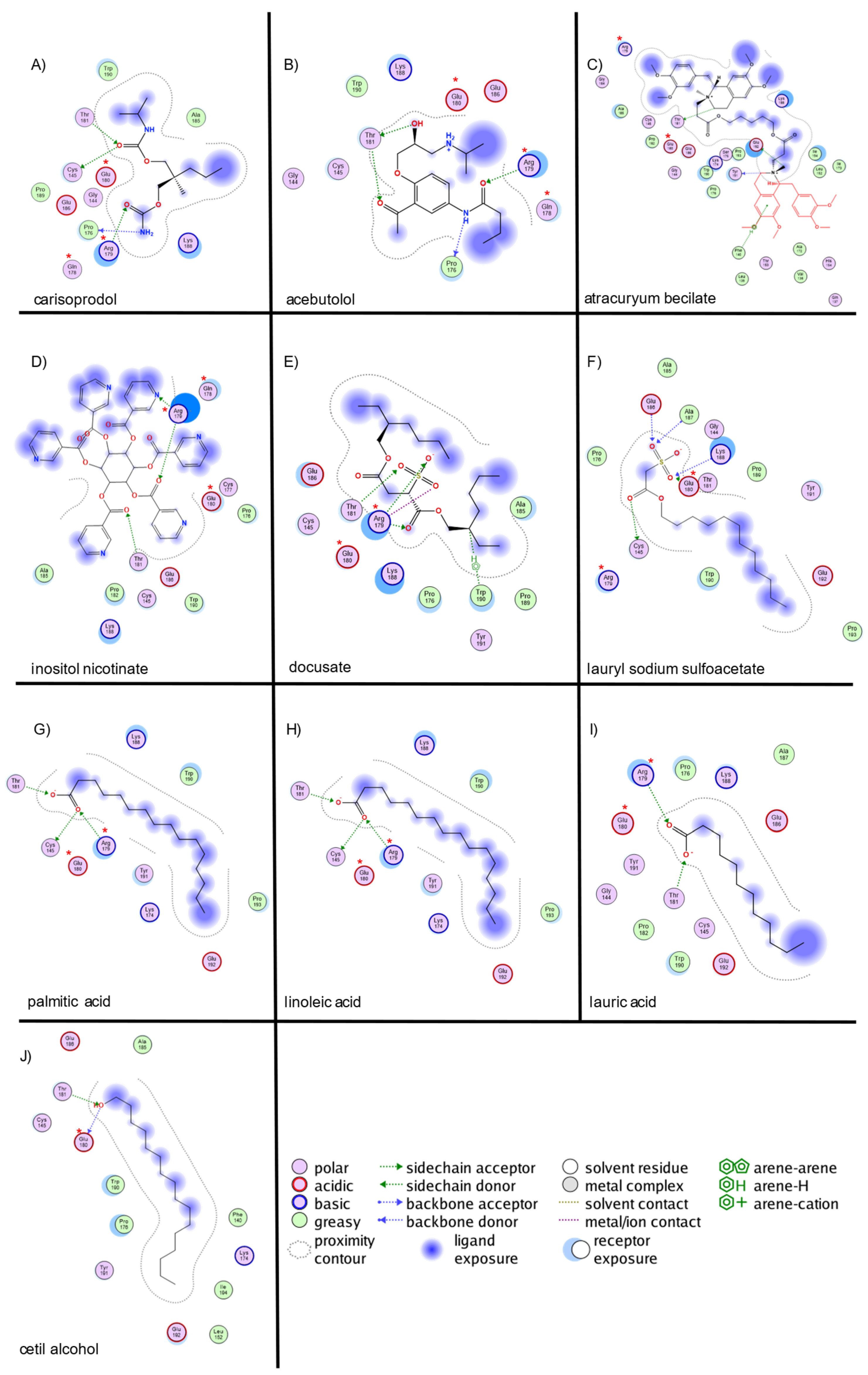

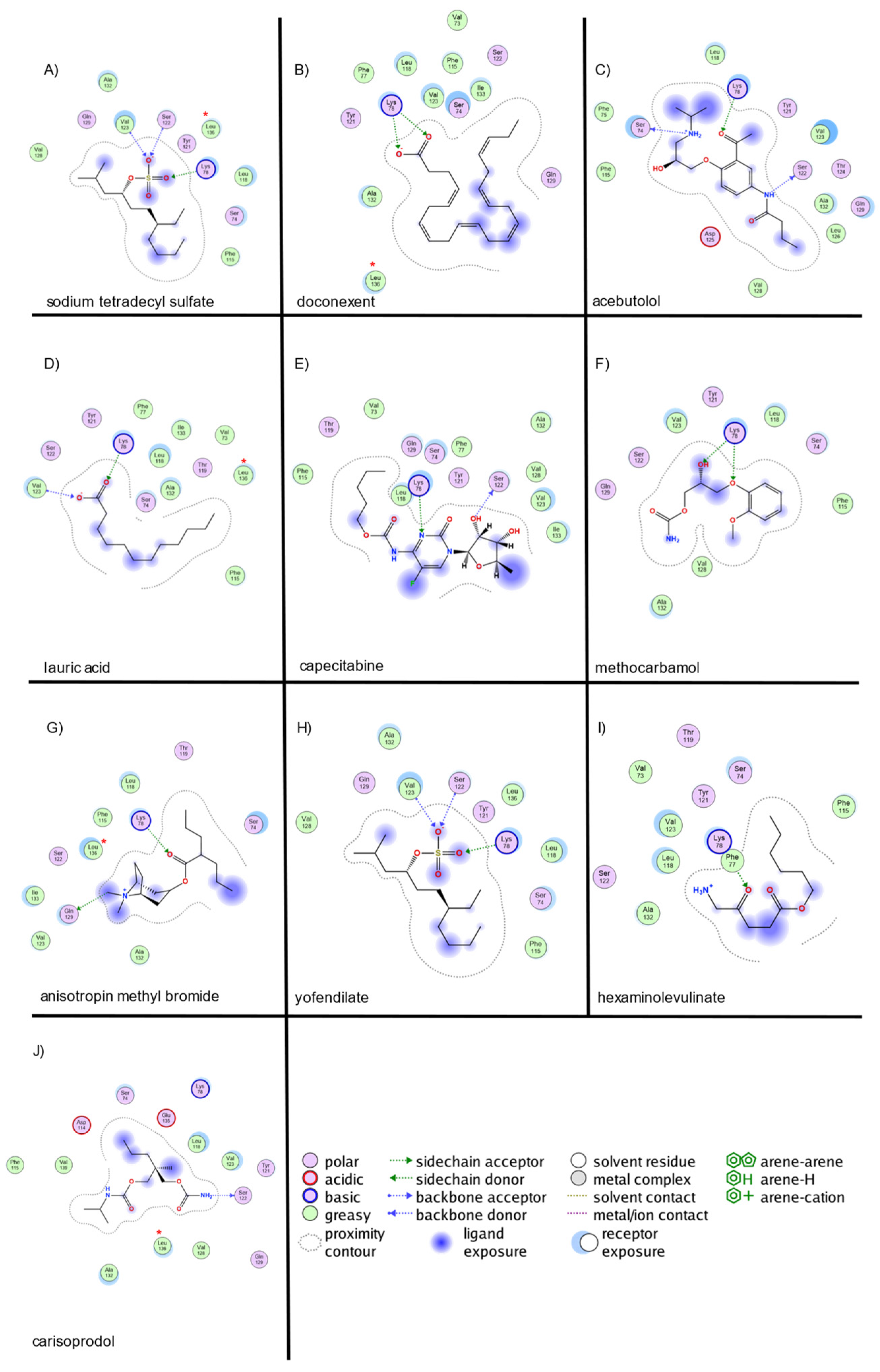
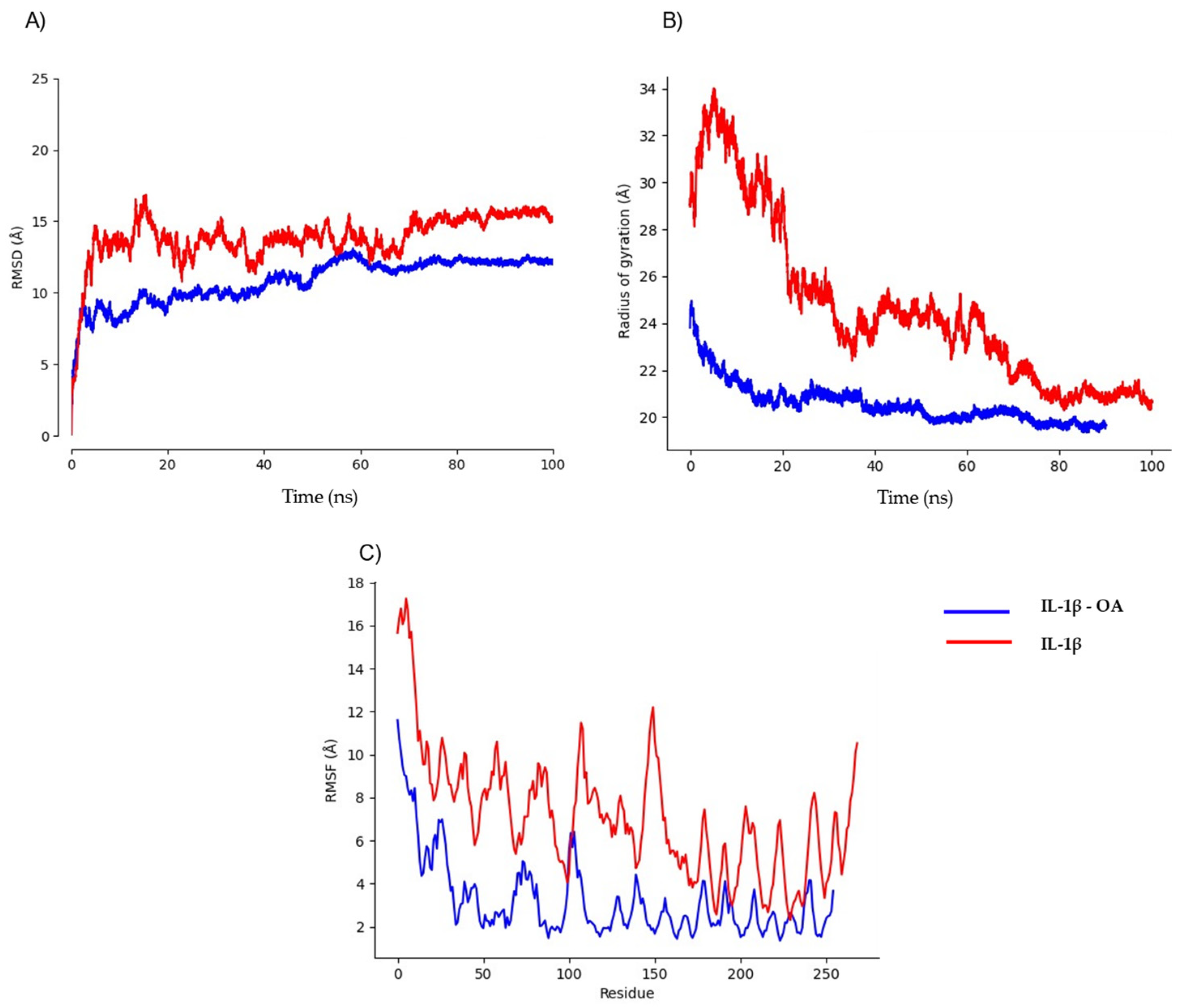
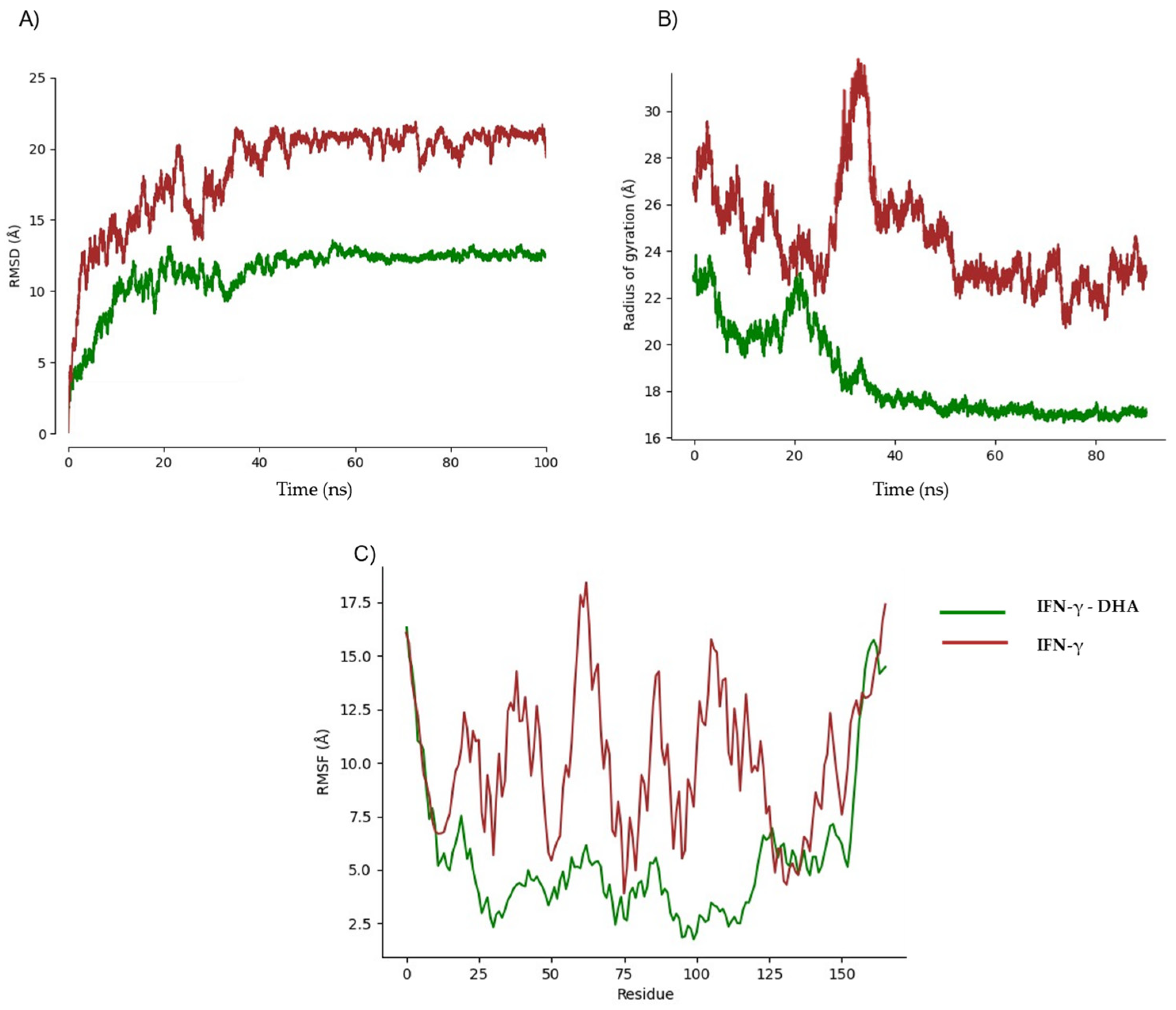

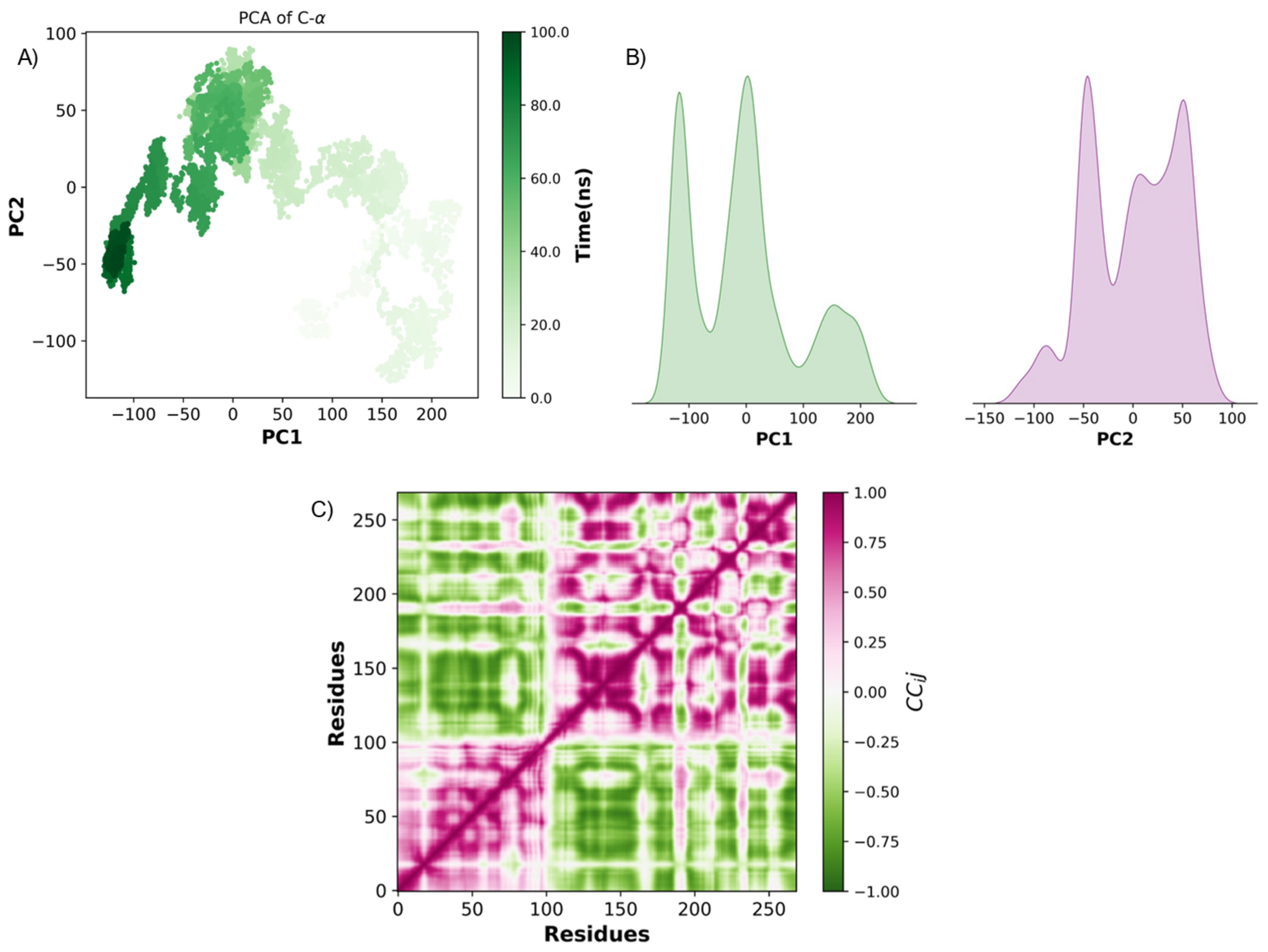
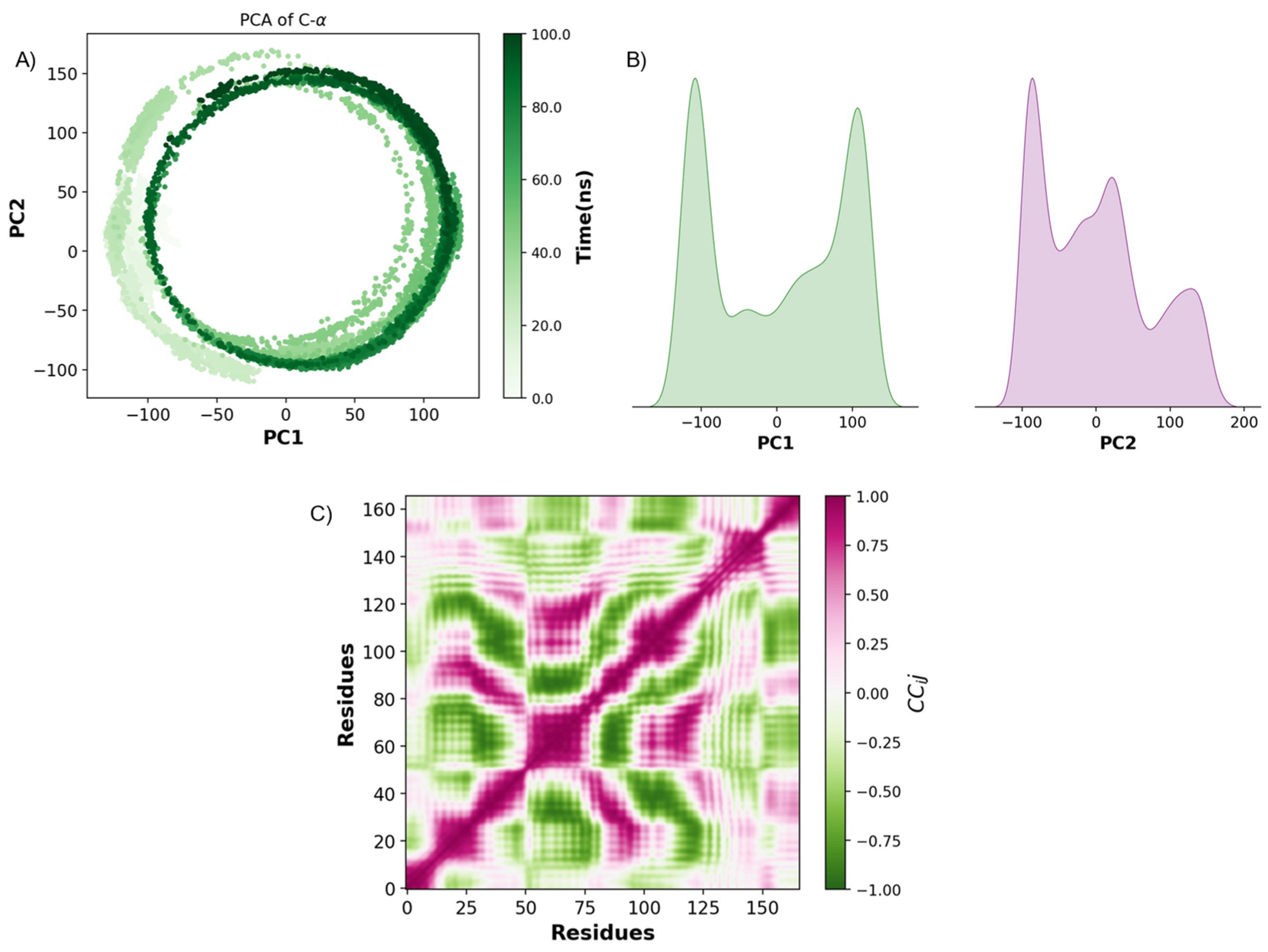
| Protein | Binding Site | Structural Characteristics | Functional Implications |
|---|---|---|---|
| TNF-α | Residues 178–180 (E-F loop, core epitope) | Located in the E-F loop; part of the monomer that forms a pore through the trimer’s center. | Critical for structural stability and epitope functionality [31,32,33]. |
| IFN-γ | Residue LEU136 (with GLU135) | Found in a region involved in truncation affecting the C-terminal; high confidence (pLDDT > 90). | Key in reducing JAK-STAT1 signaling and pro-inflammatory macrophage activation [35]. |
| IL-1β | Residue ARG120 (N-terminal). Residues in the C-terminal core region | Stabilizes the tertiary structure and receptor-binding domain; part of the pro-IL-1β precursor. Becomes part of the functional mature IL-1β. | Essential for maintaining protein stability and facilitating receptor interaction. Critical for biological activity and proteolytic maturation by Caspase-1 [36,37,38]. |
| Protein | Size | PLB | Hyd. | Side | Residues |
|---|---|---|---|---|---|
| TNF-α | 30 | 1.78 | 14 | 22 | GLY144 CYS145 PRO176 CYS177 GLN178 ARG179 GLU180 THR181 ALA185 GLU186 ALA187 LYS188 TRP190 |
| IL-1β | 84 | 1.38 | 42 | 63 | SER66 VAL67 PHE94 PHE98 GLU99 GLU100 GLU101 PRO102 ILE103 PHE105 TRP108 TYR113 VAL114 HIS115 VAL119 ARG120 PHE162 VAL163 GLN164 GLU167 PRO173 LYS209 LYS210 |
| IFN-γ | 32 | 0.86 | 21 | 23 | SER74 LYS78 PHE115 LEU118 TYR121 SER122 VAL123 ALA132 ILE133 LEU136 |
| TNF-α | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ligand Name | Freq | S-Score | Interaction Type | |||||
| H-Bond | H-Bond Residue Interaction | I-Bond | I-Bond Residue Interaction | Pi-Bond | Pi-Bond Residue Interaction | |||
| carisoprodol | 2000 | −4.5 | 5 | CYS145, PRO176, THR181, ARG179 (2 interactions) | 0 | - | 0 | - |
| acebutolol | 2000 | −4.7 | 4 | PRO176, THR181, THR181, ARG179 | 0 | - | 0 | - |
| atracuryum becilate | 2000 | −6.7 | 3 | THR181, TYR191, GLU192 | 0 | - | 1 | PHE140 |
| inositol nicotinate | 2000 | −6.7 | 3 | ARG179 (2 interactions), THR181 | 0 | - | 0 | - |
| docusate | 2000 | −6.1 | 3 | ARG179 (2interactions),THR181 | 2 | ARG179 (2 interactions) | 1 | TRP190 |
| lauryl sodium sulfoacetate | 2000 | −5.1 | 3 | GLU180 (2 interactions), THR181 | 0 | - | 0 | - |
| palmitic acid | 2000 | −4.8 | 3 | CYS145,THR181, ARG179 | 2 | ARG179 (2 interactions) | 0 | - |
| linoleic acid | 2000 | −4.4 | 3 | ARG179 (2 interactions), THR181 | 2 | ARG179 (2 interactions) | 0 | - |
| lauric acid | 2000 | −4.3 | 3 | THR181, ARG179 (2 interactions) | 2 | ARG179 (2 interactions) | 0 | - |
| cetil alcohol | 2000 | −4.3 | 2 | GLU180, THR181 | 0 | - | 0 | - |
| IL-1β | ||||||||
| tetradecyl hydrogensulfate (ester) | 1969 | −6.3 | 5 | LYS209, SER66, VAL67, ARG120 (2 interactions) | 4 | ARG120 (4 interactions) | 1 | PHE98 |
| sodium tetradecyl sulfate | 1890 | −6.1 | 5 | SER66, VAL67, ARG120 (2 interactions), LYS209 | 4 | ARG120 (4 interactions) | 0 | - |
| docusate | 2000 | −7.2 | 4 | LYS209, ARG120 (2 interactions), VAL67 | 6 | ARG120 (6 interactions) | 0 | - |
| carisoprodol | 2000 | −5.5 | 4 | VAL67, GLU101, VAL67, ARG120 | 0 | - | 0 | - |
| ioxilan | 1840 | −9.1 | 4 | GLN164, GLN164, ARG120, VAL119 | 0 | - | 0 | - |
| capecitabine | 1680 | −6.9 | 4 | GLU101, LYS209, ARG120, VAL67 | 0 | - | 0 | - |
| oleic acid | 2000 | −6.5 | 3 | ARG120(3 interactions) | 4 | ARG120 (4 interactions) | 0 | - |
| stearic acid | 2000 | −5.7 | 4 | ARG120 (3 interactions), LYS 209 | 4 | ARG120 (4 interactions) | 0 | - |
| lutein | 2000 | −7.6 | 2 | GLU101, ARG120 | 0 | - | 0 | - |
| doconexent | 2000 | −7.2 | 2 | ARG120 (2 interactions) | 5 | ARG120 (5 interactions) | 0 | - |
| IFN-γ | ||||||||
| sodium tetradecyl sulfate | 2137 | −11.4 | 3 | LYS78, SER122, VAL123 | 1 | LYS78 | 0 | - |
| doconexent | 2122 | −9.6 | 2 | LYS78 (2 interactions) | 2 | LYS78 (2 interactions) | 0 | - |
| acebutolol | 2492 | −9.8 | 3 | SER122, SER74, LYS78 | 0 | - | 0 | - |
| lauric acid | 2117 | −9.1 | 2 | VAL123, LYS78 | 1 | LYS78 | 0 | - |
| capecitabine | 2155 | −10 | 2 | SER122, LYS78 | 0 | - | 0 | - |
| methocarbamol | 1871 | −8.7 | 2 | LYS78 (2 interactions) | 0 | - | 0 | - |
| anisotropin methyl bromide | 1688 | −9.6 | 2 | GLN129, LYS78 | 0 | - | 0 | - |
| yofendilate | 2102 | −9.5 | 1 | LYS78 | 0 | - | 1 | VAL123 |
| hexaminolevulinate | 1725 | −8.5 | 1 | LYS78 | 0 | - | 0 | - |
| carisoprodol | 2830 | −8.8 | 1 | LYS78 | 0 | - | 0 | - |
| Descriptor | Oleic Acid | Linoleic Acid | DHA |
|---|---|---|---|
| ESOL Log S | −5.4 | −5.5 | −7.0 |
| ESOL Class | Moderately soluble | Moderately soluble | Poorly soluble |
| Ali Log S | −8.3 | −8.6 | −10.9 |
| Ali Class | Poorly soluble | Poorly soluble | Insoluble |
| GI absorption | High | High | Low |
| BBB permeant | No | Yes | No |
| CYP1A2 inhibitor | Yes | Yes | Yes |
| CYP2C19 inhibitor | No | No | No |
| CYP2C9 inhibitor | Yes | No | No |
| CYP2D6 inhibitor | No | No | No |
| CYP3A4 inhibitor | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Fernández, M.A.; Tristán-Flores, F.E.; Casique-Aguirre, D.; Negrete-Rodríguez, M.d.l.L.X.; Cervantes-Montelongo, J.A.; Conde-Barajas, E.; Acosta-García, G.; Silva-Martínez, G.A. Virtual Screening and Molecular Dynamics of Cytokine–Drug Complexes for Atherosclerosis Therapy. Int. J. Mol. Sci. 2025, 26, 2931. https://doi.org/10.3390/ijms26072931
Rodríguez-Fernández MA, Tristán-Flores FE, Casique-Aguirre D, Negrete-Rodríguez MdlLX, Cervantes-Montelongo JA, Conde-Barajas E, Acosta-García G, Silva-Martínez GA. Virtual Screening and Molecular Dynamics of Cytokine–Drug Complexes for Atherosclerosis Therapy. International Journal of Molecular Sciences. 2025; 26(7):2931. https://doi.org/10.3390/ijms26072931
Chicago/Turabian StyleRodríguez-Fernández, María Angélica, Fabiola Estefanía Tristán-Flores, Diana Casique-Aguirre, María de la Luz Xochilt Negrete-Rodríguez, Juan Antonio Cervantes-Montelongo, Eloy Conde-Barajas, Gerardo Acosta-García, and Guillermo Antonio Silva-Martínez. 2025. "Virtual Screening and Molecular Dynamics of Cytokine–Drug Complexes for Atherosclerosis Therapy" International Journal of Molecular Sciences 26, no. 7: 2931. https://doi.org/10.3390/ijms26072931
APA StyleRodríguez-Fernández, M. A., Tristán-Flores, F. E., Casique-Aguirre, D., Negrete-Rodríguez, M. d. l. L. X., Cervantes-Montelongo, J. A., Conde-Barajas, E., Acosta-García, G., & Silva-Martínez, G. A. (2025). Virtual Screening and Molecular Dynamics of Cytokine–Drug Complexes for Atherosclerosis Therapy. International Journal of Molecular Sciences, 26(7), 2931. https://doi.org/10.3390/ijms26072931






