RNA-Seq Reveals Adaptation Strategy in Grass Carp (Ctenopharyngodon idella) Under Hypersaline Conditions
Abstract
1. Introduction
2. Results
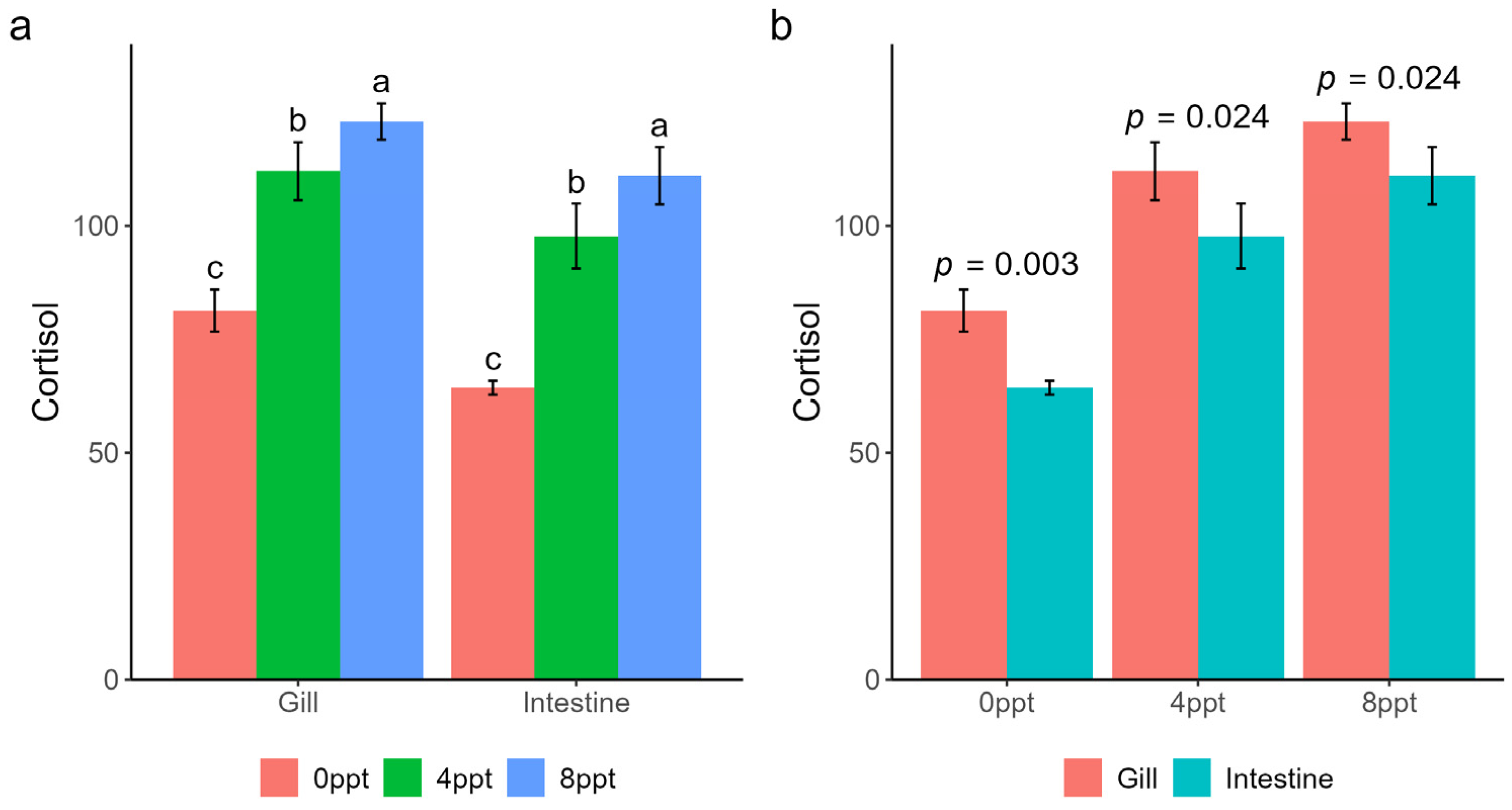
2.1. The Cortisol Concentration in Gills and Intestine
2.2. Transcriptome Sequencing and Gene Expression Analysis
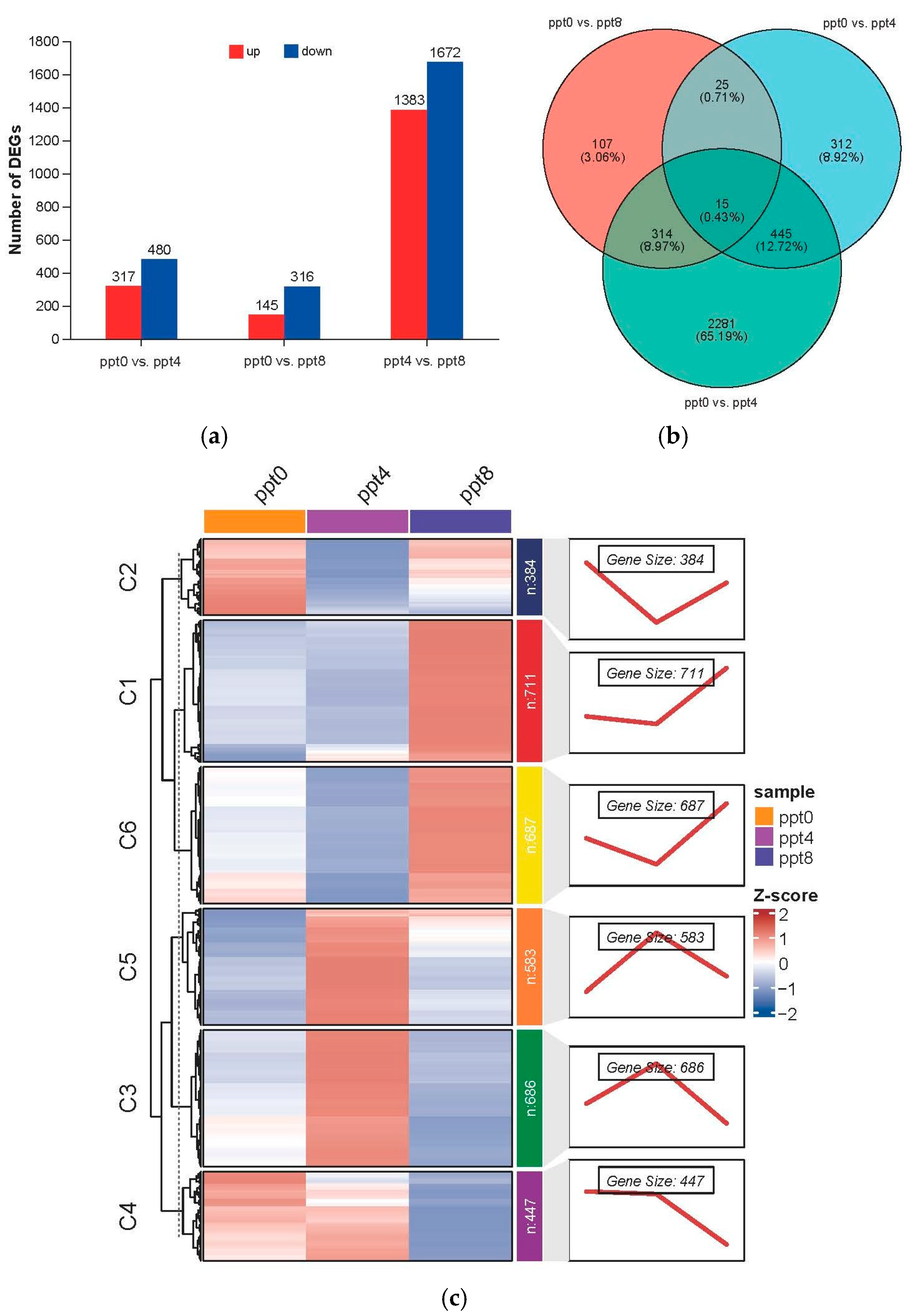
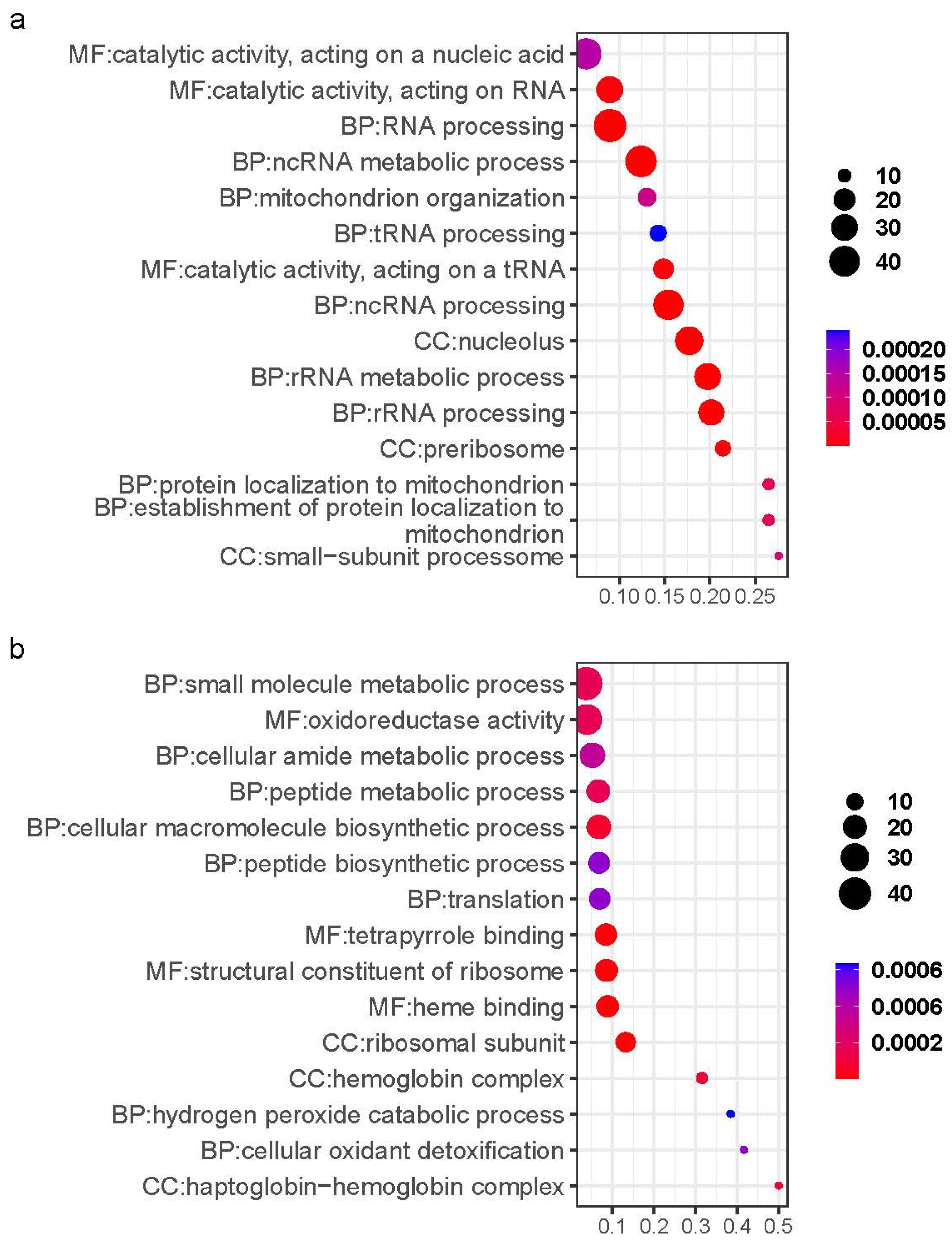
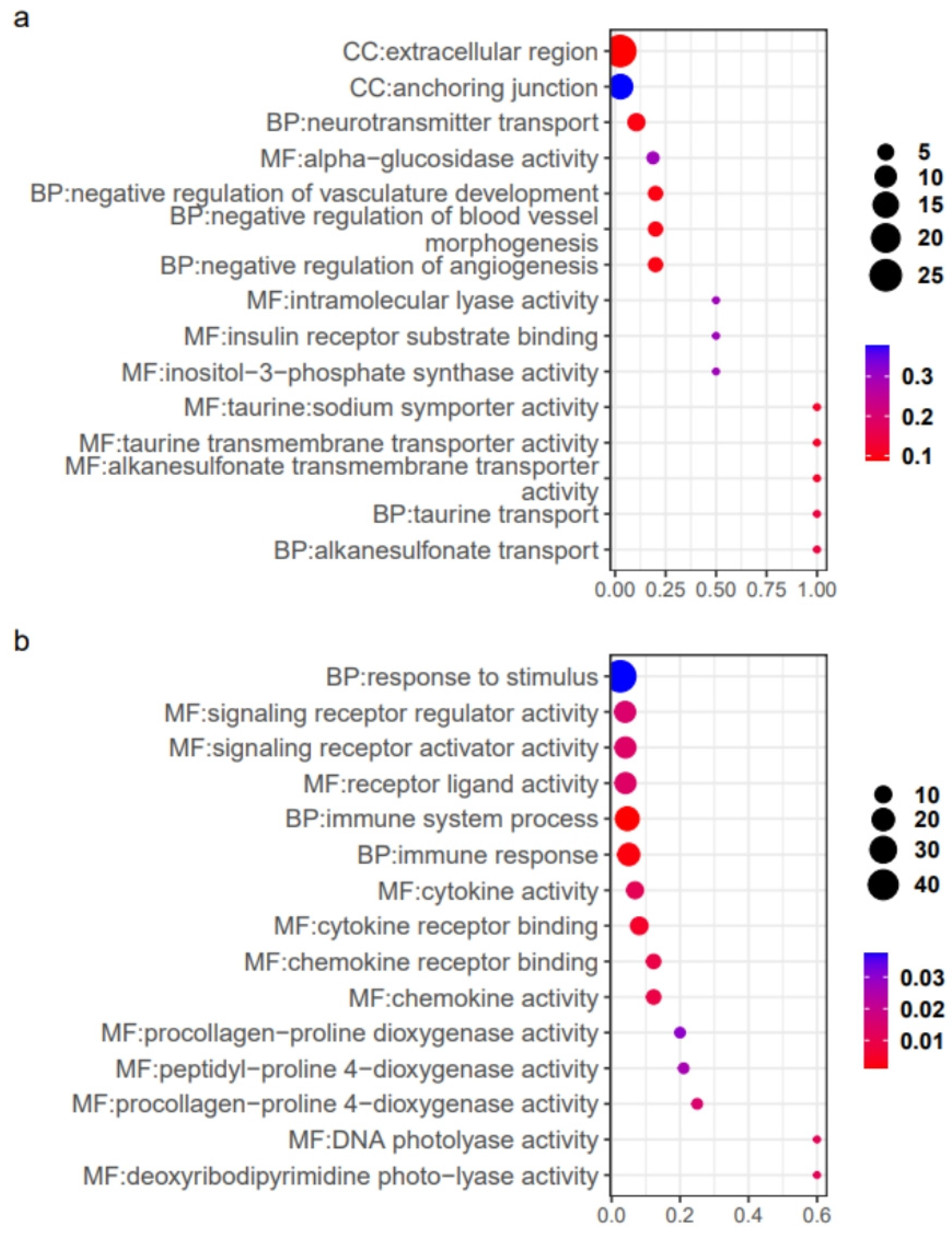
2.3. Analysis of Gill Gene Expression Patterns
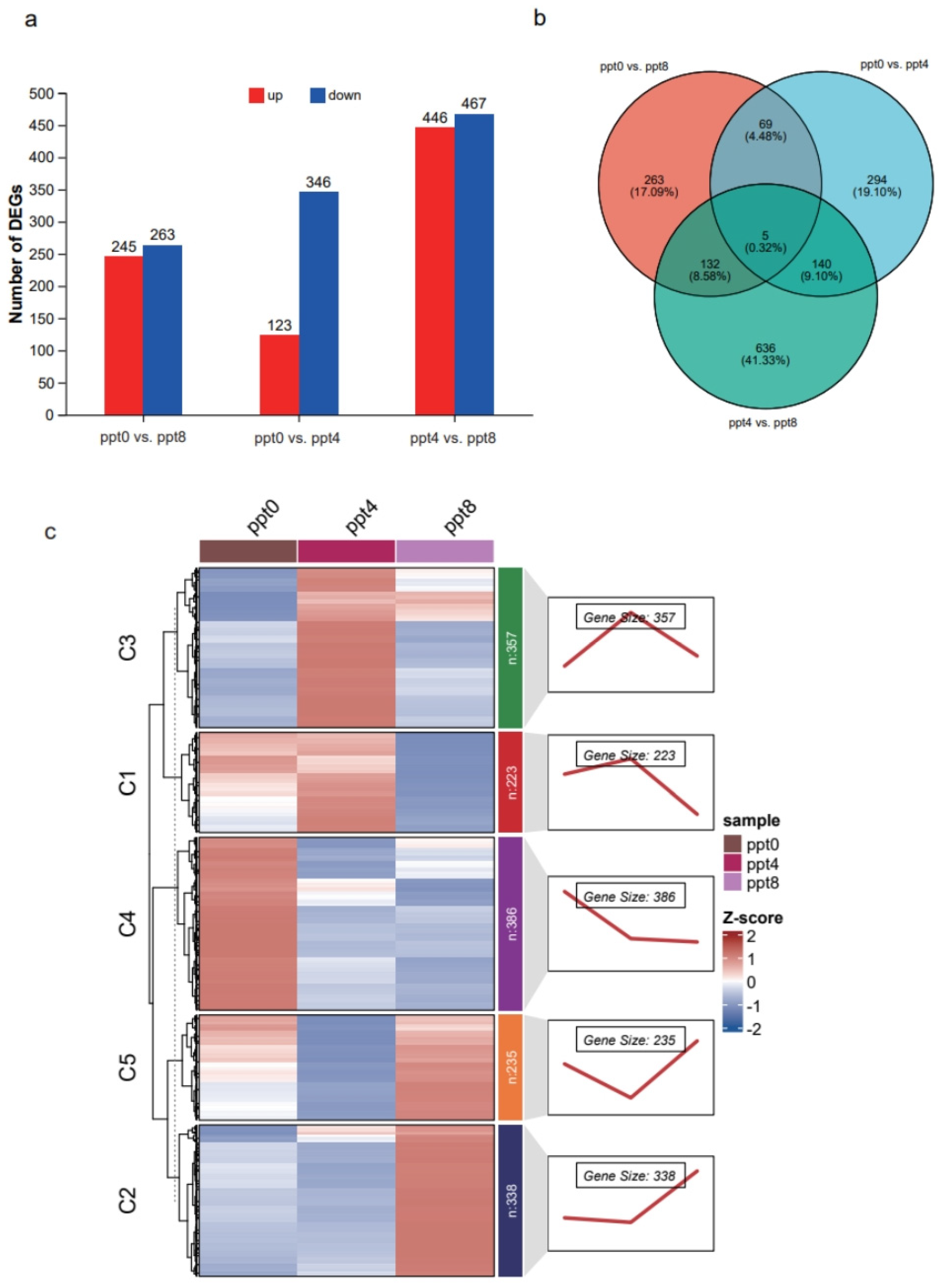
2.4. Analysis of Intestinal Gene Expression Patterns
2.5. Identification of Key Genes for Salt Tolerance
3. Discussion
3.1. The Adaptation Range of Carp to Salinity
3.2. The Effect of Cortisol on Salt Adaptation of Grass Carp
3.3. The Gene Expression Patterns of Gills Under Different Salinity
3.4. The Gene Expression Patterns of Intestines Under Different Salinity
3.5. Identification of Key Genes for Salt Adaptation in Grass Carp
4. Materials and Methods
4.1. Experimental Design
4.2. Cortisol Concentration Detection
4.3. RNA Extraction and Transcriptome Sequencing
4.4. Data Analysis
4.5. qPCR Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DEGs | Differentially expressed genes |
| AQPs | Aquaporins |
| qRT-PCR | Quantitative real-time PCR |
References
- Kefford, B.J. Why are mayflies (Ephemeroptera) lost following small increases in salinity? Three conceptual osmophysiological hypotheses. Philos. Trans. R. Soc. B Biol. Sci. 2018, 374, 20180021. [Google Scholar] [CrossRef]
- Venâncio, C.; Caon, K.; Lopes, I. Cation Composition Influences the Toxicity of Salinity to Freshwater Biota. Int. J. Environ. Res. Public Health 2023, 20, 1741. [Google Scholar] [CrossRef] [PubMed]
- Bœuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef]
- Maceina, M.J.; Shireman, J.V. Grass Carp: Effects of Salinity on Survival, Weight Loss, and Muscle Tissue Water Content. Progress. Fish-Cult. 1979, 41, 69–73. [Google Scholar] [CrossRef]
- Xu, C.; Li, E.; Suo, Y.; Su, Y.; Lu, M.; Zhao, Q.; Qin, J.G.; Chen, L. Histological and transcriptomic responses of two immune organs, the spleen and head kidney, in Nile tilapia (Oreochromis niloticus) to long-term hypersaline stress. Fish Shellfish. Immunol. 2018, 76, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Z.; He, Y.; Li, X.; Li, J. Transcriptome Analysis Reveals Impaired Fertility and Immunity Under Salinity Exposure in Juvenile Grass Carp. Front. Mar. Sci. 2021, 8, 697813. [Google Scholar]
- Zhao, Y.; Zhang, L.; Wang, C.; Xie, C. Biology and Ecology of Grass Carp in China: A Review and Synthesis. N. Am. J. Fish Manag. 2020, 40, 1379–1399. [Google Scholar] [CrossRef]
- Xie, C.; Li, J.; Li, D.; Shen, Y.; Gao, Y.; Zhang, Z. Grass Carp: The Fish that Feeds Half of China. In Aquaculture in China: Success Stories and Modern Trends; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 93–115. [Google Scholar]
- Ministry Of Agriculture And Rural Affairs, Bureau Of Fisheries. China Fishery Statistical Yearbook: 2024; China Agriculture Press: Beijing, China, 2024. [Google Scholar]
- Lin, S.; Milardi, M.; Gao, Y.; Wong, M.H. Sustainable management of non-native grass carp as a protein source, weed-control agent and sport fish. Aquac. Res. 2022, 53, 5809–5824. [Google Scholar] [CrossRef]
- McCormick, S.D.; Sundell, K.; Björnsson, B.T.; Brown, C.L.; Hiroi, J. Influence of salinity on the localization of Na+/K+-ATPase, Na+/K+/2Cl− cotransporter (NKCC) and CFTR anion channel in chloride cells of the Hawaiian goby (Stenogobius hawaiiensis). J. Exp. Biol. 2003, 206, 4575–4583. [Google Scholar] [CrossRef]
- Takvam, M.; Wood, C.M.; Kryvi, H.; Nilsen, T.O. Ion Transporters and Osmoregulation in the Kidney of Teleost Fishes as a Function of Salinity. Front. Physiol. 2021, 12, 664588. [Google Scholar]
- Reinecke, M. Influences of the environment on the endocrine and paracrine fish growth hormone–insulin-like growth factor-I system. J. Fish Biol. 2010, 76, 1233–1254. [Google Scholar] [CrossRef] [PubMed]
- Mancera, J.M.; McCormick, S.D. Influence of cortisol, growth hormone, insulin-like growth factor I and 3,3′,5-triiodo-l-thyronine on hypoosmoregulatory ability in the euryhaline teleost Fundulus heteroclitus. Fish Physiol. Biochem. 1999, 21, 25–33. [Google Scholar] [CrossRef]
- Li, J.; Xue, L.; Cao, M.; Zhang, Y.; Wang, Y.; Xu, S.; Zheng, B.; Lou, Z. Gill transcriptomes reveal expression changes of genes related with immune and ion transport under salinity stress in silvery pomfret (Pampus argenteus). Fish Physiol. Biochem. 2020, 46, 1255–1277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, M.; Lu, W.; Wang, Y.; Li, X.; Cheng, J. Transcriptomic Modulation Reveals the Specific Cellular Response in Chinese Sea Bass (Lateolabrax maculatus) Gills under Salinity Change and Alkalinity Stress. Int. J. Mol. Sci. 2023, 24, 5877. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Liu, N.; Zhang, Z.; Zhang, J. Osmoregulatory strategies of estuarine fish Scatophagus argus in response to environmental salinity changes. BMC Genom. 2022, 23, 545. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, C.; Chen, J. Salinity and temperature tolerance of brown-marbled grouper Epinephelus fuscoguttatus. Fish Physiol. Biochem. 2013, 39, 277–286. [Google Scholar] [CrossRef]
- Varsamos, S. Tolerance range and osmoregulation in hypersaline conditions in the European sea bass (Dicentrarchus labrax). J. Mar. Biol. Assoc. UK 2002, 82, 1047–1048. [Google Scholar] [CrossRef]
- Kamal, A.H.M.M.; Mair, G.C. Salinity tolerance in superior genotypes of tilapia, Oreochromis niloticus, Oreochromis mossambicus and their hybrids. Aquaculture 2005, 247, 189–201. [Google Scholar] [CrossRef]
- Qin, S.; Leng, X.; Luo, J.; Du, H.; Liu, Z.; Qiao, X.; Xiong, W.; Wei, Q. Growth and physiological characteristics of juvenile Chinese sturgeon (Acipenser sinensis) during adaptation to seawater. Aquac. Res. 2020, 51, 3813–3821. [Google Scholar] [CrossRef]
- Djiba, P.K.; Zhang, J.; Xu, Y.; Zhang, P.; Zhou, J.; Zhang, Y.; Luo, Y. Correlation between Metabolic Rate and Salinity Tolerance and Metabolic Response to Salinity in Grass Carp (Ctenopharyngodon idella). Animals 2021, 11, 3445. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Z.; Song, Y.; Yang, J.; Lu, Y.; Lai, W.; Wu, Z.; Zhao, D.; Lin, H.; Zhang, Y.; et al. Effects of salinity on growth, physiology, biochemistry and gut microbiota of juvenile grass carp (Ctenopharyngodon idella). Aquat. Toxicol. 2023, 258, 106482. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Du, J.; Xi, R.; Zhang, Q.; Li, L.; Li, D.; Takagi, Y.; Zhang, X. Effects of different culture salinities on the growth and muscle quality of grass carp (Ctenopharyngodon idellus). J. Anim. Sci. 2024, 102, e281. [Google Scholar] [CrossRef]
- Cross, D.G. The tolerance of grass carp Ctenopharyngodon idella (Val.) to seawatert. J. Fish Biol. 1970, 2, 231–233. [Google Scholar] [CrossRef]
- Fang, H.; Yang, Y.Y.; Wu, X.M.; Zheng, S.Y.; Song, Y.J.; Zhang, J.; Chang, M.X. Effects and Molecular Regulation Mechanisms of Salinity Stress on the Health and Disease Resistance of Grass Carp. Front. Immunol. 2022, 13, 917497. [Google Scholar]
- Purcell, K.M.; Hitch, A.T.; Klerks, P.L.; Leberg, P.L. Adaptation as a potential response to sea-level rise: A genetic basis for salinity tolerance in populations of a coastal marsh fish. Evol. Appl. 2008, 1, 155–160. [Google Scholar] [CrossRef]
- Yue, G.H.; Ma, K.Y.; Xia, J.H. Status of conventional and molecular breeding of salinity-tolerant tilapia. Rev. Aquac. 2024, 16, 271–286. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Yang, Q.; Liu, L.; Liu, J.; Lu, J.; Wang, Y.; Tang, R.; Li, L.; Zhang, X. Long-term crowding stress induces chronic inflammatory response and declines the immunity of grass carp (Ctenopharyngodon idella). Aquaculture 2023, 577, 739976. [Google Scholar] [CrossRef]
- McCormick, S.D. Endocrine Control of Osmoregulation in Teleost Fish1. Am. Zool. 2001, 41, 781–794. [Google Scholar] [CrossRef]
- Chang, R.J.A.; Celino-Brady, F.T.; Seale, A.P. Changes in cortisol and corticosteroid receptors during dynamic salinity challenges in Mozambique tilapia. Gen. Comp. Endocrinol. 2023, 342, 114340. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; Björnsson, B.T.; McCormick, S.D. Effects of long-term cortisol treatment on growth and osmoregulation of Atlantic salmon and brook trout. Gen. Comp. Endocrinol. 2021, 308, 113769. [Google Scholar] [CrossRef]
- Nemova, N.N.; Kaivarainen, E.I.; Rendakov, N.L.; Nikerova, K.M.; Efremov, D.A. Cortisol Content and Na+/K+-ATPase Activity under Adaptation of Juvenile Pink Salmon Oncorhynchus gorbuscha (Salmonidae) to Salinity Changes. J. Ichthyol. 2021, 61, 771–778. [Google Scholar] [CrossRef]
- Hughes, G.M.; Morgan, M. The structure of fish gills in relation to their respiratory function. Biol. Rev. 1973, 48, 419–475. [Google Scholar] [CrossRef]
- Dymowska, A.K.; Hwang, P.; Goss, G.G. Structure and function of ionocytes in the freshwater fish gill. Respir. Physiol. Neurobiol. 2012, 184, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Claiborne, J.B. Osmotic and ionic regulation in fishes. In Osmotic and Ionic Regulation; CRC Press: Boca Raton, FL, USA, 2008; pp. 295–366. [Google Scholar]
- Zhou, Z.; Hu, F.; Li, W.; Yang, X.; Hallerman, E.; Huang, Z. Effects of salinity on growth, hematological parameters, gill microstructure and transcriptome of fat greenling Hexagrammos otakii. Aquaculture 2021, 531, 735945. [Google Scholar] [CrossRef]
- Zhu, T.; Liu, Y.; Du, J.; Lei, C.; Wang, C.; Li, S.; Song, H. Effects of short-term salt exposure on gill damage, serum components and gene expression patterns in juvenile Largemouth bass (Micropterus salmoides). Comp. Biochem. Physiol. Part D Genom. Proteom. 2025, 53, 101365. [Google Scholar] [CrossRef]
- Chasiotis, H.; Kolosov, D.; Bui, P.; Kelly, S.P. Tight junctions, tight junction proteins and paracellular permeability across the gill epithelium of fishes: A review. Respir. Physiol. Neurobiol. 2012, 184, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Tipsmark, C.K.; Baltzegar, D.A.; Ozden, O.; Grubb, B.J.; Borski, R.J. Salinity regulates claudin mRNA and protein expression in the teleost gill. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, R1004–R1014. [Google Scholar] [CrossRef]
- Avella, M.; Ducoudret, O.; Pisani, D.F.; Poujeol, P. Swelling-activated transport of taurine in cultured gill cells of sea bass: Physiological adaptation and pavement cell plasticity. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R1149–R1160. [Google Scholar] [CrossRef][Green Version]
- Jiang, W.; Tian, X.; Fang, Z.; Li, L.; Dong, S.; Li, H.; Zhao, K. Metabolic responses in the gills of tongue sole (Cynoglossus semilaevis) exposed to salinity stress using NMR-based metabolomics. Sci. Total Environ. 2019, 653, 465–474. [Google Scholar] [CrossRef]
- Takeuchi, K.; Toyohara, H.; Kinoshita, M.; Sakaguchi, M. Ubiquitous increase in taurine transporter mRNA in tissues of tilapia (Oreochromis mossambicus) during high-salinity adaptation. Fish Physiol. Biochem. 2000, 23, 173–182. [Google Scholar] [CrossRef]
- Velselvi, R.; Dasgupta, S.; Varghese, T.; Sahu, N.P.; Tripathi, G.; Panmei, H.; Singha, K.P.; Krishna, G. Taurine and/or inorganic potassium as dietary osmolyte counter the stress and enhance the growth of GIFT reared in ion imbalanced low saline water. Food Chem. Mol. Sci. 2022, 4, 100058. [Google Scholar] [CrossRef] [PubMed]
- Slami, M.; Bahrekazemi, M.; Bahram, S.; Javadian, S.R. The positive effects of taurine on growth performance, immunohaematological parameters and stress response of farmed beluga (Huso huso) in both fresh water and brackish water. Aquac. Nutr. 2021, 27, 2279–2293. [Google Scholar] [CrossRef]
- Huang, M.; Yang, X.; Zhou, Y.; Ge, J.; Davis, D.A.; Dong, Y.; Gao, Q.; Dong, S. Growth, serum biochemical parameters, salinity tolerance and antioxidant enzyme activity of rainbow trout (Oncorhynchus mykiss) in response to dietary taurine levels. Mar. Life Sci. Technol. 2021, 3, 449–462. [Google Scholar] [CrossRef]
- Silva, A.L.; Wright, S.H. Integumental Taurine Transport in Mytilus Gill: Short-Term Adaptation to Reduced Salinity. J. Exp. Biol. 1992, 162, 265–279. [Google Scholar] [CrossRef]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.S.; Singer, T.D. Cystic fibrosis transmembrane conductance regulator in teleost fish. Biochim. Biophys. Acta BBA—Biomembr. 2002, 1566, 16–27. [Google Scholar] [CrossRef]
- Bodinier, C.; Lorin-Nebel, C.; Charmantier, G.; Boulo, V. Influence of salinity on the localization and expression of the CFTR chloride channel in the ionocytes of juvenile Dicentrarchus labrax exposed to seawater and freshwater. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 345–351. [Google Scholar] [CrossRef]
- Lingrel, J.B.; Van Huysse, J.; O’Brien, W.; Jewell-Motz, E.; Askew, R.; Schultheis, P. Structure-function studies of the Na, K-ATPase. Kidney Int. Suppl. 1994, 44, S32–S39. [Google Scholar] [CrossRef]
- Borgnia, M.; Nielsen, S.; Engel, A.; Agre, P. Cellular and Molecular Biology of the Aquaporin Water Channels. Annu. Rev. Biochem. 1999, 68, 425–458. [Google Scholar] [CrossRef]
- Giffard-Mena, I.; Boulo, V.; Aujoulat, F.; Fowden, H.; Castille, R.; Charmantier, G.; Cramb, G. Aquaporin molecular characterization in the sea-bass (Dicentrarchus labrax): The effect of salinity on AQP1 and AQP3 expression. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 430–444. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, S.; Lee, T. Salinity effects on expression and localization of aquaporin 3 in gills of the euryhaline milkfish (Chanos chanos). J. Exp. Zool. Part A Ecol. Integr. Physiol. 2023, 339, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yu, P.; Wen, H.; Qi, X.; Tian, Y.; Zhang, K.; Fu, Q.; Li, Y.; Li, C. Genome-Wide Characterization of Aquaporins (aqps) in Lateolabrax maculatus: Evolution and Expression Patterns During Freshwater Acclimation. Mar. Biotechnol. 2021, 23, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, K.M. New insights into the many functions of carbonic anhydrase in fish gills. Respir. Physiol. Neurobiol. 2012, 184, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Zhang, C.; Zhou, H.; Song, L.; Tu, H.; Zhao, J. Variation in pH, HCO3−, carbonic anhydrases, and HCO3− transporters in Nile tilapia during carbonate alkalinity stress. Hydrobiologia 2023, 850, 2447–2459. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Saad, M.F.; Shukry, M.; El-Keredy, A.M.S.; Nasif, O.; Van Doan, H.; Dawood, M.A.O. Physiological and ion changes of Nile tilapia (Oreochromis niloticus) under the effect of salinity stress. Aquac. Rep. 2021, 19, 100567. [Google Scholar] [CrossRef]
- Mancera, J.M.; McCormick, S.D. Osmoregulatory actions of the GH/IGF axis in non-salmonid teleosts. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1998, 121, 43–48. [Google Scholar] [CrossRef]
- Hiroi, J.; Yasumasu, S.; McCormick, S.D.; Hwang, P.; Kaneko, T. Evidence for an apical Na–Cl cotransporter involved in ion uptake in a teleost fish. J. Exp. Biol. 2008, 211, 2584–2599. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef]

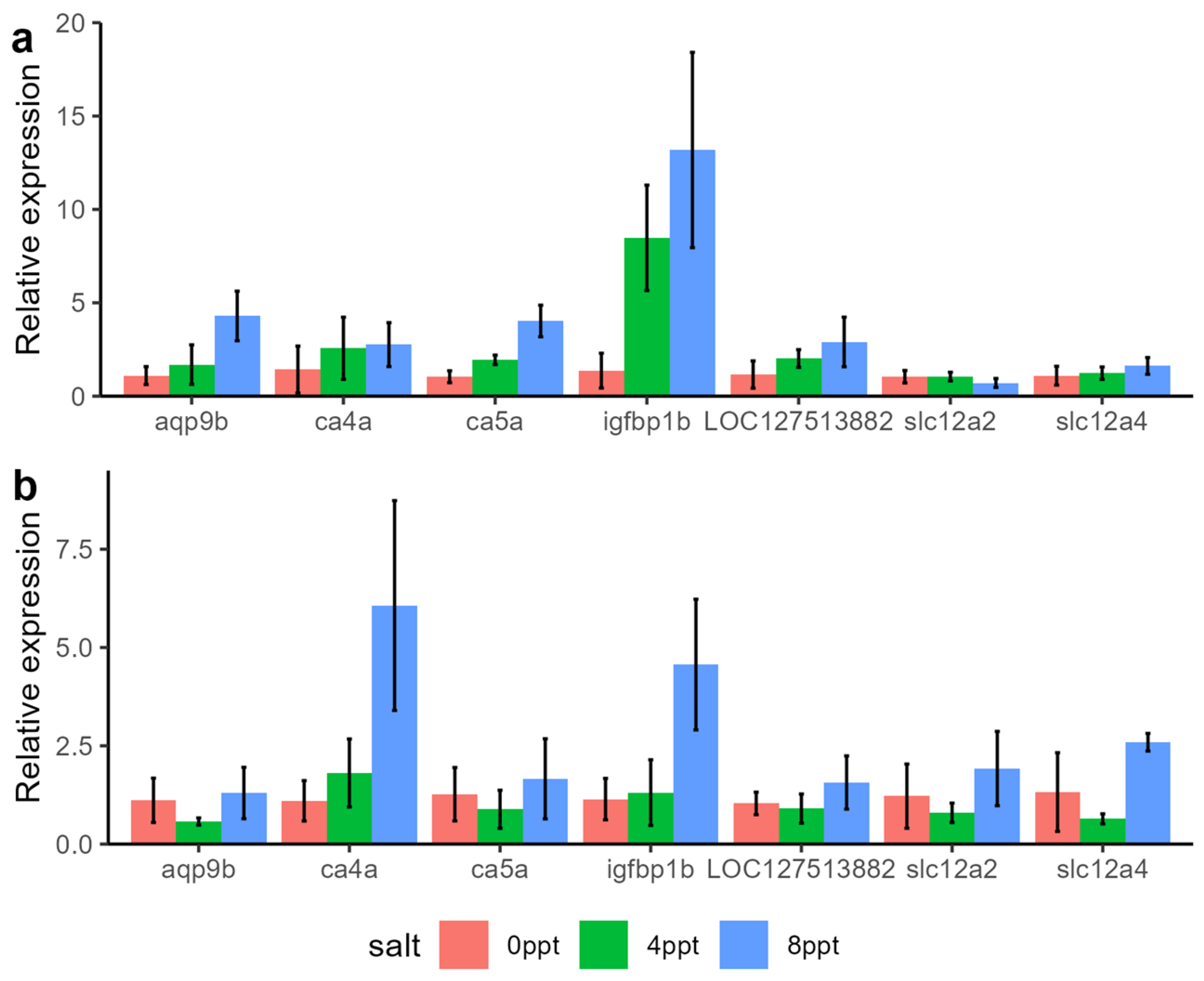
| Gene Name | Gene Description | Gill ppt0 | Gill ppt4 | Gill ppt8 | Intestine ppt0 | Intestine ppt4 | Intestine ppt8 |
|---|---|---|---|---|---|---|---|
| LOC127513882 | sodium/potassium-transporting ATPase subunit alpha-1 | 20.68 | 18.66 | 63.82 | 103.44 | 219.61 | 174.92 |
| aqp9b | aquaporin 9b | 8.54 | 9.44 | 30.48 | 0.19 | 0.07 | 0.21 |
| ca4a | carbonic anhydrase IV a | 1.01 | 0.46 | 23.79 | 1.90 | 3.34 | 6.93 |
| ca5a | carbonic anhydrase Va | 21.88 | 59.77 | 86.33 | 43.72 | 58.34 | 49.63 |
| igfbp1b | insulin-like growth factor binding protein 1b | 207.49 | 204.60 | 1250.15 | 4.94 | 11.53 | 35.71 |
| slc12a2 | solute carrier family 12 member 2 | 0.47 | 0.92 | 1.09 | 3.03 | 2.96 | 5.56 |
| slc12a4 | solute carrier family 12 member 4 | 2.98 | 2.35 | 5.53 | 1.86 | 1.61 | 3.31 |
| Primer | Sequences (5′–3′) |
|---|---|
| β-Actin-RT-F | GATGATGAAATTGCCGCACTG |
| β-Actin-RT-R | ACCGACCATGACGCCCTGATGT |
| LOC127513882-RT-F | GGGTGCCATTGTAGCCGTAA |
| LOC127513882-RT-R | CCATGTCCGTTCCCAGGTC |
| aqp9b-RT-F | CATCCACTTTGGCTTTACTC |
| aqp9b-RT-R | TGTGGCATTTACACCAGTTA |
| ca4a-RT-F | AAAGCACCATAAAGAACAACG |
| ca4a-RT-R | CCTCATAGAAGAATCCCAACA |
| ca5a-RT-F | TATTGACATCGTGGTGCGTAA |
| ca5a-RT-R | TCCTCCAGTGGTCCTCCCT |
| igfbp1b-RT-F | ACAGCAGATGTTAGGCGAGAAG |
| igfbp1b-RT-R | CAGCCGATAAATCATCAGTTCC |
| slc12a2-RT-F | TGCTGGACTGGGTAGATTGA |
| slc12a2-RT-F | GGAGGAGGGTTTGGATGA |
| slc12a4-RT-F | GTGCCCAAGTCACCGAATA |
| slc12a4-RT-F | GCGAAAGTTGTGCCTAAATAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Song, H.; Zhu, Z.; Tian, J.; Lei, C.; Du, J.; Li, S. RNA-Seq Reveals Adaptation Strategy in Grass Carp (Ctenopharyngodon idella) Under Hypersaline Conditions. Int. J. Mol. Sci. 2025, 26, 2930. https://doi.org/10.3390/ijms26072930
Zhu T, Song H, Zhu Z, Tian J, Lei C, Du J, Li S. RNA-Seq Reveals Adaptation Strategy in Grass Carp (Ctenopharyngodon idella) Under Hypersaline Conditions. International Journal of Molecular Sciences. 2025; 26(7):2930. https://doi.org/10.3390/ijms26072930
Chicago/Turabian StyleZhu, Tao, Hongmei Song, Zhu Zhu, Jing Tian, Caixia Lei, Jinxing Du, and Shengjie Li. 2025. "RNA-Seq Reveals Adaptation Strategy in Grass Carp (Ctenopharyngodon idella) Under Hypersaline Conditions" International Journal of Molecular Sciences 26, no. 7: 2930. https://doi.org/10.3390/ijms26072930
APA StyleZhu, T., Song, H., Zhu, Z., Tian, J., Lei, C., Du, J., & Li, S. (2025). RNA-Seq Reveals Adaptation Strategy in Grass Carp (Ctenopharyngodon idella) Under Hypersaline Conditions. International Journal of Molecular Sciences, 26(7), 2930. https://doi.org/10.3390/ijms26072930




