Pharmacomodulation of the Redox-Active Lead Plasmodione: Synthesis of Substituted 2-Benzylnaphthoquinone Derivatives, Antiplasmodial Activities, and Physicochemical Properties
Abstract
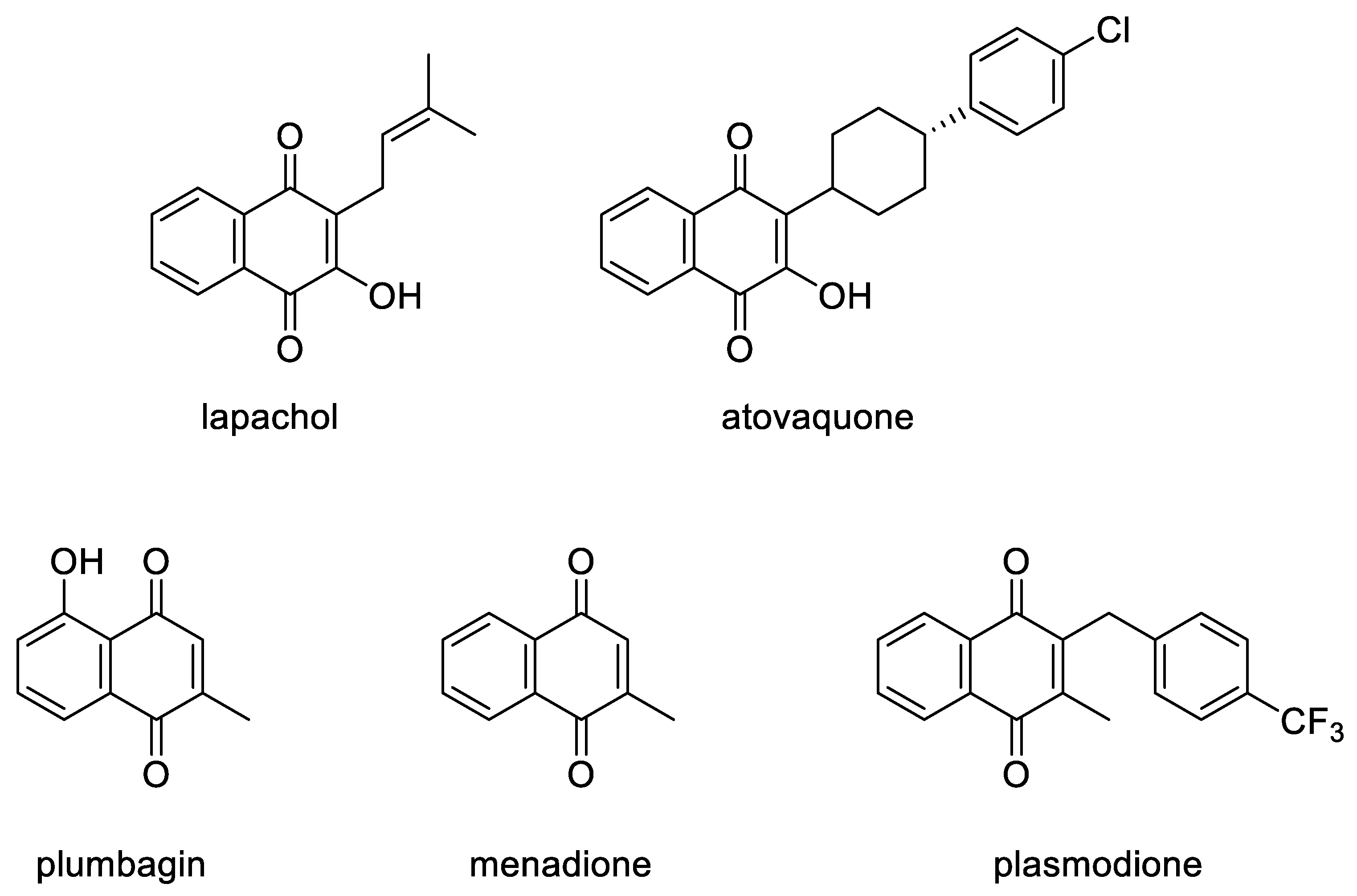
1. Introduction
2. Results and Discussion
2.1. Synthetic Chemistry
2.2. Biological Evaluation
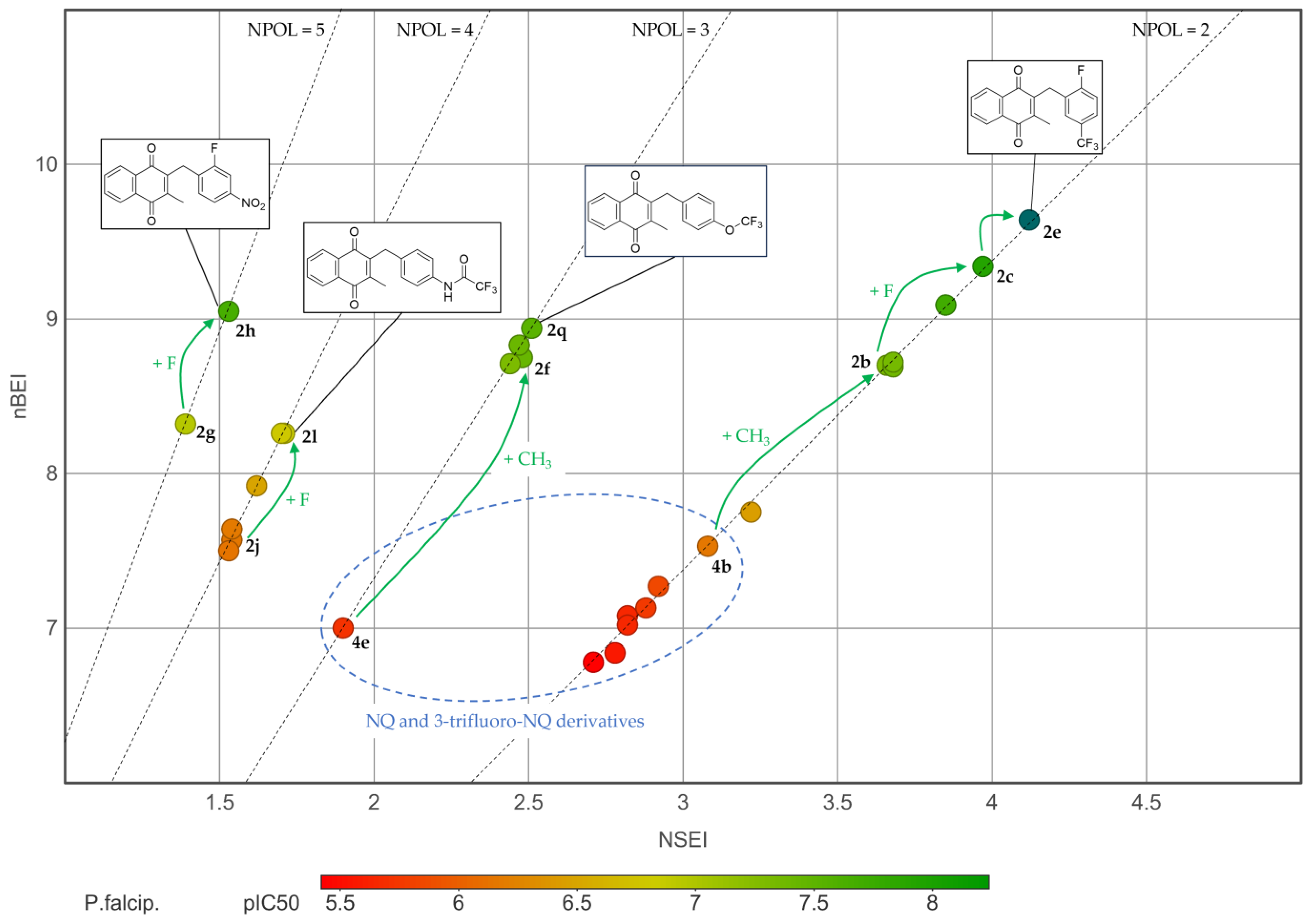
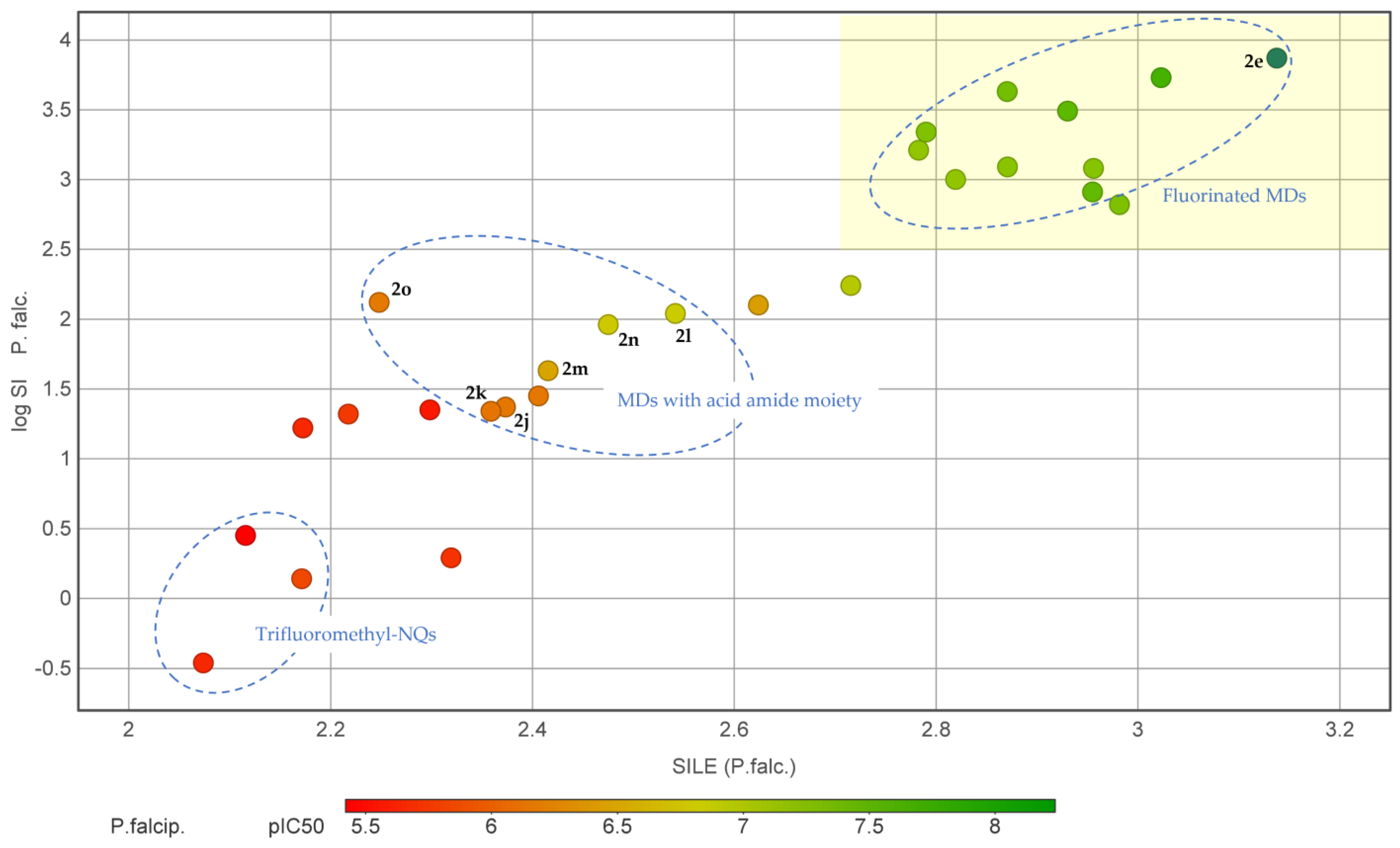
2.3. Physicochemical Investigations
2.4. Structure–Activity Relationships (SAR) of the Antiplasmodial Activity
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. General Synthetic Procedure for Benzylmenadiones 2a–i (Route A)
3.1.3. General Synthetic Procedure for Acetamidobenzylmenadiones 2j,k (Route C)
3.1.4. General Synthetic Procedure for Amidobenzylmenadiones 2l–o (Route C)
3.1.5. General Synthetic Procedure for Benzylmenadiones 2p–s (Route D)
3.1.6. General Synthetic Procedure for Benzylnaphthoquinones 4a–e (Route B)
3.1.7. General Synthetic Procedure for Trifluoromenadiones 5a–c (Route B)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Global Malaria Programme. World Malaria Report 2023. World Health Organization [Online]. 30 November 2023. Available online: https://www.who.int/publications/i/item/9789240086173 (accessed on 8 October 2024).
- WHO Global Malaria Programme. Global Technical Strategy for Malaria 2016–2030, 2021 Update. World Health Organization [Online]. 19 July 2021. Available online: https://www.who.int/publications/i/item/9789240031357 (accessed on 8 October 2024).
- Parums, D.V. Editorial: Current Status of Two Adjuvanted Malaria Vaccines and the World Health Organization (WHO) Strategy to Eradicate Malaria by 2030. Med. Sci. Monit. 2023, 29, e939357. [Google Scholar] [CrossRef]
- Poespoprodjo, J.R.; Douglas, N.M.; Ansong, D.; Kho, S.; Anstey, N.M. Malaria. Lancet 2023, 402, 2328–2345. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pertejo, Y.; Escudero-Martínez, J.M.; Reguera, R.M.; Balaña-Fouce, R.; García, P.A.; Jambrina, P.G.; San Feliciano, A.; Castro, M.-Á. Antileishmanial activity of terpenylquinones on Leishmania infantum and their effects on Leishmania topoisomerase IB. Int. J. Parasitol. Drugs Drug Resist. 2019, 11, 70–79. [Google Scholar] [CrossRef]
- Jentzsch, J.; Koko, W.S.; Al Nasr, I.S.; Khan, T.A.; Schobert, R.; Ersfeld, K.; Biersack, B. New Antiparasitic Bis-Naphthoquinone Derivatives. Chem. Biodivers. 2020, 17, e1900597. [Google Scholar] [CrossRef] [PubMed]
- Rivera, G.; Patel, N.B.; Bandyopadhyay, D. Editorial: Discovery and Development of Drugs for Neglected Diseases: Chagas Disease, Human African Trypanosomiasis, and Leishmaniasis. Front. Chem. 2021, 9, 775327. [Google Scholar] [CrossRef]
- Prieto Cardenas, L.S.; Arias Soler, K.A.; Nossa Gonzalez, D.L.; Rozo Nunez, W.E.; Cardenas-Chaparro, A.; Duchowicz, P.R.; Gomez Castano, J.A. In Silico Antiprotozoal Evaluation of 1,4-Naphthoquinone Derivatives against Chagas and Leishmaniasis Diseases Using QSAR, Molecular Docking, and ADME Approaches. Pharmaceuticals 2022, 15, 687. [Google Scholar] [CrossRef] [PubMed]
- Fotie, J. Quinones and malaria. Anti-Infect. Agents Med. Chem. 2006, 5, 357–366. [Google Scholar] [CrossRef]
- Ventura Pinto, A.; Lisboa de Castro, S. The trypanocidal activity of naphthoquinones: A review. Molecules 2009, 14, 4570–4590. [Google Scholar] [CrossRef] [PubMed]
- Andujar, I.; Rios, J.L.; Giner, R.M.; Recio, M.C. Pharmacological properties of shikonin—A review of literature since 2002. Planta Med. 2013, 79, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Futuro, D.O.; Ferreira, V.F.; Ferreira, P.G.; Nicoletti, C.D.; Borba-Santos, L.P.; Rozental, S.; Silva, F.C.D. The Antifungal Activity of Naphthoquinones: An Integrative Review. An. Acad. Bras. Cienc. 2018, 90, 1187–1214. [Google Scholar] [CrossRef]
- Patel, O.P.S.; Beteck, R.M.; Legoabe, L.J. Antimalarial application of quinones: A recent update. Eur. J. Med. Chem. 2021, 210, 113084. [Google Scholar] [CrossRef]
- Pérez-Sacau, E.; Estévez-Braun, A.; Ravelo, A.G.; Gutiérrez Yapu, D.; Giménez Turba, A. Antiplasmodial activity of naphthoquinones related to lapachol and β-lapachone. Chem. Biodivers. 2005, 2, 264–274. [Google Scholar] [CrossRef]
- Grellier, P.; Maroziene, A.; Nivinskas, H.; Sarlauskas, J.; Aliverti, A.; Cenas, N. Antiplasmodial activity of quinones: Roles of aziridinyl substituents and the inhibition of Plasmodium falciparum glutathione reductase. Arch. Biochem. Biophys. 2010, 494, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.M.; Lanteri, C.A.; O’Neil, M.T.; Johnson, J.D.; Gribble, G.W.; Trumpower, B.L. Design of antiparasitic and antifungal hydroxy-naphthoquinones that are less susceptible to drug resistance. Mol. Biochem. Parasitol. 2011, 177, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Sumsakul, W.; Plengsuriyakarn, T.; Chaijaroenkul, W.; Viyanant, V.; Karbwang, J.; Na-Bangchang, K. Antimalarial activity of plumbagin in vitro and in animal models. BMC Complement. Altern. Med. 2014, 14, 15. [Google Scholar] [CrossRef]
- Muller, T.; Johann, L.; Jannack, B.; Bruckner, M.; Lanfranchi, D.A.; Bauer, H.; Sanchez, C.; Yardley, V.; Deregnaucourt, C.; Schrevel, J.; et al. Glutathione Reductase-Catalyzed Cascade of Redox Reactions to Bioactivate Potent Antimalarial 1,4-Naphthoquinones—A New Strategy to Combat Malarial Parasites. J. Am. Chem. Soc. 2011, 133, 11557–11571. [Google Scholar] [CrossRef]
- Bielitza, M.; Belorgey, D.; Ehrhardt, K.; Johann, L.; Lanfranchi, D.A.; Gallo, V.; Schwarzer, E.; Mohring, F.; Jortzik, E.; Williams, D.L.; et al. Antimalarial NADPH-Consuming Redox-Cyclers As Superior Glucose-6-Phosphate Dehydrogenase Deficiency Copycats. Antioxid. Redox Signal. 2015, 22, 1337–1351. [Google Scholar] [CrossRef]
- Sidorov, P.; Desta, I.; Chesse, M.; Horvath, D.; Marcou, G.; Varnek, A.; Davioud-Charvet, E.; Elhabiri, M. Redox Polypharmacology as an Emerging Strategy to Combat Malarial Parasites. ChemMedChem 2016, 11, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, K.; Deregnaucourt, C.; Goetz, A.-A.; Tzanova, T.; Gallo, V.; Arese, P.; Pradines, B.; Adjalley, S.H.; Bagrel, D.; Blandin, S.; et al. The redox cycler plasmodione is a fast-acting antimalarial lead compound with pronounced activity against sexual and early asexual blood-stage parasites. Antimicrob. Agents Chemother. 2016, 60, 5146–5158. [Google Scholar] [CrossRef]
- Iacobucci, I.; Monaco, V.; Hovasse, A.; Dupouy, B.; Keumoe, R.; Cichocki, B.; Elhabiri, M.; Meunier, B.; Strub, J.-M.; Monti, M.; et al. Proteomic Profiling of Antimalarial Plasmodione Using 3-Benz(o)ylmenadione Affinity-Based Probes. ChemBioChem 2024, 25, e202400187. [Google Scholar] [CrossRef] [PubMed]
- Mounkoro, P.; Michel, T.; Golinelli-Cohen, M.-P.; Blandin, S.; Davioud-Charvet, E.; Meunier, B. A role for the succinate dehydrogenase in the mode of action of the redox-active antimalarial drug, plasmodione. Free Radic. Biol. Med. 2021, 162, 533–541. [Google Scholar] [CrossRef]
- Kumar Jha, R.; Kumar, S. Direct Functionalization of para-Quinones: A Historical Review and New Perspectives. Eur. J. Org. Chem. 2024, 27, e202400535. [Google Scholar] [CrossRef]
- Donzel, M.; Karabiyikli, D.; Cotos, L.; Elhabiri, M.; Davioud-Charvet, E. Direct C-H Radical Alkylation of 1,4-Quinones. Eur. J. Org. Chem. 2021, 2021, 3622–3633. [Google Scholar] [CrossRef]
- Rodo, E.C.; Feng, L.; Jida, M.; Ehrhardt, K.; Bielitza, M.; Boilevin, J.; Lanzer, M.; Williams, D.L.; Lanfranchi, D.A.; Davioud-Charvet, E. A Platform of Regioselective Methodologies to Access Polysubstituted 2-Methyl-1,4-naphthoquinone Derivatives: Scope and Limitations. Eur. J. Org. Chem. 2016, 2016, 1982–1993. [Google Scholar] [CrossRef]
- Li, D.; Shen, X. Iron-catalyzed regioselective alkylation of 1,4-quinones and coumarins with functionalized alkyl bromides. Org. Biomol. Chem. 2020, 18, 750–754. [Google Scholar] [CrossRef]
- Donzel, M.; Elhabiri, M.; Davioud-Charvet, E. Bioinspired Photoredox Benzylation of Quinones. J. Org. Chem. 2021, 86, 10055–10066. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Lanfranchi, D.A.; Cotos, L.; Cesar-Rodo, E.; Ehrhardt, K.; Goetz, A.-A.; Zimmermann, H.; Fenaille, F.; Blandin, S.A.; Davioud-Charvet, E. Synthesis of plasmodione metabolites and 13C-enriched plasmodione as chemical tools for drug metabolism investigation. Org. Biomol. Chem. 2018, 16, 2647–2665. [Google Scholar] [CrossRef]
- Anderson, J.M.; Kochi, J.K. Silver(I)-catalyzed oxidative decarboxylation of acids by peroxydisulfate. Role of silver(II). J. Am. Chem. Soc. 1970, 92, 1651. [Google Scholar] [CrossRef]
- Lanfranchi, D.A.; Belorgey, D.; Mueller, T.; Vezin, H.; Lanzer, M.; Davioud-Charvet, E. Exploring the trifluoromenadione core as a template to design antimalarial redox-active agents interacting with glutathione reductase. Org. Biomol. Chem. 2012, 10, 4795–4806. [Google Scholar] [CrossRef] [PubMed]
- Ilchenko, N.O.; Janson, P.G.; Szabo, K.J. Copper-mediated C-H trifluoromethylation of quinones. Chem. Commun. 2013, 49, 6614–6616. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, Y.; Ji, G.; Xu, Y.; Zhang, S.; Feng, J.; Zhang, Y.; Wang, J. Copper-Catalyzed Direct C-H Trifluoromethylation of Quinones. Org. Lett. 2013, 15, 3730–3733. [Google Scholar] [CrossRef]
- Fang, Z.; Ning, Y.; Mi, P.; Liao, P.; Bi, X. Catalytic C-H α-Trifluoromethylation of α,β-Unsaturated Carbonyl Compounds. Org. Lett. 2014, 16, 1522–1525. [Google Scholar] [CrossRef]
- Urgin, K.; Jida, M.; Ehrhardt, K.; Muller, T.; Lanzer, M.; Maes, L.; Elhabiri, M.; Davioud-Charvet, E. Pharmacomodulation of the antimalarial plasmodione: Synthesis of biaryl- and N-arylalkylamine analogues, antimalarial activities and physicochemical properties. Molecules 2017, 22, 161. [Google Scholar] [CrossRef]
- Nwaka, S.; Ramirez, B.; Brun, R.; Maes, L.; Douglas, F.; Ridley, R. Advancing drug innovation for neglected diseases-criteria for lead progression. PLoS Negl. Trop. Dis. 2009, 3, e440. [Google Scholar] [CrossRef]
- Katsuno, K.; Burrows, J.n.; Duncan, K.; van Huijsduijnen, R.H.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Samby, K.; Willis, P.a.; Burrows, J.n.; Laleu, B.; Webborn, P.j.h. Actives from MMV Open Access Boxes? A suggested way forward. PLoS Pathog. 2021, 17, e1009384. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- de Sena Pereira, V.S.; de Oliveira, C.B.S.; Fumagalli, F.; da Silva Emery, F.; da Silva, N.B.; de Andrade-Neto, V.F. Cytotoxicity, hemolysis and in vivo acute toxicity of 2-hydroxy-3-anilino-1,4-naphthoquinone derivatives. Toxicol. Rep. 2016, 3, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.P.; Hersey, A.; Montanari, D.; Overington, J. Probing the links between in vitro potency, ADMET and physicochemical parameters. Nat. Rev. Drug Discov. 2011, 10, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Meanwell, N.A. Improving Drug Candidates by Design: A Focus on Physicochemical Properties As a Means of Improving Compound Disposition and Safety. Chem. Res. Toxicol. 2011, 24, 1420–1456. [Google Scholar] [CrossRef] [PubMed]
- Tinworth, C.P.; Young, R.J. Facts, Patterns, and Principles in Drug Discovery: Appraising the Rule of 5 with Measured Physicochemical Data. J. Med. Chem. 2020, 63, 10091–10108. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Hann, M.M. Molecular obesity, potency and other addictions in drug discovery. MedChemComm 2011, 2, 349–355. [Google Scholar] [CrossRef]
- Tarcsay, A.; Keseru, G.M. Contributions of Molecular Properties to Drug Promiscuity. J. Med. Chem. 2013, 56, 1789–1795. [Google Scholar] [CrossRef]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Jalal, R.; Singh, P.P.; Majoral, J.-P.; Vishwakarma, R.A. Present drug-likeness filters in medicinal chemistry during the hit and lead optimization process: How far can they be simplified? Drug Discov. Today 2018, 23, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Flitsch, S.L.; Grigalunas, M.; Leeson, P.D.; Quinn, R.J.; Turner, N.J.; Waldmann, H. The Time and Place for Nature in Drug Discovery. JACS Au 2022, 2, 2400–2416. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Keserue, G.M.; Leeson, P.D.; Rees, D.C.; Reynolds, C.H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discov. 2014, 13, 105–121. [Google Scholar] [CrossRef]
- Johnson, T.W.; Gallego, R.A.; Edwards, M.P. Lipophilic efficiency as an important metric in drug design. J. Med. Chem. 2018, 61, 6401–6420. [Google Scholar] [CrossRef]
- Scott, J.S.; Waring, M.J. Practical application of ligand efficiency metrics in lead optimisation. Bioorg. Med. Chem. 2018, 26, 3006–3015. [Google Scholar] [CrossRef]
- Leeson, P.D.; Bento, A.P.; Gaulton, A.; Hersey, A.; Manners, E.J.; Radoux, C.J.; Leach, A.R. Target-Based Evaluation of “Drug-Like” Properties and Ligand Efficiencies. J. Med. Chem. 2021, 64, 7210–7230. [Google Scholar] [CrossRef] [PubMed]
- Abad-Zapatero, C. Ligand efficiency indices for effective drug discovery: A unifying vector formulation. Expert. Opin. Drug Discov. 2021, 16, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Nissink, J.W.M. Simple Size-Independent Measure of Ligand Efficiency. J. Chem. Inf. Model. 2009, 49, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Cavalluzzi, M.M.; Mangiatordi, G.F.; Nicolotti, O.; Lentini, G. Ligand efficiency metrics in drug discovery: The pros and cons from a practical perspective. Expert. Opin. Drug Discov. 2017, 12, 1087–1104. [Google Scholar] [CrossRef] [PubMed]
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral Druggable Space beyond the Rule of 5: Insights from Drugs and Clinical Candidates. Chem. Biol. 2014, 21, 1115–1142. [Google Scholar] [CrossRef]
- Price, E.; Weinheimer, M.; Rivkin, A.; Jenkins, G.; Nijsen, M.; Cox, P.B.; DeGoey, D. Beyond Rule of Five and PROTACs in Modern Drug Discovery: Polarity Reducers, Chameleonicity, and the Evolving Physicochemical Landscape. J. Med. Chem. 2024, 67, 5683–5698. [Google Scholar] [CrossRef]
- DeGoey, D.A.; Chen, H.-J.; Cox, P.B.; Wendt, M.D. Beyond the Rule of 5: Lessons Learned from AbbVie’s Drugs and Compound Collection. J. Med. Chem. 2018, 61, 2636–2651. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Green, D.V.S.; Luscombe, C.N.; Hill, A.P. Getting physical in drug discovery II: The impact of chromatographic hydrophobicity measurements and aromaticity. Drug Discov. Today 2011, 16, 822–830. [Google Scholar] [CrossRef]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem. Neurosci. 2016, 7, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Moving beyond Rules: The Development of a Central Nervous System Multiparameter Optimization (CNS MPO) Approach to Enable Alignment of Druglike Properties. ACS Chem. Neurosci. 2010, 1, 435–449. [Google Scholar] [CrossRef]
- Bickerton, G.R.; Paolini, G.V.; Besnard, J.; Muresan, S.; Hopkins, A.L. Quantifying the chemical beauty of drugs. Nat. Chem. 2012, 4, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, M.; Xie, L.; Xu, X.; Xu, P.; Xu, L. Computational prediction of Lee retention indices of polycyclic aromatic hydrocarbons by using machine learning. Chem. Biol. Drug Des. 2023, 101, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, P.N.; Murray, C.W. Assessing the lipophilicity of fragments and early hits. J. Comput.-Aided Mol. Des. 2011, 25, 663–667. [Google Scholar] [CrossRef]
- Young, R.J.; Leeson, P.D. Mapping the Efficiency and Physicochemical Trajectories of Successful Optimizations. J. Med. Chem. 2018, 61, 6421–6467. [Google Scholar] [CrossRef] [PubMed]
- Abad-Zapatero, C.; Blasi, D. Ligand Efficiency Indices (LEIs): More than a Simple Efficiency Yardstick. Mol. Inform. 2011, 30, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Abad-Zapatero, C.; Perisic, O.; Wass, J.; Bento, A.P.; Overington, J.; Al-Lazikani, B.; Johnson, M.E. Ligand efficiency indices for an effective mapping of chemico-biological space: The concept of an atlas-like representation. Drug Discov. Today 2010, 15, 804–811. [Google Scholar] [CrossRef]
- Skrzynska, A.; Romaniszyn, M.; Pomiklo, D.; Albrecht, L. The Application of 2-Benzyl-1,4-naphthoquinones as Pronucleophiles in Aminocatalytic Synthesis of Tricyclic Derivatives. J. Org. Chem. 2018, 83, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
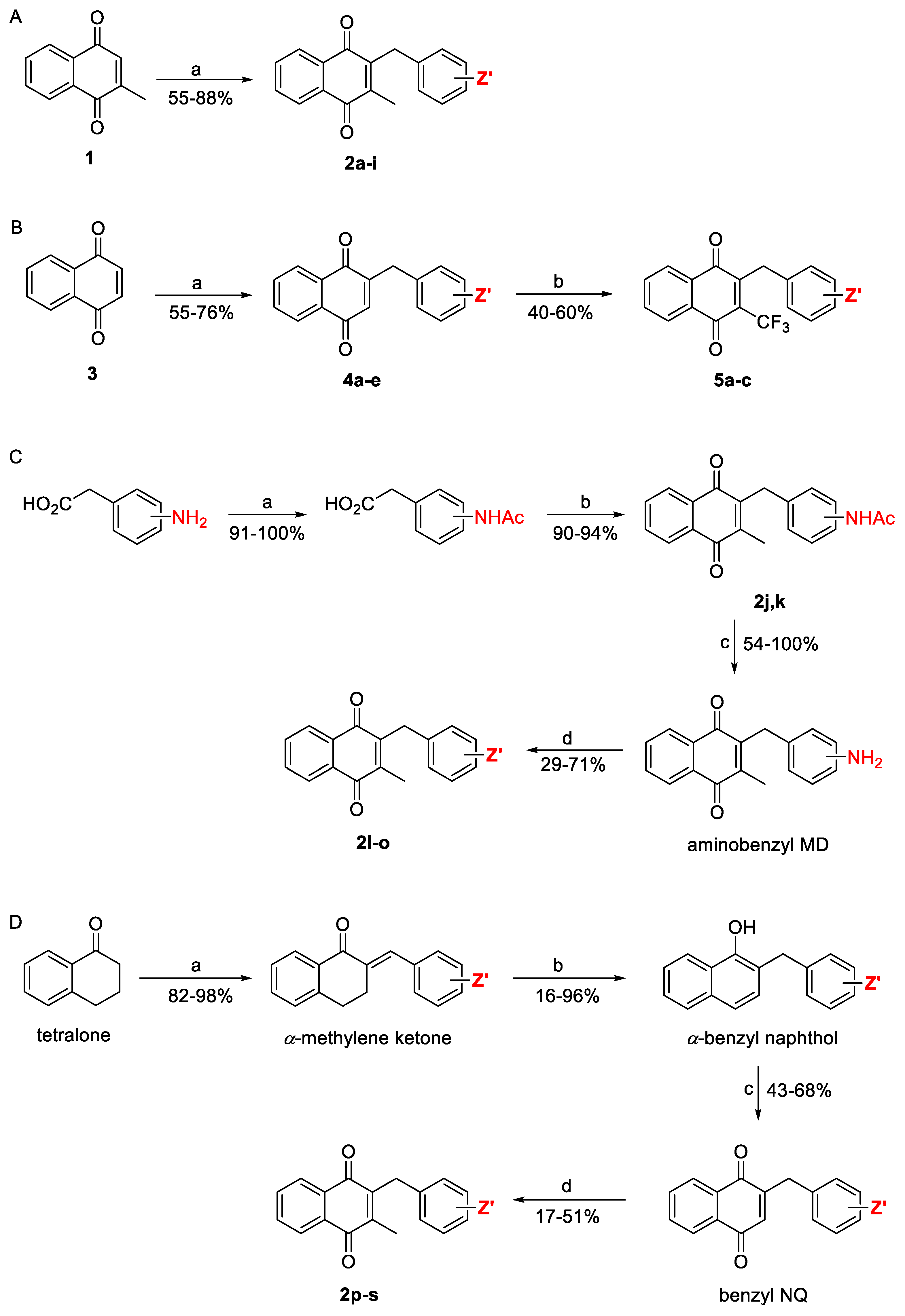

| Compd | Structure | Route | Yield (%) | Compd | Structure | Route | Yield (%) |
|---|---|---|---|---|---|---|---|
| 2a | 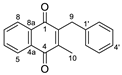 | A | 88 | 2o | 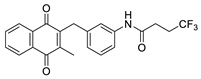 | C | 14 |
| 2b | 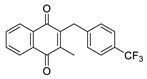 | A | 83 | 2p | 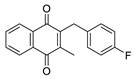 | D | 17 |
| 2c | 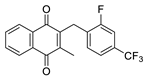 | A | 69 | 2q | 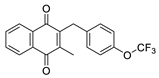 | D | <5 |
| 2d | 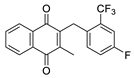 | A | 66 | 2r | 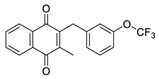 | D | <5 |
| 2e | 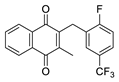 | A | 63 | 2s |  | D | <5 |
| 2f |  | A | 60 | 4a |  | B | 75 |
| 2g |  | A | 66 | 4b | 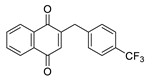 | B | 66 |
| 2h | 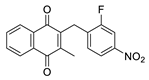 | A | 58 | 4c | 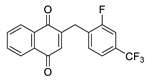 | B | 58 |
| 2i | 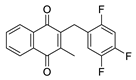 | A | 55 | 4d |  | B | 61 |
| 2j | 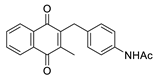 | C | 94 | 4e |  | B | 55 |
| 2k | 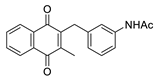 | C | 90 | 5a |  | B | 45 |
| 2l | 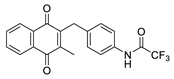 | C | 67 | 5b | 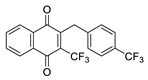 | B | 38 |
| 2m | 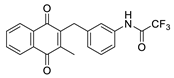 | C | 28 | 5c | 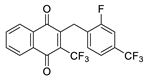 | B | 23 |
| 2n | 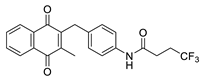 | C | 66 |
| Compd | P. falc. 1 | SI 2 | P. falc. 1 | T. b. rhod. 3 | SI 2 | T. b. rhod. 3 | Cyt L6 4 | Oral Toxicity 6 |
|---|---|---|---|---|---|---|---|---|
| IC50 μM | pIC50 5 | IC50 μM | pIC50 5 | IC50 μM | LD50 mg/kg | |||
| Chl. | 0.003 | 30367 | 8.52 | 91.1 | ||||
| Mel. | 0.006 | 4033 | 8.22 | 24.2 | ||||
| Pod. | 0.024 | |||||||
| 2a | 0.358 | 126 | 6.45 | 62.793 | 1 | 4.20 | 45.023 | 500 |
| 2b | 0.048 | 994 | 7.31 | 65.276 | 1 | 4.19 | 48.152 | 500 |
| 2c | 0.011 | 5377 | 7.94 | 81.396 | 1 | 4.09 | 61.746 | 500 |
| 2d | 0.020 | 3099 | 7.70 | 23.210 | 3 | 4.63 | 62.274 | 500 |
| 2e | 0.006 | 7495 | 8.24 | 93.021 | 0 | 4.03 | 43.038 | 2000 |
| 2f | 0.037 | 654 | 7.43 | 8.269 | 3 | 5.08 | 24,172 | 2000 |
| 2g | 0.111 | 172 | 6.96 | 28.034 | 1 | 4.55 | 19.069 | 500 |
| 2h | 0.022 | 810 | 7.67 | 25.543 | 1 | 4.59 | 17.427 | 500 |
| 2i | 0.044 | 1231 | 7.35 | 30.353 | 2 | 4.52 | 54.509 | 500 |
| 2j | 0.695 | 24 | 6.16 | 28.886 | 1 | 4.54 | 16.317 | 500 |
| 2k | 0.758 | 22 | 6.12 | 39.457 | 0 | 4.40 | 16.568 | 1000 |
| 2l | 0.147 | 109 | 6.83 | 23.384 | 1 | 4.63 | 16.109 | 1033 |
| 2m | 0.321 | 43 | 6.49 | 24.265 | 1 | 4.62 | 13.706 | 948 |
| 2n | 0.159 | 92 | 6.80 | 72.250 | 0 | 4.14 | 14.687 | 1000 |
| 2o | 0.670 | 131 | 6.17 | 50.799 | 2 | 4.29 | 87.547 | 1000 |
| 2p | 0.043 | 1194 | 7.37 | 33.225 | 2 | 4.48 | 51.124 | 500 |
| 2q | 0.029 | 4250 | 7.54 | 81.575 | 2 | 4.09 | 122.724 | 500 |
| 2r | 0.049 | 1641 | 7.31 | 26.064 | 3 | 4.58 | 80.536 | 500 |
| 2s | 0.038 | 2166 | 7.42 | 75.763 | 1 | 4.12 | 83.229 | 1000 |
| 4a | 2.755 | 22 | 5.56 | 0.318 | 193 | 6.50 | 61.551 | 500 |
| 4b | 0.686 | 28 | 6.16 | 0.503 | 38 | 6.30 | 19.189 | 500 |
| 4c | 1.762 | 21 | 5.75 | 0.790 | 47 | 6.10 | 36.997 | 500 |
| 4d | 2.307 | 17 | 5.64 | 1.744 | 22 | 5.76 | 38.379 | 500 |
| 4e | 2.006 | 2 | 5.70 | 0.522 | 8 | 6.28 | 3.944 | 2000 |
| 5a | 3.804 | 3 | 5.42 | 1.884 | 6 | 5.72 | 10.769 | 500 |
| 5b | 1.457 | 1 | 5.84 | 1.962 | 1 | 5.71 | 2.004 | 500 |
| 5c | 2.314 | 0 | 5.64 | 9.414 | 0 | 5.03 | 0.805 | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presser, A.; Blaser, G.; Pferschy-Wenzig, E.-M.; Kaiser, M.; Mäser, P.; Schuehly, W. Pharmacomodulation of the Redox-Active Lead Plasmodione: Synthesis of Substituted 2-Benzylnaphthoquinone Derivatives, Antiplasmodial Activities, and Physicochemical Properties. Int. J. Mol. Sci. 2025, 26, 2114. https://doi.org/10.3390/ijms26052114
Presser A, Blaser G, Pferschy-Wenzig E-M, Kaiser M, Mäser P, Schuehly W. Pharmacomodulation of the Redox-Active Lead Plasmodione: Synthesis of Substituted 2-Benzylnaphthoquinone Derivatives, Antiplasmodial Activities, and Physicochemical Properties. International Journal of Molecular Sciences. 2025; 26(5):2114. https://doi.org/10.3390/ijms26052114
Chicago/Turabian StylePresser, Armin, Gregor Blaser, Eva-Maria Pferschy-Wenzig, Marcel Kaiser, Pascal Mäser, and Wolfgang Schuehly. 2025. "Pharmacomodulation of the Redox-Active Lead Plasmodione: Synthesis of Substituted 2-Benzylnaphthoquinone Derivatives, Antiplasmodial Activities, and Physicochemical Properties" International Journal of Molecular Sciences 26, no. 5: 2114. https://doi.org/10.3390/ijms26052114
APA StylePresser, A., Blaser, G., Pferschy-Wenzig, E.-M., Kaiser, M., Mäser, P., & Schuehly, W. (2025). Pharmacomodulation of the Redox-Active Lead Plasmodione: Synthesis of Substituted 2-Benzylnaphthoquinone Derivatives, Antiplasmodial Activities, and Physicochemical Properties. International Journal of Molecular Sciences, 26(5), 2114. https://doi.org/10.3390/ijms26052114






