L-Carnitine and Mildronate Demonstrate Divergent Protective Effects on Mitochondrial DNA Quality Control and Inflammation Following Traumatic Brain Injury
Abstract
1. Introduction
2. Results
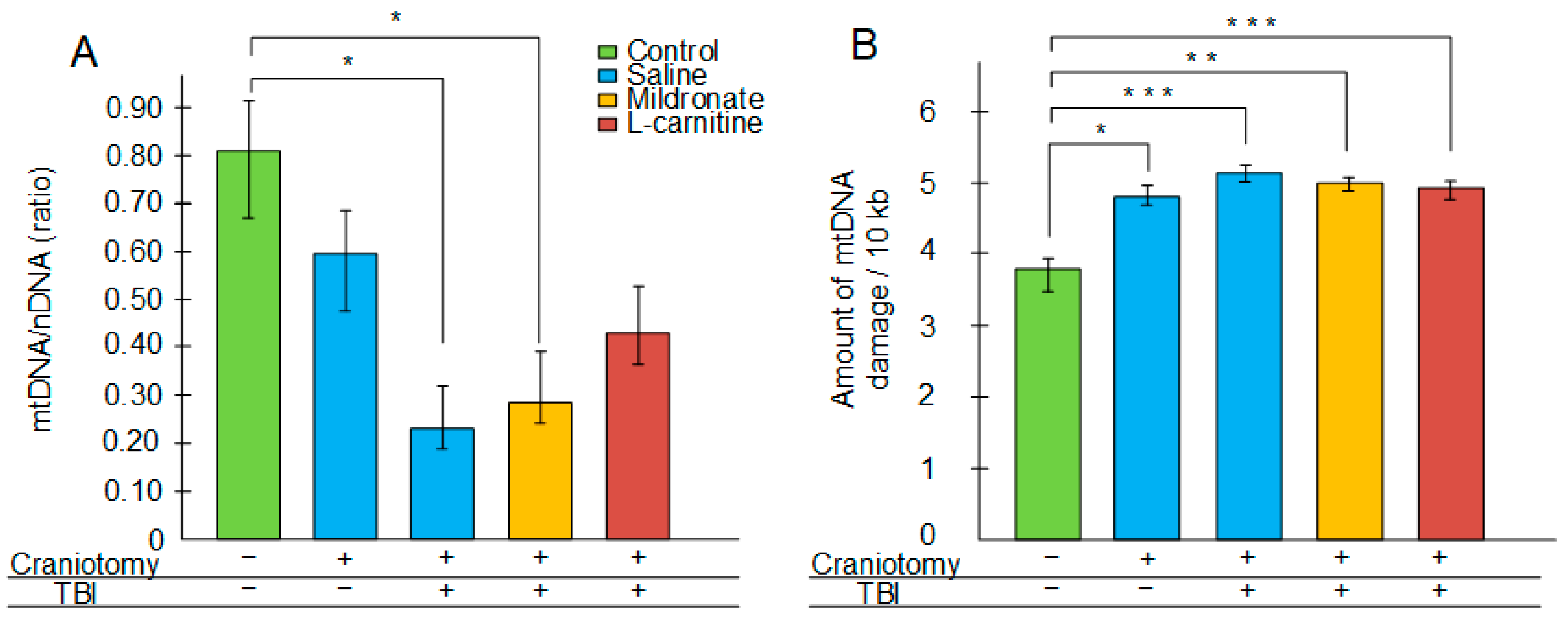
2.1. Changes in Integrity and Quantity of mtDNA in the Brain Following TBI
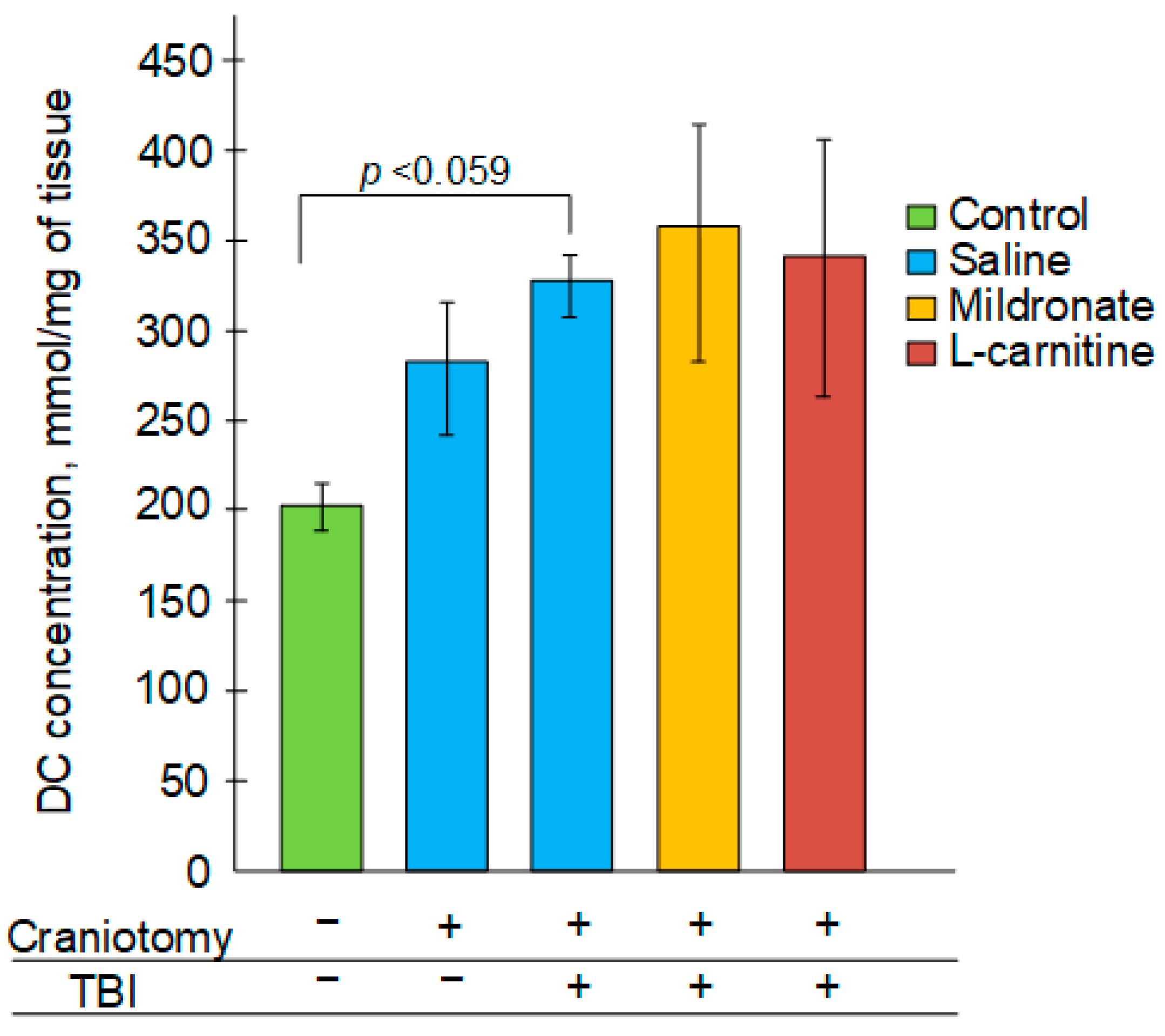
2.2. The Effect of TBI and Metabolic Modulators on the Level of Diene Conjugates in the Brain
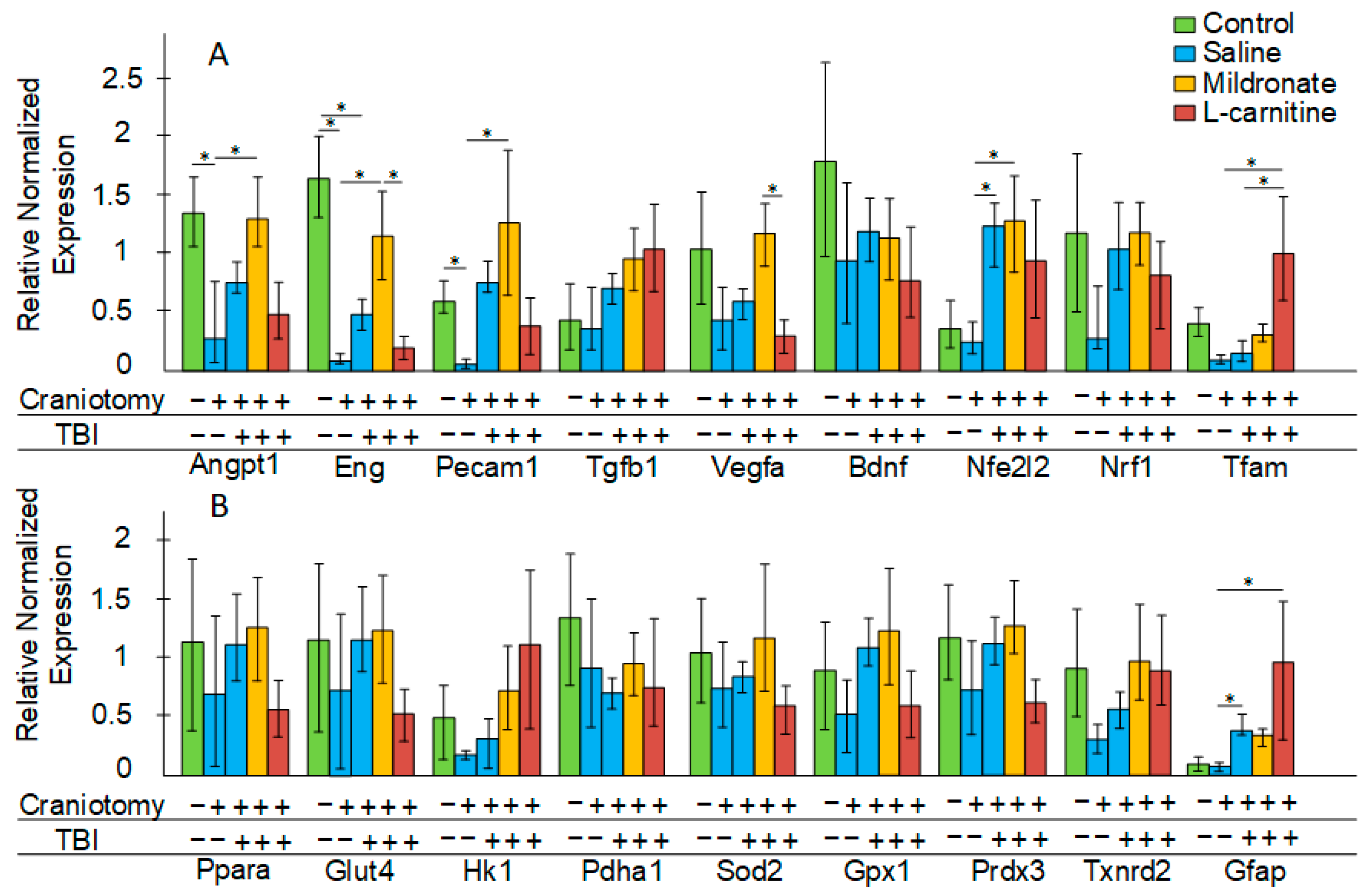
2.3. The Effect of TBI and Metabolic Modulators on Gene Expression in the Brain
2.4. The Effect of TBI and Metabolic Modulators on Gene Expression in Blood
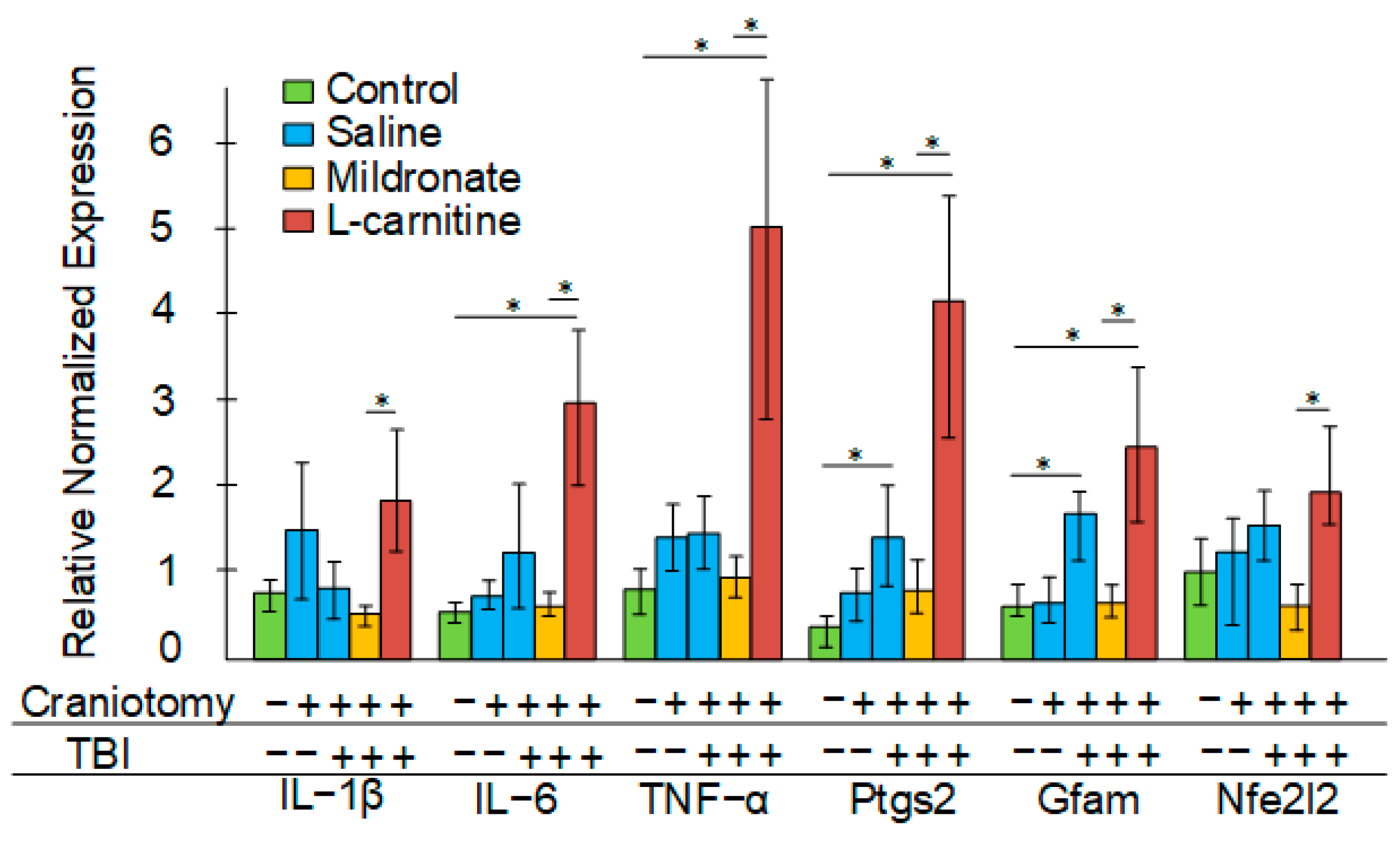
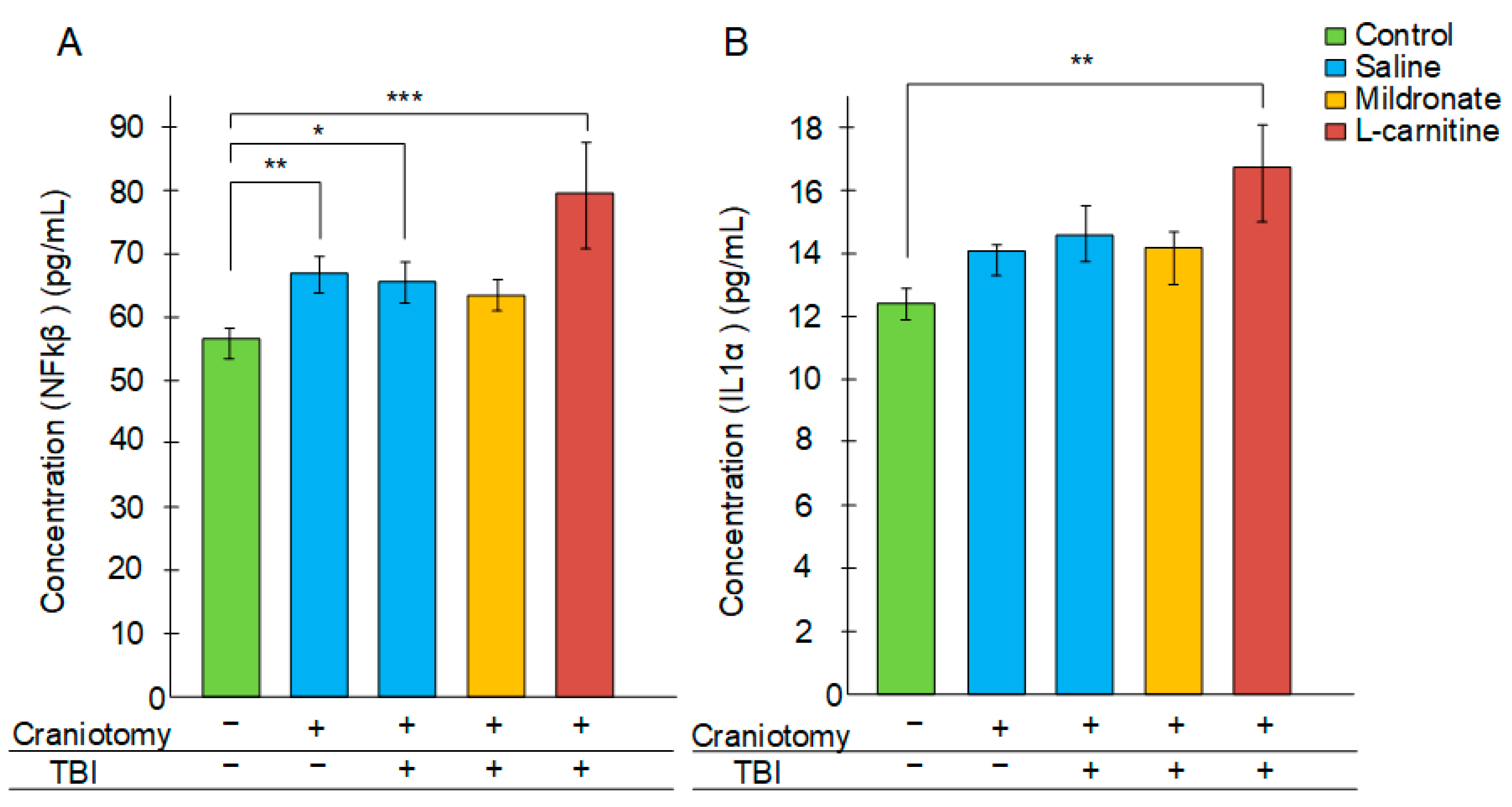
2.5. The Effect of TBI and Metabolic Switching on the Levels of Inflammatory Markers in the Blood
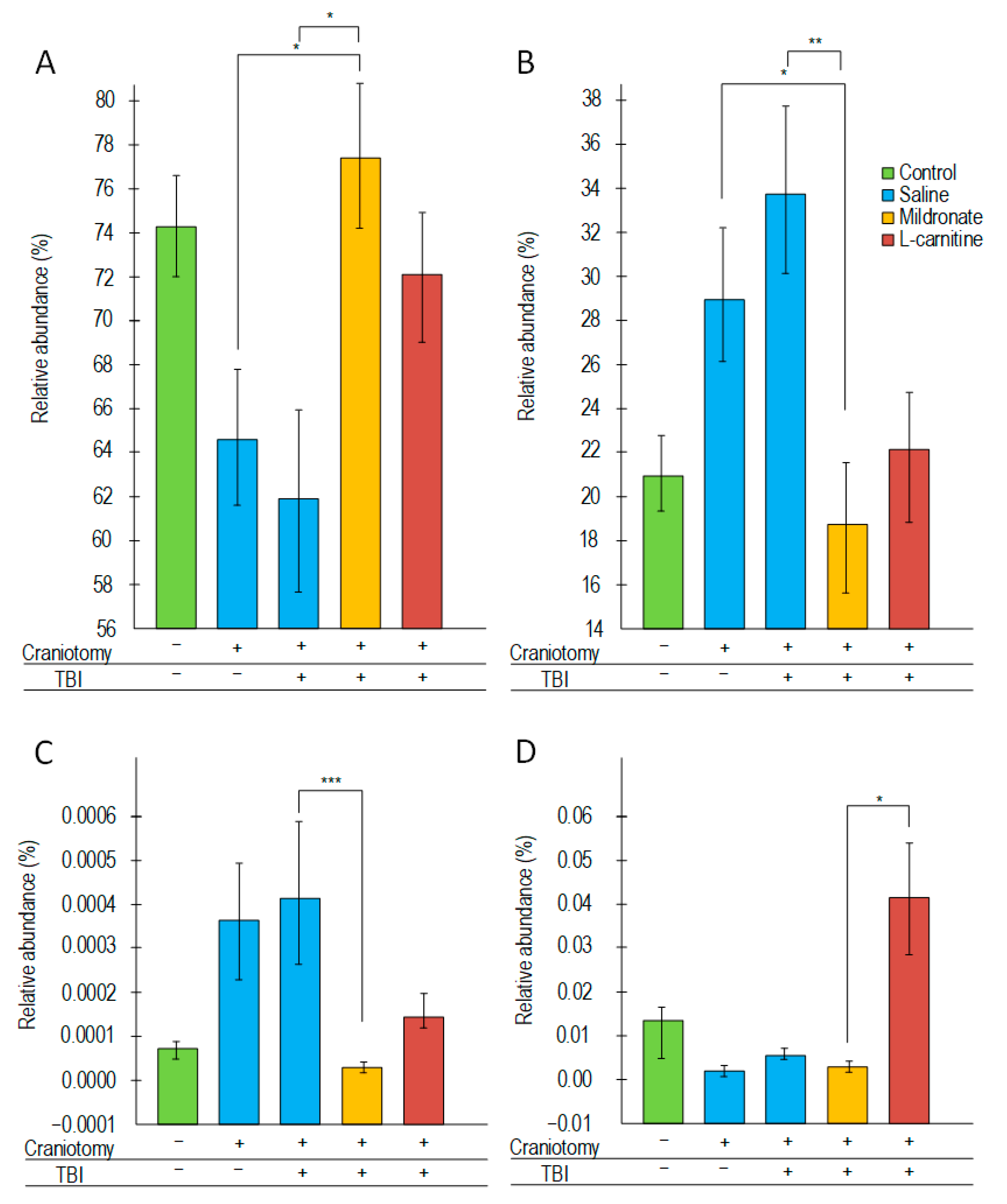
2.6. Changes in the Structure of the Bacterial Composition of the Gut Microbiome
3. Discussion
4. Materials and Methods
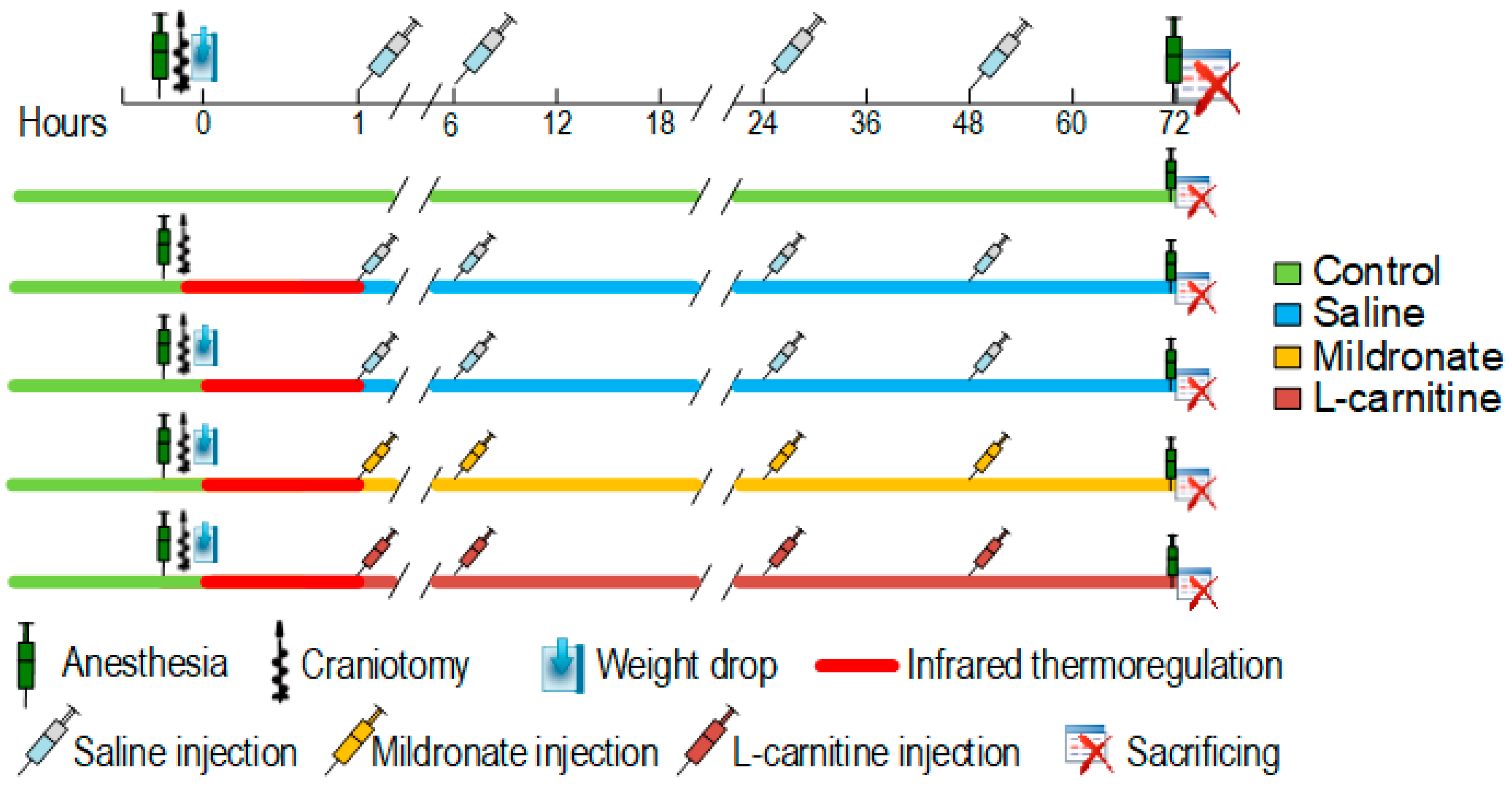
4.1. Animals and Experimental Design
4.2. Modeling of TBI
4.3. Measurement of Gene Expression
4.4. Study of mtDNA Quality Control
4.5. Assessment of the Level of Diene Conjugates
4.6. Measurement of Pro-Inflammatory Cytokines
4.7. Assessment of the Bacterial Composition of the Intestinal Microbiome
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBI | Traumatic brain injury |
| mtDNA | Mitochondrial DNA |
| BBB | Blood–brain barrier |
| ROS | Reactive oxygen species |
| CNS | Central nervous system |
| NO | Nitric oxide |
| PTSD | Post-traumatic stress disorder |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| NOS | Nitric oxide synthase |
| NFκB | Nuclear Factor Kappa B |
| Angpt1 | Angiopoietin 1 |
| Eng | Endoglin |
| Pecam | Platelet/endothelial cell adhesion molecule 1 |
| Vegfa | Vascular endothelial growth factor A |
| Tgfb1 | Transforming growth factor beta 1 |
| Bdnf | Brain-derived neurotrophic factor |
| Nfe2l2 | Nuclear factor erythroid 2-like 2 |
| Nrf1 | Nuclear respiratory factor 1 |
| Tfam | Transcription factor A |
| Ppara | Peroxisome proliferator-activated receptor alpha |
| Glut4 | Glucose transporter type 4 |
| Hk1 | Hexokinase-1 |
| Pdha1 | Pyruvate dehydrogenase E1 component subunit alpha |
| Sod2 | Superoxide dismutase 2 |
| Gpx1 | Glutathione peroxidase 1 |
| Prdx3 | Peroxiredoxin 3 |
| Txnrd2 | Thioredoxin reductase 2 |
| Gfap | Glial fibrillary acidic protein |
| Ptgs2 | Prostaglandin-endoperoxide synthase 2 |
| Il1b | Interleukin 1 beta |
| Il6 | Interleukin 6 |
| Tnf | Tumor necrosis factor |
References
- Dumurgier, J.; Tzourio, C. Epidemiology of neurological diseases in older adults. Rev. Neurol. 2020, 176, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Butkova, T.V.; Malsagova, K.A.; Nakhod, V.I.; Petrovskiy, D.V.; Izotov, A.A.; Balakin, E.I.; Yurku, K.A.; Umnikov, A.S.; Pustovoyt, V.I.; Kaysheva, A.L. Candidate Molecular Biomarkers of Traumatic Brain Injury: A Systematic Review. Biomolecules 2024, 14, 1283. [Google Scholar] [CrossRef]
- Mira, R.G.; Quintanilla, R.A.; Cerpa, W. Mild Traumatic Brain Injury Induces Mitochondrial Calcium Overload and Triggers the Upregulation of NCLX in the Hippocampus. Antioxidants 2023, 12, 403. [Google Scholar] [CrossRef]
- Cheng, G.; Kong, R.; Zhang, L.; Zhang, J. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br. J. Pharmacol. 2012, 167, 699–719. [Google Scholar] [CrossRef]
- Isaev, N.K.; Genrikhs, E.E.; Stelmashook, E.V. Methylene blue and its potential in the treatment of traumatic brain injury, brain ischemia, and Alzheimer’s disease. Prog. Neurobiol. 2024, 35, 585–595. [Google Scholar] [CrossRef]
- Gureev, A.P.; Nesterova, V.V.; Sadovnikova, I.S. Long-range PCR as a tool for evaluating mitochondrial DNA damage: Principles, benefits, and limitations of the technique. DNA Repair. 2025, 146, 103812. [Google Scholar] [CrossRef]
- Lifshitz, J.; McIntosh, T.K. Age-associated mitochondrial DNA deletions are not evident chronically after experimental brain injury in the rat. J. Neurotrauma 2003, 20, 139–149. [Google Scholar] [CrossRef]
- McDonald, R.P.; Horsburgh, K.J.; Graham, D.I.; Nicoll, J.A. Mitochondrial DNA deletions in acute brain injury. NeuroReport 1999, 10, 1875–1878. [Google Scholar] [CrossRef]
- Rickards, L.; Lynn, A.; Barker, M.E.; Russell, M.; Ranchordas, M.K. Comparison of the polyphenol content and in vitro antioxidant capacity of fruit-based nutritional supplements commonly consumed by athletic and recreationally active populations. J. Int. Soc. Sports Nutr. 2022, 19, 336–348. [Google Scholar] [CrossRef]
- Wang, W.; Pan, D.; Liu, Q.; Chen, X.; Wang, S. L-Carnitine in the Treatment of Psychiatric and Neurological Manifestations: A Systematic Review. Nutrients 2024, 16, 1232. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Shokouhi, G.; Hamishehkar, H.; Soleimanpour, H.; Sanaie, S.; Porhomayon, J.; Rasouli, F.; Nader, N.D. A pilot trial of l -carnitine in patients with traumatic brain injury: Effects on biomarkers of injury. J. Crit. Care 2018, 45, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Salta, S.; Bravo, J.; Silva, A.P.; Summavielle, T. Acetyl-L-Carnitine Prevents Methamphetamine-Induced Structural Damage on Endothelial Cells via ILK-Related MMP-9 Activity. Mol. Neurobiol. 2016, 53, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Sayed-Ahmed, M.M.; Al-Omar, F.A.; Al-Yahya, A.A.; Aleisa, A.M.; Al-Shabanah, O.A. Carnitine esters prevent oxidative stress damage and energy depletion following transient forebrain ischaemia in the rat hippocampus. Clin. Exp. Pharmacol. Physiol. 2006, 33, 725–733. [Google Scholar] [CrossRef]
- Leverve, X.; Batandier, C.; Fontaine, E. Choosing the right substrate. Novartis Found. Symp. 2007, 280, 108–121. [Google Scholar]
- Suzuki, Y.; Kitagawa, Y.; Matsuoka, Y.; Fukuda, J.; Mizushima, Y. Severe cerebral and systemic necrotizing vasculitis developing during pregnancy in a case of systemic lupus erythematosus. J. Rheumatol. 1990, 17, 1408–1411. [Google Scholar]
- Dambrova, M. Mildronate cardioprotective action through carnitine-lowering effect. Trends Cardiovasc. Med. 2002, 12, 275–279. [Google Scholar] [CrossRef]
- Liu, F.; Sui, X.; Wang, Q.; Li, J.; Yang, W.; Yang, Y.; Xiao, Z.; Sun, Y.; Guo, X.; Yang, X.; et al. Insights into the pharmacodynamics and pharmacokinetics of meldonium after exposure to acute high altitude. Front. Pharmacol. 2023, 14, 1119046. [Google Scholar] [CrossRef]
- Dzintare, M.; Baumane, L.; Meirena, D.; Lauberte, L.; Kalvinsh, I.; Sjakste, N. Involvement of Nitric Oxide Production in the Mildronate Mechanism of Action. Pharmacol. Rev. Commun. 2002, 12, 163–170. [Google Scholar] [CrossRef]
- Sjakste, N.; Baumane, L.; Boucher, J.; Dzintare, M.; Meirena, D.; Sjakste, J.; Lauberte, L.; Kalvinsh, I. Effects of gamma-butyrobetaine and mildronate on nitric oxide production in lipopolysaccharide-treated rats. Basic. Clin. Pharmacol. Toxicol. 2004, 94, 46–50. [Google Scholar] [CrossRef]
- Demir, D.; Bektaşoğlu, P.K.; Koyuncuoğlu, T.; Kandemir, C.; Akakın, D.; Yüksel, M.; Çelikoğlu, E.; Yeğen, B.Ç.; Gürer, B. Neuroprotective effects of mildronate in a rat model of traumatic brain injury. Injury 2019, 50, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.T.; Yarnell, A.; Kean, W.S.; Gold, E.; Lewis, B.; Ren, M.; McMullen, D.C.; Jacobowitz, D.M.; Pollard, H.B.; O’Neill, J.T.; et al. Craniotomy: True sham for traumatic brain injury, or a sham of a sham? J. Neurotrauma 2011, 28, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Osier, N.; Dixon, C.E. The Controlled Cortical Impact Model of Experimental Brain Trauma: Overview, Research Applications, and Protocol. Methods Mol. Biol. 2016, 1462, 177–192. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Nourhaghighi, N.; Teichert-Kuliszewska, K.; Davis, J.; Stewart, D.J.; Nag, S. Altered expression of angiopoietins during blood-brain barrier breakdown and angiogenesis. Mod. Pathol. 2003, 83, 1211–1222. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, L.; Zhang, R.; Su, H. The roles of endoglin gene in cerebrovascular diseases. Neuroimmunol. Neuroinflammation 2017, 4, 199–210. [Google Scholar] [CrossRef]
- Wimmer, I.; Tietz, S.; Nishihara, H.; Deutsch, U.; Sallusto, F.; Gosselet, F.; Lyck, R.; Muller, W.A.; Lassmann, H.; Engelhardt, B. PECAM-1 Stabilizes Blood-Brain Barrier Integrity and Favors Paracellular T-Cell Diapedesis Across the Blood-Brain Barrier During Neuroinflammation. Front. Immunol. 2019, 10, 711. [Google Scholar] [CrossRef]
- Hwang, I.K.; Kim, D.W.; Yoo, K.; Jung, B.; Song, J.; Jung, J.; Choi, S.Y.; Kang, T.; Lee, J.; Kwon, Y.; et al. Ischemia-induced changes of platelet endothelial cell adhesion molecule-1 in the hippocampal CA1 region in gerbils. Brain Res. 2005, 1048, 251–257. [Google Scholar] [CrossRef]
- Carlos, T.M.; Clark, R.S.B.; Franicola-Higgins, D.; Schiding, J.K.; Kochanek, P.M. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J. Leukoc. Biol. 1997, 61, 279–285. [Google Scholar] [CrossRef]
- Michinaga, S.; Tanabe, A.; Nakaya, R.; Fukutome, C.; Inoue, A.; Iwane, A.; Minato, Y.; Tujiuchi, Y.; Miyake, D.; Mizuguchi, H.; et al. Angiopoietin-1/Tie-2 signal after focal traumatic brain injury is potentiated by BQ788, an ETB receptor antagonist, in the mouse cerebrum: Involvement in recovery of blood–brain barrier function. J. Neurochem. 2020, 154, 330–348. [Google Scholar] [CrossRef]
- Qi, R.; Sammler, E.; Gonzalez-Hunt, C.P.; Barraza, I.; Pena, N.; Rouanet, J.P.; Naaldijk, Y.; Goodson, S.; Fuzzati, M.; Blandini, F.; et al. A blood-based marker of mitochondrial DNA damage in Parkinson’s disease. Sci. Transl. Med. 2023, 15, eabo1557. [Google Scholar] [CrossRef] [PubMed]
- Sanders, L.H.; Howlett, E.H.; McCoy, J.; Greenamyre, J.T. Mitochondrial DNA damage as a peripheral biomarker for mitochondrial toxin exposure in rats. Toxicol. Sci. 2014, 142, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Sadovnikova, I.S.; Chernyshova, E.V.; Tsvetkova, A.D.; Babenkova, P.I.; Nesterova, V.V.; Krutskikh, E.P.; Volodina, D.E.; Samoylova, N.A.; Andrianova, N.V.; et al. Beta-Hydroxybutyrate Mitigates Sensorimotor and Cognitive Impairments in a Photothrombosis-Induced Ischemic Stroke in Mice. Int. J. Mol. Sci. 2024, 25, 5710. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Silachev, D.N.; Sadovnikova, I.S.; Krutskikh, E.P.; Chernyshova, E.V.; Volodina, D.E.; Samoylova, N.A.; Potanina, D.V.; Burakova, I.Y.; Smirnova, Y.D.; et al. The Ketogenic Diet but not Hydroxycitric Acid Keeps Brain Mitochondria Quality Control and mtDNA Integrity Under Focal Stroke. Mol. Neurobiol. 2023, 60, 4288–4303. [Google Scholar] [CrossRef]
- Haugen, A.C.; Di Prospero, N.A.; Parker, J.S.; Fannin, R.D.; Chou, J.; Meyer, J.N.; Halweg, C.; Collins, J.B.; Durr, A.; Fischbeck, K.; et al. Altered gene expression and DNA damage in peripheral blood cells from Friedreich’s ataxia patients: Cellular model of pathology. PLoS Genet. 2010, 6, e1000812. [Google Scholar] [CrossRef]
- Darbinian, N.; Darbinyan, A.; Merabova, N.; Selzer, M.E.; Amini, S. HIV-1 and HIV-1-Tat Induce Mitochondrial DNA Damage in Human Neurons. J. HIV AIDS 2020, 6, 176. [Google Scholar] [CrossRef]
- Karunadharma, P.P.; Nordgaard, C.L.; Olsen, T.W.; Ferrington, D.A. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Investig. Opthalmol. Vis. Sci. 2010, 51, 5470–5479. [Google Scholar] [CrossRef]
- Fesharaki-Zadeh, A. Oxidative Stress in Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 13000. [Google Scholar] [CrossRef]
- Kolesnikova, L.I.; Semyonova, N.V.; Grebenkina, L.A.; Darenskaya, M.A.; Suturina, L.V.; Gnusina, S.V. Integral indicator of oxidative stress in human blood. Bull. Exp. Biol. Med. 2014, 157, 715–717. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Srivastava, A.; Pawar, N.; Sagarkar, S.; Sakharkar, A.J. Repeated mild traumatic brain injury induces persistent variations in mitochondrial DNA copy number in mesocorticolimbic neurocircuitry of the rat. Neurosci. Res. 2020, 155, 34–42. [Google Scholar] [CrossRef]
- Bersani, F.S.; Morley, C.; Lindqvist, D.; Epel, E.S.; Picard, M.; Yehuda, R.; Flory, J.; Bierer, L.M.; Makotkine, I.; Abu-Amara, D.; et al. Mitochondrial DNA copy number is reduced in male combat veterans with PTSD. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bounes, F.V.; Faure, G.; Rouget, A.; Conil, J.-M.; Georges, B.; Geeraerts, T.; Fourcade, O.; Minville, V.; Delmas, C. Plasma free carnitine in severe trauma: Influence of the association with traumatic brain injury. Injury 2018, 49, 538–542. [Google Scholar] [CrossRef]
- Voronkov, A.V.; Pozdnyakov, D.I. Neuroprotective effect of L-carnitine. Focus on changing mitochondrial function. Res. Results Pharmacol. 2020, 6, 29–42. [Google Scholar] [CrossRef]
- Liu, S.-H.; Zhang, Y.-C. Effect of levocarnitine on cerebral ischemia-reperfusion rats via activating Nrf2/ARE signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8168–8174. [Google Scholar] [CrossRef]
- Hiskens, M.I.; Li, K.M.; Schneiders, A.G.; Fenning, A.S. Repetitive mild traumatic brain injury-induced neurodegeneration and inflammation is attenuated by acetyl-L-carnitine in a preclinical model. Front. Pharmacol. 2023, 14, 1254382. [Google Scholar] [CrossRef]
- Bordoni, L.; Sawicka, A.K.; Szarmach, A.; Winklewski, P.J.; Olek, R.A.; Gabbianelli, R. A Pilot Study on the Effects of l-Carnitine and Trimethylamine-N-Oxide on Platelet Mitochondrial DNA Methylation and CVD Biomarkers in Aged Women. Int. J. Mol. Sci. 2020, 21, 1047. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Yang, F.; Zhao, R.; Pan, X.; Liang, J.; Tian, L.; Li, X.; Liu, L.; Xing, Y.; et al. Gut Microbiota-Dependent Marker TMAO in Promoting Cardiovascular Disease: Inflammation Mechanism, Clinical Prognostic, and Potential as a Therapeutic Target. Front. Pharmacol. 2019, 10, 1360. [Google Scholar] [CrossRef]
- Nam, H.S. Gut Microbiota and Ischemic Stroke: The Role of Trimethylamine N-Oxide. J. Stroke 2019, 21, 151–159. [Google Scholar] [CrossRef]
- Annunziata, G.; Ciampaglia, R.; Capò, X.; Guerra, F.; Sureda, A.; Tenore, G.C.; Novellino, E. Polycystic ovary syndrome and cardiovascular risk. Could trimethylamine N-oxide (TMAO) be a major player? A potential upgrade forward in the DOGMA theory. Biomed. Pharmacother. 2021, 143, 112171. [Google Scholar] [CrossRef]
- Nicassio, L.; Fracasso, F.; Sirago, G.; Musicco, C.; Picca, A.; Marzetti, E.; Calvani, R.; Cantatore, P.; Gadaleta, M.N.; Pesce, V. Dietary supplementation with acetyl- l -carnitine counteracts age-related alterations of mitochondrial biogenesis, dynamics and antioxidant defenses in brain of old rats. Exp. Gerontol. 2017, 98, 99–109. [Google Scholar] [CrossRef]
- Terpolilli, N.A.; Kim, S.-W.; Thal, S.C.; Kuebler, W.M.; Plesnila, N. Inhaled nitric oxide reduces secondary brain damage after traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 2012, 33, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.; Pagnussat, A.S.; Seo, J.H.; Navaratna, D.; Leung, W.; Lok, J.; Guo, S.; Waeber, C.; Salbego, C.G.; Lo, E.H. Pro-angiogenic effects of resveratrol in brain endothelial cells: Nitric oxide-mediated regulation of vascular endothelial growth factor and metalloproteinases. J. Cereb. Blood Flow Metab. 2012, 32, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Dusserre, N.; L’Heureux, N.; Bell, K.S.; Stevens, H.Y.; Yeh, J.; Otte, L.A.; Loufrani, L.; Frangos, J.A. PECAM-1 interacts with nitric oxide synthase in human endothelial cells: Implication for flow-induced nitric oxide synthase activation. Arter. Thromb. Vasc. Biol. 2004, 24, 1796–1802. [Google Scholar] [CrossRef][Green Version]
- Verdam, F.J.; Fuentes, S.; de Jonge, C.; Zoetendal, E.G.; Erbil, R.; Greve, J.W.; Buurman, W.A.; de Vos, W.M.; Rensen, S.S. Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 2013, 21, E607–E615. [Google Scholar] [CrossRef]
- Hou, J.-Y.; Xu, H.; Cao, G.-Z.; Tian, L.-L.; Wang, L.-H.; Zhu, N.-Q.; Zhang, J.-J.; Yang, H.-J. Multi-omics reveals Dengzhan Shengmai formulation ameliorates cognitive impairments in D-galactose-induced aging mouse model by regulating CXCL12/CXCR4 and gut microbiota. Front. Pharmacol. 2023, 14, 1175970. [Google Scholar] [CrossRef]
- Beitnere, U.; van Groen, T.; Kumar, A.; Jansone, B.; Klusa, V.; Kadish, I. Mildronate improves cognition and reduces amyloid-β pathology in transgenic Alzheimer’s disease mice. J. Neurosci. Res. 2014, 92, 338–346. [Google Scholar] [CrossRef]
- e Luz, E.W.M.; Vieira, L.R.; Semedo, J.G.; Bona, S.R.; Forgiarini, L.F.; Pereira, P.; Cavalcante, A.A.M.; Marroni, N.A.P.; Picada, J.N. Neurobehavioral effects of l-carnitine and its ability to modulate genotoxicity and oxidative stress biomarkers in mice. Pharmacol. Biochem. Behav. 2013, 110, 40–45. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Chen, M.-K.; Yang, B.-Y.; Huang, X.-J.; Zhang, X.-R.; He, L.-Q.; Zhang, J.; Hua, Z.-C. Use of 16S rRNA Gene-Targeted Group-Specific Primers for Real-Time PCR Analysis of Predominant Bacteria in Mouse Feces. Appl. Environ. Microbiol. 2015, 81, 6749–6756. [Google Scholar] [CrossRef]
| Gene | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|
| 18s | CGGCTACCACATCCAAGGAA | GCTGGAATTACTGTGGCT |
| Angpt1 | AGCAATCCTTAGCATAGGGGC | TGTGTAACCGTTCAGCGTGG |
| Bdnf | AAGGACGCGGACTTGTACAC | CGCTAATACTGTCACACACGC |
| Eng | CAACTTAGCTCTGCGCCCTA | GGTGGAGGCTTGGGATACTC |
| Gapdh | GGCTCCCTAGGCCCCTCCTG | TCCCAACTCGGCCCCCAACA |
| Gfap | CGAAGAAAACCGCATCACCA | CCGCATCTCCACCGTCTTTA |
| Glut4 | CCTCCCGCCCTTAGTTG | CTGCAAAGCGTAGGTACCA |
| Gpx1 | AGTCCACCGTGTATGCCTTC | GTGTCCGAACTGATTGCACG |
| Hk1 | GTTCGAGAAGATGGTGAGCG | AGAGTTCCCATCCCGTTTCA |
| Nfe2l2 | CTCTCTGAACTCCTGGACGG | GGGTCTCCGTAAATGGAAG |
| Nrf1 | AGCACGGAGTGACCCAAA | TGTACGTGGCTACATGGACCT |
| Pdha1 | GTTTTGGGCGTGGCTTCG | GGCTTGCCGGCTTCTG |
| Pecam1 | GGAACGAGAGCCACAGAGAC | TTCCATTAAGGGAGCCTTCCG |
| Ppara | AGAGCCCCATCTGTCCTCTC | ACTGGTAGTCTGCAAAACCAAA |
| Prdx3 | GGTTGCTCGTCATGCAAGTG | CCACAGTATGTCTGTCAAACA |
| Sod2 | CAGACCTGCCTTACGACTATGG | CTCGGTGGCGTTGAGATTGTT |
| Tfam | ATTCCGAAGTGTTTTTCCAGCA | TCTGAAAGTTTTCGATCTGGGT |
| Tgfb1 | CATGACATGAACCGGCCCTT | GAAGTTGGCATGGTAGCCCT |
| Txnrd2 | GATCTCTTGGTGATCGGTGGG | CGGGGAGAGGGTTCCACATA |
| Vegfa | TATTCAGCGGACTCACCAGC | AACCAACCTCCTCAAACCGT |
| Angpt1 | AGCAATCCTTAGCATAGGGGC | TGTGTAACCGTTCAGCGTGG |
| Bdnf | AAGGACGCGGACTTGTACAC | CGCTAATACTGTCACACACGC |
| Eng | CAACTTAGCTCTGCGCCCTA | GGTGGAGGCTTGGGATACTC |
| Group | ID Primers | The Nucleotide Sequence (5′-3′) |
|---|---|---|
| Bacteroidetes | Bac960F | GTTTAATTCGATGATACGCGAG |
| Bac1100R | TTAASCCGACACCTCACGG | |
| Firmicutes | Firm934F | GGAGYATGTGGTTTAATTCGAAGCA |
| Firm1060R | AGCTGACGACAACCATGCAC | |
| Actinobacteria | Act664F | TGTAGCGGTGGAATGCGC |
| Act941R | AATTAAGCCACATGCTCCGCT | |
| Betaproteobacteria | Beta979F | AACGCGAAAAACCTTACCTACC |
| Beta1130R | TGCCCTTTCGTAGCAACTAGTG | |
| Gammaproteobacteria | Gamma877F | GCTAACGCATTAAGTRYCCCG |
| Gamma1066 R | GCCATGCRGCACCTGTCT | |
| Epsilonproteobacteria | Epsilon940F | TAGGCTTGACATTGATAGAATC |
| Epsilon1129 R | CTTACGAAGGCAGTCTCCTTA | |
| Deferribacteres | Defer1115F | CTATTTCCAGTTGCTAACGG |
| Defer1265R | GAGHTGCTTCCCTCTGATTATG | |
| Saccharibacteria | Sac1031F | AAGAGAACTGTGCCTTCGG |
| Sac1218R | GCGTAAGGGAAATACTGACC | |
| Tenericutes | Ten662F | ATGTGTAGCGGTAAAATGCGTAA |
| Ten862R | CMTACTTGCGTACGTACTACT | |
| Verrucomicrobia | Ver1165F | TCAKGTCAGTATGGCCCTTAT |
| Ver1263R | CAGTTTTYAGGATTTCCTCCGCC | |
| Universal | 926F | AAACTCAAAKGAATTGACGG |
| 1062R | CTCACRRCACGAGCTGAC |
| ID Primer | Forward Primer 5′-3′ | ID Primer | Reverse Primer 5′-3′ |
|---|---|---|---|
| ChrM: Pr. 1 | TAAATTTCGTGCCAGCCACC | ChrM: Pr. 1 (long) | ATGCTACCTTTGCACGGTCA |
| ChrM: Pr. 2 | ACGAGGGTCCAACTGTCTCTTA | ChrM: Pr. 2 (short) | AGCTCCATAGGGTCTTCTCGT |
| ChrM: Pr. 2 (long) | CCGGCTGCGTATTCTACGTT | ||
| ChrM: Pr. 3 | CTAGCAGAAACAAACCGGGC | ChrM: Pr. 3 (long) | TTAGGGCTTTGAAGGCTCGC |
| ChrM: Pr. 7 | TCATTCTTCTACTATCCCCAATCC | ChrM: Pr. 7 (long) | TGGTTTGGGAGATTGGTTGATG |
| ChrM: Pr. 8 | CCCCAATCCCTCCTTCCAAC | ChrM: Pr. 8 (long) | GGTGGGGAGTAGCTCCTTCTT |
| ChrM: Pr. 9 | AAGAAGGAGCTACTCCCCACC | ChrM: Pr. 9 (long) | GTTGACACGTTTTACGCCGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gureev, A.P.; Nesterova, V.V.; Babenkova, P.I.; Ivanov, M.E.; Plotnikov, E.Y.; Silachev, D.N. L-Carnitine and Mildronate Demonstrate Divergent Protective Effects on Mitochondrial DNA Quality Control and Inflammation Following Traumatic Brain Injury. Int. J. Mol. Sci. 2025, 26, 2902. https://doi.org/10.3390/ijms26072902
Gureev AP, Nesterova VV, Babenkova PI, Ivanov ME, Plotnikov EY, Silachev DN. L-Carnitine and Mildronate Demonstrate Divergent Protective Effects on Mitochondrial DNA Quality Control and Inflammation Following Traumatic Brain Injury. International Journal of Molecular Sciences. 2025; 26(7):2902. https://doi.org/10.3390/ijms26072902
Chicago/Turabian StyleGureev, Artem P., Veronika V. Nesterova, Polina I. Babenkova, Mikhail E. Ivanov, Egor Y. Plotnikov, and Denis N. Silachev. 2025. "L-Carnitine and Mildronate Demonstrate Divergent Protective Effects on Mitochondrial DNA Quality Control and Inflammation Following Traumatic Brain Injury" International Journal of Molecular Sciences 26, no. 7: 2902. https://doi.org/10.3390/ijms26072902
APA StyleGureev, A. P., Nesterova, V. V., Babenkova, P. I., Ivanov, M. E., Plotnikov, E. Y., & Silachev, D. N. (2025). L-Carnitine and Mildronate Demonstrate Divergent Protective Effects on Mitochondrial DNA Quality Control and Inflammation Following Traumatic Brain Injury. International Journal of Molecular Sciences, 26(7), 2902. https://doi.org/10.3390/ijms26072902








