Salivary Metabolomics as a Diagnostic Tool: Distinct Metabolic Profiles Across Orofacial Pain Subtypes
Abstract
1. Introduction
2. Results
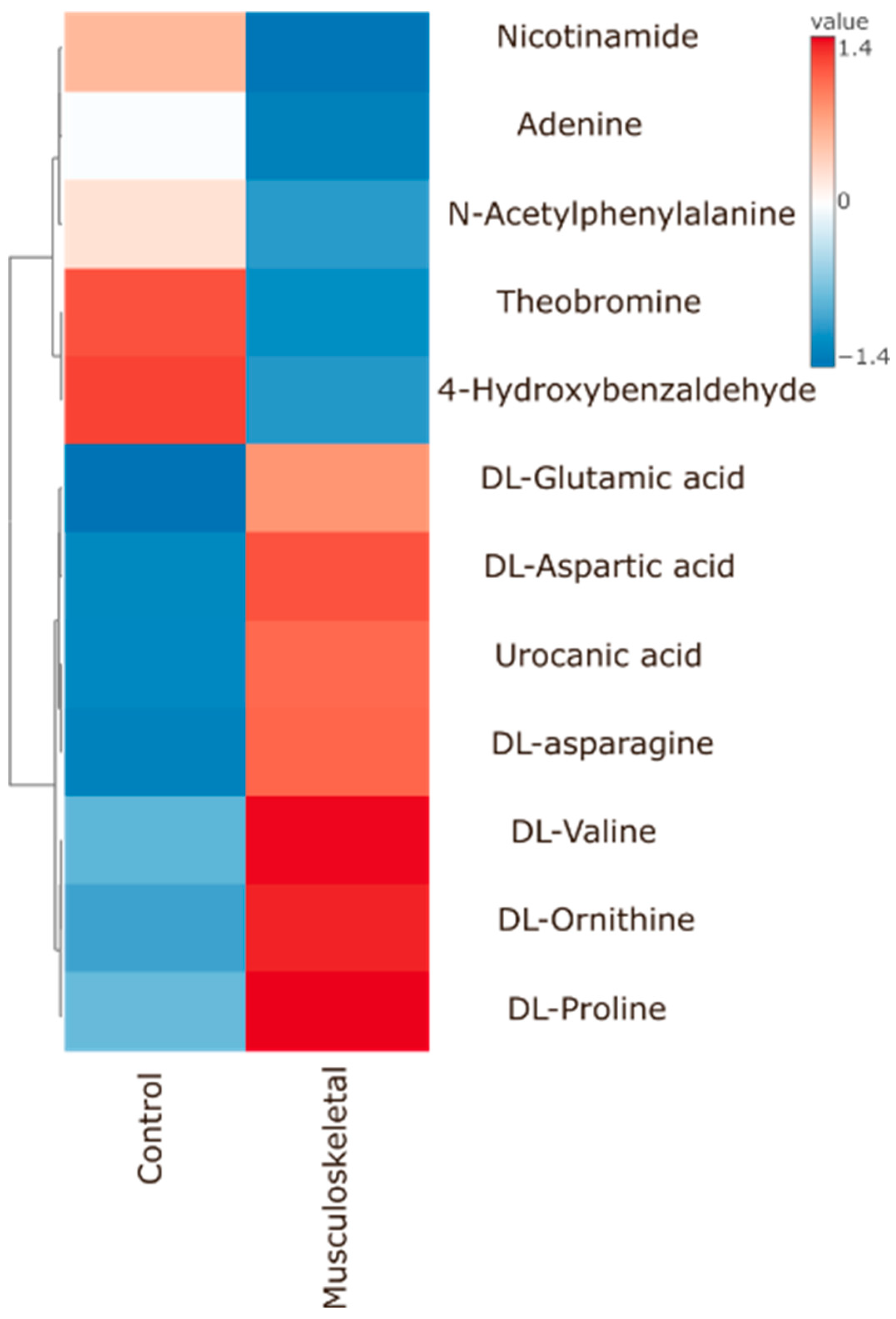
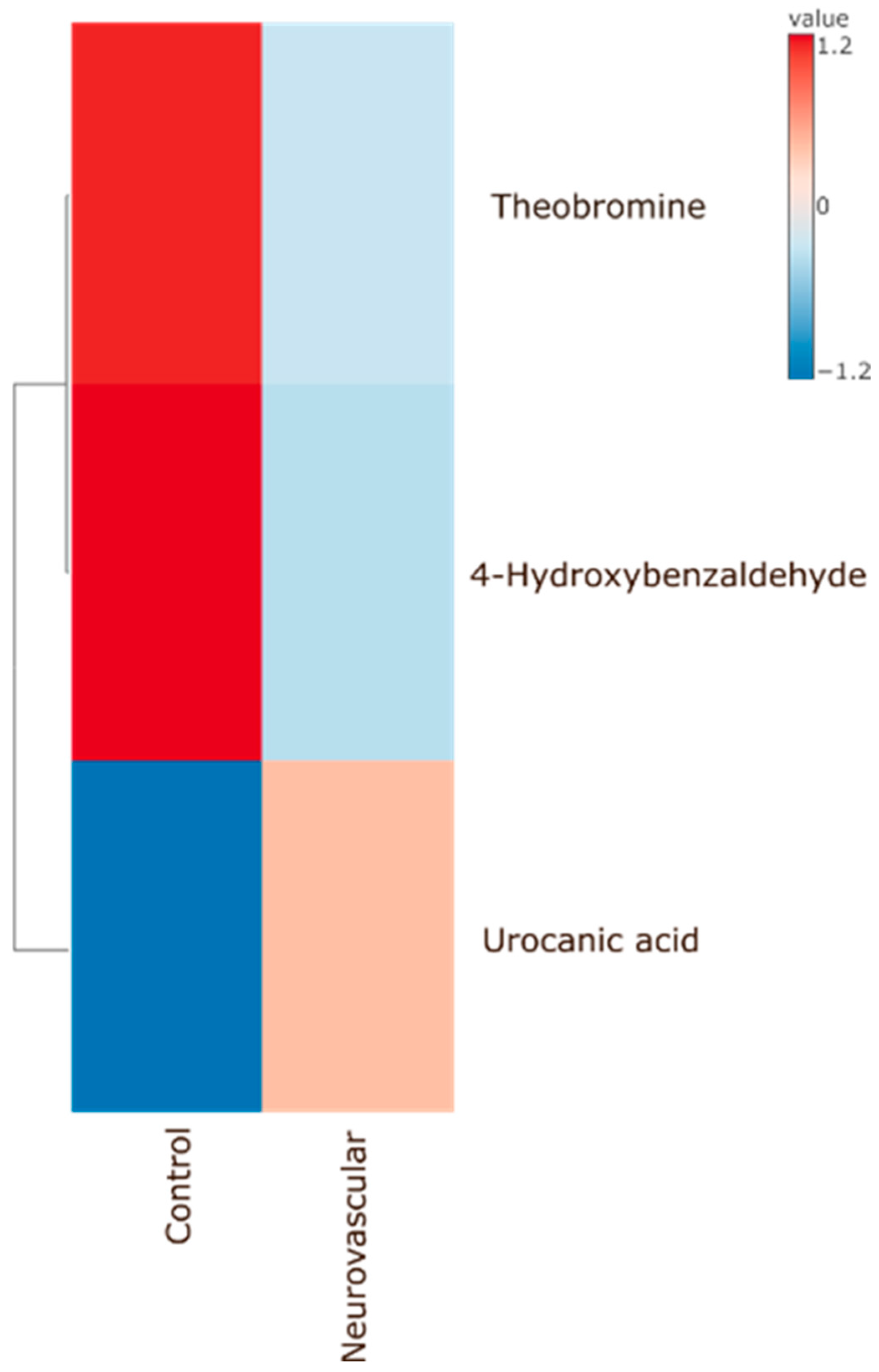
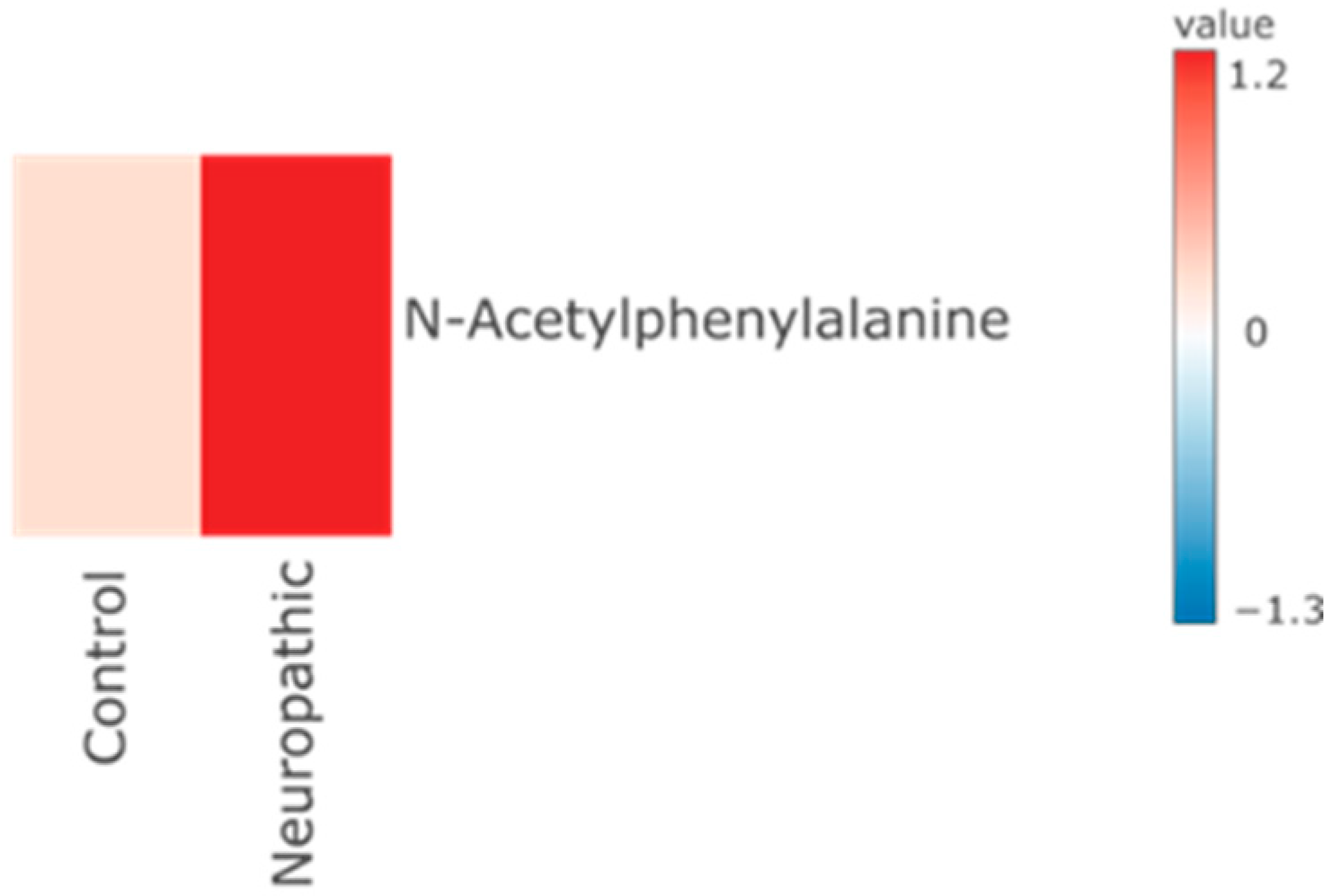
Significant Metabolites to Pain (As Compared to Control) According to Pain Types
3. Discussion
4. Materials and Methods
4.1. Criteria and Collection of Medical Records
4.2. Orofacial Pain Diagnosis
4.2.1. Musculoskeletal Group
4.2.2. Neurovascular Group
4.2.3. Neuropathic Group
4.3. Collection of Medical Records
4.4. Saliva Collection
4.5. Sample Preparation
4.6. Statistical Analysis and Data Visualization
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Macfarlane, T.V.; Kincey, J.; Worthington, H.V. The association between psychological factors and oro-facial pain: A community-based study. Eur. J. Pain 2002, 6, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Sharav, Y.; Benoliel, R. Orofacial Pain and Headache; Elsevier Ltd.: Amsterdam, The Netherlands, 2008; Chapter 3; pp. 45–56. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (HIS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef] [PubMed]
- Benoliel, R.; May, A.; Svensson, P.; Pigg, M.; Law, A.; Nixdorf, D.; Renton, T.; Sharav, Y.; Ernberg, M.; Peck, C.; et al. International Classification of Orofacial Pain, 1st edition (ICOP). Cephalalgia 2020, 40, 129–221. [Google Scholar] [CrossRef]
- D’andrea, G.; D’amico, D.; Bussone, G.; Bolner, A.; Aguggia, M.; Saracco, M.G.; Galloni, E.; De Riva, V.; D’arrigo, A.; Colavito, D.; et al. Tryptamine levels are low in plasma of chronic migraine and chronic tension-type headache. Neurol. Sci. 2014, 35, 1941–1945. [Google Scholar] [CrossRef]
- Sanches, M.L.; Sforça, M.L.; Turco, E.G.L.; Faber, J.; Smith, R.L.; de Moraes, L.O.C. 1H-NMR-Based salivary metabolomics from females with temporomandibular disorders—A pilot study. Clin. Chim. Acta 2020, 510, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Mäntyselkä, P.; Ali-Sisto, T.; Kautiainen, H.; Niskanen, L.; Viinamäki, H.; Velagapudi, V.; Lehto, S.M. The association between musculoskeletal pain and circulating ornithine: A population-based study. Pain Med. 2017, 18, 1145–1151. [Google Scholar] [CrossRef]
- Livshits, G.; Malkin, I.; Bowyer, R.C.E.; Verdi, S.; Bell, J.T.; Menni, C.; Williams, F.M.K.; Steves, C.J. Multi-OMICS analyses of frailty and chronic widespread musculoskeletal pain suggest involvement of shared neurological pathways. Pain 2018, 159, 2565–2572. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered kynurenine pathway metabolites in serum of chronic migraine patients. J. Headache Pain 2016, 17, 47. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Perugino, F.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered serum levels of kynurenine metabolites in patients affected by cluster headache. J. Headache Pain 2016, 17, 27. [Google Scholar] [CrossRef]
- D’andrea, G.; Bussone, G.; Di Fiore, P.; Perini, F.; Gucciardi, A.; Bolner, A.; Aguggia, M.; Saracco, G.; Galloni, E.; Giordano, G.; et al. Pathogenesis of chronic cluster headache and bouts: Role of tryptamine, arginine metabolism and α1-agonists. Neurol. Sci. 2017, 38, 37–43. [Google Scholar] [CrossRef]
- D’andrea, G.; D’amico, D.; Bussone, G.; Bolner, A.; Aguggia, M.; Saracco, M.G.; Galloni, E.; De Riva, V.; Colavito, D.; Leon, A.; et al. The role of tyrosine metabolism in the pathogenesis of chronic migraine. Cephalalgia 2013, 33, 932–937. [Google Scholar] [CrossRef]
- Lionetto, L.; Guglielmetti, M.; Cipolla, F.; Bernardini, S.; Koehler, B.E.; Capi, M.; De Bernardini, D.; Curto, M.; Manetti, R.; Nicoletti, F.; et al. Polyamines serum levels in episodic and chronic migraine. Expert Rev. Neurother. 2021, 21, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Aczél, T.; Körtési, T.; Kun, J.; Urbán, P.; Bauer, W.; Herczeg, R.; Farkas, R.; Kovács, K.; Vásárhelyi, B.; Karvaly, G.B.; et al. Identification of disease- and headache-specific mediators and pathways in migraine using blood transcriptomic and metabolomic analysis. J. Headache Pain 2021, 22, 117. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Testarelli, L. Assessment of growth factors, cytokines, and cellular markers in saliva of patients with trigeminal neuralgia. Molecules 2021, 26, 2964. [Google Scholar] [CrossRef]
- Moreau, C.; El Habnouni, C.; Lecron, J.-C.; Morel, F.; Delwail, A.; Le Gall-Ianotto, C.; Le Garrec, R.; Misery, L.; Piver, E.; Vaillant, L.; et al. Salivary metabolome indicates a shift in tyrosine metabolism in patients with burning mouth syndrome: A prospective case–control study. Pain 2023, 164, e144–e156. [Google Scholar] [CrossRef]
- Ye, L.; Dai, Q.; Hou, F.; Wu, C.; Qiu, X.; Yuan, P.; Chen, F.; Meng, Y.; Feng, X.; Jiang, L. Salivary metabolomics of burning mouth syndrome: A cross-sectional study. Arch. Oral Biol. 2022, 144, 105552. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B.; Langefeld, C.; Olivier, M.; Cox, L.A. Integrated omics: Tools, advances and future approaches. J. Mol. Endocrinol. 2019, 62, R21–R45. [Google Scholar] [CrossRef]
- Gardner, A.; Carpenter, G.; So, P.-W. Salivary metabolomics: From diagnostic biomarker discovery to investigating biological function. Metabolites 2020, 10, 47. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- López-López, Á.; López-Gonzálvez, Á.; Barker-Tejeda, T.C.; Barbas, C. A review of validated biomarkers obtained through metabolomics. Expert Rev. Mol. Diagn. 2018, 18, 557–575. [Google Scholar] [CrossRef]
- Roi, A.; Rusu, L.C.; Roi, C.I.; Luca, R.E.; Boia, S.; Munteanu, R.I. A New Approach for the Diagnosis of Systemic and Oral Diseases Based on Salivary Biomolecules. Dis. Markers 2019, 2019, 8761860. [Google Scholar] [CrossRef]
- Teckchandani, S.; Gowda, G.A.N.; Raftery, D.; Curatolo, M. Metabolomics in chronic pain research. Eur. J. Pain 2021, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Rodriguez-Saona, L.; Hackshaw, K.V. Metabolomics in central sensitivity syndromes. Metabolites 2020, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Aroke, E.N.; Powell-Roach, K.L. The Metabolomics of Chronic Pain Conditions: A Systematic Review. Biol. Res. Nurs. 2020, 22, 458–471. [Google Scholar] [CrossRef]
- D’andrea, G.; Gucciardi, A.; Perini, F.; Leon, A. The role of neurotransmitters and neuromodulators in the pathogenesis of cluster headache: A review. Neurol. Sci. 2019, 40, 39–44. [Google Scholar] [CrossRef] [PubMed]
- D’andrea, G.; Gucciardi, A.; Giordano, G.; Bussone, G.; Leon, A. Study of arginine metabolism in medication overuse chronic migraine: Possible defect in NO synthesis. Neurol. Sci. 2022, 43, 2745–2749. [Google Scholar] [CrossRef]
- Alexander, G.M.; Reichenberger, E.; Peterlin, B.L.; Perreault, M.J.; Grothusen, J.R.; Schwartzman, R.J. Plasma amino acids changes in complex regional pain syndrome. Pain Res. Treat. 2013, 2013, 742407. [Google Scholar] [CrossRef]
- Gunn, J.; Hill, M.M.; Cotton, B.M.; Deer, T.R. An analysis of biomarkers in patients with chronic pain. Pain Physician 2020, 23, E41–E49. [Google Scholar] [CrossRef]
- Pereira, V.; Goudet, C. Emerging trends in pain modulation by metabotropic glutamate receptors. Front. Mol. Neurosci. 2019, 11, 464. [Google Scholar] [CrossRef]
- Krief, G.; Haviv, Y.; Deutsch, O.; Keshet, N.; Almoznino, G.; Zacks, B.; Palmon, A.; Aframian, D.J. Proteomic profiling of whole-saliva reveals correlation between Burning Mouth Syndrome and the neurotrophin signaling pathway. Sci. Rep. 2019, 9, 4794. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Sharav, Y.; Haviv, Y.; Almoznino, G.; Benoliel, R. Neurovascular Orofacial Pain. In Contemporary Oral Medicine: A Comprehensive Approach to Clinical Practice; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Haviv, Y.; Khan, J.; Zini, A.; Almoznino, G.; Sharav, Y.; Benoliel, R. Trigeminal neuralgia (part I): Revisiting the clinical phenotype. Cephalalgia 2016, 36, 730–746. [Google Scholar] [CrossRef]
- Haviv, Y.; Zadik, Y.; Sharav, Y.; Benoliel, R. Painful Traumatic Trigeminal Neuropathy: An Open Study on the Pharmacotherapeutic Response to Stepped Treatment. J. Oral Facial Pain Headache 2014, 28, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Benoliel, R.; Gaul, C. Persistent idiopathic facial pain. Cephalalgia 2017, 37, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Findler, M.; Michaeli, E.; Haviv, Y. [BURNING MOUTH SYNDROME—A FLAME HARD TO EXTINGUISH]. Harefuah 2016, 155, 506–509. [Google Scholar]
- Benoliel, R.; Eliav, E.; Elishoov, H.; Sharav, Y. Diagnosis and treatment of persistent pain after trauma to the head and neck. J. Oral Maxillofac. Surg. 1994, 52, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Haviv, Y.; Rettman, A.; Aframian, D.; Sharav, Y.; Benoliel, R. Myofascial Pain: An Open Study on the Pharmacotherapeutic Response to Stepped Treatment with Tricyclic Antidepressants and Gabapentin. J. Oral Facial Pain Headache 2015, 29, 144–151. [Google Scholar] [CrossRef]
- Aframian, D.J.; Helcer, M.; Livni, D.; Markitziu, A. Pilocarpine for the treatment of salivary glands’ impairment caused by radioiodine therapy for thyroid cancer. Oral Dis. 2006, 12, 297–300. [Google Scholar] [CrossRef]
- Lapidot-Cohen, T.; Rosental, L.; Brotman, Y. Liquid Chromatography–Mass Spectrometry (LC-MS)-Based Analysis for Lipophilic Compound Profiling in Plants. Curr. Protoc. Plant Biol. 2020, 5, e20109. [Google Scholar] [CrossRef]
- Hummel, J.; Segu, S.; Li, Y.; Irgang, S.; Jueppner, J.; Giavalisco, P. Ultra Performance Liquid Chromatography and High Resolution Mass Spectrometry for the Analysis of Plant Lipids. Front. Plant Sci. 2011, 2, 54. [Google Scholar] [CrossRef]

| p-Values | ||||||
|---|---|---|---|---|---|---|
| control vs. Mig/TMD | ||||||
| 4-Hydroxybenzaldehyde | 0.0237 | 0.0213 | ||||
| DL-Valine | 0.0324 | 0.0009 | ||||
| N-Acetylphenylalanine | 0.0389 | 0.0155 | ||||
| Theobromine | 0.0036 | 0.0001 | ||||
| control vs. TMD | ||||||
| Adenine | 0.0182 | |||||
| DL-Ornithine | 0.0013 | |||||
| L-Histidine | 0.0428 | |||||
| Nicotinamide | 0.0262 | |||||
| control vs. PTN/TN/TTH | ||||||
| D-(+)-Pyroglutamic Acid | 0.0068 | 0.0084 | 0.0008 | |||
| DL-Glutamic acid | 0.0326 | 0.0249 | 0.013 | |||
| control vs. TTH | ||||||
| DL-Asparagine | 0.0008 | |||||
| control vs. PTN/TMD/TN/TTH | ||||||
| DL-Aspartic acid | 0.0326 | 0.0082 | 0.0227 | 0.0021 | ||
| DL-Citrulline | 0.0268 | <0.0001 | 0.0189 | 0.0246 | ||
| DL-Isoleucine | 0.0145 | 0.0002 | 0.0068 | 0.0027 | ||
| control vs. BMS/Mig/PTN/TMD/TN/TTH | ||||||
| DL-Glutamine | 0.0434 | 0.0026 | 0.0015 | <0.0001 | 0.0003 | 0.0009 |
| control vs. PTN/TMD/TN | ||||||
| DL-Phenylalanine | 0.0045 | 0.0354 | 0.0048 | |||
| control vs. BMS/TMD | ||||||
| DL-Proline | 0.0329 | 0.0002 | ||||
| control vs. Mig/PTN/TMD/TN/TTH | ||||||
| DL-Tryptophan | 0.0061 | 0.0156 | 0.0044 | 0.0128 | 0.0396 | |
| control vs. TN | ||||||
| DL-Tyrosine | 0.0063 | |||||
| control vs. BMS/Mig/TMD/TN/TTH | ||||||
| N-Acetyl-L-aspartic acid | 0.0222 | 0.0408 | 0.0249 | 0.0324 | 0.0246 | |
| control vs. BMS/Mig/PTN/TN | ||||||
| Spermidine | 0.0116 | 0.0016 | 0.0008 | 0.0156 | ||
| control vs. Mig/TMD/TTH | ||||||
| Urocanic acid | 0.0172 | 0.0002 | 0.0474 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasinska, W.; Birenzweig, Y.; Sharav, Y.; Aframian, D.J.; Rettman, A.; Hanut, A.; Brotman, Y.; Haviv, Y. Salivary Metabolomics as a Diagnostic Tool: Distinct Metabolic Profiles Across Orofacial Pain Subtypes. Int. J. Mol. Sci. 2025, 26, 2260. https://doi.org/10.3390/ijms26052260
Jasinska W, Birenzweig Y, Sharav Y, Aframian DJ, Rettman A, Hanut A, Brotman Y, Haviv Y. Salivary Metabolomics as a Diagnostic Tool: Distinct Metabolic Profiles Across Orofacial Pain Subtypes. International Journal of Molecular Sciences. 2025; 26(5):2260. https://doi.org/10.3390/ijms26052260
Chicago/Turabian StyleJasinska, Weronika, Yonatan Birenzweig, Yair Sharav, Doron J. Aframian, Andra Rettman, Aiham Hanut, Yariv Brotman, and Yaron Haviv. 2025. "Salivary Metabolomics as a Diagnostic Tool: Distinct Metabolic Profiles Across Orofacial Pain Subtypes" International Journal of Molecular Sciences 26, no. 5: 2260. https://doi.org/10.3390/ijms26052260
APA StyleJasinska, W., Birenzweig, Y., Sharav, Y., Aframian, D. J., Rettman, A., Hanut, A., Brotman, Y., & Haviv, Y. (2025). Salivary Metabolomics as a Diagnostic Tool: Distinct Metabolic Profiles Across Orofacial Pain Subtypes. International Journal of Molecular Sciences, 26(5), 2260. https://doi.org/10.3390/ijms26052260






