Enhancement of NK Cell Cytotoxic Activity and Immunoregulatory Effects of a Natural Product Supplement Across a Wide Age Span: A 30-Day In Vivo Human Study
Abstract
1. Introduction
2. Results
2.1. AgePro Uptake Increases the Cytotoxic Activity of NK Cells and Enhances K562 Killing In Vitro in a Ratio-Dependent Manner
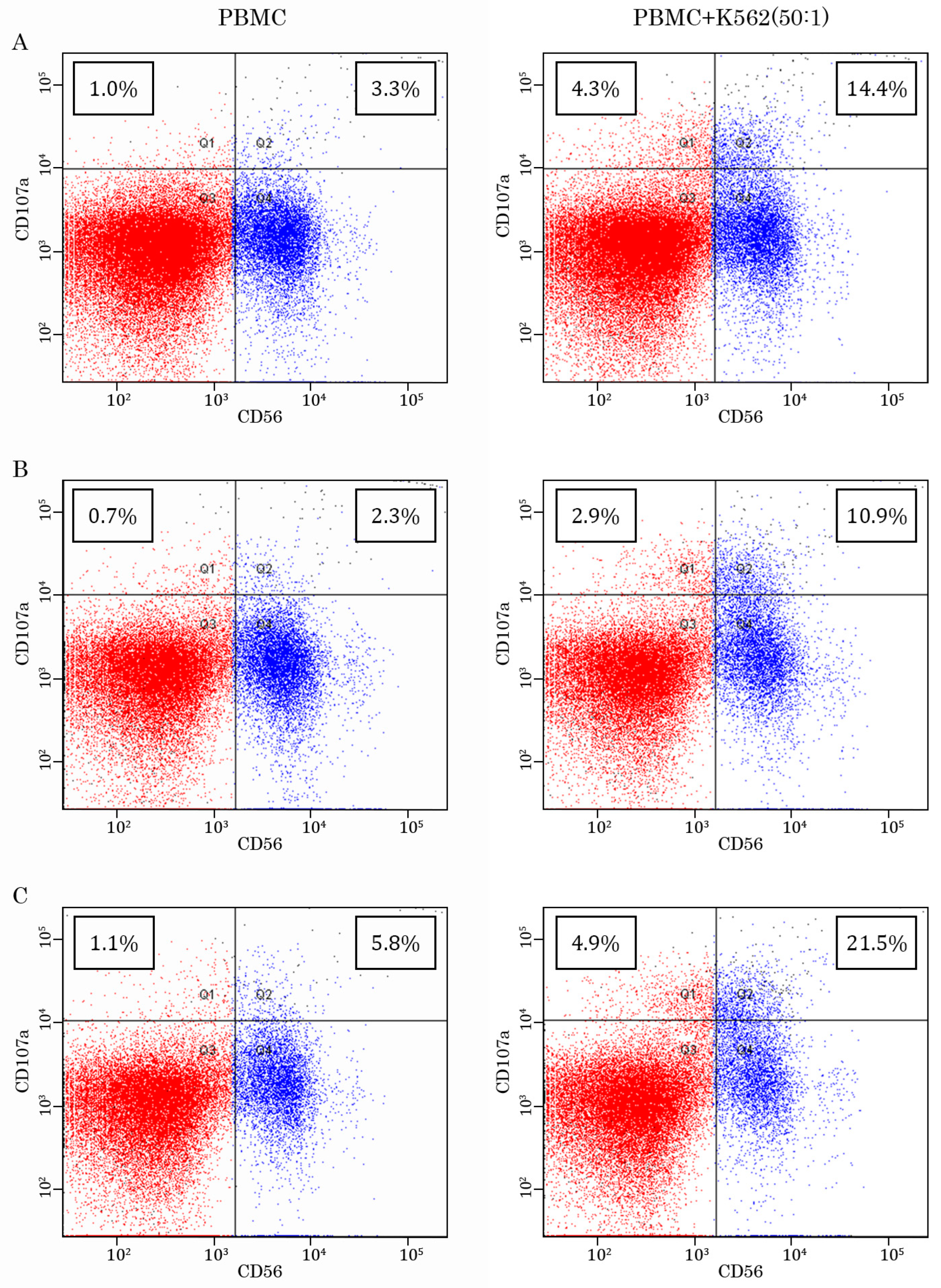
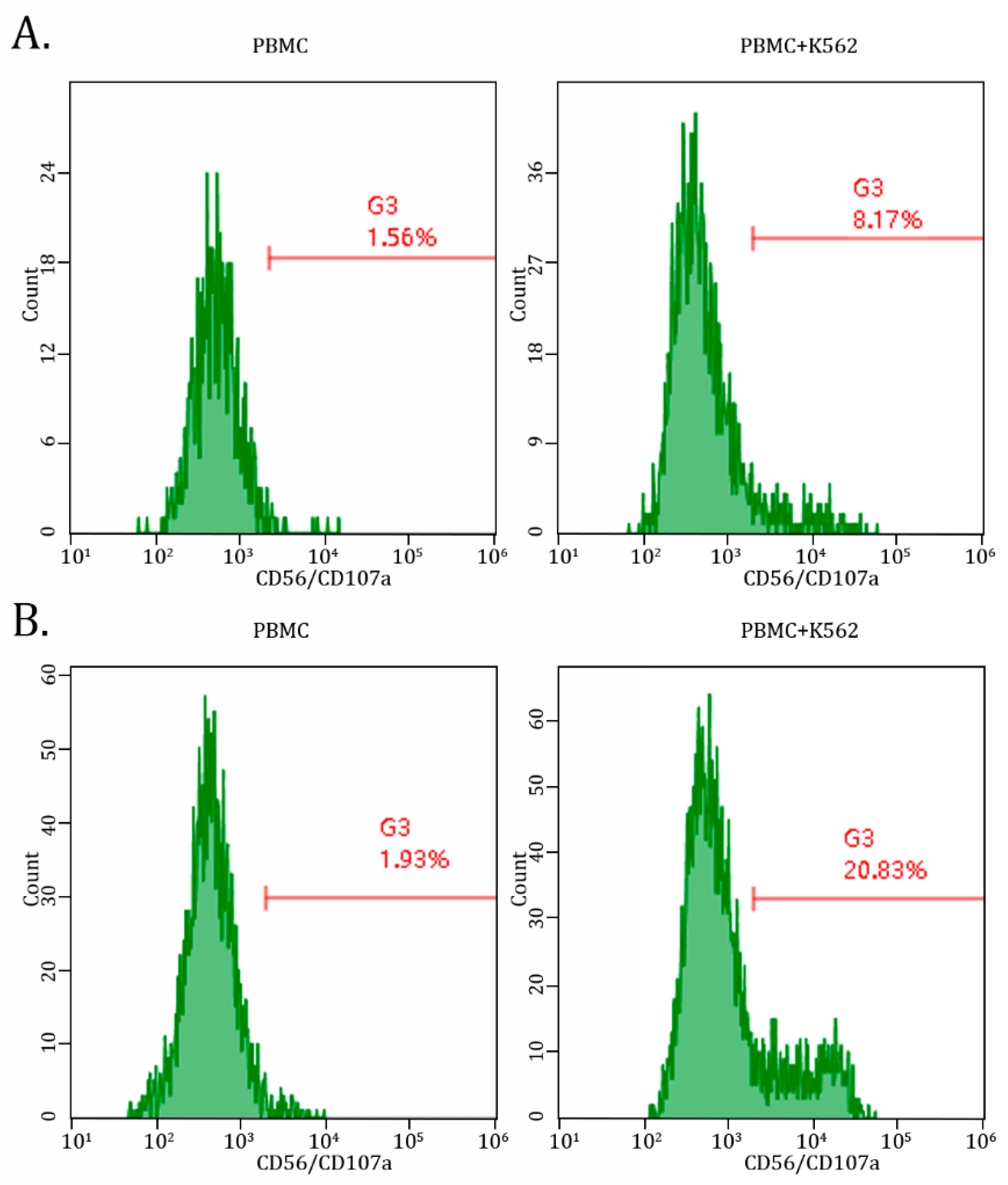
2.2. AgePro Increases CD107a Expression in NK Cells
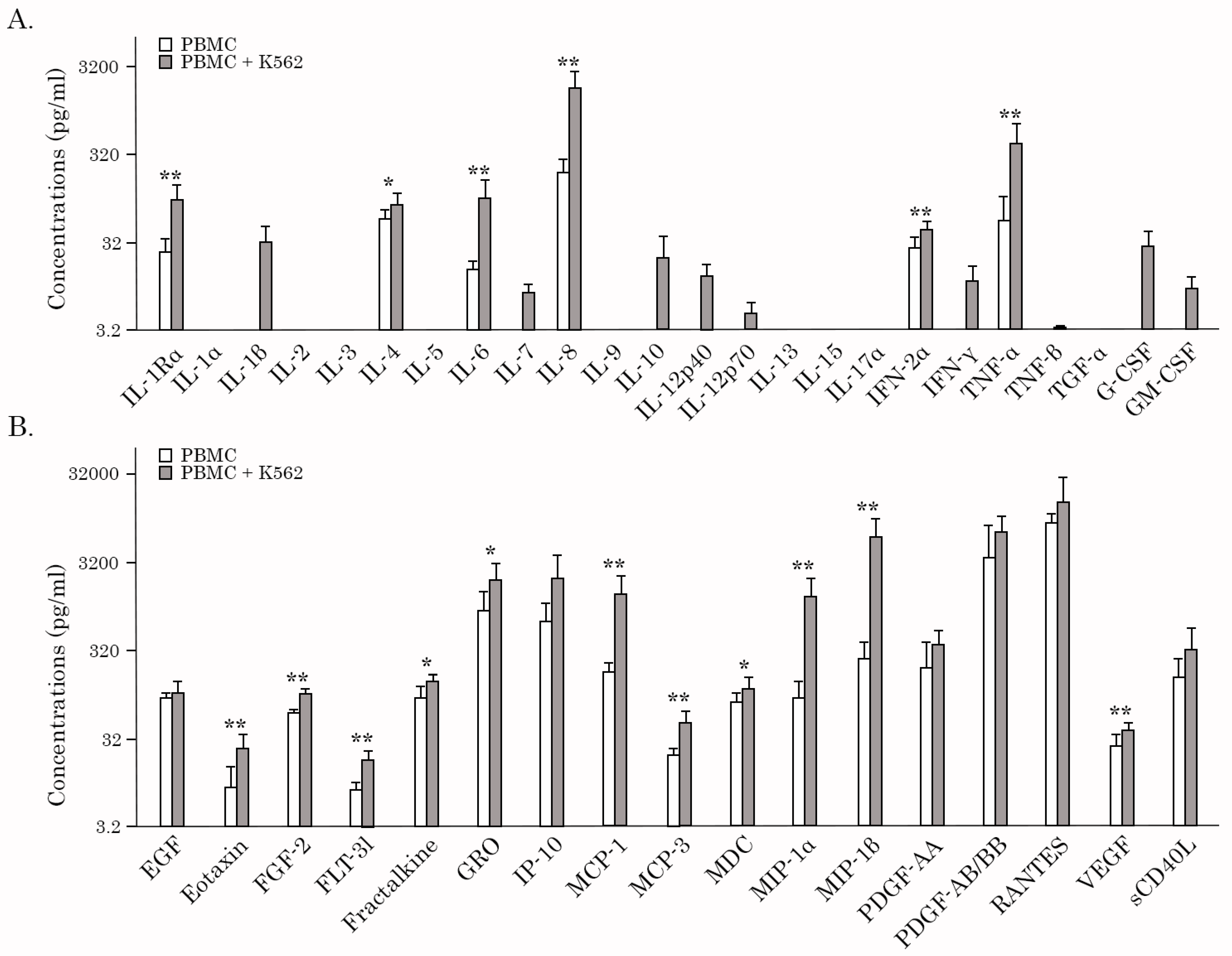
2.3. AgePro Stimulates the Production of Cytokines/Chemokines by PBMCs Cultured with K562 Target Cells
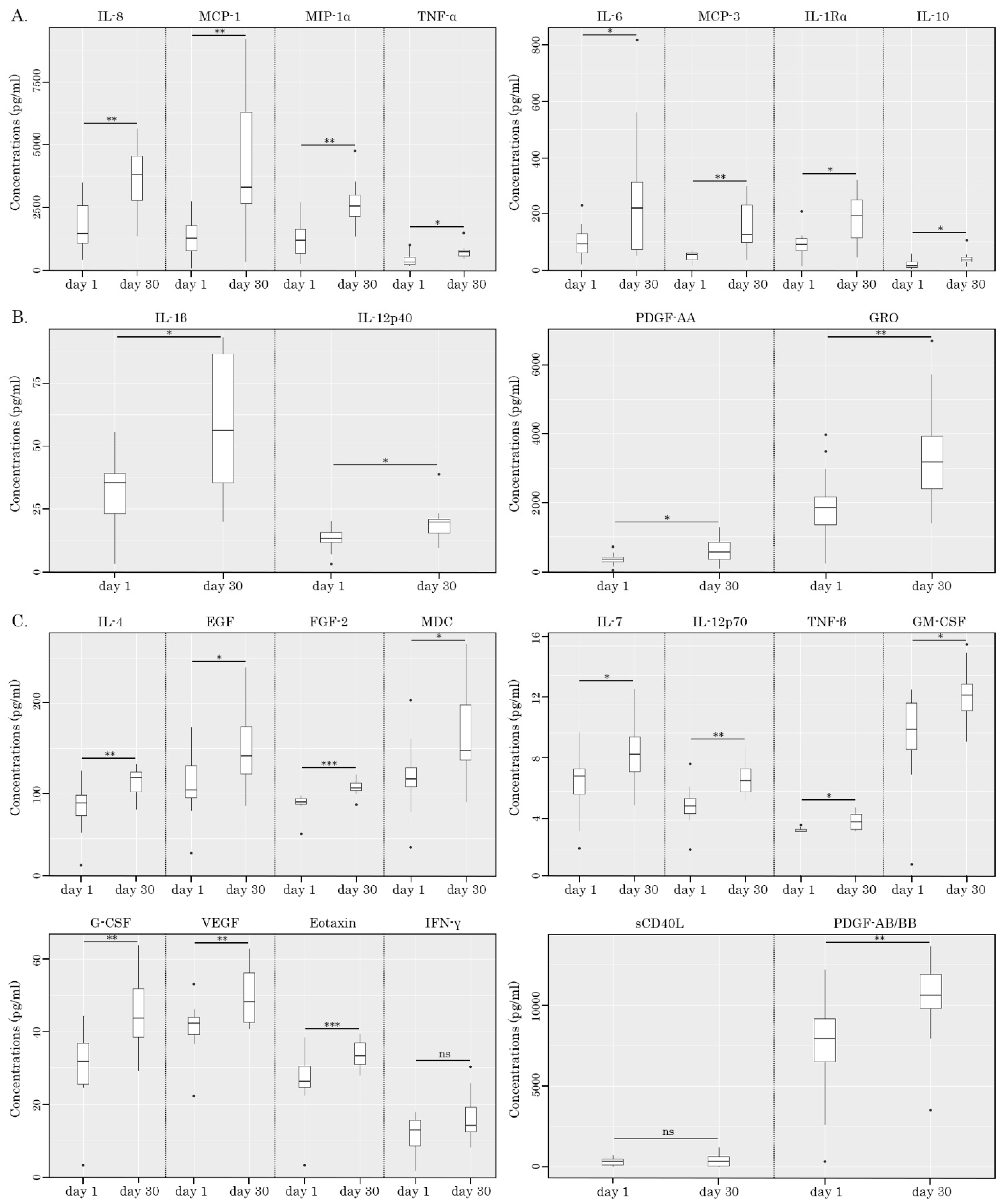
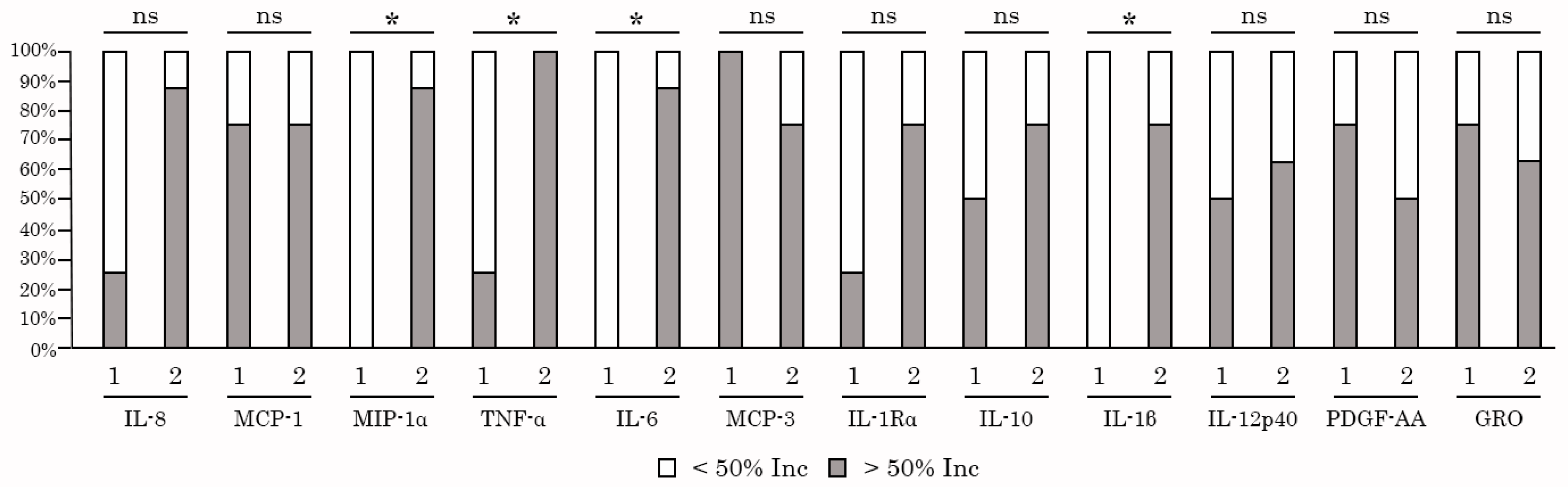
2.4. NK-Based Cytotoxicity Correlates with Increased Production of TNF-α, IL-6, and IL-1β
2.5. AgePro Does not Induce Spontaneous Activation of PBMC
3. Discussion
- The cytotoxicity assays based on the direct measurement of the killing of previously labeled (e.g., with carboxyfluorescein diacetate succinimidyl ester-labeled (CFSE)) target cells (e.g., K562 human myeloid leukemia cells). Due to the reduced expression of HLA class I molecules present in K562 cells and increased expression of ligands for activatory NK receptors, these cells are considered the most suitable target cells to examine the functional activity of NK cells and NK cell-mediated cytotoxicity [46]. Indeed, the loss or aberrant expression of MHC-I molecules on tumor cells was shown as the most critical signal for NK cell recognition [47,48];
- CD107a degranulation assay designed to count the amount of secreted cytotoxic granules, including perforin and granzyme, which trigger target cell death [49];
- Assays aimed to examine the cytokines release activity. Based on these assays, impaired NKA was found to be a driving force for several human diseases. Notably, low or absent NKA is currently established as a significant characteristic of hemophagocytic lymphohistiocytosis (HLH) [50,51]. Impaired NKA was also shown for liver diseases, including hepatocellular carcinomas (HCCs) and cirrhosis [52], other types of human malignancies, and viral infections, as well.
4. Materials and Methods
4.1. Blood Sample Preparation
4.2. Cells and Cell Culture
4.3. Antibodies
4.4. PBMC NK Cytotoxicity Assay
4.5. CD107a Degranulation Assay
4.6. Multiplex Analysis of Cytokines
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fraker, L.D.; Halter, S.A.; Forbes, J.T. Effects of orally administered retinol on natural killer cell activity in wild type BALB/c and congenitally athymic BALB/c mice. Cancer Immunol. Immunother. 1986, 21, 114–118. [Google Scholar] [CrossRef]
- Recchia, F.; Saggio, G.; Cesta, A.; Candeloro, G.; Di Blasio, A.; Amiconi, G.; Lombardo, M.; Nuzzo, A.; Lalli, A.; Alesse, E.; et al. Phase II study of interleukin-2 and 13-cis-retinoic acid as maintenance therapy in metastatic colorectal cancer. Cancer Immunol. Immunother. 2007, 56, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Tamura, J.; Kubota, K.; Murakami, H.; Sawamura, M.; Matsushima, T.; Tamura, T.; Saitoh, T.; Kurabayshi, H.; Naruse, T. Immunomodulation by vitamin B12: Augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin. Exp. Immunol. 1999, 116, 28–32. [Google Scholar] [CrossRef]
- Partearroyo, T.; Úbeda, N.; Montero, A.; Achón, M.; Varela-Moreiras, G. Vitamin B12 and folic acid imbalance modifies NK cytotoxicity, lymphocytes B and lymphoproliferation in aged rats. Nutrients 2013, 5, 4836–4848. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Ghoneum, M. In vivo effect of ascorbic acid on enhancement of human natural killer cell activity. Nutr. Res. 1993, 13, 753–764. [Google Scholar] [CrossRef]
- Kim, H.; Jang, M.; Kim, Y.; Choi, J.; Jeon, J.; Kim, J.; Hwang, Y.I.; Kang, J.S.; Lee, W.J. Red ginseng and vitamin C increase immune cell activity and decrease lung inflammation induced by influenza A virus/H1N1 infection. J. Pharm. Pharmacol. 2016, 68, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Dambaeva, S.; Kim, M.W.; Han, A.R.; Fukui, A.; Gilman-Sachs, A.; Beaman, K.; Kwak-Kim, J. 1,25-Dihydroxy-vitamin D3 regulates NK-cell cytotoxicity, cytokine secretion, and degranulation in women with recurrent pregnancy losses. Eur. J. Immunol. 2015, 45, 3188–3199. [Google Scholar] [CrossRef]
- Guo, T.L.; Chi, R.P.; Hernandez, D.M.; Auttachoat, W.; Zheng, J.F. Decreased 7,12-dimethylbenz[a]anthracene-induced carcinogenesis coincides with the induction of antitumor immunities in adult female B6C3F1 mice pretreated with genistein. Carcinogenesis 2007, 28, 2560–2566. [Google Scholar] [CrossRef]
- Yadav, V.S.; Mishra, K.P.; Singh, D.P.; Mehrotra, S.; Singh, V.K. Immunomodulatory effects of curcumin. Immunopharmacol. Immunotoxicol. 2005, 27, 485–497. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anticancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef]
- Su, T.; Shen, H.; He, M.; Yang, S.; Gong, X.; Huang, C.; Guo, L.; Wang, H.; Feng, S.; Mi, T.; et al. Quercetin promotes the proportion and maturation of NK cells by binding to MYH9 and improves cognitive functions in aged mice. Immun. Ageing 2024, 21, 29. [Google Scholar]
- Grudzien, M.; Rapak, A. Effect of Natural Compounds on NK Cell Activation. J. Immunol. Res. 2018, 2018, 4868417. [Google Scholar] [CrossRef] [PubMed]
- Bouamama, S.; Bouamama, A. Quercetin handles cellular oxidant/antioxidant systems and mitigates immunosenescence hallmarks in human PBMCs: An in vitro study. J. Biochem. Mol. Toxicol. 2023, 37, e23354. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Zhang, B.; Wu, C.Y.; Chiu, E.; Day, A.; O’Connor, R.S.; Yackoubov, D.; Simantov, R.; McKenna, D.H.; Cao, Q.; et al. Nicotinamide enhances natural killer cell function and yields remissions in patients with non-Hodgkin lymphoma. Sci. Transl. Med. 2023, 15, eade3341. [Google Scholar] [PubMed]
- Okabe, K.; Yaku, K.; Uchida, Y.; Fukamizu, Y.; Sato, T.; Sakurai, T.; Tobe, K.; Nakagawa, T. Oral Administration of Nicotinamide Mononucleotide Is Safe and Efficiently Increases Blood Nicotinamide Adenine Dinucleotide Levels in Healthy Subjects. Front. Nutr. 2022, 9, 868640. [Google Scholar]
- Takeda, K.; Okumura, K. Nicotinamide mononucleotide augments the cytotoxic activity of natural killer cells in young and elderly mice. Biomed. Res. 2021, 42, 173–179. [Google Scholar]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar]
- Ghosh, S.; Iacucci, M. Diverse Immune Effects of Bovine Colostrum and Benefits in Human Health and Disease. Nutrients 2021, 13, 3798. [Google Scholar] [CrossRef]
- Vetvicka, V.; Fernandez-Botran, R. Non-specific immunostimulatory effects of transfer factor. Int. Clin. Pathol. J. 2020, 8, 1–6. [Google Scholar]
- Vetvicka, V.; Vetvickova, J. Antigen-Specific Immunomodulatory Effects of Transfer Factor. Austin J. Clin. Pathol. 2020, 7, 1062. [Google Scholar]
- Vetvicka, V.; Vetvickova, J. Effects of Transfer Factor Supplementation on Immune Reactions in Mice. J. Nutr. Health Sci. 2019, 6, 301. [Google Scholar]
- Han, X.; Vollmer, D.; Enioutina, E.Y. Immunomodulatory Effects of Modified Bovine Colostrum, Whey, and Their Combination with Other Natural Products: Effects on Human Peripheral Blood Mononuclear Cells. Curr. Ther. Res. Clin. Exp. 2023, 99, 100720. [Google Scholar] [CrossRef] [PubMed]
- Kandarian, F.; Sunga, G.M.; Arango-Saenz, D.; Rossetti, M.A. Flow Cytometry-Based Cytotoxicity Assay for the Assessment of Human NK Cell Activity. J. Vis. Exp. 2017, 126, 56191. [Google Scholar]
- Lecoeur, H.; Fevrier, M.; Garcia, S.; Riviere, Y.; Gougeon, M.L. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J. Immunol. Methods 2001, 253, 177–187. [Google Scholar] [CrossRef]
- Krzewski, K.; Gil-Krzewska, A.; Nguyen, V.; Peruzzi, G.; Coligan, J.E. LAMP1/CD107a is required for efficient perforin delivery to lytic granules and NK-cell cytotoxicity. Blood 2013, 121, 4672–4683. [Google Scholar] [CrossRef] [PubMed]
- Furuta, K.; Yang, X.-L.; Chen, J.-S.; Hamilton, S.; August, J.T. Differential Expression of the Lysosome-Associated Membrane Proteins in Normal Human Tissues. Arch. Biochem. Biophys. 1999, 365, 75–82. [Google Scholar] [CrossRef]
- Savoy, S.K.A.; Boudreau, J.E. The evolutionary arms race between virus and NK cells: Diversity enables population-level virus control. Viruses 2019, 11, 959. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fu, T.; Jiang, Y.Z.; Shao, Z.M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Liu, M.; Liang, S.; Zhang, C. NK cells in autoimmune diseases: Protective or pathogenic? Front. Immunol. 2021, 12, 624687. [Google Scholar] [CrossRef]
- Kucuksezer, U.C.; Aktas Cetin, E.; Esen, F.; Tahrali, I.; Akdeniz, N.; Gelmez, M.Y.; Deniz, G. The Role of Natural Killer Cells in Autoimmune Diseases. Front. Immunol. 2021, 12, 622306. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Imani, S.; Tao, L.; Deng, Y.; Cai, Y. Natural killer cells: Friend or foe in metabolic diseases? Front. Immunol. 2021, 12, 614429. [Google Scholar]
- Segerberg, F.; Lundtoft, C.; Reid, S.; Hjorton, K.; Leonard, D.; Nordmark, G.; Carlsten, M.; Hagberg, N. Autoantibodies to Killer Cell Immunoglobulin-Like Receptors in Patients With Systemic Lupus Erythematosus Induce Natural Killer Cell Hyporesponsiveness. Front. Immunol. 2019, 10, 2164. [Google Scholar]
- Berhani, O.; Glasner, A.; Kahlon, S.; Duev-Cohen, A.; Yamin, R.; Horwitz, E.; Enk, J.; Moshel, O.; Varvak, A.; Porgador, A.; et al. Human anti-NKp46 antibody for studies of NKp46-dependent NK cell function and its applications for type 1 diabetes and cancer research. Eur. J. Immunol. 2019, 49, 228–241. [Google Scholar]
- Hudspeth, K.; Pontarini, E.; Tentorio, P.; Cimino, M.; Donadon, M.; Torzilli, G.; Lugli, E.; Della Bella, S.; Gershwin, M.E.; Mavilio, D. The role of natural killer cells in autoimmune liver disease: A comprehensive review. J. Autoimmun. 2013, 46, 55–65. [Google Scholar]
- Björkström, N.K.; Ljunggren, H.G.; Michaëlsson, J. Emerging insights into natural killer cells in human peripheral tissues. Nat. Rev. Immunol. 2016, 16, 310–320. [Google Scholar]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar]
- Han, X.; Vollmer, D.; Yan, X.; Zhang, Y.; Zang, M.; Zhang, C.; Sherwin, C.M.; Enioutina, E.Y. Immunomodulatory Effects of Modified Colostrum, Whey, and Their Combination With Other Natural Products: Effects on Natural Killer Cells. Curr. Ther. Res. Clin. Exp. 2024, 101, 100750. [Google Scholar] [PubMed]
- Demidenko, O.; Barardo, D.; Budovskii, V.; Finnemore, R.; Palmer, F.R.; Kennedy, B.K.; Budovskaya, Y.V. Rejuvant®, a potential life-extending compound formulation with alpha-ketoglutarate and vitamins, conferred an average 8 year reduction in biological aging, after an average of 7 months of use, in the TruAge DNA methylation test. Aging 2021, 13, 24485–24499. [Google Scholar]
- Wirth, A.; Wolf, B.; Huang, C.K.; Glage, S.; Hofer, S.J.; Bankstahl, M.; Bär, C.; Thum, T.; Kahl, K.G.; Sigrist, S.J.; et al. Novel aspects of age-protection by spermidine supplementation are associated with preserved telomere length. Geroscience 2021, 43, 673–690. [Google Scholar]
- Han, X.; Vollmer, D. Two Natural Product Compositions Improve Lifespan and Healthspan in Caenorhabditis Elegans by Targeting the Hallmarks of Aging. Preprints 2024. [Google Scholar] [CrossRef]
- Shafer, D.; Smith, M.R.; Borghaei, H.; Millenson, M.M.; Li, T.; Litwin, S.; Anad, R.; Al-Saleem, T. Low NK cell counts in peripheral blood are associated with inferior overall survival in patients with follicular lymphoma. Leuk. Res. 2013, 37, 1213–1215. [Google Scholar] [CrossRef] [PubMed]
- Seymour, F.; Cavenagh, J.D.; Mathews, J.; Gribben, J.G. NK cells CD56bright and CD56dim subset cytokine loss and exhaustion is associated with impaired survival in myeloma. Blood Adv. 2022, 6, 5152–5159. [Google Scholar] [CrossRef] [PubMed]
- Nersesian, S.; Schwartz, S.L.; Grantham, S.R.; MacLean, L.K.; Lee, S.N.; Pugh-Toole, M.; Boudreau, J.E. NK cell infiltration is associated with improved overall survival in solid cancers: A systematic review and meta-analysis. Transl. Oncol. 2021, 14, 100930. [Google Scholar] [CrossRef]
- Ichise, H.; Tsukamoto, S.; Hirashima, T.; Konishi, Y.; Oki, C.; Tsukiji, S.; Iwano, S.; Miyawaki, A.; Sumiyama, K.; Terai, K.; et al. Functional visualization of NK cell-mediated killing of metastatic single tumor cells. Elife 2022, 11, e76269. [Google Scholar] [CrossRef]
- Vyas, M.; Requesens, M.; Nguyen, T.H.; Peigney, D.; Azin, M.; Demehri, S. Natural killer cells suppress cancer metastasis by eliminating circulating cancer cells. Front. Immunol. 2023, 13, 1098445. [Google Scholar] [CrossRef] [PubMed]
- West, W.H.; Cannon, G.B.; Kay, H.D.; Bonnard, G.D.; Herberman, R.B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: Characterization of effector cells. J. Immunol. 1977, 118, 355–361. [Google Scholar] [CrossRef]
- Melaiu, O.; Lucarini, V.; Cifaldi, L.; Fruci, D. Influence of the tumor microenvironment on NK cell function in solid tumors. Front. Immunol. 2019, 10, 3038. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, X.; Wei, H. The adverse impact of tumor microenvironment on NK-Cell. Front. Immunol. 2021, 12, 633361. [Google Scholar] [CrossRef]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wu, L.; Wang, J.; Tang, R.; Li, S.; Chen, J.; Gao, Z.; Pei, R.; Wang, Z. Application of an improved flow cytometry-based NK cell activity assay in adult hemophagocytic lymphohistiocytosis. Int. J. Hematol. 2017, 105, 828–834. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.S.; Lee, J.M.; Park, K.H.; Choi, A.R.; Yoon, J.H.; Ryu, H.; Oh, E.J. Natural Killer Cell Function Tests by Flowcytometry-Based Cytotoxicity and IFN-γ Production for the Diagnosis of Adult Hemophagocytic Lymphohistiocytosis. Int. J. Mol. Sci. 2019, 20, 5413. [Google Scholar] [CrossRef]
- Kim, J.; Phan, M.T.; Kweon, S.; Yu, H.; Park, J.; Kim, K.H.; Hwang, I.; Han, S.; Kwon, M.J.; Cho, D. A Flow Cytometry-Based Whole Blood Natural Killer Cell Cytotoxicity Assay Using Overnight Cytokine Activation. Front. Immunol. 2020, 11, 1851. [Google Scholar] [CrossRef]
- Aktas, E.; Kucuksezer, U.C.; Bilgic, S.; Erten, G.; Deniz, G. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 2009, 254, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Cohnen, A.; Chiang, S.C.; Stojanovic, A.; Schmidt, H.; Claus, M.; Saftig, P.; Janßen, O.; Cerwenka, A.; Bryceson, Y.T.; Watzl, C. Surface CD107a/LAMP-1 protects natural killer cells from degranulation-associated damage. Blood 2013, 122, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Poursine-Laurent, J.; Truscott, S.M.; Lybarger, L.; Song, Y.J.; Yang, L.; French, A.R.; Sunwoo, J.B.; Lemieux, S.; Hansen, T.H.; et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 2005, 436, 709–713. [Google Scholar] [CrossRef]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Cuturi, M.C.; Anegón, I.; Sherman, F.; Loudon, R.; Clark, S.C.; Perussia, B.; Trinchieri, G. Production of hematopoietic colony-stimulating factors by human natural killer cells. J. Exp. Med. 1989, 169, 569–583. [Google Scholar] [CrossRef]
- Smyth, M.J.; Zachariae, C.O.; Norihisa, Y.; Ortaldo, J.R.; Hishinuma, A.; Matsushima, K. IL-8 gene expression and production in human peripheral blood lymphocyte subsets. J. Immunol. 1991, 146, 3815–3823. [Google Scholar] [CrossRef]
- Warren, H.S.; Kinnear, B.F.; Phillips, J.H.; Lanier, L.L. Production of IL-5 by human NK cells and regulation of IL-5 secretion by IL-4, IL-10, and IL-12. J. Immunol. 1995, 154, 5144–5152. [Google Scholar] [CrossRef]
- Bluman, E.M.; Bartynski, K.J.; Avalos, B.R.; Caligiuri, M.A. Human natural killer cells produce abundant macrophage inflammatory protein-1 alpha in response to monocyte-derived cytokines. J. Clin. Investig. 1996, 97, 2722–2727. [Google Scholar] [CrossRef]
- Oliva, A.; Kinter, A.L.; Vaccarezza, M.; Rubbert, A.; Catanzaro, A.; Moir, S.; Monaco, J.; Ehler, L.; Mizell, S.; Jackson, R.; et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J. Clin. Investig. 1998, 102, 223–231. [Google Scholar] [PubMed]
- Fehniger, T.A.; Shah, M.H.; Turner, M.J.; VanDeusen, J.B.; Whitman, S.P.; Cooper, M.A.; Suzuki, K.; Wechser, M.; Goodsaid, F.; Caligiuri, M.A. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: Implications for the innate immune response. J. Immunol. 1999, 162, 4511–4520. [Google Scholar] [CrossRef] [PubMed]
- Roda, J.M.; Parihar, R.; Magro, C.; Nuovo, G.J.; Tridandapani, S.; Carson, W.E., 3rd. Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated tumor cells. Cancer Res. 2006, 66, 517–526. [Google Scholar]
- Mannino, M.H.; Zhu, Z.; Xiao, H.; Bai, Q.; Wakefield, M.R.; Fang, Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett. 2015, 367, 103–107. [Google Scholar] [PubMed]
- Konjević, G.M.; Vuletić, A.M.; Martinović, K.M.M.; Larsen, A.K.; Jurišić, V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar]
- Shibata, Y.; Foster, L.A.; Kurimoto, M.; Okamura, H.; Nakamura, R.M.; Kawajiri, K.; Justice, J.P.; Van Scott, M.R.; Myrvik, Q.N.; Metzger, W.J. Immunoregulatory roles of IL-10 in innate immunity: IL-10 inhibits macrophage production of IFN-gamma-inducing factors but enhances NK cell production of IFN-gamma. J. Immunol. 1998, 161, 4283–4288. [Google Scholar]
- Park, J.Y.; Lee, S.H.; Yoon, S.-R.; Park, Y.-J.; Jung, H.; Kim, T.-D.; Choi, I. IL-15-induced IL-10 increases the cytolytic activity of human natural killer cells. Mol. Cells 2011, 32, 265–272. [Google Scholar]
- Tahtinen, S.; Tong, A.J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [PubMed]
- Jedema, I.; van der Werff, N.M.; Barge, R.M.; Willemze, R.; Falkenburg, J.H. New CFSE-based assay to determine susceptibility to lysis by cytotoxic T cells of leukemic precursor cells within a heterogeneous target cell population. Blood 2004, 103, 2677–2682. [Google Scholar]
- Claus, M.; Greil, J.; Watzl, C. Comprehensive analysis of NK cell function in whole blood samples. J. Immunol. Methods 2009, 341, 154–164. [Google Scholar]
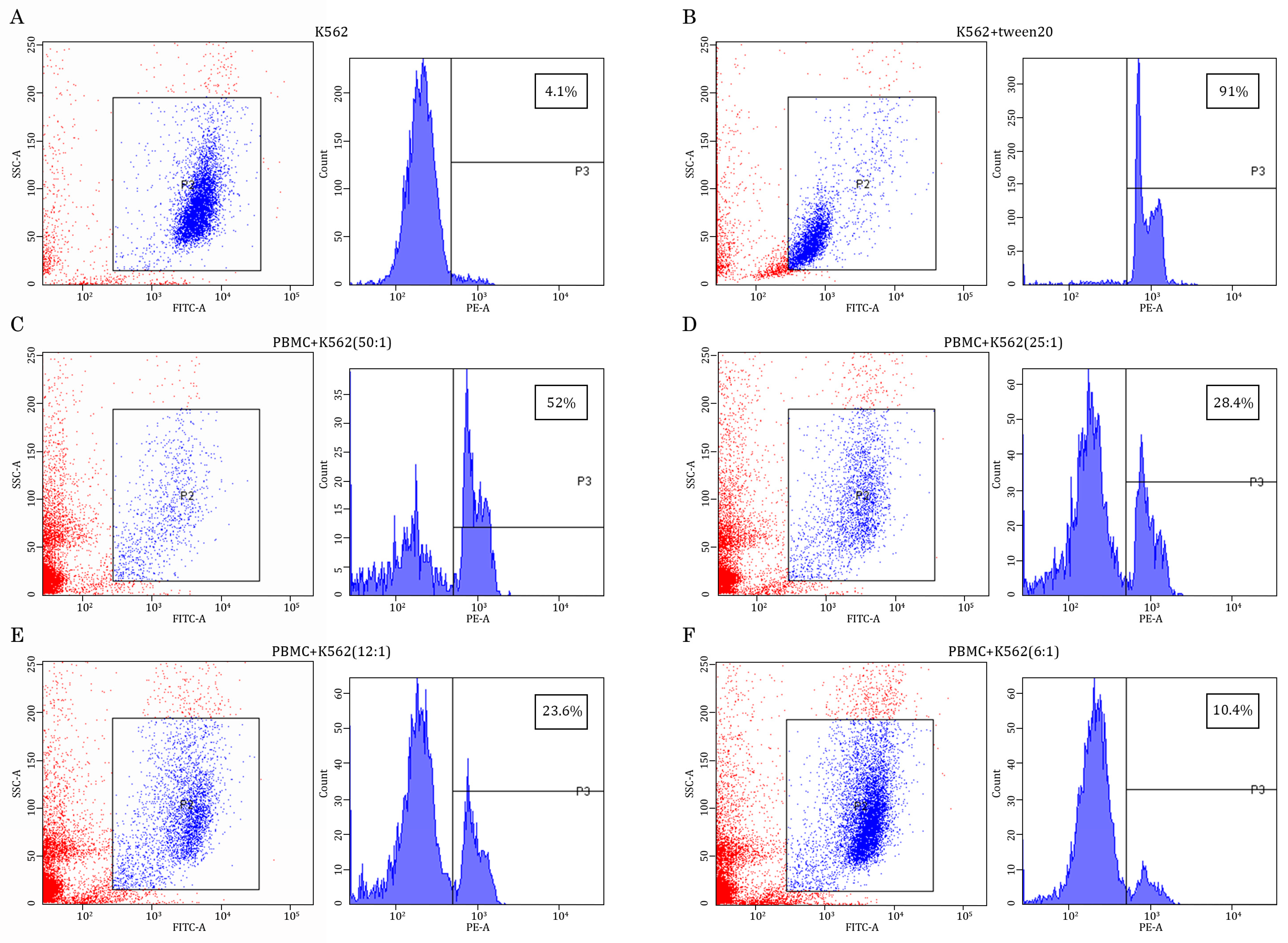
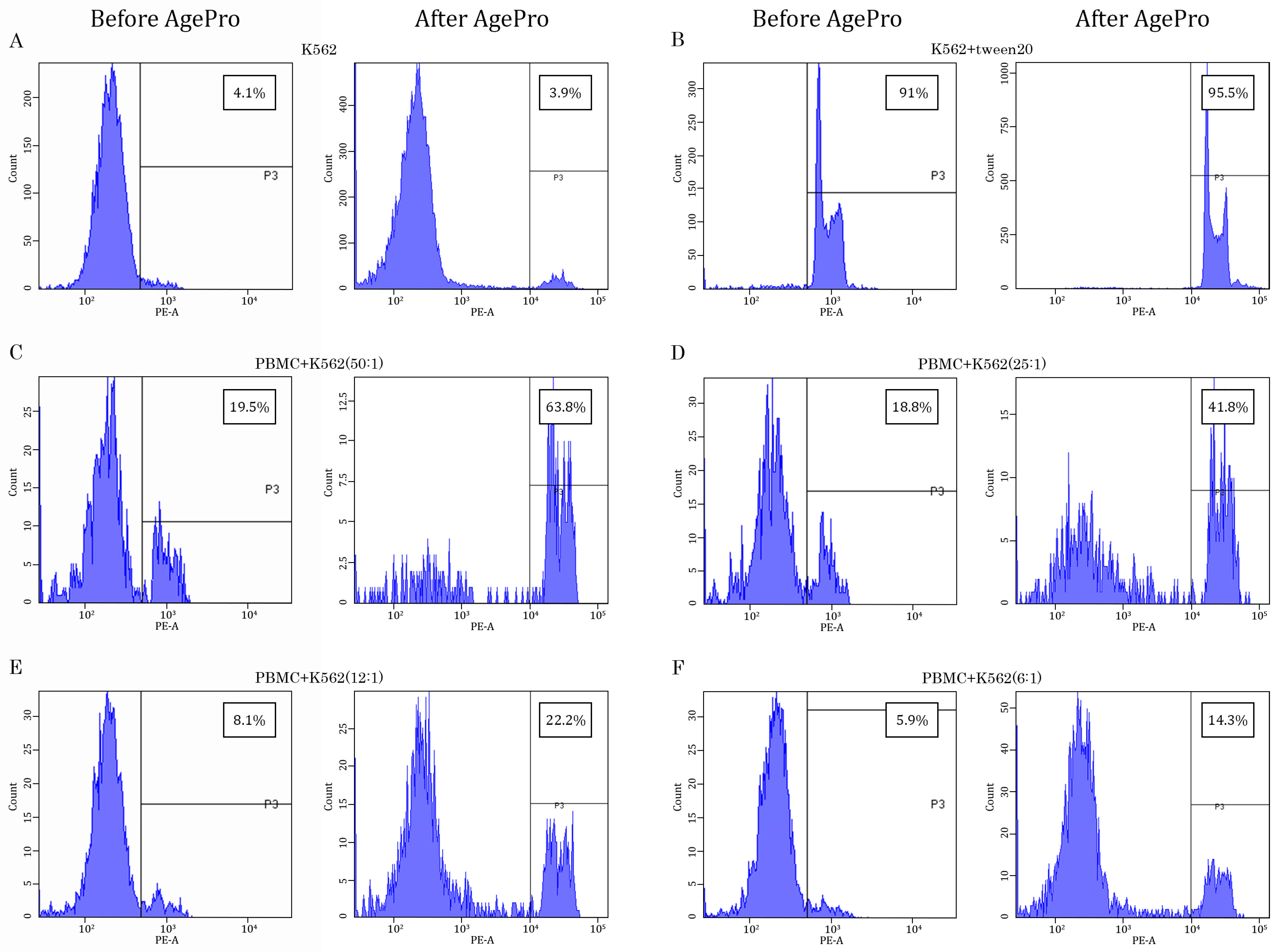
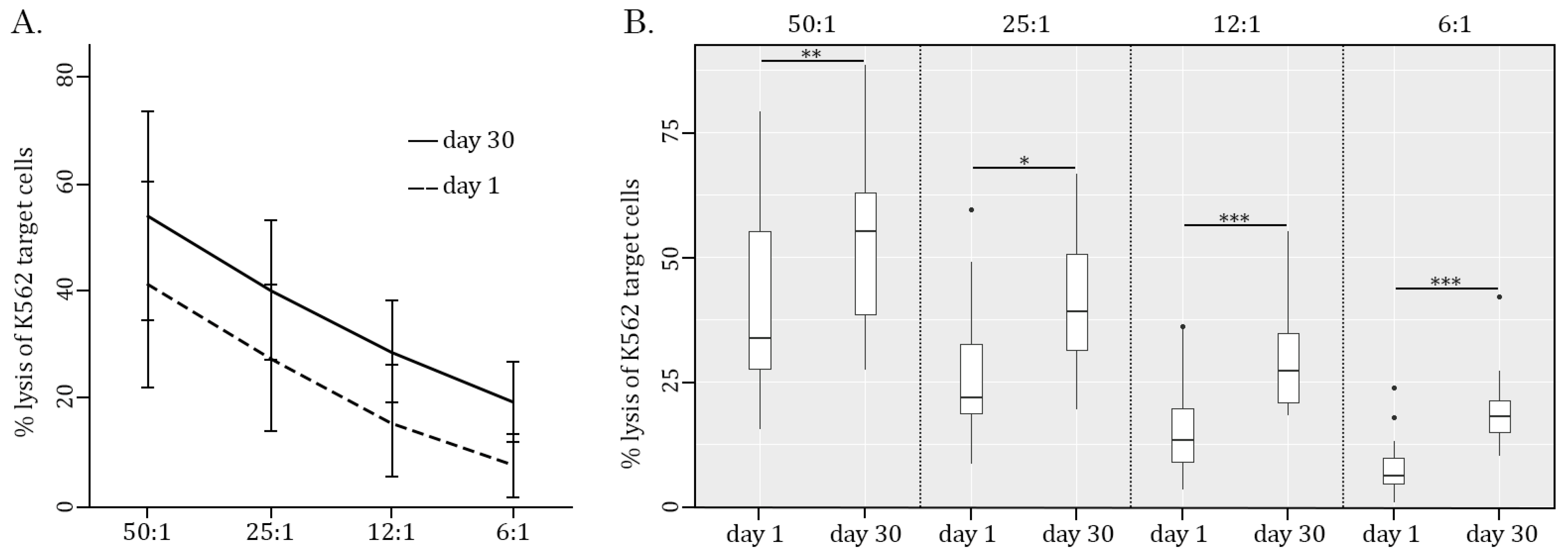
| Criteria | Avg Age | Day 1 | Day 30 | % Inc | p | r | |
|---|---|---|---|---|---|---|---|
| E:T ratio 50:1 | All | 45.9 ± 18.0 | 41.1 ± 19.1 | 54 ± 19.3 | 31% | 0.0008 | |
| Age <= 35 | 32 ± 3.6 | 42.7 ± 17.5 | 55.3 ± 19.3 | 30% | 0.03 | ||
| Age >= 50 | 61.5 ± 14.2 | 39.3 ± 21.8 | 52.5 ± 20.5 | 34% | 0.02 | ||
| E:T ratio 25:1 | All | 45.9 ± 18.0 | 21.9 (18.7–32.7) | 39.2 (31.5–50.5) | 79% | 0.03 | 0.46 |
| Age <= 35 | 32 ± 3.6 | 21.9 (20.2–30.1) | 40.1 (30.1–50.9) | 83% | 0.008 | 0.47 | |
| Age >= 50 | 61.5 ± 14.2 | 28.6 ± 17.4 | 39.0 ± 9.81 | 36% | 0.08 ns | ||
| E:T ratio 12:1 | All | 45.9 ± 18.0 | 13.3 (8.9–19.7) | 27.3 (20.9–34.7) | 105% | 0.00002 | 0.58 |
| Age <= 35 | 32 ± 3.6 | 13.3 (10.9–18.5) | 27.3 (23.2–33.0) | 105% | 0.004 | 0.68 | |
| Age >= 50 | 61.5 ± 14.2 | 15.6 ± 12.6 | 27.3 ± 8.15 | 75% | 0.0009 | ||
| E:T ratio 6:1 | All | 45.9 ± 18.0 | 6.2 (4.6–9.7) | 18.1 (15–21.3) | 192% | 0.00008 | 0.72 |
| Age <= 35 | 32 ± 3.6 | 6.2 (4.6–8.7) | 19.3 (15.9–24.7) | 211% | 0.004 | 0.84 | |
| Age >= 50 | 61.5 ± 14.2 | 7.6 (2.0–14.3) | 17.3 (13.6–18.9) | 128% | 0.04 | 0.45 | |
| CD107a Expression | PBMC | PBMC + IL-2 | PBMC + K562 | PBMC + K562 + IL-2 |
|---|---|---|---|---|
| Mean | 3.09 | 4.34 | 6.77 | 6.80 |
| Standard deviation | 0.91 | 1.42 | 2.08 | 2.08 |
| % increased relative to PBMC | - | 41% | 120% | 120% |
| p | - | 0.13 | 0.007 | 0.02 |
| Cytokine | Day 1 | Day 30 | % Inc | p | r |
|---|---|---|---|---|---|
| IL-1β | 32.8 ± 15.6 | 56.9 ± 27.4 | 74% | 0.044 | |
| IL-1Rα | 77.9 ± 52.0 | 199 ± 93.7 | 156% | 0.0025 | |
| IL-4 | 89.4 (75.2–98.0) | 118 (102–124) | 32% | 0.0024 | 0.54 |
| IL-6 | 92.7 (61.4–128) | 220 (74.1–313) | 137% | 0.027 | 0.4 |
| IL-7 | 6.45 ± 2.23 | 8.61 ± 2.12 | 34% | 0.032 | |
| IL-8 | 1766 ± 1044 | 3645 ± 1253 | 106% | 0.0024 | |
| IL-10 | 14.4 (9.94–30.2) | 34.8 (29.8–45.7) | 142% | 0.012 | 0.45 |
| IL-12p40 | 13.0 ± 4.79 | 19.6 ± 7.19 | 51% | 0.0296 | |
| IL-12p70 | 4.99 ± 1.42 | 6.90 ± 1.16 | 38% | 0.0066 | |
| IFN-γ | 11.5 ± 5.34 | 16.3 ± 6.41 | 41% | 0.054 ns | |
| TNF-α | 292 (202–509) | 720 (551–784) | 147% | 0.012 | 0.52 |
| TNF-β | 3.2 (3.2–3.34) | 3.86 (3.34–4.43) | 21% | 0.014 | 0.53 |
| G-CSF | 31.8 (25.6–36.8) | 43.6 (38.4–51.9) | 37% | 0.0068 | 0.59 |
| GM-CSF | 9.61 ± 3.25 | 12.6 ± 2.09 | 31% | 0.024 | |
| EGF | 109 ± 36.2 | 150 ± 43.5 | 38% | 0.011 | |
| FGF-2 | 90.4 (88.1–94.0) | 106 (103–112) | 17% | 0.00098 | 0.74 |
| PDGF-AA | 355 (290–415) | 576 (355–857) | 62% | 0.027 | 0.37 |
| PDGF-AB/BB | 7921 (6514–9160) | 10,588 (9800–11,888) | 34% | 0.0049 | 0.55 |
| VEGF | 41.1 ± 7.2 | 49.6 ± 7.66 | 21% | 0.0054 | |
| sCD40L | 335 ± 230 | 412 ± 400 | 23% | 0.48 ns | |
| GRO | 1946 ± 1079 | 3418 ± 1630 | 76% | 0.0075 | |
| MCP-1 | 1254 (765–1766) | 3297 (2647–6300) | 163% | 0.0068 | 0.61 |
| MIP-1α | 1301 ± 862 | 2638 ± 891 | 103% | 0.0041 | |
| MCP-3 | 55.7 (37.0–60.9) | 126 (97.8–232) | 126% | 0.0015 | 0.72 |
| Eotaxin | 26.2 (24.5–30.5) | 33.3 (30.9–36.9) | 27% | 0.00098 | 0.54 |
| MDC | 118 ± 39.6 | 163 ± 49.6 | 38% | 0.014 |
| Cytokine/Chemokine | Spearman’s Rank Correlation rho (r) | p |
|---|---|---|
| MIP-1a | 0.769697 | 0.014 * |
| TNF-alpha | 0.6969697 | 0.031 * |
| IL-6 | 0.7575758 | 0.016 * |
| IL-1beta | 0.4060606 | 0.247 ns |
| Day 1 | Day 30 | Day 1/Day 30 | ||||
|---|---|---|---|---|---|---|
| PBMC | PBMC + K562 | PBMC | PBMC + K562 | PBMC | PBMC + K562 | |
| Mean ± SD | 1.95 (1.84–2.06) | 8.78 (7.96–9.45) | 2.46 (1.88–3.2) | 18.40 (16.3–20.7) | - | - |
| Fold increase | - | 4.5 | - | 7.5 | 1.2 | 2.1 |
| p | - | 0.0022 | - | 0.0022 | 0.44 ns | 0.031 |
| r | 0.83 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boichuk, S.; Galembikova, A.; Vollmer, D. Enhancement of NK Cell Cytotoxic Activity and Immunoregulatory Effects of a Natural Product Supplement Across a Wide Age Span: A 30-Day In Vivo Human Study. Int. J. Mol. Sci. 2025, 26, 2897. https://doi.org/10.3390/ijms26072897
Boichuk S, Galembikova A, Vollmer D. Enhancement of NK Cell Cytotoxic Activity and Immunoregulatory Effects of a Natural Product Supplement Across a Wide Age Span: A 30-Day In Vivo Human Study. International Journal of Molecular Sciences. 2025; 26(7):2897. https://doi.org/10.3390/ijms26072897
Chicago/Turabian StyleBoichuk, Sergei, Aigul Galembikova, and David Vollmer. 2025. "Enhancement of NK Cell Cytotoxic Activity and Immunoregulatory Effects of a Natural Product Supplement Across a Wide Age Span: A 30-Day In Vivo Human Study" International Journal of Molecular Sciences 26, no. 7: 2897. https://doi.org/10.3390/ijms26072897
APA StyleBoichuk, S., Galembikova, A., & Vollmer, D. (2025). Enhancement of NK Cell Cytotoxic Activity and Immunoregulatory Effects of a Natural Product Supplement Across a Wide Age Span: A 30-Day In Vivo Human Study. International Journal of Molecular Sciences, 26(7), 2897. https://doi.org/10.3390/ijms26072897







