Effect of Bovine Follicular Fluid Small Extracellular Vesicles Isolated by Ultracentrifugation and Chromatography on In Vitro Oocyte Maturation and Embryo Development
Abstract
1. Introduction
2. Results
2.1. Isolation and Characterization of Small Extracellular Vesicles from Follicular Fluid (ffsEVs)
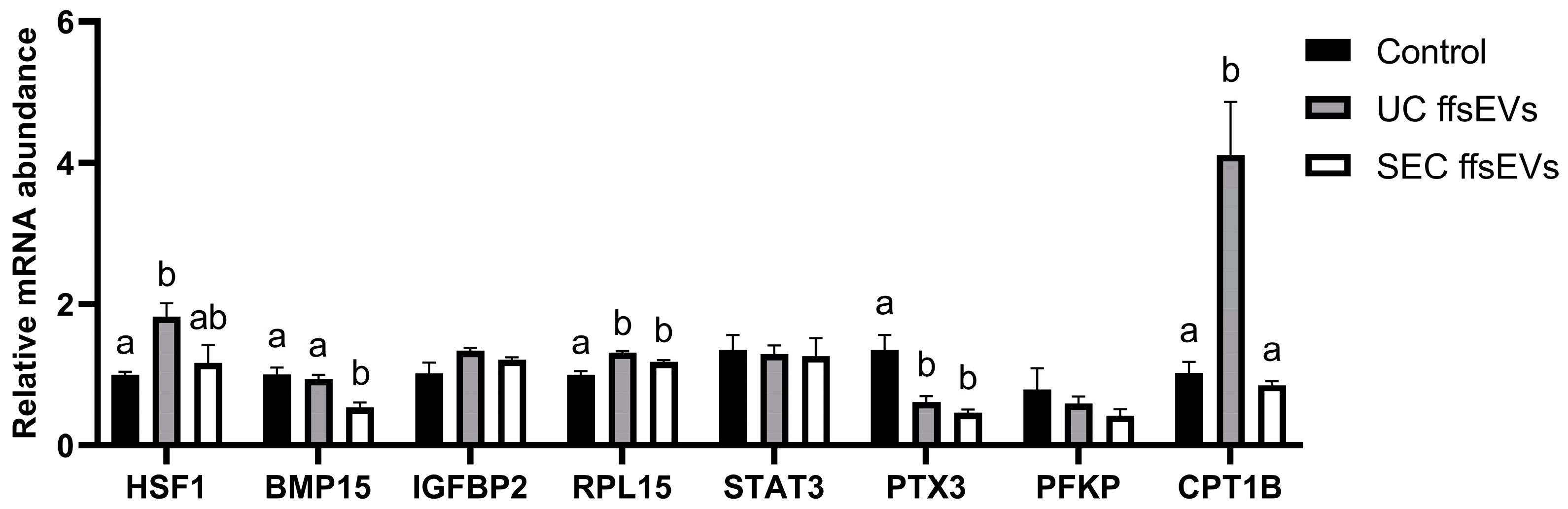
2.2. Transcript Level Analysis of Mature Oocytes
2.3. In Vitro-Fertilized Embryo Development
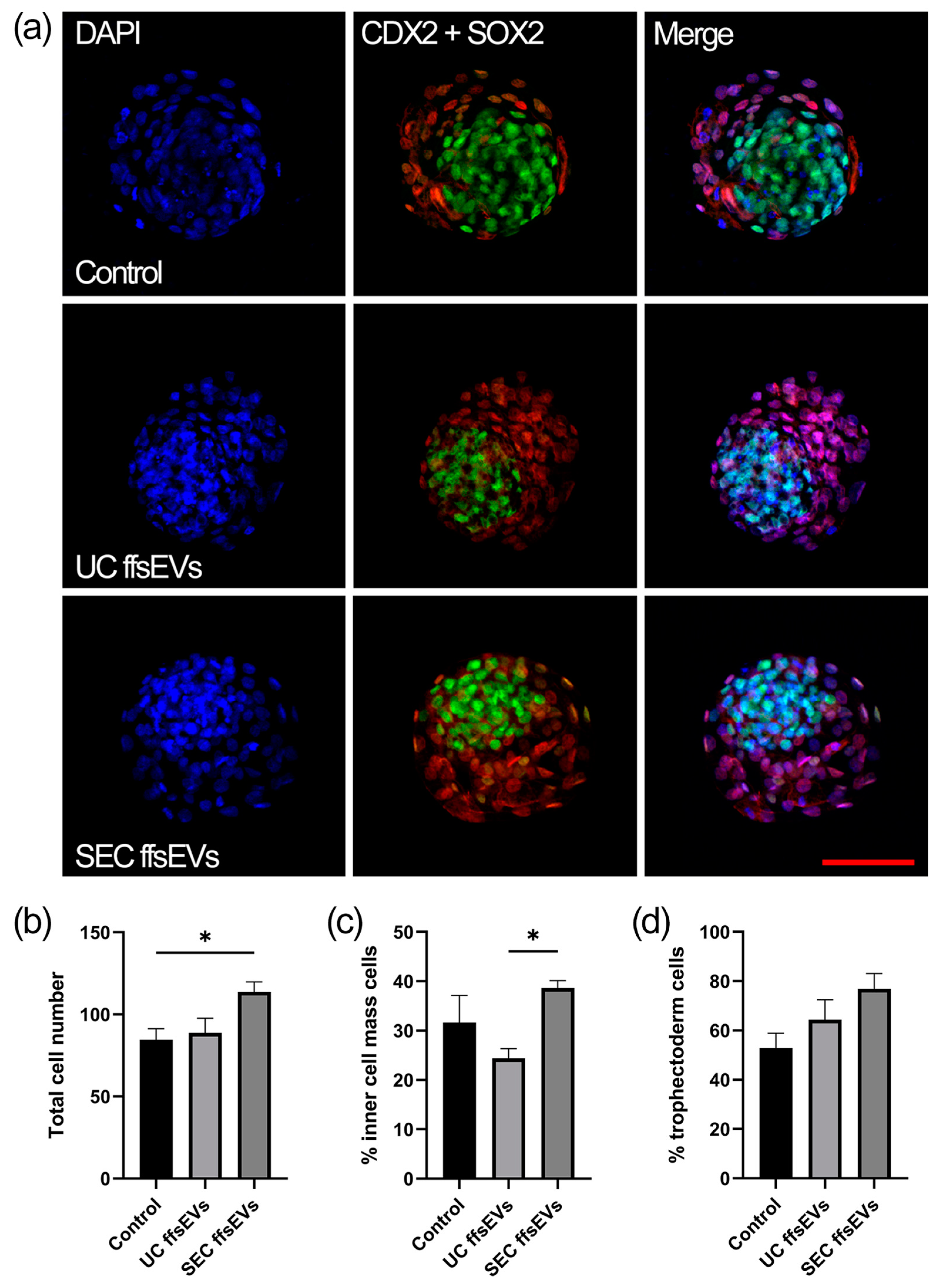
2.4. Total Cell Number and Cell Localization
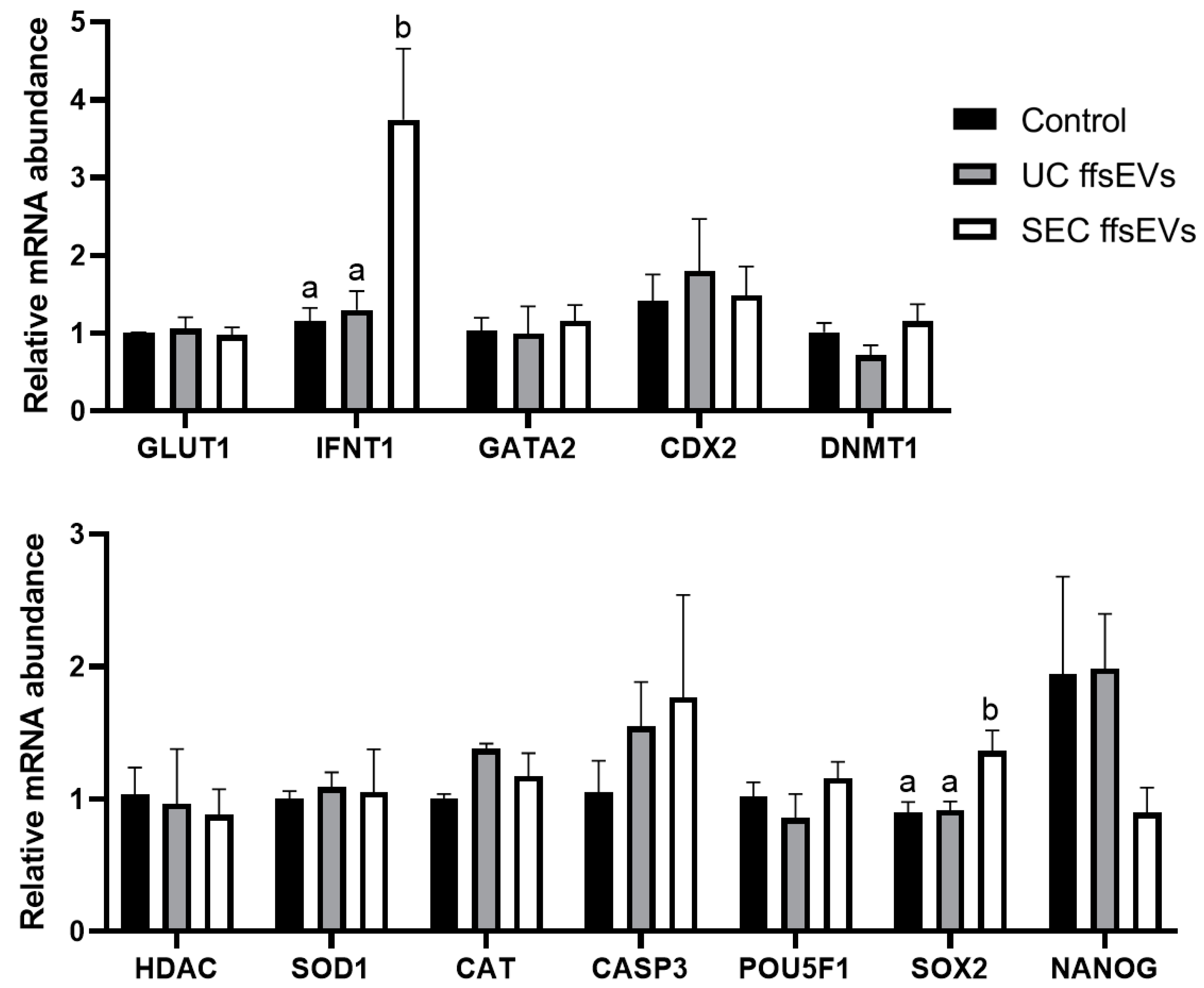
2.5. Transcript Level Analysis of Embryos
3. Discussion
4. Materials and Methods
4.1. Follicular Fluid Collection and Small Extracellular Vesicle (sEV) Isolation
4.2. Nanoparticle Tracking Analysis (NTA)
4.3. Transmission Scanning Electron Microscopy (STEM)
4.4. Total Protein Quantification and Western Blot
4.5. In Vitro Oocyte Maturation and ffsEVs Supplementation
4.6. ffsEVs Uptake Assay
4.7. In Vitro Fertilization and Embryo Culture
4.8. Quantitative Real-Time Reverse Transcription PCR (Two-Step RT-qPCR)
4.9. Total Cell Number, Cell Localization, and Confocal Microscopy
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galli, C.; Lazzari, G. Practical Aspects of IVM/IVF in Cattle. Anim. Reprod. Sci. 1996, 42, 371–379. [Google Scholar] [CrossRef]
- Salgado-Cruz, E.; Lopera Vasquez, R. Aspectos Esenciales Sobre Las Técnicas de Fertilización in vitro En Bovinos. Rev. De Investig. Vet. Del Perú 2020, 31, e17138. [Google Scholar] [CrossRef]
- Wu, B.; Zan, L. Enhance Beef Cattle Improvement by Embryo Biotechnologies. Reprod. Domest. Anim. 2012, 47, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Herrick, J.R. Assisted Reproductive Technologies for Endangered Species Conservation: Developing Sophisticated Protocols with Limited Access to Animals with Unique Reproductive Mechanisms. Biol. Reprod. 2019, 100, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.; Fair, T. The ART of Studying Early Embryo Development: Progress and Challenges in Ruminant Embryo Culture. Theriogenology 2014, 81, 49–55. [Google Scholar] [CrossRef]
- Fernández, A.; Díaz, T.; Muñoz, G. Producción in vitro de Embriones Bovinos. Rev. De La Fac. De Cienc. Veterinarias. Univ. Cent. De Venez. 2007, 48, 51–60. [Google Scholar]
- Qu, P.; Qing, S.; Liu, R.; Qin, H.; Wang, W.; Qiao, F.; Ge, H.; Liu, J.; Zhang, Y.; Cui, W.; et al. Effects of Embryo-Derived Exosomes on the Development of Bovine Cloned Embryos. PLoS ONE 2017, 12, e0174535. [Google Scholar] [CrossRef] [PubMed]
- Urrego, R.; Rodriguez-Osorio, N.; Niemann, H. Epigenetic Disorders and Altered Gene Expression after Use of Assisted Reproductive Technologies in Domestic Cattle. Epigenetics 2014, 9, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Mucci, N.; Aller, J.; Kaiser, G.G.; Hozbor, F.; Cabodevila, J.; Alberio, R.H. Effect of Estrous Cow Serum during Bovine Embryo Culture on Blastocyst Development and Cryotolerance after Slow Freezing or Vitrification. Theriogenology 2006, 65, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R. Fertilization Studies and Assisted Fertilization in Mammals: Their Development and Future. J. Reprod. Dev. 2012, 58, 25–32. [Google Scholar] [CrossRef]
- Aplin, J.D.; Ruane, P.T. Embryo–Epithelium Interactions during Implantation at a Glance. J. Cell Sci. 2017, 130, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.A.; Rizos, D.; Rodriguez-Martinez, H.; Funahashi, H. Oocyte-Cumulus Cells Crosstalk: New Comparative Insights. Theriogenology 2023, 205, 87–93. [Google Scholar] [CrossRef]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular Vesicles: Roles in Gamete Maturation, Fertilization and Embryo Implantation. Hum. Reprod. Update 2015, 22, 182–193. [Google Scholar] [CrossRef]
- Sandoval Montiel, A.A.; Hernández Cortés, P.; Guerrero Reyes, J.; Anaya Ruiz, M.; Rosas Murrieta, N.H. Las Vesículas Extracelulares y Su Papel En La Salud y El Cáncer. Alianzas Y Tend. BUAP 2017, 2, 7–11. [Google Scholar]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of Extracellular Vesicles (EV): Exosomes, Microvesicles, Retrovirus-like Vesicles, and Apoptotic Bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jia, Y.; Mao, C.; Liu, S. Small Extracellular Vesicles: Non-Negligible Vesicles in Tumor Progression, Diagnosis, and Therapy. Cancer Lett. 2024, 580, 216481. [Google Scholar] [CrossRef] [PubMed]
- Stremersch, S.; De Smedt, S.C.; Raemdonck, K. Therapeutic and Diagnostic Applications of Extracellular Vesicles. J. Control. Release 2016, 244, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.; Meirelles, F.; Perecin, F.; da Silveira, J. Cellular and Extracellular Vesicular Origins of MiRNAs within the Bovine Ovarian Follicle. Reprod. Domest. Anim. 2017, 52, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-T.; Hong, X.; Christenson, L.K.; McGinnis, L.K. Extracellular Vesicles from Bovine Follicular Fluid Support Cumulus Expansion. Biol. Reprod. 2015, 93, 117. [Google Scholar] [CrossRef]
- Uzbekova, S.; Almiñana, C.; Labas, V.; Teixeira-Gomes, A.-P.; Combes-Soia, L.; Tsikis, G.; Carvalho, A.V.; Uzbekov, R.; Singina, G. Protein Cargo of Extracellular Vesicles From Bovine Follicular Fluid and Analysis of Their Origin From Different Ovarian Cells. Front. Vet. Sci. 2020, 7, 584948. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; Veeramachaneni, D.N.R.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-Secreted Vesicles in Equine Ovarian Follicular Fluid Contain MiRNAs and Proteins: A Possible New Form of Cell Communication Within the Ovarian Follicle1. Biol. Reprod. 2012, 86, 71. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.M.H.; Hoelker, M.; Noferesti, S.S.; Salilew-Wondim, D.; Tholen, E.; Looft, C.; Rings, F.; Uddin, M.J.; Spencer, T.E.; Schellander, K.; et al. Exosomal and Non-Exosomal Transport of Extra-Cellular MicroRNAs in Follicular Fluid: Implications for Bovine Oocyte Developmental Competence. PLoS ONE 2013, 8, e78505. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; Andrade, G.M.; del Collado, M.; Sampaio, R.V.; Sangalli, J.R.; Silva, L.A.; Pinaffi, F.V.L.; Jardim, I.B.; Cesar, M.C.; Nogueira, M.F.G.; et al. Supplementation with Small-Extracellular Vesicles from Ovarian Follicular Fluid during in vitro Production Modulates Bovine Embryo Development. PLoS ONE 2017, 12, e0179451. [Google Scholar] [CrossRef]
- de Ávila, A.C.F.C.M.; Bridi, A.; Andrade, G.M.; del Collado, M.; Sangalli, J.R.; Nociti, R.P.; da Silva Junior, W.A.; Bastien, A.; Robert, C.; Meirelles, F.V.; et al. Estrous Cycle Impacts MicroRNA Content in Extracellular Vesicles That Modulate Bovine Cumulus Cell Transcripts during in vitro Maturation. Biol. Reprod. 2020, 102, 362–375. [Google Scholar] [CrossRef] [PubMed]
- da Silva Rosa, P.M.; Bridi, A.; de Ávila Ferronato, G.; Prado, C.M.; Bastos, N.M.; Sangalli, J.R.; Meirelles, F.V.; Perecin, F.; da Silveira, J.C. Corpus Luteum Presence in the Bovine Ovary Increase Intrafollicular Progesterone Concentration: Consequences in Follicular Cells Gene Expression and Follicular Fluid Small Extracellular Vesicles MiRNA Contents. J. Ovarian Res. 2024, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhu, L.; Shen, H.; Lu, J.; Zou, Q.; Huang, C.; Li, H.; Huang, B. Exosomal MiRNA-17-5p Derived from Human Umbilical Cord Mesenchymal Stem Cells Improves Ovarian Function in Premature Ovarian Insufficiency by Regulating SIRT7. Stem Cells 2020, 38, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhang, J.; Xu, B.; He, Y.; Liu, W.; Li, J.; Zhang, S.; Lin, X.; Su, D.; Wu, T.; et al. HucMSC-Derived Exosomes Mitigate the Age-Related Retardation of Fertility in Female Mice. Mol. Ther. 2020, 28, 1200–1213. [Google Scholar] [CrossRef]
- Rodrigues, T.A.; Tuna, K.M.; Alli, A.A.; Tribulo, P.; Hansen, P.J.; Koh, J.; Paula-Lopes, F.F. Follicular Fluid Exosomes Act on the Bovine Oocyte to Improve Oocyte Competence to Support Development and Survival to Heat Shock. Reprod. Fertil. Dev. 2019, 31, 888–897. [Google Scholar] [CrossRef]
- Morales Dalanezi, F.; Mogollon Garcia, H.D.; de Andrade Ferrazza, R.; Fagali Franchi, F.; Kubo Fontes, P.; de Souza Castilho, A.C.; Gouveia Nogueira, M.F.; dos Santos Schmidt, E.M.; Sartori, R.; Pinheiro Ferreira, J.C. Extracellular Vesicles of Follicular Fluid from Heat-Stressed Cows Modify the Gene Expression of in vitro-Matured Oocytes. Anim. Reprod. Sci. 2019, 205, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Mol, E.A.; Goumans, M.-J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher Functionality of Extracellular Vesicles Isolated Using Size-Exclusion Chromatography Compared to Ultracentrifugation. Nanomedicine 2017, 13, 2061–2065. [Google Scholar] [CrossRef]
- Soares, M.; Pinto, M.M.; Nobre, R.J.; de Almeida, L.P.; da Graça Rasteiro, M.; Almeida-Santos, T.; Ramalho-Santos, J.; Sousa, A.P. Isolation of Extracellular Vesicles from Human Follicular Fluid: Size-Exclusion Chromatography versus Ultracentrifugation. Biomolecules 2023, 13, 278. [Google Scholar] [CrossRef]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of Small Extracellular Vesicles Isolated from Plasma by Ultracentrifugation or Size-exclusion Chromatography: Yield, Purity and Functional Potential. J. Extracell. Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef]
- Lane, R.E.; Korbie, D.; Trau, M.; Hill, M.M. Purification Protocols for Extracellular Vesicles. Extracell. Vesicles Methods Protoc. 2017, 1660, 111–130. [Google Scholar]
- Guan, S.; Yu, H.; Yan, G.; Gao, M.; Sun, W.; Zhang, X. Characterization of Urinary Exosomes Purified with Size Exclusion Chromatography and Ultracentrifugation. J. Proteome Res. 2020, 19, 2217–2225. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Gabryś, J.; Kij-Mitka, B.; Sawicki, S.; Kochan, J.; Nowak, A.; Łojko, J.; Karnas, E.; Bugno-Poniewierska, M. Extracellular Vesicles from Follicular Fluid May Improve the Nuclear Maturation Rate of in vitro Matured Mare Oocytes. Theriogenology 2022, 188, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Macaulay, A.D.; Gilbert, I.; Scantland, S.; Fournier, E.; Ashkar, F.; Bastien, A.; Saadi, H.A.S.; Gagné, D.; Sirard, M.-A.; Khandjian, É.W.; et al. Cumulus Cell Transcripts Transit to the Bovine Oocyte in Preparation for Maturation. Biol. Reprod. 2016, 94, 16. [Google Scholar] [CrossRef]
- Russell, D.L.; Gilchrist, R.B.; Brown, H.M.; Thompson, J.G. Bidirectional Communication between Cumulus Cells and the Oocyte: Old Hands and New Players? Theriogenology 2016, 86, 62–68. [Google Scholar] [CrossRef]
- Latham, K.E. Mechanisms and Control of Embryonic Genome Activation in Mammalian Embryos. Int. Rev. Cytol. 1999, 193, 71–124. [Google Scholar] [PubMed]
- Sanchez, F.; Adriaenssens, T.; Romero, S.; Smitz, J. Quantification of Oocyte-Specific Transcripts in Follicle-Enclosed Oocytes during Antral Development and Maturation in vitro. Mol. Hum. Reprod. 2009, 15, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Barna, J.; Csermely, P.; Vellai, T. Roles of Heat Shock Factor 1 beyond the Heat Shock Response. Cell. Mol. Life Sci. 2018, 75, 2897–2916. [Google Scholar] [CrossRef] [PubMed]
- Le Masson, F.; Razak, Z.; Kaigo, M.; Audouard, C.; Charry, C.; Cooke, H.; Westwood, J.T.; Christians, E.S. Identification of Heat Shock Factor 1 Molecular and Cellular Targets during Embryonic and Adult Female Meiosis. Mol. Cell. Biol. 2011, 31, 3410–3423. [Google Scholar] [CrossRef]
- Metchat, A.; Åkerfelt, M.; Bierkamp, C.; Delsinne, V.; Sistonen, L.; Alexandre, H.; Christians, E.S. Mammalian Heat Shock Factor 1 Is Essential for Oocyte Meiosis and Directly Regulates Hsp90α Expression. J. Biol. Chem. 2009, 284, 9521–9528. [Google Scholar] [CrossRef] [PubMed]
- Bierkamp, C.; Luxey, M.; Metchat, A.; Audouard, C.; Dumollard, R.; Christians, E. Lack of Maternal Heat Shock Factor 1 Results in Multiple Cellular and Developmental Defects, Including Mitochondrial Damage and Altered Redox Homeostasis, and Leads to Reduced Survival of Mammalian Oocytes and Embryos. Dev. Biol. 2010, 339, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Christians, E.; Davis, A.A.; Thomas, S.D.; Benjamin, I.J. Maternal Effect of Hsf1 on Reproductive Success. Nature 2000, 407, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Caixeta, E.S.; Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G.; Price, C.A.; Machado, M.F.; Lima, P.F.; Buratini, J. Bone Morphogenetic Protein 15 and Fibroblast Growth Factor 10 Enhance Cumulus Expansion, Glucose Uptake, and Expression of Genes in the Ovulatory Cascade during in vitro Maturation of Bovine Cumulus–Oocyte Complexes. Reproduction 2013, 146, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Paulini, F.; Melo, E.O. The Role of Oocyte-Secreted Factors GDF9 and BMP15 in Follicular Development and Oogenesis. Reprod. Domest. Anim. 2011, 46, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, Y.; Zhang, L.; Wang, B.; Liu, J.; Luo, Y.; Guo, Z.; Quan, F.; Zhang, Y. Oocyte-Secreted Factors in Oocyte Maturation Media Enhance Subsequent Development of Bovine Cloned Embryos. Mol. Reprod. Dev. 2014, 81, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Jitjumnong, J.; Tang, P.-C. Bone Morphogenetic Protein 15 (BMP-15) Improves in vitro Mouse Folliculogenesis. Animals 2023, 13, 980. [Google Scholar] [CrossRef]
- Yao, J.; Ren, X.; Ireland, J.J.; Coussens, P.M.; Smith, T.P.L.; Smith, G.W. Generation of a Bovine Oocyte CDNA Library and Microarray: Resources for Identification of Genes Important for Follicular Development and Early Embryogenesis. Physiol Genom. 2004, 19, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Baum, E.Z.; Wormington, W.M. Coordinate Expression of Ribosomal Protein Genes during Xenopus Development. Dev. Biol. 1985, 111, 488–498. [Google Scholar] [CrossRef]
- Plaks, V.; Gershon, E.; Zeisel, A.; Jacob-Hirsch, J.; Neeman, M.; Winterhager, E.; Rechavi, G.; Domany, E.; Dekel, N. Blastocyst Implantation Failure Relates to Impaired Translational Machinery Gene Expression. Reproduction 2014, 148, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Camaioni, A.; Klinger, F.G.; Campagnolo, L.; Salustri, A. The Influence of Pentraxin 3 on the Ovarian Function and Its Impact on Fertility. Front. Immunol. 2018, 9, 2808. [Google Scholar] [CrossRef]
- Nagyova, E.; Kalous, J.; Nemcova, L. Increased Expression of Pentraxin 3 after in vivo and in vitro Stimulation with Gonadotropins in Porcine Oocyte-Cumulus Complexes and Granulosa Cells. Domest. Anim. Endocrinol. 2016, 56, 29–35. [Google Scholar] [CrossRef]
- Salustri, A.; Garlanda, C.; Hirsch, E.; De Acetis, M.; Maccagno, A.; Bottazzi, B.; Doni, A.; Bastone, A.; Mantovani, G.; Peccoz, P.B.; et al. PTX3 Plays a Key Role in the Organization of the Cumulus Oophorus Extracellular Matrix and in in vivo Fertilization. Development 2004, 131, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hao, C.; Shen, X.; Zhang, Y.; Liu, X. RUNX2, GPX3 and PTX3 Gene Expression Profiling in Cumulus Cells Are Reflective Oocyte/Embryo Competence and Potentially Reliable Predictors of Embryo Developmental Competence in PCOS Patients. Reprod. Biol. Endocrinol. 2013, 11, 109. [Google Scholar] [CrossRef]
- Li, S.-H.; Lin, M.-H.; Hwu, Y.-M.; Lu, C.-H.; Yeh, L.-Y.; Chen, Y.-J.; Lee, R.K.-K. Correlation of Cumulus Gene Expression of GJA1, PRSS35, PTX3, and SERPINE2 with Oocyte Maturation, Fertilization, and Embryo Development. Reprod. Biol. Endocrinol. 2015, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Gao, M.; Yang, R.; Zhao, Z.; Mi, J.; Sun, H.; Xiao, H.; Fang, X. The Effect of CPT1B Gene on Lipid Metabolism and Its Polymorphism Analysis in Chinese Simmental Cattle. Anim. Biotechnol. 2022, 33, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lazo, L.; Brisard, D.; Elis, S.; Maillard, V.; Uzbekov, R.; Labas, V.; Desmarchais, A.; Papillier, P.; Monget, P.; Uzbekova, S. Fatty Acid Synthesis and Oxidation in Cumulus Cells Support Oocyte Maturation in Bovine. Mol. Endocrinol. 2014, 28, 1502–1521. [Google Scholar] [CrossRef]
- Mogas, T. Update on the Vitrification of Bovine Oocytes and in vitro-Produced Embryos. Reprod. Fertil. Dev. 2019, 31, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Anastasi, M.R.; Zhang, V.J.; Russell, D.L.; Robker, R.L. Regulation of Fatty Acid Oxidation in Mouse Cumulus-Oocyte Complexes during Maturation and Modulation by PPAR Agonists. PLoS ONE 2014, 9, e87327. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, E.L.; Fuentes, F.B.; Felmer, R.N.; Yeste, M.; Arias, M.E. Extracellular Vesicles in Mammalian Reproduction: A Review. Zygote 2022, 30, 440–463. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.E.; Risopatrón, J.; Sánchez, R.; Felmer, R. Intracytoplasmic Sperm Injection Affects Embryo Developmental Potential and Gene Expression in Cattle. Reprod. Biol. 2015, 15, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ledezma, J.J.; Mathialagan, N.; Villanueva, C.; Sikes, J.D.; Roberts, R.M. Expression of Bovine Trophoblast Interferons by in vitro-derived Blastocysts Is Correlated with Their Morphological Quality and Stage of Development. Mol. Reprod. Dev. 1993, 36, 1–6. [Google Scholar] [CrossRef]
- Aguilera, C.; Wong, Y.S.; Gutierrez-Reinoso, M.A.; Velásquez, A.E.; Melo-Báez, B.; Cabezas, J.; Caamaño, D.; Navarrete, F.; Castro, F.O.; Rodriguez-Alvarez, L.l. Embryo-Maternal Communication Mediated by Extracellular Vesicles in the Early Stages of Embryonic Development Is Modified by in vitro Conditions. Theriogenology 2024, 214, 43–56. [Google Scholar] [CrossRef]
- Qiao, F.; Ge, H.; Ma, X.; Zhang, Y.; Zuo, Z.; Wang, M.; Zhang, Y.; Wang, Y. Bovine Uterus-Derived Exosomes Improve Developmental Competence of Somatic Cell Nuclear Transfer Embryos. Theriogenology 2018, 114, 199–205. [Google Scholar] [CrossRef]
- Bai, H.; Kawahara, M.; Takahashi, M.; Imakawa, K. Recent Progress of Interferon-Tau Research and Potential Direction beyond Pregnancy Recognition. J. Reprod. Dev. 2022, 68, 2022–2061. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J. Genetic Control of Early Cell Lineages in the Mammalian Embryo. Annu. Rev. Genet. 2018, 52, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zernicka-Goetz, M. Principles of Self-Organization of the Mammalian Embryo. Cell 2020, 183, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Goissis, M.D.; Cibelli, J.B. Functional Characterization of SOX2 in Bovine Preimplantation Embryos1. Biol. Reprod. 2014, 90, 30. [Google Scholar] [CrossRef]
- Luo, L.; Shi, Y.; Wang, H.; Wang, Z.; Dang, Y.; Li, S.; Wang, S.; Zhang, K. Base Editing in Bovine Embryos Reveals a Species-Specific Role of SOX2 in Regulation of Pluripotency. PLoS Genet. 2022, 18, e1010307. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, A.E.; Veraguas, D.; Cabezas, J.; Manríquez, J.; Castro, F.O.; Rodríguez-Alvarez, L.L. The Expression Level of SOX2 at the Blastocyst Stage Regulates the Developmental Capacity of Bovine Embryos up to Day-13 of in vitro Culture. Zygote 2019, 27, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeel, E.; Balaban, B.; Ziebe, S.; Lundin, K.; Cuesta, M.J.G.; Klein, B.M.; Helmgaard, L.; Arce, J.-C. Association between Blastocyst Morphology and Outcome of Single-Blastocyst Transfer. Reprod. Biomed. Online 2013, 27, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Y.; Yu, Y.; Zhang, X.-W. Overall Blastocyst Quality, Trophectoderm Grade, and Inner Cell Mass Grade Predict Pregnancy Outcome in Euploid Blastocyst Transfer Cycles. Chin. Med. J. 2018, 131, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Kemper, J.M.; Hammond, E.R.; Xu, F.; Liu, G.; Xue, L.; Bai, X.; Liao, H.; Xue, S.; Zhao, S.; et al. Blastocyst Quality and Reproductive and Perinatal Outcomes: A Multinational Multicentre Observational Study. Hum. Reprod. 2023, 38, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.-B.; Kang, Y.-K.; Choi, Y.-H.; Park, J.S.; Kim, H.-N.; Oh, K.B.; Son, D.-S.; Park, H.; Lee, K.-K.; Han, Y.-M. Aberrant Allocations of Inner Cell Mass and Trophectoderm Cells in Bovine Nuclear Transfer Blastocysts1. Biol. Reprod. 2002, 67, 487–492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Soom, A.; Ysebaert, M.-T.; de Kruif, A. Relationship between Timing of Development, Morula Morphology, and Cell Allocation to Inner Cell Mass and Trophectoderm in in vitro-Produced Bovine Embryos. Mol. Reprod. Dev. 1997, 47, 47–56. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A Comparison of Methods for TheI and Separation of Extracellular Vesicles from Protein and Lipid Particles in Human Serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.S.; Catita, J.; Rosa, I.M.; da Cruz E Silva, O.A.B.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, B.; Han, D.; Guo, Z.; Deng, J.; Li, W.; Huang, L.; Liu, J.; Cai, Z.; Bian, J.; Huang, S. Altered Small Non-coding RNA Expression Profiles of Extracellular Vesicles in the Prostatic Fluid of Patients with Chronic Pelvic Pain Syndrome. Exp. Ther. Med. 2022, 23, 382. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Pang, R.T.K.; Liu, W.; Li, Q.; Cheng, R.; Yeung, W.S.B. Polymer-Based Precipitation Preserves Biological Activities of Extracellular Vesicles from an Endometrial Cell Line. PLoS ONE 2017, 12, e0186534. [Google Scholar] [CrossRef]
- Arias, M.E.; Sánchez, R.; Felmer, R. Effect of Anisomycin, a Protein Synthesis Inhibitor, on the in vitro Developmental Potential, Ploidy and Embryo Quality of Bovine ICSI Embryos. Zygote 2016, 24, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.E.; Vargas, T.; Gallardo, V.; Aguila, L.; Felmer, R. Simple and Efficient Chemically Defined in vitro Maturation and Embryo Culture System for Bovine Embryos. Animals 2022, 12, 3057. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, C.; Arias, M.E.; Vargas, T.; Paredes, M.; Sánchez, R.; Felmer, R. Stability of Reference Genes for Normalization of Reverse Transcription Quantitative Real-Time PCR (RT-QPCR) Data in Bovine Blastocysts Produced by IVF, ICSI and SCNT. Zygote 2014, 22, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Aguila, L.; Nociti, R.P.; Sampaio, R.V.; Therrien, J.; Meirelles, F.V.; Felmer, R.N.; Smith, L.C. Haploid Androgenetic Development of Bovine Embryos Reveals Imbalanced WNT Signaling and Impaired Cell Fate Differentiation. Biol. Reprod. 2023, 109, 821–838. [Google Scholar] [CrossRef]



| Treatment | COCs No. | Cleavage | Total Blastocysts | Expanded | Hatched |
|---|---|---|---|---|---|
| No. and (%) | No. and (%) | No. and (%) 1 | No. and (%) 1 | ||
| Control | 280 | 227 (81.07 ± 8.65) | 71 (25.36 ± 5.05) | 25 (35.21 ±12.06) | 4 (5.63 ± 6.66) |
| UC ffsEVs | 230 | 199 (86.52 ± 6.29) | 81 (35.22 ± 7.35) | 37 (45.68 ± 10.72) | 2 (2.47 ± 3.76) |
| SEC ffsEVs | 307 | 261 (85.52 ± 6.41) | 89 (28.99 ± 5.68) | 37 (41.57 ± 17.00) | 9 (10.11 ± 10.84) |
| Gene | NCBI Code | Sequence (5′-3′) | Ta (°C) | Product Size (bp) |
|---|---|---|---|---|
| HSF1 | NM_001076809.1 | F: CCAGCAACAGAAAGTCGTCA | 54 | 92 |
| R: GGGGGATCTTTCTCTTCACC | ||||
| BMP15 | NM_001031752.1 | F: ATCATGCCATCATCCAGAACC | 53 | 73 |
| R: TAAGGGACACAGGAAGGCTGA | ||||
| RPL15 | NM_001077866.2 | F: CAAACGCCCAGTTCCTAAGG | 55 | 76 |
| R: TCGAGCAAACTTGAGCTGGTT | ||||
| CPT1B | NM_001034349.2 | F: TCTCCAGCAAGTTCTCCAGC | 54 | 126 |
| R: ATCTCTCCAGCCCTTAGCCA | ||||
| IGFBP2 | NM_174555.1 | F: AGCATGGCCTGTACAACCTC | 55 | 95 |
| R: GATCAGCTTCCCGGTGTTAG | ||||
| STAT3 | NM_001012671.2 | F: TGGCATAGCTTCCTCTGTAT | 50 | 183 |
| R: CTTAGGGTCTCCTTCAACCT | ||||
| PTX3 | NM_001076259.2 | F: CATATGCCAGTTGGGAAGGT | 51 | 139 |
| R: GCCTTCTCCAGTCTCCCTTT | ||||
| PFKP | NM_001193220.3 | F: AAGCACGAGGAGTTCTGTGTC | 55 | 122 |
| R: TCACACGTATCGGTGATGGT | ||||
| HPRT1 | NM_001034035.2 | F: TGCTGAGGATTTGGAGGAGG | 54 | 154 |
| R: CAACAGGTCGGCAAAGAACT | ||||
| GAPDH | NM_001034034.2 | F: GGAGCCAAACGGGTCATCATCTC | 57 | 233 |
| R: GAGGGGCCATCCACAGTCTTCT |
| Gene | NCBI Code | Sequence (5′-3′) | Ta (°C) | Product Size (bp) |
|---|---|---|---|---|
| GLUT1 | M60448.1 | F: CCCAGGTGTTCGGCCTGGAC | 59 | 214 |
| R: TGCAGGTCGCGGGTCACGTC | ||||
| CDX2 | NM_001206299.1 | F: GCCACCATGTACGTGAGCTAC | 56 | 140 |
| R: ACATGGTATCCGCCGTAGTC | ||||
| GATA2 | NM_001192114.3 | F: GAGGACTGTAAGCGTAAAGG | 54 | 140 |
| R: AAGAACCAAGTCTCCCCAT | ||||
| DNMT1 | NM_182651.2 | F: CGCATGGGCTACCAGTGCACCTT | 58 | 312 |
| R: GGGCTCCCCGTTGTATGAAATCT | ||||
| INFT1 | NM_001015511.4 | F: TCATTCGGGCCAGGAGCCTG | 57 | 108 |
| R: TGGCCCTGGTGCTGGTCAGC | ||||
| CAT | NM_001035386.2 | F: ACCCTCGTGGCTTGCCAG | 54 | 192 |
| R: ACTCAGGACGCAGGCCTCC | ||||
| HDAC1 | NM_001037444.2 | F: GGCTCTGACTCCTTGTCTGG | 53 | 103 |
| R: GCATAGGCAGGTTGAAGCTC | ||||
| POU5F1 | NM_174580.3 | F: CGAAAGAGAAAGCGGACGGAG | 53 | 178 |
| R: TTGATCGTTTGCCCTTCTGG | ||||
| SOX2 | NM_001105463.2 | F: TTTGTCCGAGACGGAGAAGC | 55 | 146 |
| R: CTCCCGGCAGTGTGTACTTA | ||||
| CASP3 | NM_001077840.1 | F: TACTTGGGAAGGTGTGAGAAAACTAA | 55 | 71 |
| R: AACCCGTCTCCCTTTATATTGCT | ||||
| SOD1 | NM_174615.2 | F: GCTGTACCAGTGCAGGTC | 54 | 195 |
| R: CATGGACCACCATCGTGC | ||||
| SF3A1 | NM_001081510.1 | F: GCGGGAGGAAGAAGTAGGAG | 57 | 125 |
| R: TCAGCAAGAGGGACACAAA | ||||
| HMBS | NM_001046207.1 | F: CTTTGGAGAGGAATGAAGTGG | 53 | 80 |
| R: AATGGTGAAGCCAGGAGGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-García, F.; Muñoz-Acuña, E.; Valencia, C.; Aguila, L.; Felmer, R.; Arias, M.E. Effect of Bovine Follicular Fluid Small Extracellular Vesicles Isolated by Ultracentrifugation and Chromatography on In Vitro Oocyte Maturation and Embryo Development. Int. J. Mol. Sci. 2025, 26, 2880. https://doi.org/10.3390/ijms26072880
Pérez-García F, Muñoz-Acuña E, Valencia C, Aguila L, Felmer R, Arias ME. Effect of Bovine Follicular Fluid Small Extracellular Vesicles Isolated by Ultracentrifugation and Chromatography on In Vitro Oocyte Maturation and Embryo Development. International Journal of Molecular Sciences. 2025; 26(7):2880. https://doi.org/10.3390/ijms26072880
Chicago/Turabian StylePérez-García, Felipe, Erwin Muñoz-Acuña, Cecilia Valencia, Luis Aguila, Ricardo Felmer, and María Elena Arias. 2025. "Effect of Bovine Follicular Fluid Small Extracellular Vesicles Isolated by Ultracentrifugation and Chromatography on In Vitro Oocyte Maturation and Embryo Development" International Journal of Molecular Sciences 26, no. 7: 2880. https://doi.org/10.3390/ijms26072880
APA StylePérez-García, F., Muñoz-Acuña, E., Valencia, C., Aguila, L., Felmer, R., & Arias, M. E. (2025). Effect of Bovine Follicular Fluid Small Extracellular Vesicles Isolated by Ultracentrifugation and Chromatography on In Vitro Oocyte Maturation and Embryo Development. International Journal of Molecular Sciences, 26(7), 2880. https://doi.org/10.3390/ijms26072880







