Increased Herpesvirus Entry Mediator Expression on Circulating Monocytes and Subsets Predicts Poor Outcomes in Pancreatic Ductal Adenocarcinoma Patients
Abstract
1. Introduction
2. Results
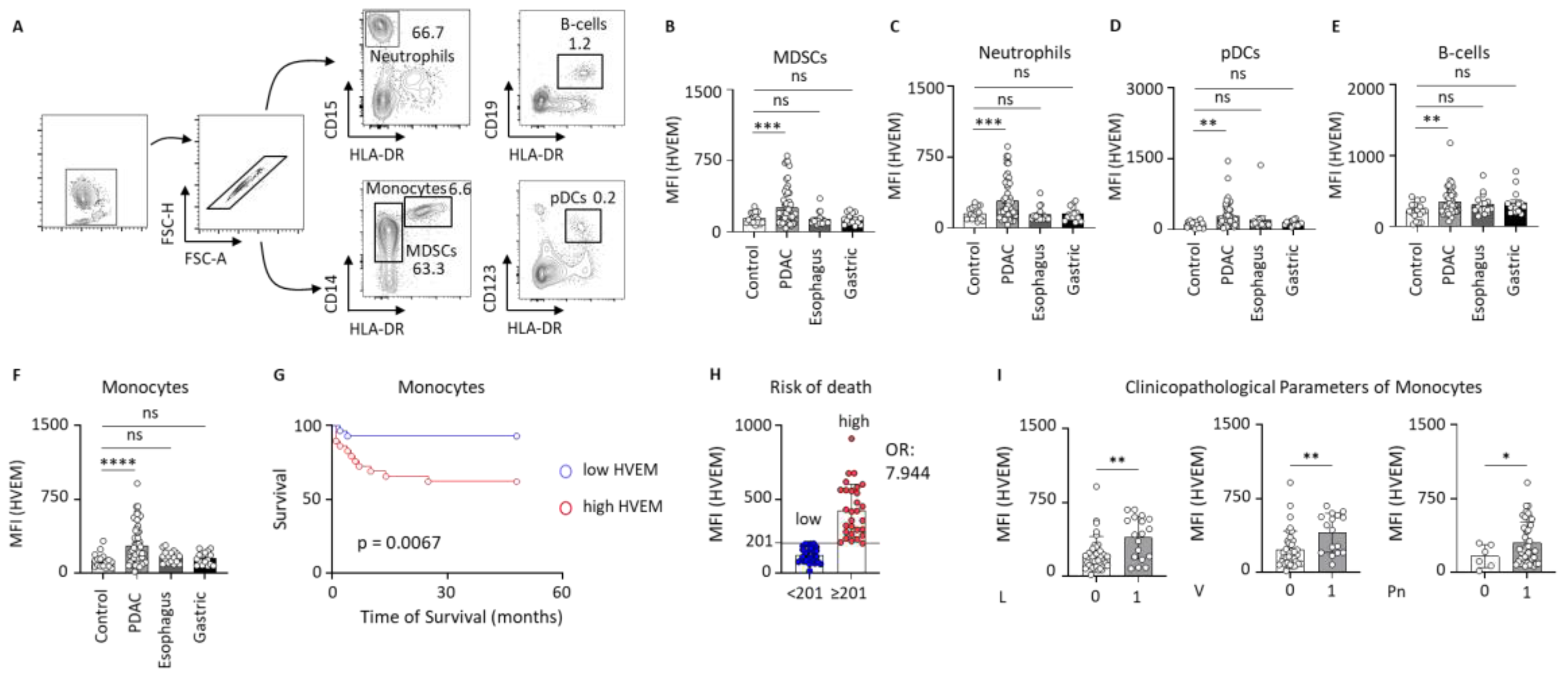
2.1. HVEM Expression on Circulating Leucocytes in Clinical Control and Gastrointestinal Cancer Patients
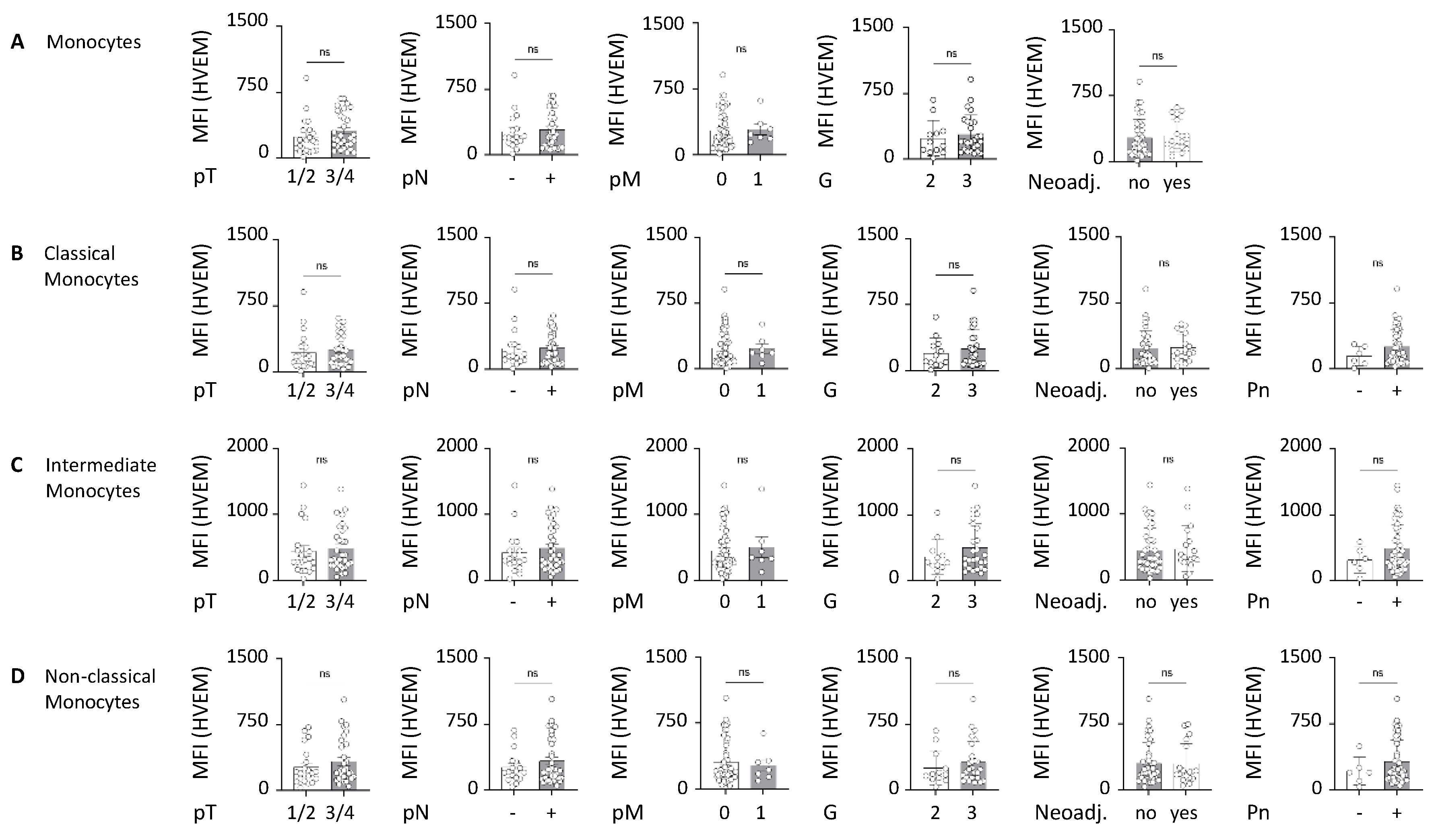
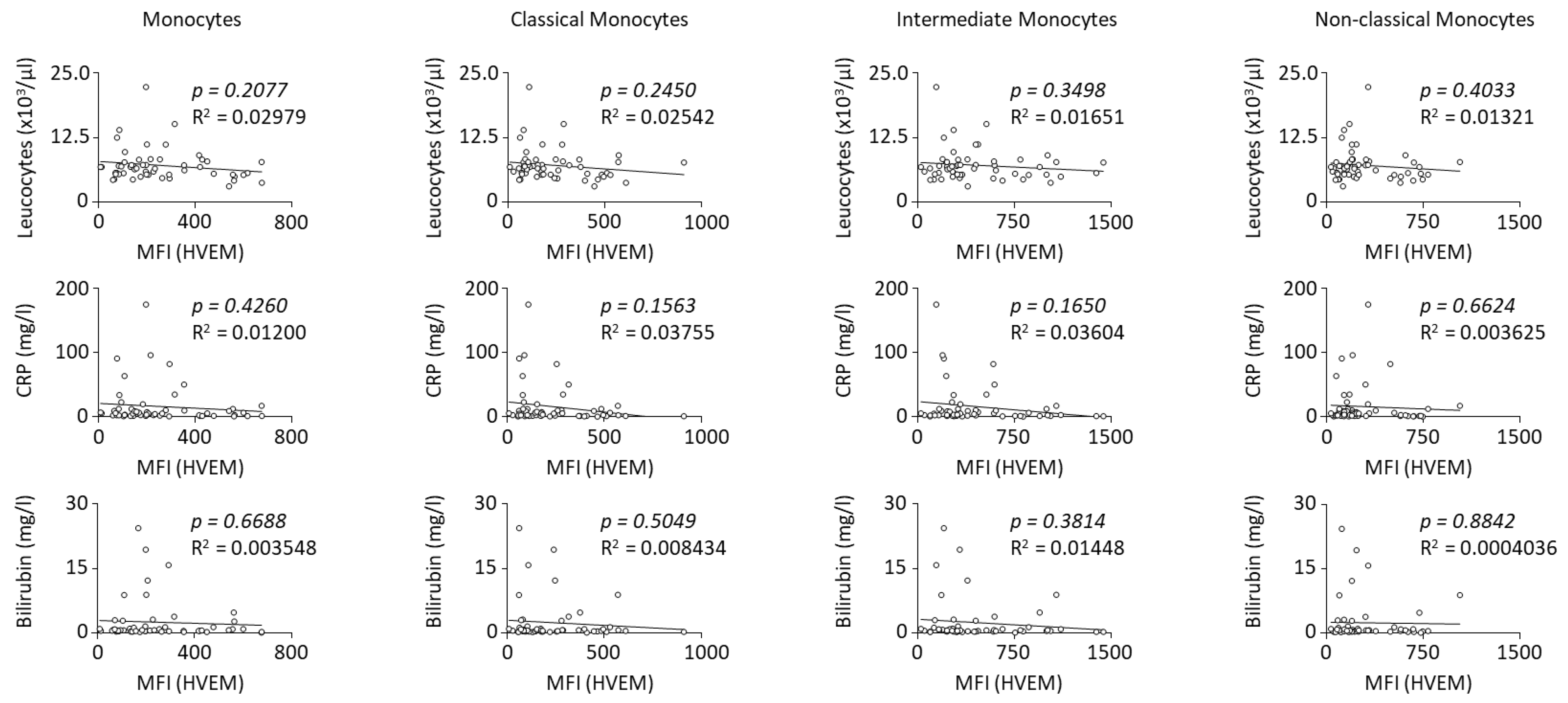
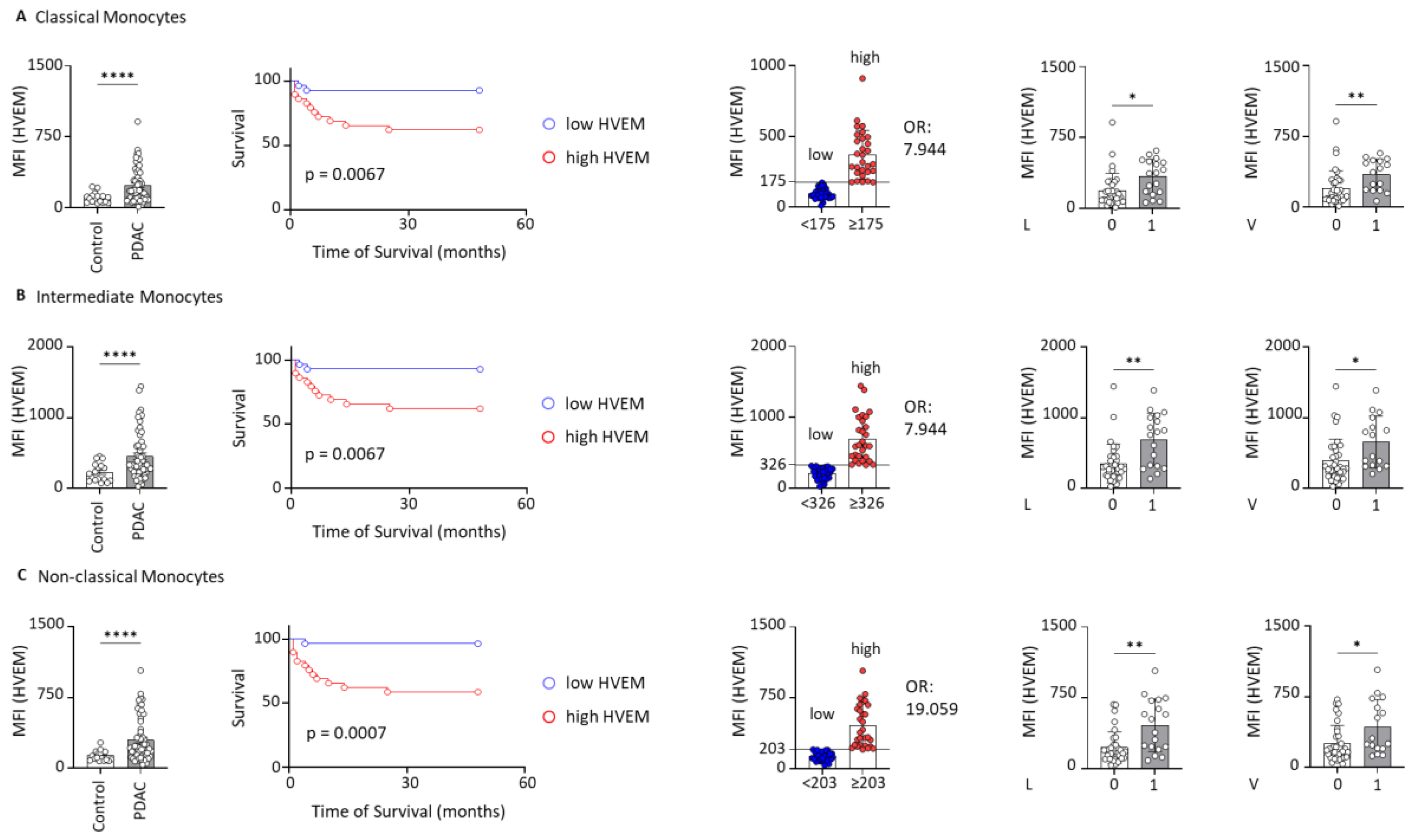
2.2. HVEM Expression on Monocyte Subsets Correlates with Worst Outcomes
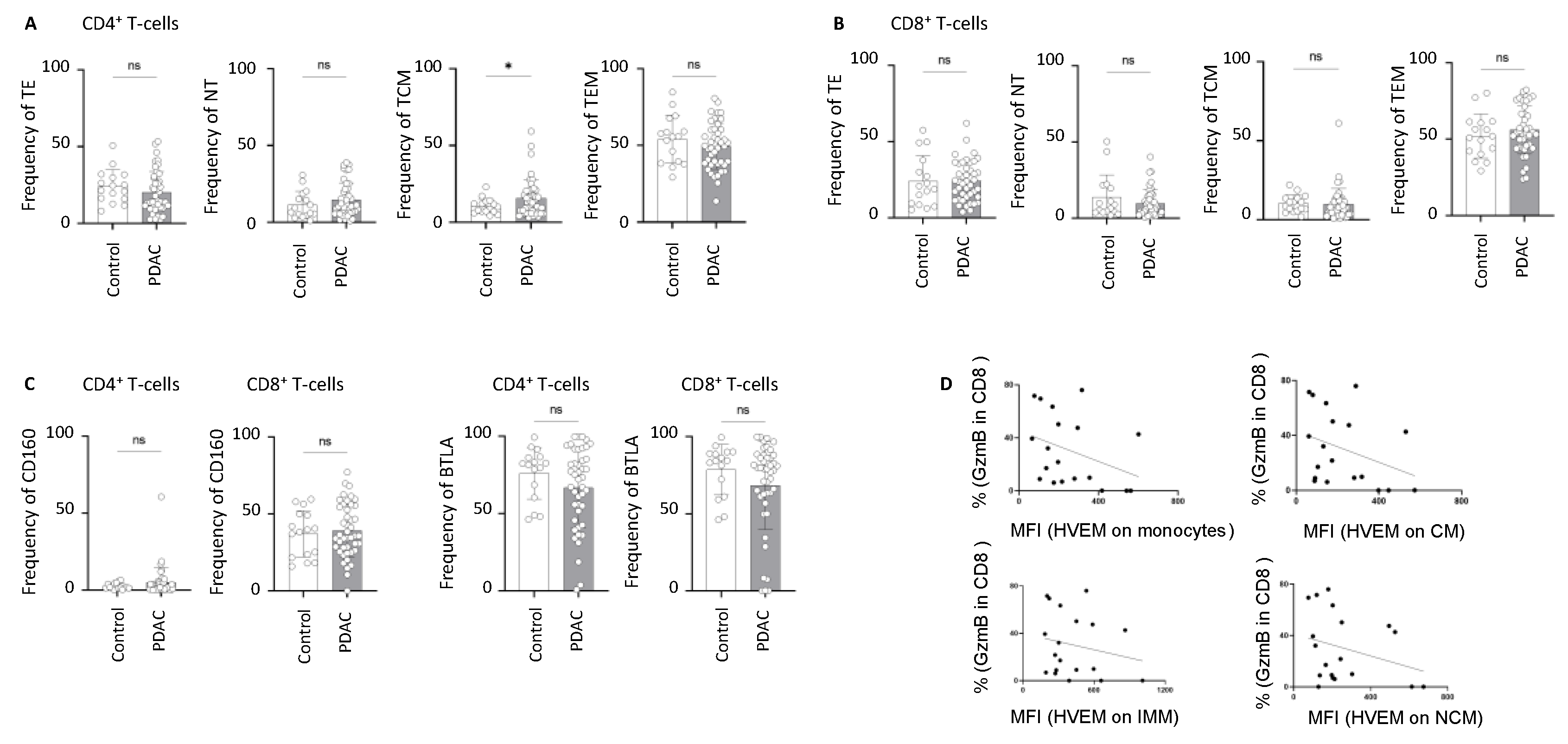
2.3. Alterations in the Frequency of BTLA- and CD160-Expressing T-Cells in PDAC
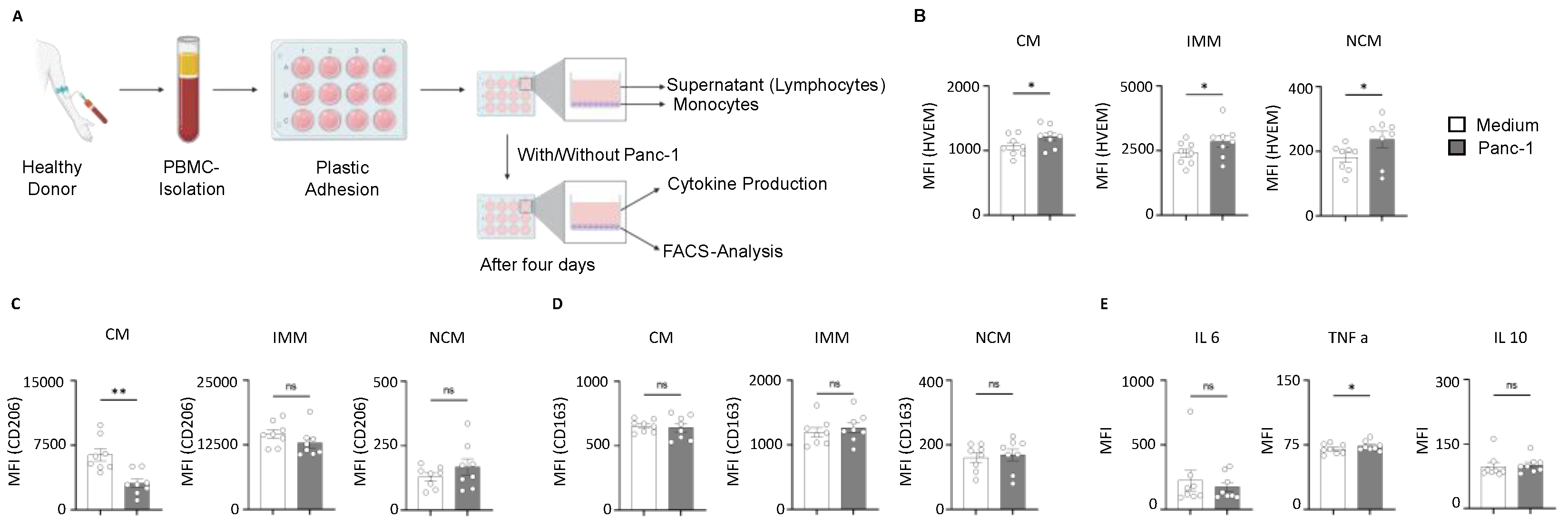
2.4. HVEM Expression on Monocyte Subsets Correlates with the Phagocytic Activity and Cytokine Production During Pancreatic Cancer
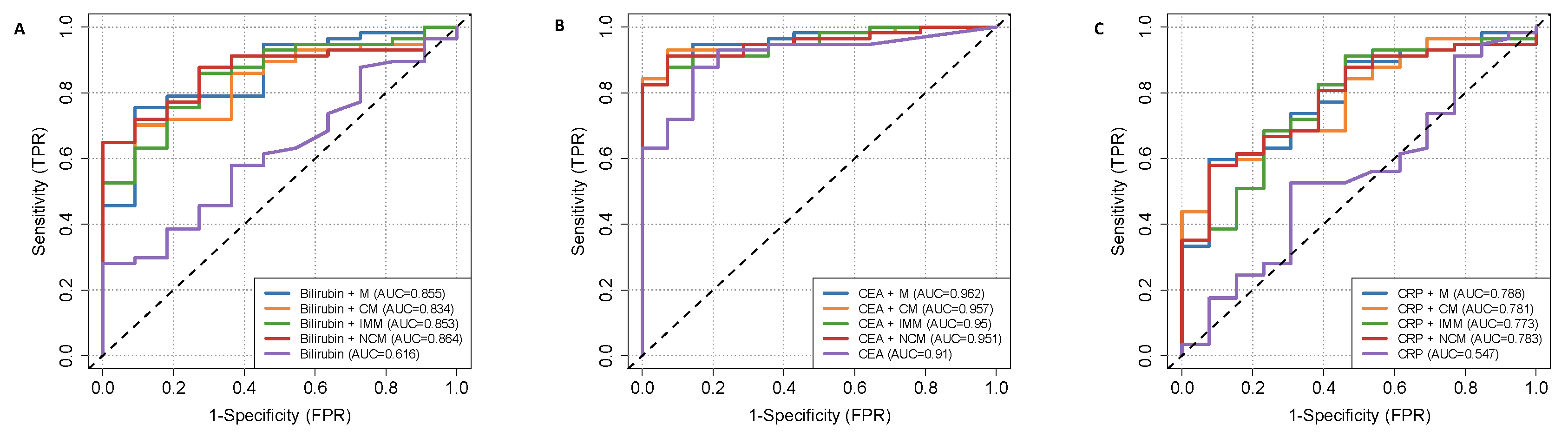
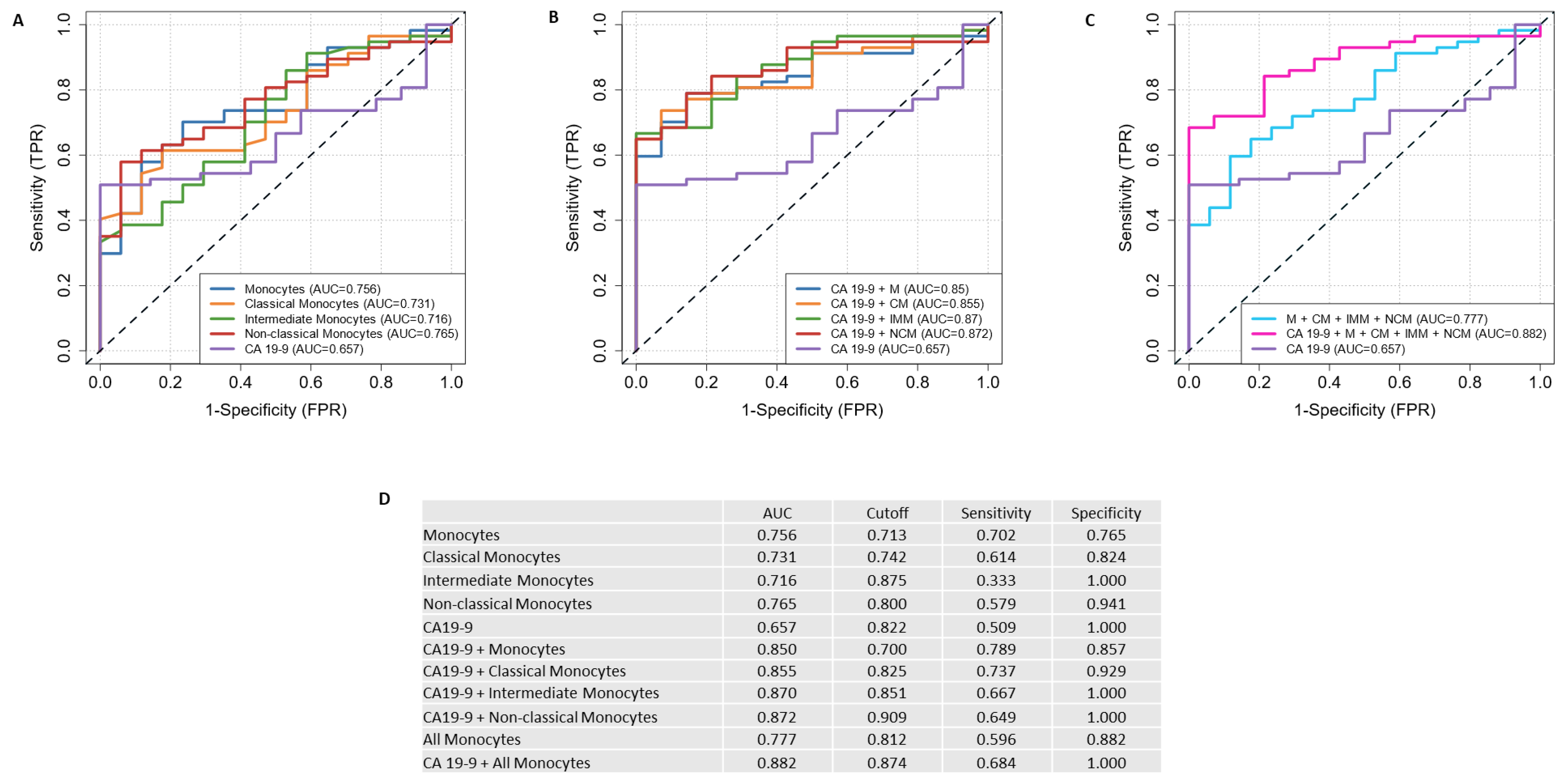
2.5. Discriminating Power of HVEM-Expressing Monocytes and Their Subsets with Serum CA19-9 to Discriminate PDAC Patients from Clinical Control Patients
3. Discussion
4. Materials and Methods
4.1. PDAC and Control Cohort
4.2. Sample Processing
4.3. Flow Cytometry
4.4. Enzyme-Linked Immunosorbent Assay (ESSAY)
4.5. Human PBMC Isolation and Monocytes Functional Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PDAC | Pancreatic ductal adenocarcinoma |
| PanIN | Pancreatic intraepithelial neoplasias |
| HVEM | Herpesvirus entry mediator |
| BTLA IL6 IL10 | B- and T-lymphocyte attenuator Interleukin 6 Interleukin 10 |
| MDSCs | Myeloid-derived suppressor cells |
| pDCs | plasmacytoid Dendritic cells |
| MFI | Mean fluorescence intensity |
| CM | Classical monocytes |
| IMM | Intermediate monocytes |
| NCM | Non-classical monocytes |
| TE | Effector T-cells |
| NT | Naive T-cells |
| TCM | Central Memory T-cells |
| TEM | Effector memory T-cells |
| AUC | Area under the curve |
| ROC | Receiver operating characteristic |
| TAM | Tumor-associated macrophages |
| HR | Hazard ratio |
| 95% CI | 95% confidence interval |
Appendix A
| Expression of CD270 on Monocytes | Low | High | p-Value | |
|---|---|---|---|---|
| Number | 28 | 29 | ||
| Mean age (in years [range]) | 71 (48–87) | 70 (43–90) | ||
| Sex | Male | 11 | 13 | 0.67 |
| Female | 17 | 16 | ||
| pT category | (y)pT1 | 2 | 1 | 0.73 |
| (y)pT2 | 9 | 9 | ||
| (y)pT3 | 10 | 11 | ||
| (y)pT4 | 3 | 1 | ||
| Unresectable | 4 | 7 | ||
| pN category | (y)pN0 | 9 | 8 | 0.94 |
| (y)pN1,2 | 15 | 14 | ||
| Unresectable | 4 | 7 | ||
| Vascular invasion | V0 | 20 | 16 | 0.38 |
| V1 | 4 | 6 | ||
| Unresectable | 4 | 7 | ||
| Lymphatic invasion | L0 | 20 | 14 | 0.13 |
| L1 | 4 | 8 | ||
| Unresectable | 4 | 7 | ||
| Perineural invasion | Pn0 | 3 | 3 | 0.91 |
| Pn1 | 21 | 19 | ||
| Unresectable | 4 | 7 | ||
| Grading | G2 | 7 | 5 | 0.63 |
| G3 | 13 | 13 | ||
| Unresectable/neoadjuvant treatment | 8 | 11 | ||
| Distant metastasis | No | 25 | 25 | 0.72 |
| Yes | 3 | 4 | ||
| UICC stage | (y)I | 5 | 4 | 0.91 |
| (y)II | 8 | 9 | ||
| (y)III | 10 | 8 | ||
| (y)IV | 3 | 4 | ||
| Unresectable | 2 | 4 | ||
| Neoadjuvant treatment | No | 21 | 18 | 0.30 |
| Yes | 7 | 11 | ||
| ASA score | 1 | 0 | 1 | 0.08 |
| 2 | 4 | 9 | ||
| 3 Unresectable | 22 2 | 19 0 |
| Expression of CD270 on CMs | Low | High | p-Value | |
|---|---|---|---|---|
| Number | 28 | 29 | ||
| Mean age (in years [range]) | 71 (48–87) | 70 (43–90) | ||
| Sex | Male | 11 | 13 | 0.67 |
| Female | 17 | 16 | ||
| pT category | (y)pT1 | 2 | 1 | 0.59 |
| (y)pT 2 | 9 | 9 | ||
| (y)pT 3 | 11 | 10 | ||
| (y)pT 4 | 2 | 2 | ||
| Unresectable | 4 | 7 | ||
| pN category | (y)pN0 | 9 | 8 | 0.94 |
| (y)pN1,2 | 15 | 14 | ||
| Unresectable | 4 | 7 | ||
| Vascular invasion | V0 | 22 | 14 | 0.02 |
| V1 | 2 | 8 | ||
| Unresectable | 4 | 7 | ||
| Lymphatic invasion | L0 | 20 | 14 | 0.13 |
| L1 | 4 | 8 | ||
| Unresectable | 4 | 7 | ||
| Perineural invasion | Pn0 | 3 | 3 | 0.91 |
| Pn1 | 21 | 19 | ||
| Unresectable | 4 | 7 | ||
| Grading | G2 | 7 | 5 | 0.49 |
| G3 | 12 | 14 | ||
| Unresectable/neoadjuvant treatment | 9 | 10 | ||
| Distant metastasis | No | 26 | 24 | 0.25 |
| Yes | 2 | 5 | ||
| UICC stage | (y)I | 5 | 4 | 0.13 |
| (y)II | 8 | 9 | ||
| (y)III | 11 | 7 | ||
| (y)IV | 2 | 5 | ||
| Unresectable | 2 | 4 | ||
| Neoadjuvant treatment | No | 21 | 18 | 0.29 |
| Yes | 7 | 11 | ||
| ASA score | 1 | 0 | 1 | 0.08 |
| 2 | 4 | 9 | ||
| 3 Unresectable | 22 2 | 19 0 |
| Expression of CD270 on IMMs | Low | High | p-Value | |
|---|---|---|---|---|
| Number | 28 | 29 | ||
| Mean age (in years [range]) | 69 (48–84) | 71 (43–90) | ||
| Sex | Male | 11 | 13 | 0.67 |
| Female | 17 | 16 | ||
| pT category | (y)pT1 | 2 | 1 | 0.31 |
| (y)pT2 | 9 | 9 | ||
| (y)pT3 | 11 | 10 | ||
| (y)pT4 | 3 | 1 | ||
| Unresectable | 3 | 8 | ||
| pN category | (y)pN0 | 9 | 8 | 0.88 |
| (y)pN1,2 | 16 | 13 | ||
| Unresectable | 3 | 8 | ||
| Vascular invasion | V0 | 22 | 14 | 0.08 |
| V1 | 3 | 7 | ||
| Unresectable | 3 | 8 | ||
| Lymphatic invasion | L0 | 21 | 13 | 0.09 |
| L1 | 4 | 8 | ||
| Unresectable | 3 | 8 | ||
| Perineural invasion | Pn0 | 3 | 3 | 0.82 |
| Pn1 | 22 | 18 | ||
| Unresectable | 3 | 8 | ||
| Grading | G2 | 7 | 5 | 0.49 |
| G3 | 12 | 14 | ||
| Unresectable/neoadjuvant treatment | 9 | 10 | ||
| Distant metastasis | No | 26 | 24 | 0.25 |
| Yes | 2 | 5 | ||
| UICC stage | (y)I | 5 | 4 | 0.13 |
| (y)II | 8 | 9 | ||
| (y)III | 11 | 7 | ||
| (y)IV | 2 | 5 | ||
| Unresectable | 2 | 4 | ||
| Neoadjuvant treatment | No | 20 | 19 | 0.63 |
| Yes | 8 | 10 | ||
| ASA score | 1 | 0 | 1 | 0.08 |
| 2 | 4 | 9 | ||
| 3 Unresectable | 22 2 | 19 0 |
| Expression of CD270 on NCMs | Low | High | p-Value | |
|---|---|---|---|---|
| Number | 28 | 29 | ||
| Mean age (in years [range]) | 71 (48–84) | 70 (43–90) | ||
| Sex | Male | 11 | 13 | 0.67 |
| Female | 17 | 16 | ||
| pT category | (y)pT1 | 2 | 1 | 0.03 |
| (y)pT2 | 9 | 9 | ||
| (y)pT3 | 9 | 12 | ||
| (y)pT4 | 4 | 0 | ||
| Unresectable | 4 | 7 | ||
| pN category | (y)pN0 | 8 | 9 | 0.59 |
| (y)pN1,2 | 16 | 13 | ||
| Unresectable | 4 | 7 | ||
| Vascular invasion | V0 | 20 | 13 | 0.07 |
| V1 | 4 | 9 | ||
| Unresectable | 4 | 7 | ||
| Lymphatic invasion | L0 | 21 | 13 | 0.03 |
| L1 | 3 | 9 | ||
| Unresectable | 4 | 7 | ||
| Perineural invasion | Pn0 | 4 | 2 | 0.45 |
| Pn1 | 20 | 20 | ||
| Unresectable | 4 | 7 | ||
| Grading | G2 | 8 | 4 | 0.16 |
| G3 | 11 | 15 | ||
| Unresectable/neoadjuvant treatment | 9 | 10 | ||
| Distant metastasis | No | 25 | 25 | 0.72 |
| Yes | 3 | 4 | ||
| UICC stage | (y)I | 4 | 5 | 0.10 |
| (y)II | 7 | 10 | ||
| (y)III | 12 | 6 | ||
| (y)IV | 3 | 4 | ||
| Unresectable | 2 | 4 | ||
| Neoadjuvant treatment | No | 19 | 20 | 0.92 |
| Yes | 9 | 9 | ||
| ASA score | 1 | 1 | 0 | 0.21 |
| 2 | 5 | 8 | ||
| 3 Unresectable | 20 2 | 21 0 |



References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [PubMed]
- WHO. Estimated Number of Deaths from 2022 to 2050, Both Sexes, Age [0–85+]. Available online: https://gco.iarc.who.int/tomorrow/en/dataviz/bars?populations=905_908_909_935_903_904&single_unit=50000&sexes=0&cancers=13&years=2050&types=1&key=total&show_bar_mode_prop=1 (accessed on 29 August 2024).
- Bethesda, M.; National Cancer Institute. SEER Cancer Stat Facts: Pancreatic Cancer. Survival Statistics. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 29 August 2024).
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [PubMed]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402 e1. [Google Scholar] [PubMed]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 2140–2141. [Google Scholar]
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic cancer: Advances and challenges. Cell 2023, 186, 1729–1754. [Google Scholar]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar]
- Fahrmann, J.F.; Schmidt, C.M.; Mao, X.; Irajizad, E.; Loftus, M.; Zhang, J.; Patel, N.; Vykoukal, J.; Dennison, J.B.; Long, J.P.; et al. Lead-Time Trajectory of CA19-9 as an Anchor Marker for Pancreatic Cancer Early Detection. Gastroenterology 2021, 160, 1373–1383.e6. [Google Scholar]
- Li, H.B.; Yang, Z.H.; Guo, Q.Q. Immune checkpoint inhibition for pancreatic ductal adenocarcinoma: Limitations and prospects: A systematic review. Cell Commun. Signal. 2021, 19, 117. [Google Scholar]
- Ye, X.; Yu, Y.; Zheng, X.; Ma, H. Clinical immunotherapy in pancreatic cancer. Cancer Immunol. Immunother. 2024, 73, 64. [Google Scholar]
- Wojciechowicz, K.; Spodzieja, M.; Wardowska, A. The BTLA-HVEM complex—The future of cancer immunotherapy. Eur. J. Med. Chem. 2024, 268, 116231. [Google Scholar] [CrossRef] [PubMed]
- Ward-Kavanagh, L.K.; Lin, W.W.; Sedy, J.R.; Ware, C.F. The TNF Receptor Superfamily in Co-stimulating and Co-inhibitory Responses. Immunity 2016, 44, 1005–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, S.; Augustine, M.M.; Xu, H.; Wang, J.; Sun, J.; Broadwater, M.; Ruff, W.; Luo, L.; Zhu, G.; et al. Neuron-specific SALM5 limits inflammation in the CNS via its interaction with HVEM. Sci. Adv. 2016, 2, e1500637. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Barbosa, J.I.; Schneider, P.; Weigert, A.; Lee, K.M.; Kim, T.J.; Perez-Simon, J.A.; Del Rio, M.L. HVEM, a cosignaling molecular switch, and its interactions with BTLA, CD160 and LIGHT. Cell. Mol. Immunol. 2019, 16, 679–682. [Google Scholar] [CrossRef]
- Cheung, T.C.; Oborne, L.M.; Steinberg, M.W.; Macauley, M.G.; Fukuyama, S.; Sanjo, H.; D’Souza, C.; Norris, P.S.; Pfeffer, K.; Murphy, K.M.; et al. T cell intrinsic heterodimeric complexes between HVEM and BTLA determine receptivity to the surrounding microenvironment. J. Immunol. 2009, 183, 7286–7296. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Granda-Diaz, R.; Martinez-Perez, A.; Aguilar-Garcia, C.; Rodrigo, J.P.; Garcia-Pedrero, J.M.; Gonzalez, S. Beyond the anti-PD-1/PD-L1 era: Promising role of the BTLA/HVEM axis as a future target for cancer immunotherapy. Mol. Cancer 2023, 22, 142. [Google Scholar] [CrossRef]
- Cheung, T.C.; Steinberg, M.W.; Oborne, L.M.; Macauley, M.G.; Fukuyama, S.; Sanjo, H.; D’Souza, C.; Norris, P.S.; Pfeffer, K.; Murphy, K.M.; et al. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc. Natl. Acad. Sci. USA 2009, 106, 6244–6249. [Google Scholar] [CrossRef]
- Gavrieli, M.; Murphy, K.M. Association of Grb-2 and PI3K p85 with phosphotyrosile peptides derived from BTLA. Biochem. Biophys. Res. Commun. 2006, 345, 1440–1445. [Google Scholar] [CrossRef]
- Aubergeon, L.; Sawaf, M.; Felten, R.; Gottenberg, J.E.; Dumortier, H.; Monneaux, F. High BTLA Expression Likely Contributes to Contraction of the Regulatory T Cell Subset in Lupus Disease. Front. Immunol. 2021, 12, 767099. [Google Scholar] [CrossRef]
- Shang, Y.; Guo, G.; Cui, Q.; Li, J.; Ruan, Z.; Chen, Y. The expression and anatomical distribution of BTLA and its ligand HVEM in rheumatoid synovium. Inflammation 2012, 35, 1102–1112. [Google Scholar] [CrossRef]
- Lan, X.; Li, S.; Gao, H.; Nanding, A.; Quan, L.; Yang, C.; Ding, S.; Xue, Y. Increased BTLA and HVEM in gastric cancer are associated with progression and poor prognosis. Onco Targets Ther. 2017, 10, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; He, M.; Zhang, G.L.; Zhao, Q. Distinct Changes of BTLA and HVEM Expressions in Circulating CD4(+) and CD8(+) T Cells in Hepatocellular Carcinoma Patients. J. Immunol. Res. 2018, 2018, 4561571. [Google Scholar] [CrossRef] [PubMed]
- Malissen, N.; Macagno, N.; Granjeaud, S.; Granier, C.; Moutardier, V.; Gaudy-Marqueste, C.; Habel, N.; Mandavit, M.; Guillot, B.; Pasero, C.; et al. HVEM has a broader expression than PD-L1 and constitutes a negative prognostic marker and potential treatment target for melanoma. Oncoimmunology 2019, 8, e1665976. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Katsuta, E.; Yan, L.; Tokumaru, Y.; Katz, M.H.G.; Takabe, K. Transcriptomic Profile of Lymphovascular Invasion, a Known Risk Factor of Pancreatic Ductal Adenocarcinoma Metastasis. Cancers 2020, 12, 2033. [Google Scholar] [CrossRef]
- Chen, J.W.; Bhandari, M.; Astill, D.S.; Wilson, T.G.; Kow, L.; Brooke-Smith, M.; Toouli, J.; Padbury, R.T. Predicting patient survival after pancreaticoduodenectomy for malignancy: Histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB 2010, 12, 101–108. [Google Scholar] [CrossRef]
- Hong, S.M.; Goggins, M.; Wolfgang, C.L.; Schulick, R.D.; Edil, B.H.; Cameron, J.L.; Handra-Luca, A.; Herman, J.M.; Hruban, R.H. Vascular invasion in infiltrating ductal adenocarcinoma of the pancreas can mimic pancreatic intraepithelial neoplasia: A histopathologic study of 209 cases. Am. J. Surg. Pathol. 2012, 36, 235–241. [Google Scholar] [CrossRef]
- Liaskou, E.; Zimmermann, H.W.; Li, K.K.; Oo, Y.H.; Suresh, S.; Stamataki, Z.; Qureshi, O.; Lalor, P.F.; Shaw, J.; Syn, W.K.; et al. Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology 2013, 57, 385–398. [Google Scholar] [CrossRef]
- Hansen, F.J.; David, P.; Akram, M.; Knoedler, S.; Mittelstadt, A.; Merkel, S.; Podolska, M.J.; Swierzy, I.; Rossdeutsch, L.; Klosch, B.; et al. Circulating Monocytes Serve as Novel Prognostic Biomarker in Pancreatic Ductal Adenocarcinoma Patients. Cancers 2023, 15, 363. [Google Scholar] [CrossRef]
- Jakubzick, C.V.; Randolph, G.J.; Henson, P.M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 2017, 17, 349–362. [Google Scholar] [CrossRef]
- Poh, A.R.; Ernst, M. Tumor-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: Therapeutic Opportunities and Clinical Challenges. Cancers 2021, 13, 2860. [Google Scholar] [CrossRef]
- Caronni, N.; La Terza, F.; Vittoria, F.M.; Barbiera, G.; Mezzanzanica, L.; Cuzzola, V.; Barresi, S.; Pellegatta, M.; Canevazzi, P.; Dunsmore, G.; et al. IL-1beta(+) macrophages fuel pathogenic inflammation in pancreatic cancer. Nature 2023, 623, 415–422. [Google Scholar]
- Hussain, Z.; Bertran, T.; Finetti, P.; Lohmann, E.; Mamessier, E.; Bidaut, G.; Bertucci, F.; Rego, M.; Tomasini, R. Macrophages reprogramming driven by cancer-associated fibroblasts under FOLFIRINOX treatment correlates with shorter survival in pancreatic cancer. Cell Commun. Signal. 2024, 22, 1. [Google Scholar] [PubMed]
- Sanford, D.E.; Belt, B.A.; Panni, R.Z.; Mayer, A.; Deshpande, A.D.; Carpenter, D.; Mitchem, J.B.; Plambeck-Suess, S.M.; Worley, L.A.; Goetz, B.D.; et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: A role for targeting the CCL2/CCR2 axis. Clin. Cancer Res. 2013, 19, 3404–3415. [Google Scholar] [PubMed]
- Gu, L.; Li, H.; Chen, L.; Ma, X.; Li, X.; Gao, Y.; Zhang, Y.; Xie, Y.; Zhang, X. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: Evidence from a systematic review and meta-analysis. Oncotarget 2016, 7, 31926–31942. [Google Scholar] [PubMed]
- Lin, S.; Fang, Y.; Mo, Z.; Lin, Y.; Ji, C.; Jian, Z. Prognostic value of lymphocyte to monocyte ratio in pancreatic cancer: A systematic review and meta-analysis including 3338 patients. World J. Surg. Oncol. 2020, 18, 186. [Google Scholar]
- Williams, H.; Mack, C.; Baraz, R.; Marimuthu, R.; Naralashetty, S.; Li, S.; Medbury, H. Monocyte Differentiation and Heterogeneity: Inter-Subset and Interindividual Differences. Int. J. Mol. Sci. 2023, 24, 8757. [Google Scholar] [CrossRef]
- Mukherjee, R.; Kanti Barman, P.; Kumar Thatoi, P.; Tripathy, R.; Kumar Das, B.; Ravindran, B. Non-Classical monocytes display inflammatory features: Validation in Sepsis and Systemic Lupus Erythematous. Sci. Rep. 2015, 5, 13886. [Google Scholar] [CrossRef]
- Ma, B.; Meng, H.; Tian, Y.; Wang, Y.; Song, T.; Zhang, T.; Wu, Q.; Cui, Y.; Li, H.; Zhang, W.; et al. High expression of HVEM is associated with improved prognosis in intrahepatic cholangiocarcinoma. Oncol. Lett. 2021, 21, 69. [Google Scholar]
- Kamal, A.M.; Wasfey, E.F.; Elghamry, W.R.; Sabry, O.M.; Elghobary, H.A.; Radwan, S.M. Genetic signature of CTLA-4, BTLA, TIM-3 and LAG-3 molecular expression in colorectal cancer patients: Implications in diagnosis and survival outcomes. Clin. Biochem. 2021, 96, 13–18. [Google Scholar]
- Migita, K.; Sho, M.; Shimada, K.; Yasuda, S.; Yamato, I.; Takayama, T.; Matsumoto, S.; Wakatsuki, K.; Hotta, K.; Tanaka, T.; et al. Significant involvement of herpesvirus entry mediator in human esophageal squamous cell carcinoma. Cancer 2014, 120, 808–817. [Google Scholar]
- Tsang, J.Y.S.; Chan, K.W.; Ni, Y.B.; Hlaing, T.; Hu, J.; Chan, S.K.; Cheung, S.Y.; Tse, G.M. Expression and Clinical Significance of Herpes Virus Entry Mediator (HVEM) in Breast Cancer. Ann. Surg. Oncol. 2017, 24, 4042–4050. [Google Scholar] [PubMed]
- Hokuto, D.; Sho, M.; Yamato, I.; Yasuda, S.; Obara, S.; Nomi, T.; Nakajima, Y. Clinical impact of herpesvirus entry mediator expression in human hepatocellular carcinoma. Eur. J. Cancer 2015, 51, 157–165. [Google Scholar] [PubMed]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Gonzalez-Rodriguez, A.P.; Payer, Á.R.; Gonzalez-Garcia, E.; Lopez-Soto, A.; Gonzalez, S. BTLA/HVEM Axis Induces NK Cell Immunosuppression and Poor Outcome in Chronic Lymphocytic Leukemia. Cancers 2021, 13, 1766. [Google Scholar] [CrossRef]
- Tang, M.; Cao, X.; Li, Y.; Li, G.Q.; He, Q.H.; Li, S.J.; Chen, J.; Xu, G.L.; Zhang, K.Q. High expression of herpes virus entry mediator is associated with poor prognosis in clear cell renal cell carcinoma. Am. J. Cancer Res. 2019, 9, 975–987. [Google Scholar] [PubMed]
- Han, M.Z.; Wang, S.; Zhao, W.B.; Ni, S.L.; Yang, N.; Kong, Y.; Huang, B.; Chen, A.J.; Li, X.G.; Wang, J.; et al. Immune checkpoint molecule herpes virus entry mediator is overexpressed and associated with poor prognosis in human glioblastoma. EBioMedicine 2019, 43, 159–170. [Google Scholar]
- David, P.; Westmeier, J.; Drabczyk-Pluta, M.; Werner, T.; Ickler, J.; Francois, S.; Bayer, W.; Sutter, K.; Del Rio, M.L.; Rodriguez-Barbosa, J.I.; et al. The Role of the Inhibitory Ligand HVEM and Its Receptors CD160 and BTLA in the Regulation of Anti-retroviral T Cell Responses. Front. Virol. 2022, 2, 836291. [Google Scholar]
- del Rio, M.L.; Jones, N.D.; Buhler, L.; Norris, P.; Shintani, Y.; Ware, C.F.; Rodriguez-Barbosa, J.I. Selective blockade of herpesvirus entry mediator-B and T lymphocyte attenuator pathway ameliorates acute graft-versus-host reaction. J. Immunol. 2012, 188, 4885–4896. [Google Scholar]
- Guruprasad, P.; Carturan, A.; Zhang, Y.; Cho, J.H.; Kumashie, K.G.; Patel, R.P.; Kim, K.H.; Lee, J.S.; Lee, Y.; Kim, J.H.; et al. The BTLA-HVEM axis restricts CAR T cell efficacy in cancer. Nat. Immunol. 2024, 25, 1020–1032. [Google Scholar]
- Chen, Y.L.; Lin, H.W.; Chien, C.L.; Lai, Y.L.; Sun, W.Z.; Chen, C.A.; Cheng, W.F. BTLA blockade enhances Cancer therapy by inhibiting IL-6/IL-10-induced CD19(high) B lymphocytes. J. Immunother. Cancer 2019, 7, 313. [Google Scholar]
- Stecher, C.; Battin, C.; Leitner, J.; Zettl, M.; Grabmeier-Pfistershammer, K.; Holler, C.; Zlabinger, G.J.; Steinberger, P. PD-1 Blockade Promotes Emerging Checkpoint Inhibitors in Enhancing T Cell Responses to Allogeneic Dendritic Cells. Front. Immunol. 2017, 8, 572. [Google Scholar]
- Nielsen, M.C.; Andersen, M.N.; Moller, H.J. Monocyte isolation techniques significantly impact the phenotype of both isolated monocytes and derived macrophages in vitro. Immunology 2020, 159, 63–74. [Google Scholar]

| PDAC Patients | ||
|---|---|---|
| Number | 57 | |
| Mean age (in years [range]) | 70 (43–90) | |
| Sex (male:female) | 24:33 | |
| pT category | (y)pT1 | 3 |
| (y)pT2 | 18 | |
| (y)pT3 | 21 | |
| (y)pT4 | 4 | |
| Unresectable | 11 | |
| pN category | (y)pN0 | 17 |
| (y)pN1,2 | 29 | |
| Unresectable | 11 | |
| Vascular invasion | V0 | 36 |
| V1 | 10 | |
| Unresectable | 11 | |
| Lymphatic invasion | L0 | 34 |
| L1 | 12 | |
| Unresectable | 11 | |
| Perineural invasion | Pn0 | 6 |
| Pn1 | 40 | |
| Unresectable | 11 | |
| Grading | G2 | 12 |
| G3 | 26 | |
| Unresectable/neoadjuvant treatment | 19 | |
| Distant metastasis | No | 50 |
| Yes | 7 | |
| UICC stage | (y)I | 9 |
| (y)II | 17 | |
| (y)III | 18 | |
| (y)IV | 7 | |
| Unresectable | 6 | |
| Neoadjuvant treatment | No | 39 |
| Yes | 18 | |
| ASA score | 1 | 1 |
| 2 | 13 | |
| 3 | 41 | |
| Unresectable | 2 |
| Clinical Control Patients | |
|---|---|
| Number | 17 |
| Mean age (in years [range]) | 68 (39–85) |
| Sex (male:female) | 7:10 |
| Adipositas | 1 |
| Cholecystectomy | 3 |
| Hernia | 7 |
| Lipoma | 1 |
| Diaphragmatic hernia | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuchenreuther, I.; Clausen, F.-N.; Mazurie, J.; Paul, S.; Czubayko, F.; Mittelstädt, A.; Koch, A.-K.; Karabiber, A.; Hansen, F.J.; Arnold, L.-S.; et al. Increased Herpesvirus Entry Mediator Expression on Circulating Monocytes and Subsets Predicts Poor Outcomes in Pancreatic Ductal Adenocarcinoma Patients. Int. J. Mol. Sci. 2025, 26, 2875. https://doi.org/10.3390/ijms26072875
Kuchenreuther I, Clausen F-N, Mazurie J, Paul S, Czubayko F, Mittelstädt A, Koch A-K, Karabiber A, Hansen FJ, Arnold L-S, et al. Increased Herpesvirus Entry Mediator Expression on Circulating Monocytes and Subsets Predicts Poor Outcomes in Pancreatic Ductal Adenocarcinoma Patients. International Journal of Molecular Sciences. 2025; 26(7):2875. https://doi.org/10.3390/ijms26072875
Chicago/Turabian StyleKuchenreuther, Isabelle, Finn-Niklas Clausen, Johanne Mazurie, Sushmita Paul, Franziska Czubayko, Anke Mittelstädt, Ann-Kathrin Koch, Alara Karabiber, Frederik J. Hansen, Lisa-Sophie Arnold, and et al. 2025. "Increased Herpesvirus Entry Mediator Expression on Circulating Monocytes and Subsets Predicts Poor Outcomes in Pancreatic Ductal Adenocarcinoma Patients" International Journal of Molecular Sciences 26, no. 7: 2875. https://doi.org/10.3390/ijms26072875
APA StyleKuchenreuther, I., Clausen, F.-N., Mazurie, J., Paul, S., Czubayko, F., Mittelstädt, A., Koch, A.-K., Karabiber, A., Hansen, F. J., Arnold, L.-S., Weisel, N., Merkel, S., Brunner, M., Krautz, C., Vera, J., Grützmann, R., Weber, G. F., & David, P. (2025). Increased Herpesvirus Entry Mediator Expression on Circulating Monocytes and Subsets Predicts Poor Outcomes in Pancreatic Ductal Adenocarcinoma Patients. International Journal of Molecular Sciences, 26(7), 2875. https://doi.org/10.3390/ijms26072875








