In Utero Alcohol and Unsuitable Home Environmental Exposure Combined with FMR1 Full Mutation Allele Cause Severe Fragile X Syndrome Phenotypes
Abstract
:1. Introduction
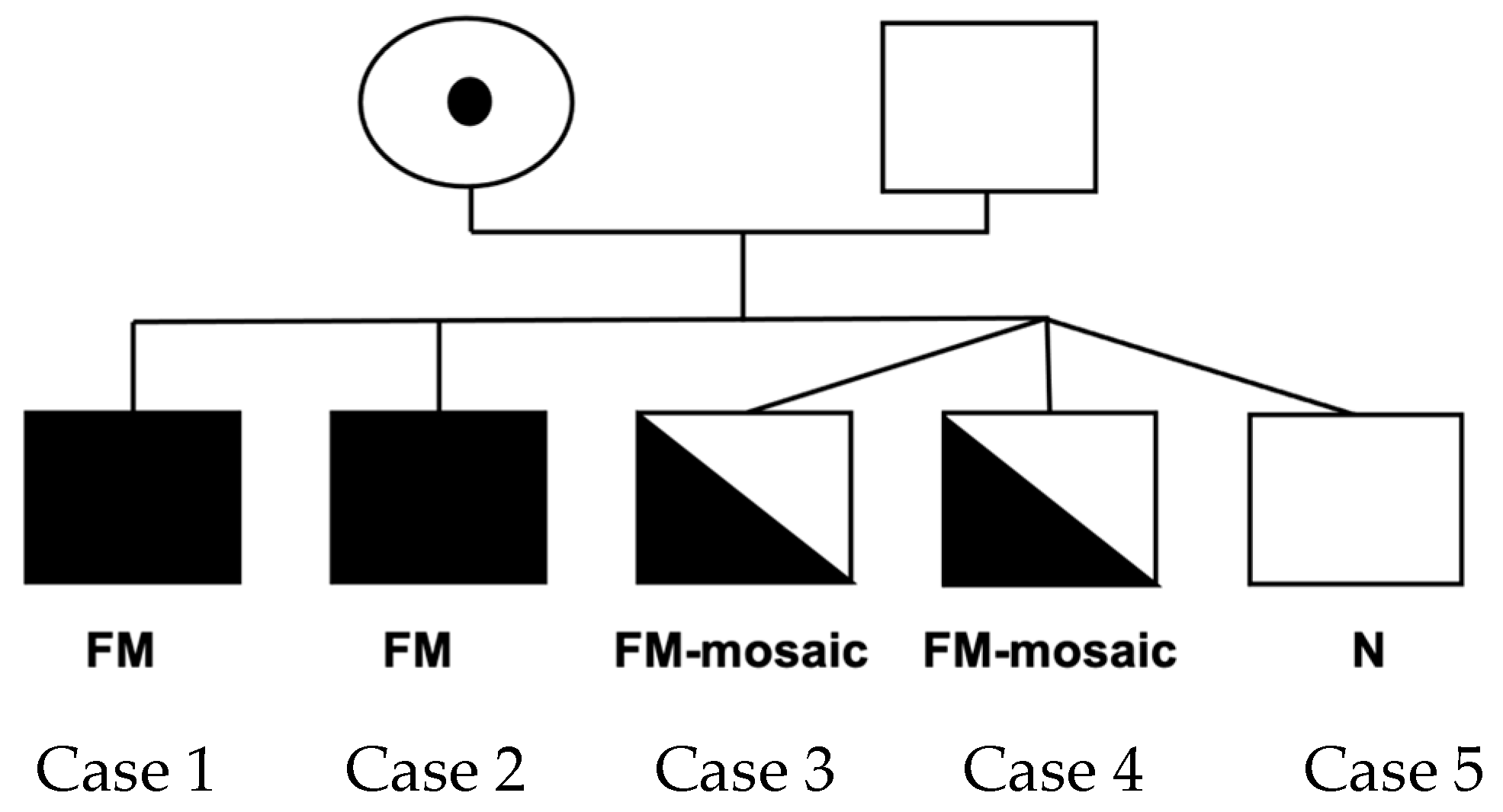
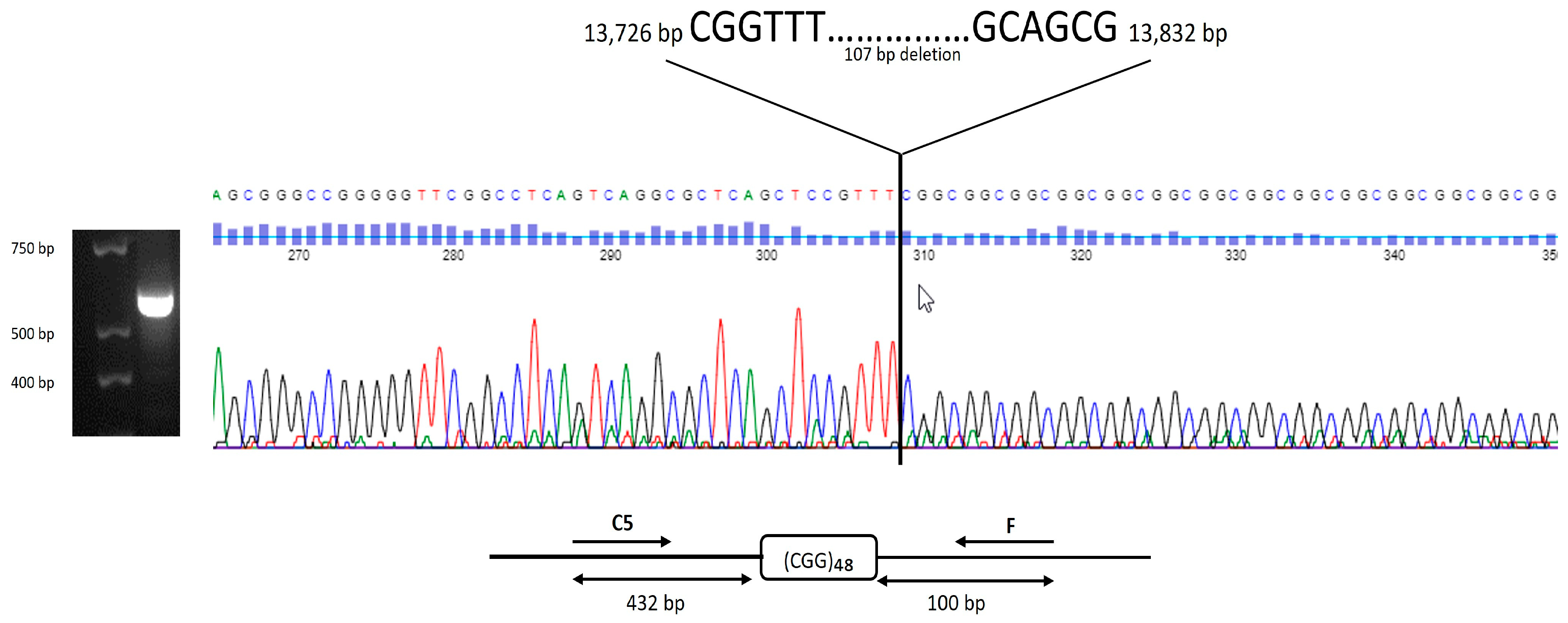
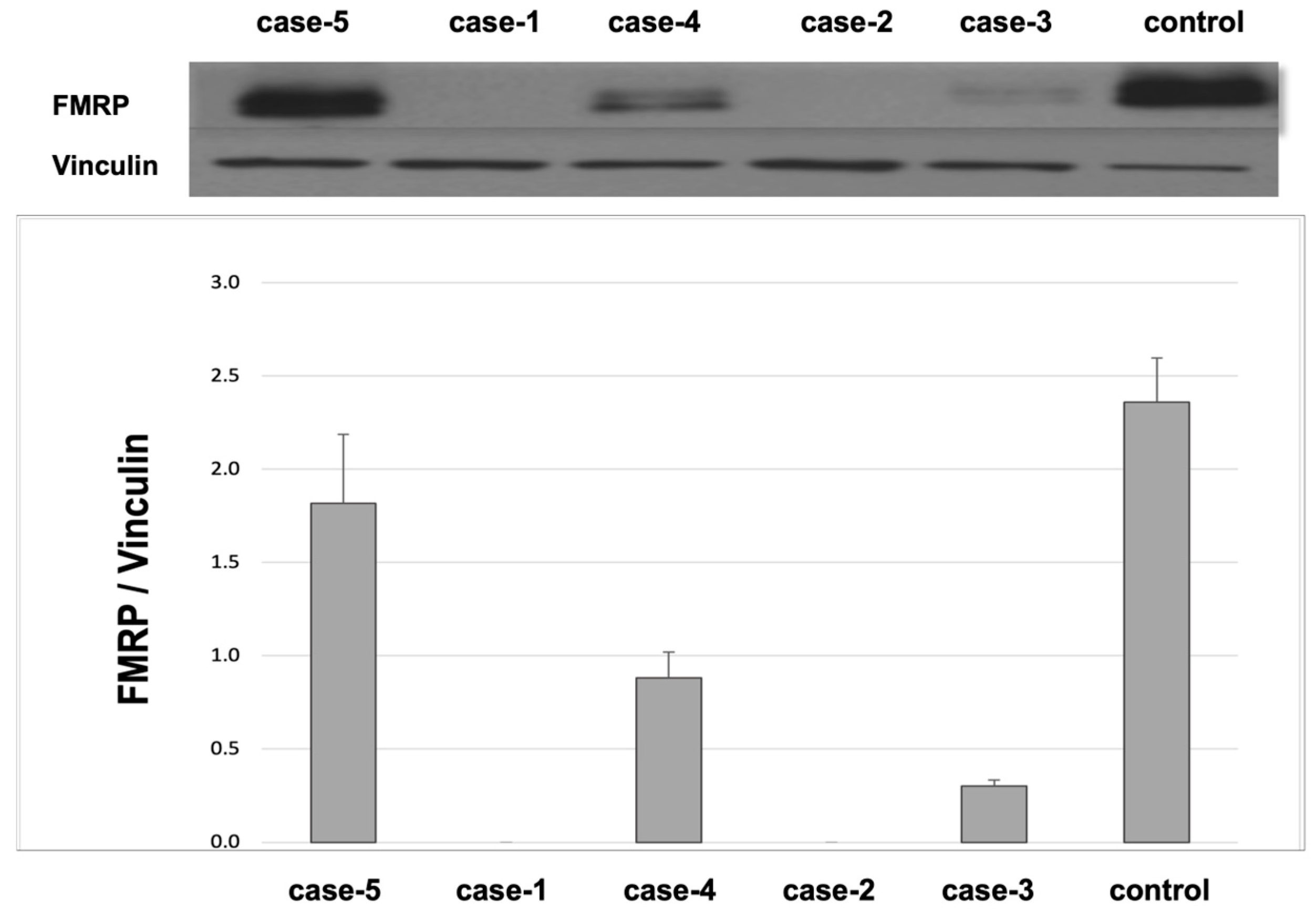
2. Results
Cases
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Molecular Measures
4.3. Clinical Measures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, D.B., Jr.; Raspa, M.; Olmsted, M.; Holiday, D.B. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. Am. J. Med. Genet. Part A 2008, 146A, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, P.J. The fragile X prevalence paradox. J. Med. Genet. 2008, 45, 498–499. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, J.S.; Nelson, D.L.; Zhang, F.; Pieretti, M.; Caskey, C.T.; Saxe, D.; Warren, S.T. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum. Mol. Genet. 1992, 1, 397–400. [Google Scholar] [CrossRef]
- Bassell, G.J.; Warren, S.T. Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron 2008, 60, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, A.; Hallahan, B. Fragile X-associated disorders: A clinical overview. J. Neurol. 2012, 259, 401–413. [Google Scholar] [CrossRef]
- Jiraanont, P.; Zafarullah, M.; Sulaiman, N.; Espinal, G.M.; Randol, J.L.; Durbin-Johnson, B.; Schneider, A.; Hagerman, R.J.; Hagerman, P.J.; Tassone, F. FMR1 Protein Expression Correlates with Intelligence Quotient in Both Peripheral Blood Mononuclear Cells and Fibroblasts from Individuals with an FMR1 Mutation. J. Mol. Diagn. JMD 2024, 26, 498–509. [Google Scholar] [CrossRef]
- Schneider, A.; Winarni, T.I.; Cabal-Herrera, A.M.; Bacalman, S.; Gane, L.; Hagerman, P.; Tassone, F.; Hagerman, R. Elevated FMR1-mRNA and lowered FMRP—A double-hit mechanism for psychiatric features in men with FMR1 premutations. Transl. Psychiatry 2020, 10, 205. [Google Scholar] [CrossRef]
- Baker, E.K.; Arpone, M.; Aliaga, S.M.; Bretherton, L.; Kraan, C.M.; Bui, M.; Slater, H.R.; Ling, L.; Francis, D.; Hunter, M.F.; et al. Incomplete silencing of full mutation alleles in males with fragile X syndrome is associated with autistic features. Mol. Autism 2019, 10, 21. [Google Scholar] [CrossRef]
- Field, M.; Dudding-Byth, T.; Arpone, M.; Baker, E.K.; Aliaga, S.M.; Rogers, C.; Hickerton, C.; Francis, D.; Phelan, D.G.; Palmer, E.E.; et al. Significantly Elevated FMR1 mRNA and Mosaicism for Methylated Premutation and Full Mutation Alleles in Two Brothers with Autism Features Referred for Fragile X Testing. Int. J. Mol. Sci. 2019, 20, 3907. [Google Scholar] [CrossRef]
- Jiraanont, P.; Kumar, M.; Tang, H.T.; Espinal, G.; Hagerman, P.J.; Hagerman, R.J.; Chutabhakdikul, N.; Tassone, F. Size and methylation mosaicism in males with Fragile X syndrome. Expert. Rev. Mol. Diagn. 2017, 17, 1023–1032. [Google Scholar] [CrossRef]
- Saldarriaga, W.; González-Teshima, L.Y.; Forero-Forero, J.V.; Tang, H.-T.; Tassone, F. Mosaicism in Fragile X syndrome: A family case series. J. Intellect. Disabil. 2021, 26, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.F.; dos Santos, J.M.; Gonçalves, A.P.; Tassone, F.; Mendoza-Morales, G.; Ribeiro, M.G.; Kahn, E.; Boy, R.; Pimentel, M.M.; Santos-Rebouças, C.B. Finding FMR1 mosaicism in Fragile X syndrome. Expert. Rev. Mol. Diagn. 2016, 16, 501–507. [Google Scholar] [CrossRef]
- Pretto, D.; Yrigollen, C.M.; Tang, H.T.; Williamson, J.; Espinal, G.; Iwahashi, C.K.; Durbin-Johnson, B.; Hagerman, R.J.; Hagerman, P.J.; Tassone, F. Clinical and molecular implications of mosaicism in FMR1 full mutations. Front. Genet. 2014, 5, 318. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Hessl, D.; Dyer-Friedman, J.; Johnston, C.; Wisbeck, J.; Taylor, A.; Reiss, A. Biological and environmental contributions to adaptive behavior in fragile X syndrome. Am. J. Med. Genet. Part A 2003, 117, 21–29. [Google Scholar] [CrossRef]
- Hessl, D.; Dyer-Friedman, J.; Glaser, B.; Wisbeck, J.; Barajas, R.G.; Taylor, A.; Reiss, A. The influence of environmental and genetic factors on behavior problems and autistic symptoms in boys and girls with fragile X syndrome. Pediatrics 2001, 108, e88. [Google Scholar] [CrossRef]
- Hemingway, S.J. Diagnostic Guide for Fetal Alcohol Spectrum Disorders: The 4-Digit Diagnostic Code, 4th ed.; University of Washington: Seattle, WA, USA, 2024. [Google Scholar]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.-S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.; et al. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef] [PubMed]
- Hagan, J.F., Jr.; Balachova, T.; Bertrand, J.; Chasnoff, I.; Dang, E.; Fernandez-Baca, D.; Kable, J.; Kosofsky, B.; Senturias, Y.N.; Singh, N.; et al. Neurobehavioral Disorder Associated With Prenatal Alcohol Exposure. Pediatrics 2016, 138, e20151553. [Google Scholar] [CrossRef]
- Cook, J.L.; Green, C.R.; Lilley, C.M.; Anderson, S.M.; Baldwin, M.E.; Chudley, A.E.; Conry, J.L.; LeBlanc, N.; Loock, C.A.; Lutke, J.; et al. Fetal alcohol spectrum disorder: A guideline for diagnosis across the lifespan. Can. Med. Assoc. J. 2016, 188, 191–197. [Google Scholar] [CrossRef]
- Popova, S.; Charness, M.E.; Burd, L.; Crawford, A.; Hoyme, H.E.; Mukherjee, R.A.S.; Riley, E.P.; Elliott, E.J. Fetal alcohol spectrum disorders. Nat. Rev. Dis. Primers 2023, 9, 11. [Google Scholar] [CrossRef]
- Feldmann, R. The present and historical prevalence of foetal alcohol syndrome in children living in wine-producing communities. Acta Paediatr. 2020, 109, 1928–1929. [Google Scholar] [CrossRef]
- Carpita, B.; Migli, L.; Chiarantini, I.; Battaglini, S.; Montalbano, C.; Carmassi, C.; Cremone, I.M.; Dell’Osso, L. Autism Spectrum Disorder and Fetal Alcohol Spectrum Disorder: A Literature Review. Brain Sci. 2022, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.A.; Nash, K.; Koren, G.; Rovet, J. Autism characteristics in children with fetal alcohol spectrum disorders. Child. Neuropsychol. 2013, 19, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, R.; Layton, M.; Yacoub, E.; Turk, J. Autism and autistic traits in people exposed to heavy prenatal alcohol: Data from a clinical series of 21 individuals and nested case control study. Adv. Ment. Health Intellect. Disabil. 2011, 5, 42–49. [Google Scholar] [CrossRef]
- Baggio, S.; Zenki, K.; Martins Silva, A.; dos Santos, T.G.; Rech, G.; Lazzarotto, G.; Dias, R.D.; Mussulini, B.H.; Rico, E.P.; de Oliveira, D.L. Fetal alcohol spectrum disorders model alters the functionality of glutamatergic neurotransmission in adult zebrafish. NeuroToxicology 2020, 78, 152–160. [Google Scholar] [CrossRef]
- Ehrhart, F.; Roozen, S.; Verbeek, J.; Koek, G.; Kok, G.; van Kranen, H.; Evelo, C.T.; Curfs, L.M.G. Review and gap analysis: Molecular pathways leading to fetal alcohol spectrum disorders. Mol. Psychiatry 2019, 24, 10–17. [Google Scholar] [CrossRef]
- Lussier, A.A.; Weinberg, J.; Kobor, M.S. Epigenetics studies of fetal alcohol spectrum disorder: Where are we now? Epigenomics 2017, 9, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.; Hoem, G.; Hagerman, P. Fragile X and autism: Intertwined at the molecular level leading to targeted treatments. Mol. Autism 2010, 1, 12. [Google Scholar] [CrossRef]
- Kraan, C.M.; Godler, D.E.; Amor, D.J. Epigenetics of fragile X syndrome and fragile X-related disorders. Dev. Med. Child Neurol. 2019, 61, 121–127. [Google Scholar] [CrossRef]
- Steinhausen, H.C.; Von Gontard, A.; Spohr, H.L.; Hauffa, B.P.; Eiholzer, U.; Backes, M.; Willms, J.; Malin, Z. Behavioral phenotypes in four mental retardation syndromes: Fetal alcohol syndrome, Prader-Willi syndrome, fragile X syndrome, and tuberosis sclerosis. Am. J. Med. Genet. 2002, 111, 381–387. [Google Scholar] [CrossRef]
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Popova, S. Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017, 171, 948–956. [Google Scholar] [CrossRef]
- May, P.A.; Chambers, C.D.; Kalberg, W.O.; Zellner, J.; Feldman, H.; Buckley, D.; Kopald, D.; Hasken, J.M.; Xu, R.; Honerkamp-Smith, G.; et al. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA 2018, 319, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Protic, D.; Rajaratnam, A.; Salcedo-Arellano, M.J.; Aydin, E.Y.; Schneider, A. Fragile X-Associated Neuropsychiatric Disorders (FXAND). Front. Psychiatry 2018, 9, 564. [Google Scholar] [CrossRef]
- Kogan, C.S.; Turk, J.; Hagerman, R.J.; Cornish, K.M. Impact of the Fragile X mental retardation 1 (FMR1) gene premutation on neuropsychiatric functioning in adult males without fragile X-associated Tremor/Ataxia syndrome: A controlled study. Am. J. Med. Genetics. Part. B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2008, 147b, 859–872. [Google Scholar] [CrossRef]
- Chen, E.; Sharma, M.R.; Shi, X.; Agrawal, R.K.; Joseph, S. Fragile X mental retardation protein regulates translation by binding directly to the ribosome. Mol. Cell 2014, 54, 407–417. [Google Scholar] [CrossRef]
- Hou, L.; Antion, M.D.; Hu, D.; Spencer, C.M.; Paylor, R.; Klann, E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron 2006, 51, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Ku, L.; Wilkinson, K.D.; Warren, S.T.; Feng, Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001, 29, 2276–2283. [Google Scholar] [CrossRef]
- Willemsen, R.; Levenga, J.; Oostra, B.A. CGG repeat in the FMR1 gene: Size matters. Clin. Genet. 2011, 80, 214–225. [Google Scholar] [CrossRef]
- Qin, M.; Kang, J.; Burlin, T.V.; Jiang, C.; Smith, C.B. Postadolescent changes in regional cerebral protein synthesis: An in vivo study in the FMR1 null mouse. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 5087–5095. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Schmidt, K.C.; Zametkin, A.J.; Bishu, S.; Horowitz, L.M.; Burlin, T.V.; Xia, Z.; Huang, T.; Quezado, Z.M.; Smith, C.B. Altered cerebral protein synthesis in fragile X syndrome: Studies in human subjects and knockout mice. J. Cereb. Blood Flow. Metab. 2013, 33, 499–507. [Google Scholar] [CrossRef]
- Kaminen-Ahola, N. Fetal alcohol spectrum disorders: Genetic and epigenetic mechanisms. Prenat. Diagn. 2020, 40, 1185–1192. [Google Scholar] [CrossRef]
- Astley Hemingway, S.J.; Bledsoe, J.M.; Brooks, A.; Davies, J.K.; Jirikowic, T.; Olson, E.M.; Thorne, J.C. Twin study confirms virtually identical prenatal alcohol exposures can lead to markedly different fetal alcohol spectrum disorder outcomes-fetal genetics influences fetal vulnerability. Adv. Pediatr. Res. 2018, 5, 23. [Google Scholar] [CrossRef] [PubMed]
- Aishworiya, R.; Chi, M.H.; Zafarullah, M.; Mendoza, G.; Ponzini, M.D.; Kim, K.; Biag, H.M.B.; Thurman, A.J.; Abbeduto, L.; Hessl, D.; et al. Intercorrelation of Molecular Biomarkers and Clinical Phenotype Measures in Fragile X Syndrome. Cells 2023, 12, 1920. [Google Scholar] [CrossRef]
- Payán-Gómez, C.; Ramirez-Cheyne, J.; Saldarriaga, W. Variable Expressivity in Fragile X Syndrome: Towards the Identification of Molecular Characteristics That Modify the Phenotype. Appl. Clin. Genet. 2021, 14, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.D.; Zhao, X. The molecular biology of FMRP: New insights into fragile X syndrome. Nat. Rev. Neurosci. 2021, 22, 209–222. [Google Scholar] [CrossRef]
- Bleuzé, L.; Triaca, V.; Borreca, A. FMRP-Driven Neuropathology in Autistic Spectrum Disorder and Alzheimer’s disease: A Losing Game. Front. Mol. Biosci. 2021, 8, 699613. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Warren, S.T. Understanding the molecular basis of fragile X syndrome. Human. Mol. Genet. 2000, 9, 901–908. [Google Scholar] [CrossRef]
- Jin, P.; Warren, S.T. New insights into fragile X syndrome: From molecules to neurobehaviors. Trends Biochem. Sci. 2003, 28, 152–158. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Zhou, S.; Yang, L.; Shi, Q.; Li, Y.; Zhang, K.; Yang, L.; Zhao, M.; Yang, Q. Imbalance between Glutamate and GABA in Fmr1 Knockout Astrocytes Influences Neuronal Development. Genes 2016, 7, 45. [Google Scholar] [CrossRef]
- Hammond, L.S.; Macias, M.M.; Tarleton, J.C.; Pai, G.S. Fragile X syndrome and deletions in FMR1: New case and review of the literature. Am. J. Med. Genet. 1997, 72, 430–434. [Google Scholar] [CrossRef]
- Grasso, M.; Faravelli, F.; Nigro, C.L.; Chiurazzi, P.; Sperandeo, M.P.; Argusti, A.; Pomponi, M.G.; Lecora, M.; Sebastio, G.F.; Perroni, L.; et al. Mosaicism for the full mutation and a microdeletion involving the CGG repeat and flanking sequences in the FMR1 gene in eight fragile X patients. Am. J. Med. Genet. 1999, 85, 311–316. [Google Scholar] [CrossRef]
- Grønskov, K.; Hjalgrim, H.; Bjerager, M.O.; Brøndum-Nielsen, K. Deletion of all CGG repeats plus flanking sequences in FMR1 does not abolish gene expression. Am. J. Hum. Genet. 1997, 61, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Mannermaa, A.; Pulkkinen, L.; Kajanoja, E.; Ryynanen, M.; Saarikoski, S. Deletion in the FMR1 gene in a fragile-X male. Am. J. Med. Genet. 1996, 64, 293–295. [Google Scholar] [CrossRef]
- Mila, M.; Castellvi Bel, S.; Sanchez, A.; Lazaro, C.; Villa, M.; Estivill, X. Mosaicism for the fragile X syndrome full mutation and deletions within the CGG repeat of the FMR1 gene. J. Med. Genet. 1996, 33, 338–340. [Google Scholar] [CrossRef]
- Schmucker, B.; Ballhausen, W.G.; Pfeiffer, R.A. Mosaicism of a microdeletion of 486 bp involving the CGG repeat of the FMR1 gene due to misalignment of GTT tandem repeats at chi-like elements flanking both breakpoints and a full mutation. Human Genet. 1996, 98, 409–414. [Google Scholar] [CrossRef] [PubMed]
- de Graaff, E.; de Vries, B.B.; Willemsen, R.; van Hemel, J.O.; Mohkamsing, S.; Oostra, B.A.; van den Ouweland, A.M. The fragile X phenotype in a mosaic male with a deletion showing expression of the FMR1 protein in 28% of the cells. Am. J. Med. Genet. 1996, 64, 302–308. [Google Scholar] [CrossRef]
- Han, X.-D.; Powell, B.R.; Phalin, J.L.; Chehab, F.F. Mosaicism for a full mutation, premutation, and deletion of the CGG repeats results in 22% FMRP and elevated FMR1 mRNA levels in a high-functioning fragile X male. Am. J. Med. Genet. Part A 2006, 140A, 1463–1471. [Google Scholar] [CrossRef]
- Beilina, A.; Tassone, F.; Schwartz, P.H.; Sahota, P.; Hagerman, P.J. Redistribution of transcription start sites within the FMR1 promoter region with expansion of the downstream CGG-repeat element. Hum. Mol. Genet. 2004, 13, 543–549. [Google Scholar] [CrossRef]
- Budimirovic, D.B.; Schlageter, A.; Filipovic-Sadic, S.; Protic, D.D.; Bram, E.; Mahone, E.M.; Nicholson, K.; Culp, K.; Javanmardi, K.; Kemppainen, J.; et al. A Genotype-Phenotype Study of High-Resolution FMR1 Nucleic Acid and Protein Analyses in Fragile X Patients with Neurobehavioral Assessments. Brain Sci. 2020, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Loesch, D.Z.; Bui, Q.M.; Dissanayake, C.; Clifford, S.; Gould, E.; Bulhak-Paterson, D.; Tassone, F.; Taylor, A.K.; Hessl, D.; Hagerman, R.; et al. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neurosci. Biobehav. Rev. 2007, 31, 315–326. [Google Scholar] [CrossRef]
- Olney, J.W.; Wozniak, D.F.; Jevtovic-Todorovic, V.; Ikonomidou, C. Glutamate signaling and the fetal alcohol syndrome. Ment. Retard. Dev. Disabil. Res. Rev. 2001, 7, 267–275. [Google Scholar] [CrossRef]
- Salcedo-Arellano, M.J.; Lozano, R.; Tassone, F.; Hagerman, R.J.; Saldarriaga, W. Alcohol use dependence in fragile X syndrome. Intractable Rare Dis. Res. 2016, 5, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Aishworiya, R.; Biag, H.M.B.; Salcedo-Arellano, M.J.; Musa, Z.; Schneider, A.; Clark, C.; Santos, E.; Tassone, F.; Hagerman, R. Fragile X Syndrome and Fetal Alcohol Syndrome: Occurrence of Dual Diagnosis in a Set of Triplets. J. Dev. Behav. Pediatr. JDBP 2023, 44, e470–e475. [Google Scholar] [CrossRef] [PubMed]
- Filipovic-Sadic, S.; Sah, S.; Chen, L.; Krosting, J.; Sekinger, E.; Zhang, W.; Hagerman, P.J.; Stenzel, T.T.; Hadd, A.G.; Latham, G.J.; et al. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin. Chem. 2010, 56, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Pan, R.; Amiri, K.; Taylor, A.K.; Hagerman, P.J. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J. Mol. Diagn. JMD 2008, 10, 43–49. [Google Scholar] [CrossRef]
- Zafarullah, M.; Li, J.; Salemi, M.R.; Phinney, B.S.; Durbin-Johnson, B.P.; Hagerman, R.; Hessl, D.; Rivera, S.M.; Tassone, F. Blood Proteome Profiling Reveals Biomarkers and Pathway Alterations in Fragile X PM at Risk for Developing FXTAS. Int. J. Mol. Sci. 2023, 24, 13477. [Google Scholar] [CrossRef]
- Roid, G.H.; Koch, C. Leiter-3: Nonverbal Cognitive and Neuropsychological Assessment. In Handbook of Nonverbal Assessment; McCallum, R.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 127–150. [Google Scholar]
- Esler, A.N.; Bal, V.H.; Guthrie, W.; Wetherby, A.; Ellis Weismer, S.; Lord, C. The Autism Diagnostic Observation Schedule, Toddler Module: Standardized Severity Scores. J. Autism Dev. Disord. 2015, 45, 2704–2720. [Google Scholar] [CrossRef]
- Cicchetti, D.V.; Carter, A.S.; Gray, S.A.O. Vineland Adaptive Behavior Scales. In Encyclopedia of Autism Spectrum Disorders; Volkmar, F.R., Ed.; Springer: New York, NY, USA, 2013; pp. 3281–3284. [Google Scholar]
- Farmer, C.; Aman, M.G. Aberrant Behavior Checklist. In Encyclopedia of Autism Spectrum Disorders; Volkmar, F.R., Ed.; Springer: New York, NY, USA, 2020; pp. 1–8. [Google Scholar]
| Measurement | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Leiter-3 | |||||
| Non-Verbal (NV)-IQ | 69 | 57 | 61 | 55 | 84 |
| ADOS-2 a | |||||
| Social Affect (SA) Total | 19 | 15 | 13 | 9 | 3 |
| Restricted and Repetitive Behavior (RRB) Total | 1 | 0 | 7 | 7 | 5 |
| SA + RRB Total | 20 | 15 | 20 | 15 | 8 |
| Comparison Score | 8 | 7 | 8 | 7 | 4 |
| Classification | ASD | ASD | ASD | ASD | ASD |
| ABC-C b | |||||
| Irritability | 40 | 40 | 38 | 39 | 36 |
| Lethargy/Social Withdrawal | 22 | 10 | 4 | 5 | 3 |
| Stereotypy | 10 | 10 | 10 | 10 | 12 |
| Hyperactivity | 31 | 41 | 37 | 43 | 37 |
| Inappropriate Speech | 5 | 6 | 4 | 7 | 6 |
| Composite Score | 108 | 107 | 93 | 104 | 94 |
| Vineland | |||||
| Communication Standard Score | 28 | 50 | 62 | 60 | 62 |
| Daily Living Skills Standard Score | 59 | 67 | 72 | 71 | 64 |
| Socialization Standard Score | 38 | 50 | 48 | 50 | 48 |
| Adaptive Behaviour Composite | 43 | 57 | 62 | 61 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winarni, T.I.; Aishworiya, R.; Culpepper, H.; Zafarullah, M.; Mendoza, G.; Wilaisakditipakorn, T.J.; Likhitweerawong, N.; Law, J.; Hagerman, R.; Tassone, F. In Utero Alcohol and Unsuitable Home Environmental Exposure Combined with FMR1 Full Mutation Allele Cause Severe Fragile X Syndrome Phenotypes. Int. J. Mol. Sci. 2025, 26, 2840. https://doi.org/10.3390/ijms26072840
Winarni TI, Aishworiya R, Culpepper H, Zafarullah M, Mendoza G, Wilaisakditipakorn TJ, Likhitweerawong N, Law J, Hagerman R, Tassone F. In Utero Alcohol and Unsuitable Home Environmental Exposure Combined with FMR1 Full Mutation Allele Cause Severe Fragile X Syndrome Phenotypes. International Journal of Molecular Sciences. 2025; 26(7):2840. https://doi.org/10.3390/ijms26072840
Chicago/Turabian StyleWinarni, Tri Indah, Ramkumar Aishworiya, Hannah Culpepper, Marwa Zafarullah, Guadalupe Mendoza, Tanaporn Jasmine Wilaisakditipakorn, Narueporn Likhitweerawong, Julie Law, Randi Hagerman, and Flora Tassone. 2025. "In Utero Alcohol and Unsuitable Home Environmental Exposure Combined with FMR1 Full Mutation Allele Cause Severe Fragile X Syndrome Phenotypes" International Journal of Molecular Sciences 26, no. 7: 2840. https://doi.org/10.3390/ijms26072840
APA StyleWinarni, T. I., Aishworiya, R., Culpepper, H., Zafarullah, M., Mendoza, G., Wilaisakditipakorn, T. J., Likhitweerawong, N., Law, J., Hagerman, R., & Tassone, F. (2025). In Utero Alcohol and Unsuitable Home Environmental Exposure Combined with FMR1 Full Mutation Allele Cause Severe Fragile X Syndrome Phenotypes. International Journal of Molecular Sciences, 26(7), 2840. https://doi.org/10.3390/ijms26072840






