Metabolic Dysfunction-Associated Steatotic Liver Disease Induced by Microplastics: An Endpoint in the Liver–Eye Axis
Abstract
1. Microplastics and the Liver
1.1. Miceoplastics and Nanoplastics
1.2. Liver Damage
Chronic Inflammation, Oxidative Stress, and Cellular Senescence
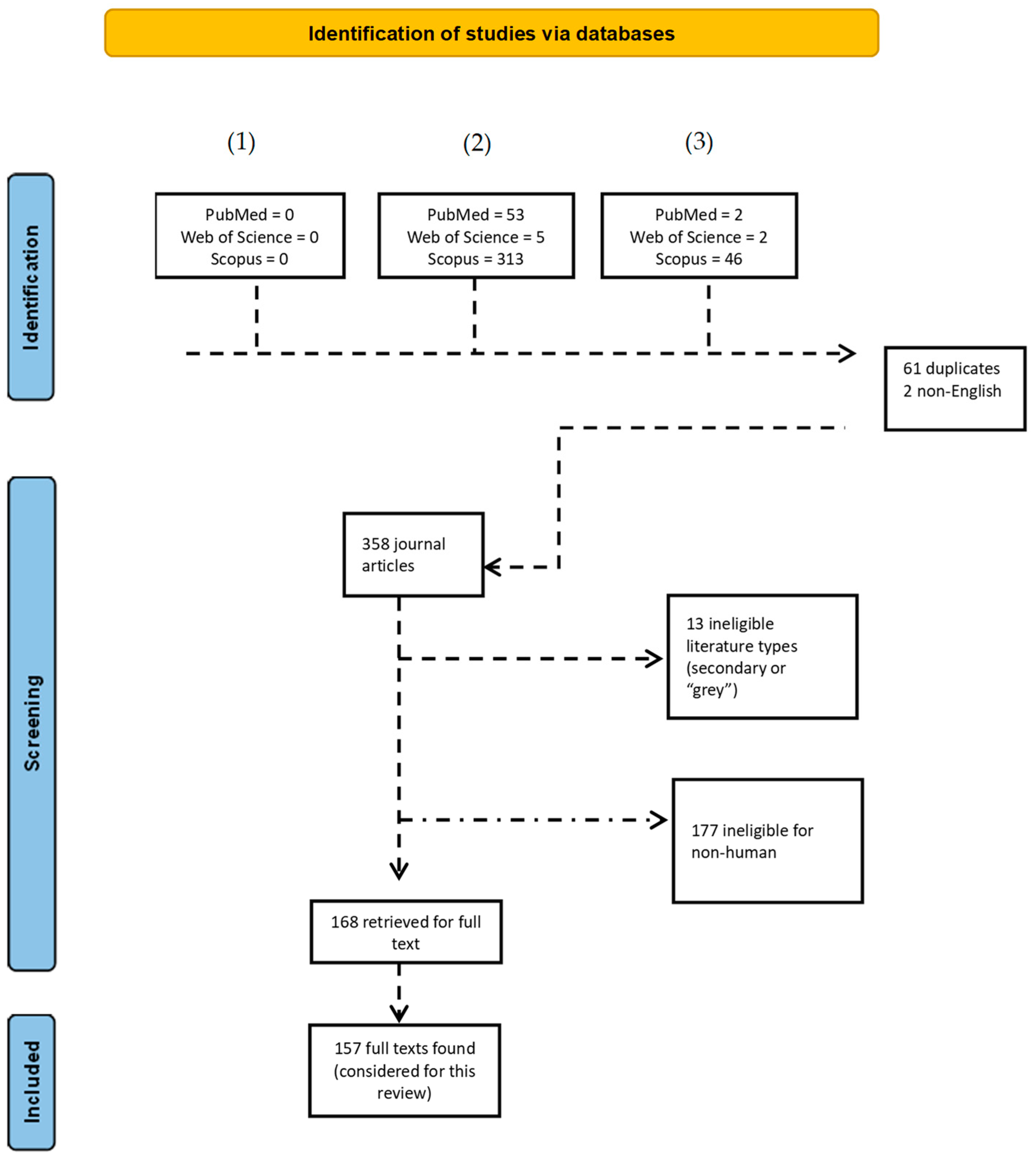
2. Laboratory Assessment
2.1. Histology and Histologic Scoring Systems for Chronic Liver Disease
2.2. Assessing Microplastics
2.2.1. Macroscopy and Microscopy: Visual Assessment of Microplastics
2.2.2. Identifying Microplastic and Nanoplastic Particles
2.3. Microplastic-Induced Histopathological Lesions in the Liver
3. Toxic Exposure
4. Liver–Eye Axis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Proposal for a Regulation of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52022PC0677 (accessed on 3 March 2025).
- Chiang, C.C.; Yeh, H.; Shiu, R.F.; Chin, W.C.; Yen, T.H. Impact of microplastics and nanoplastics on liver health: Current understanding and future research directions. World J. Gastroenterol. 2024, 30, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.W. Bisphenol A leaching from polycarbonate baby bottles into baby food causes potential health issues. Clin. Exp. Pediatr. 2022, 65, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Rani, A. Types and Sources of Microplastics; The Ubiquitous Environment Contaminant: A Review. J. Polym. Mater. 2022, 39, 17–35. [Google Scholar] [CrossRef]
- Mirani, A.; Kianfar, E.; Maleknia, L.; Javanbakht, M. Recent advances in nicotine electrochemical biosensors: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100753. [Google Scholar] [CrossRef]
- Sarkar, S.; Diab, H.; Thompson, J. Microplastic Pollution: Chemical Characterization and Impact on Wildlife. Int. J. Environ. Res. Public Health 2023, 20, 1745. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, P. Micro(nano)plastics: Invisible compounds with a visible impact. F1000Research 2024, 13, 69. [Google Scholar] [CrossRef]
- Ashrafy, A.; Liza, A.A.; Islam, M.N.; Billah, M.M.; Arafat, S.T.; Rahman, M.M.; Rahman, S.M. Microplastics Pollution: A Brief Review of Its Source and Abundance in Different Aquatic Ecosystems. J. Hazard. Mater. Adv. 2023, 9, 100215. [Google Scholar] [CrossRef]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Asgari Lajayer, B.; Biglari Quchan Atigh, Z.; Nayeri, S.; Ahmadabadi, M.; Taghipour, L.; Senapathi, V.; Astatkie, T.; Price, G.W. Micro (nano) plastics uptake, toxicity and detoxification in plants: Challenges and prospects. Ecotoxicol. Environ. Saf. 2023, 268, 115676. [Google Scholar] [CrossRef]
- Ivleva, N.P. Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives. Chem. Rev. 2021, 121, 11886–11936. [Google Scholar] [CrossRef]
- Moore, S.; Paalanen, L.; Melymuk, L.; Katsonouri, A.; Kolossa-Gehring, M.; Tolonen, H. The Association between ADHD and Environmental Chemicals-A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 2849. [Google Scholar] [CrossRef] [PubMed]
- Botelho, R.M.; Silva, A.L.M.; Borbely, A.U. The Autism Spectrum Disorder and Its Possible Origins in Pregnancy. Int. J. Environ. Res. Public Health 2024, 21, 244. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, J.; Kim, H.; Ko, I.O.; Jo, E.K.; Choi, E.J.; Lee, H.J.; Shim, I.; Woo, H.J.; Choi, J.; Kim, G.H.; et al. Pre/post-natal exposure to microplastic as a potential risk factor for autism spectrum disorder. Environ. Int. 2022, 161, 107121. [Google Scholar] [CrossRef] [PubMed]
- Thi, L.A.P.; Nguyen, V.-H.; Do, X.D.; Dang, T.H.L.; Do, H.T.; Nguyen, T.L. Microplastics occurrence, contamination, and effects on human health—A critical review. In Microplastics; Elsevier: Amsterdam, The Netherlands, 2025; pp. 451–479. [Google Scholar]
- Wang, G.; Lin, Y.; Shen, H. Exposure to Polystyrene Microplastics Promotes the Progression of Cognitive Impairment in Alzheimer’s Disease: Association with Induction of Microglial Pyroptosis. Mol. Neurobiol. 2024, 61, 900–907. [Google Scholar] [CrossRef]
- Liang, J.; Ji, F.; Abdullah, A.L.B.; Qin, W.; Zhu, T.; Tay, Y.J.; Li, Y.; Han, M. Micro/nano-plastics impacts in cardiovascular systems across species. Sci. Total Environ. 2024, 942, 173770. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, Y.; Bai, L.; Cui, J. Microplastics: An often-overlooked issue in the transition from chronic inflammation to cancer. J. Transl. Med. 2024, 22, 959. [Google Scholar] [CrossRef]
- Zhu, M.; Li, P.; Xu, T.; Zhang, G.; Xu, Z.; Wang, X.; Zhao, L.; Yang, H. Combined exposure to lead and microplastics increased risk of glucose metabolism in mice via the Nrf2/NF-kappaB pathway. Environ. Toxicol. 2024, 39, 2502–2511. [Google Scholar] [CrossRef]
- Xu, R.; Cao, J.W.; Lv, H.L.; Geng, Y.; Guo, M.Y. Polyethylene microplastics induced gut microbiota dysbiosis leading to liver injury via the TLR2/NF-kappaB/NLRP3 pathway in mice. Sci. Total Environ. 2024, 917, 170518. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, K.; Huang, L.; Niu, X.; Li, L.; Gao, L.; Xia, Z. Polystyrene microplastics induce liver fibrosis and lipid deposition in mice through three hub genes revealed by the RNA-seq. Sci. Rep. 2025, 15, 2583. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Zhou, N.; Chen, Y.; Ling, Z.; Xiang, P. Microplastics in the human body: A comprehensive review of exposure, distribution, migration mechanisms, and toxicity. Sci. Total Environ. 2024, 946, 174215. [Google Scholar] [CrossRef]
- Lee, Y.; Sung, M.; Sung, S.E.; Choi, J.H.; Kang, K.K.; Park, J.W.; Kim, Y.J.; Lee, S. The histopathological and functional consequences of microplastic exposure. Discov. Appl. Sci. 2025, 7, 72. [Google Scholar] [CrossRef]
- Yee, M.S.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of Microplastics and Nanoplastics on Human Health. Nanomater 2021, 11, 496. [Google Scholar] [CrossRef]
- Alpízar Salazar, M.; Olguín Reyes, S.E.; Medina Estévez, A.; Saturno Lobos, J.A.; De Aldecoa Castillo, J.M.; Carrera Aguas, J.C.; Alaniz Monreal, S.; Navarro Rodríguez, J.A.; Alpízar Sánchez, D.M. Natural History of Metabolic Dysfunction-Associated Steatotic Liver Disease: From Metabolic Syndrome to Hepatocellular Carcinoma. Medicina 2025, 61, 88. [Google Scholar] [CrossRef] [PubMed]
- Samy, A.M.; Kandeil, M.A.; Sabry, D.; Abdel-Ghany, A.A.; Mahmoud, M.O. From NAFLD to NASH: Understanding the spectrum of non-alcoholic liver diseases and their consequences. Heliyon 2024, 10, e30387. [Google Scholar] [CrossRef]
- Stoess, C.; Choi, Y.K.; Onyuru, J.; Friess, H.; Hoffman, H.M.; Hartmann, D.; Feldstein, A.E. Cell Death in Liver Disease and Liver Surgery. Biomedicines 2024, 12, 559. [Google Scholar] [CrossRef]
- Fan, J.G.; Xu, X.Y.; Yang, R.X.; Nan, Y.M.; Wei, L.; Jia, J.D.; Zhuang, H.; Shi, J.P.; Li, X.Y.; Sun, C.; et al. Guideline for the Prevention and Treatment of Metabolic Dysfunction-associated Fatty Liver Disease (Version 2024). J. Clin. Transl. Hepatol. 2024, 12, 955–974. [Google Scholar] [CrossRef]
- Yang, A.H.; Tincopa, M.A.; Tavaglione, F.; Ajmera, V.H.; Richards, L.M.; Amangurbanova, M.; Butcher, C.; Hernandez, C.; Madamba, E.; Singh, S.; et al. Prevalence of steatotic liver disease, advanced fibrosis and cirrhosis among community-dwelling overweight and obese individuals in the USA. Gut 2024, 73, 2045–2053. [Google Scholar] [CrossRef]
- Panganiban, J.; Kehar, M.; Ibrahim, S.H.; Hartmann, P.; Sood, S.; Hassan, S.; Ramirez, C.M.; Kohli, R.; Censani, M.; Mauney, E.; et al. Metabolic dysfunction-associated steatotic liver disease (MASLD) in children with obesity: An Obesity Medicine Association (OMA) and expert joint perspective 2025. Obes. Pillars 2025, 100164. [Google Scholar] [CrossRef]
- Leal-Lassalle, H.; Estévez-Vázquez, O.; Cubero, F.J.; Nevzorova, Y.A. Metabolic and alcohol-associated liver disease (MetALD): A representation of duality. NPJ Gut Liver 2025, 2, 1. [Google Scholar] [CrossRef]
- Mladenic, K.; Lenartic, M.; Marinovic, S.; Polic, B.; Wensveen, F.M. The “Domino effect” in MASLD: The inflammatory cascade of steatohepatitis. Eur. J. Immunol. 2024, 54, e2149641. [Google Scholar] [CrossRef]
- Sandireddy, R.; Sakthivel, S.; Gupta, P.; Behari, J.; Tripathi, M.; Singh, B.K. Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases. Front. Cell Dev. Biol. 2024, 12, 1433857. [Google Scholar] [CrossRef]
- Vesković, M.; Šutulović, N.; Hrnčić, D.; Stanojlović, O.; Macut, D.; Mladenović, D. The Interconnection between Hepatic Insulin Resistance and Metabolic Dysfunction-Associated Steatotic Liver Disease—The Transition from an Adipocentric to Liver-Centric Approach. Curr. Issues Mol. Biol. 2023, 45, 9084–9102. [Google Scholar] [CrossRef] [PubMed]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The interplay between cytokines, inflammation, and antioxidants: Mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef] [PubMed]
- Scarlata, G.G.M.; Colaci, C.; Scarcella, M.; Dallio, M.; Federico, A.; Boccuto, L.; Abenavoli, L. The Role of Cytokines in the Pathogenesis and Treatment of Alcoholic Liver Disease. Diseases 2024, 12, 69. [Google Scholar] [CrossRef]
- Bridgeman, L.; Cimbalo, A.; López-Rodríguez, D.; Pamies, D.; Frangiamone, M. Exploring toxicological pathways of microplastics and nanoplastics: Insights from animal and cellular models. J. Hazard. Mater. 2025, 490, 137795. [Google Scholar] [CrossRef]
- Feng, L.; Chen, C.; Xiong, X.; Wang, X.; Li, X.; Kuang, Q.; Wei, X.; Gao, L.; Niu, X.; Li, Q.; et al. PS-MPs promotes the progression of inflammation and fibrosis in diabetic nephropathy through NLRP3/Caspase-1 and TGF-β1/Smad2/3 signaling pathways. Ecotoxicol. Environ. Saf. 2024, 273, 116102. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, K.; Xiang, Y.; Ma, B.; Li, H.; Li, Y.; Shi, Y.; Li, S.; Bai, Y. Role of MCP-1 as an inflammatory biomarker in nephropathy. Front. Immunol. 2023, 14, 1303076. [Google Scholar] [CrossRef]
- Takenoshita, Y.; Tokito, A.; Jougasaki, M. Inhibitory Effects of Eicosapentaenoic Acid on Vascular Endothelial Growth Factor-Induced Monocyte Chemoattractant Protein-1, Interleukin-6, and Interleukin-8 in Human Vascular Endothelial Cells. Int. J. Mol. Sci. 2024, 25, 2749. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- de Magalhaes, J.P. Cellular senescence in normal physiology. Science 2024, 384, 1300–1301. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Signal Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Mahmud, F.; Sarker, D.B.; Jocelyn, J.A.; Sang, Q.A. Molecular and Cellular Effects of Microplastics and Nanoplastics: Focus on Inflammation and Senescence. Cells 2024, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Shiwakoti, S.; Park, E.Y.; Kang, K.W.; Schini-Kerth, V.B.; Park, S.H.; Ji, H.Y.; Park, J.S.; Ko, J.Y.; Oak, M.H. SGLT2 inhibition ameliorates nano plastics-induced premature endothelial senescence and dysfunction. Sci. Rep. 2023, 13, 6256. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ming, J.; Liu, Y.; Hu, G.; Liao, Q. Epigenetic modification of miR-217 promotes intervertebral disc degeneration by targeting the FBXO21-ERK signalling pathway. Arthritis. Res. Ther. 2022, 24, 261. [Google Scholar] [CrossRef]
- Lett, Z.; Hall, A.; Skidmore, S.; Alves, N.J. Environmental microplastic and nanoplastic: Exposure routes and effects on coagulation and the cardiovascular system. Environ. Pollut. 2021, 291, 118190. [Google Scholar] [CrossRef]
- Palmer, M.; Kleiner, D.E.; Goodman, Z.; Brunt, E.; Avigan, M.I.; Regev, A.; Hayashi, P.H.; Lewis, J.H.; Mehta, R.; Harrison, S.A.; et al. Liver biopsy for assessment of suspected drug-induced liver injury in metabolic dysfunction-associated steatohepatitis clinical trials: Expert consensus from the Liver Forum. Aliment. Pharmacol. Ther. 2024, 59, 201–216. [Google Scholar] [CrossRef]
- Wang, S.; Friedman, S.L. Found in translation-Fibrosis in metabolic dysfunction-associated steatohepatitis (MASH). Sci. Transl. Med. 2023, 15, eadi0759. [Google Scholar] [CrossRef]
- Wang, D.K.; Miao, J.X.; Zhang, L.H.; Zhang, L. Research advances in the diagnosis and treatment of MASLD/MASH. Ann. Med. 2025, 57, 2445780. [Google Scholar] [CrossRef]
- Ampuero, J.; Aller, R.; Gallego-Duran, R.; Crespo, J.; Calleja, J.L.; Garcia-Monzon, C.; Gomez-Camarero, J.; Caballeria, J.; Lo Iacono, O.; Ibanez, L.; et al. The biochemical pattern defines MASLD phenotypes linked to distinct histology and prognosis. J. Gastroenterol. 2024, 59, 586–597. [Google Scholar] [CrossRef]
- Sergi, C.M. NAFLD (MASLD)/NASH (MASH): Does It Bother to Label at All? A Comprehensive Narrative Review. Int. J. Mol. Sci. 2024, 25, 8462. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.; Syed, T.; Siddiqui, M. Non-invasive diagnosis of metabolic dysfunction associated steatotic liver disease (MASLD). In Hepatology; Elsevier: Amsterdam, The Netherlands, 2025; pp. 111–140. [Google Scholar]
- Sanyal, A.J.; Shankar, S.S.; Yates, K.P.; Bolognese, J.; Daly, E.; Dehn, C.A.; Neuschwander-Tetri, B.; Kowdley, K.; Vuppalanchi, R.; Behling, C.; et al. Diagnostic performance of circulating biomarkers for non-alcoholic steatohepatitis. Nat. Med. 2023, 29, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, K.; Mingrone, G.; George, J.; Mantzoros, C.S. Accurate non-invasive detection of MASH with fibrosis F2-F3 using a lightweight machine learning model with minimal clinical and metabolomic variables. Metabolism 2025, 163, 156082. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Kleiner, D.E.; Wilson, L.A.; Belt, P.; Neuschwander-Tetri, B.A.; Network, N.C.R. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011, 53, 810–820. [Google Scholar] [CrossRef]
- Hjelkrem, M.; Stauch, C.; Shaw, J.; Harrison, S.A. Validation of the non-alcoholic fatty liver disease activity score. Aliment. Pharmacol. Ther. 2011, 34, 214–218. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, B.; Wang, H. Analytical methods for microplastics in the environment: A review. Environ. Chem. Lett. 2023, 21, 383–401. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Schwabl, P.; Koppel, S.; Konigshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Karrman, A.; Rotander, A.; Hassellov, M. Comparison between manta trawl and in situ pump filtration methods, and guidance for visual identification of microplastics in surface waters. Environ. Sci. Pollut. Res. Int. 2020, 27, 5559–5571. [Google Scholar] [CrossRef]
- Lavers, J.L.; Oppel, S.; Bond, A.L. Factors influencing the detection of beach plastic debris. Mar. Environ. Res. 2016, 119, 245–251. [Google Scholar] [CrossRef]
- Caputo, F.; Vogel, R.; Savage, J.; Vella, G.; Law, A.; Della Camera, G.; Hannon, G.; Peacock, B.; Mehn, D.; Ponti, J.; et al. Measuring particle size distribution and mass concentration of nanoplastics and microplastics: Addressing some analytical challenges in the sub-micron size range. J. Colloid Interface Sci. 2021, 588, 401–417. [Google Scholar] [CrossRef] [PubMed]
- von Moos, N.; Burkhardt-Holm, P.; Kohler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.W.; Xu, E.G.; Du, F.N.; Li, R.L.; Liu, J.F.; Shi, H.H. Analysis of environmental nanoplastics: Progress and challenges. Chem. Eng. J. 2021, 410, 128208. [Google Scholar] [CrossRef]
- Schwaferts, C.; Niessner, R.; Elsner, M.; Ivleva, N.P. Methods for the analysis of submicrometer- and nanoplastic particles in the environment. Trac-Trends Anal. Chem. 2019, 112, 52–65. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, B.; Li, Z.; Zhong, Y.; Wang, B.; Zhang, B.; Du, J.; Ye, R.; Xian, H.; Min, W.; et al. Polystyrene nanoplastic exposure induces excessive mitophagy by activating AMPK/ULK1 pathway in differentiated SH-SY5Y cells and dopaminergic neurons in vivo. Part. Fibre Toxicol. 2023, 20, 44. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frige, C.; et al. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Huynh, K. Presence of microplastics in carotid plaques linked to cardiovascular events. Nat. Rev. Cardiol. 2024, 21, 279. [Google Scholar] [CrossRef]
- Wang, T.; Yi, Z.; Liu, X.; Cai, Y.; Huang, X.; Fang, J.; Shen, R.; Lu, W.; Xiao, Y.; Zhuang, W.; et al. Multimodal detection and analysis of microplastics in human thrombi from multiple anatomically distinct sites. EBioMedicine 2024, 103, 105118. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Yang, Y.; Du, Z.; Li, L.; Zhang, M.; Ni, S.; Yue, Z.; Yang, K.; Wang, Y.; et al. Microplastics in three types of human arteries detected by pyrolysis-gas chromatography/mass spectrometry (Py-GC/MS). J. Hazard. Mater. 2024, 469, 133855. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, F.; Jiang, Z.; Du, Z.; Liu, S.; Zhang, M.; Jin, Y.; Qin, Y.; Yang, X.; Wang, C.; et al. Microplastics are associated with elevated atherosclerotic risk and increased vascular complexity in acute coronary syndrome patients. Part. Fibre Toxicol. 2024, 21, 34. [Google Scholar] [CrossRef]
- Anuar, S.T.; Altarawnah, R.S.; Mohd Ali, A.A.; Lee, B.Q.; Khalik, W.; Yusof, K.; Ibrahim, Y.S. Utilizing Pyrolysis-Gas Chromatography/Mass Spectrometry for Monitoring and Analytical Characterization of Microplastics in Polychaete Worms. Polymers 2022, 14, 3054. [Google Scholar] [CrossRef] [PubMed]
- Brits, M.; van Velzen, M.J.M.; Sefiloglu, F.Ö.; Scibetta, L.; Groenewoud, Q.; Garcia-Vallejo, J.J.; Vethaak, A.D.; Brandsma, S.H.; Lamoree, M.H. Quantitation of micro and nanoplastics in human blood by pyrolysis-gas chromatography–mass spectrometry. Microplastics Nanoplastics 2024, 4, 12. [Google Scholar] [CrossRef]
- Vasudeva, M.; Warrier, A.K.; Kartha, V.B.; Unnikrishnan, V.K. Advances in microplastic characterization: Spectroscopic techniques and heavy metal adsorption insights. TrAC Trends Anal. Chem. 2025, 183, 118111. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Massarelli, C.; Uricchio, V.F. Fourier Transform Infrared Spectroscopy to Assess the Degree of Alteration of Artificially Aged and Environmentally Weathered Microplastics. Polymers 2023, 15, 911. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zeng, Y.; Cai, Z.; Wu, J.; Chan, L.L.; Zhu, J.; Zhou, J. Polystyrene microplastics alter the intestinal microbiota function and the hepatic metabolism status in marine medaka (Oryzias melastigma). Sci. Total Environ. 2021, 759, 143558. [Google Scholar] [CrossRef]
- Tian, R.; Guan, M.; Chen, L.; Wan, Y.; He, L.; Zhao, Z.; Gao, T.; Zong, L.; Chang, J.; Zhang, J. Mechanism insights into the histopathological changes of polypropylene microplastics induced gut and liver in zebrafish. Ecotoxicol. Environ. Saf. 2024, 280, 116537. [Google Scholar] [CrossRef]
- Zhuang, J.; Chen, Q.; Xu, L.; Chen, X. Combined exposure to polyvinyl chloride and polystyrene microplastics induces liver injury and perturbs gut microbial and serum metabolic homeostasis in mice. Ecotoxicol. Environ. Saf. 2023, 267, 115637. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Ali, W.; Zhuang, T.; Sun, J.; Wang, T.; Song, J.; Ma, Y.; Yuan, Y.; Bian, J.; et al. Co-exposure of polyvinyl chloride microplastics with cadmium promotes nonalcoholic fatty liver disease in female ducks through oxidative stress and glycolipid accumulation. Poult. Sci. 2024, 103, 104152. [Google Scholar] [CrossRef]
- Guraka, A.; Souch, G.; Duff, R.; Brown, D.; Moritz, W.; Kermanizadeh, A. Microplastic-induced hepatic adverse effects evaluated in advanced quadruple cell human primary models following three weeks of repeated exposure. Chemosphere 2024, 364, 143032. [Google Scholar] [CrossRef]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Puschel, K.; Huber, S.; Fischer, E.K. Microplastics detected in cirrhotic liver tissue. EBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef]
- Patton, G.N.; Lee, H.J. Chemical Insights into Topical Agents in Intraocular Pressure Management: From Glaucoma Etiopathology to Therapeutic Approaches. Pharmaceutics 2024, 16, 274. [Google Scholar] [CrossRef] [PubMed]
- Kaluzhny, Y.; Klausner, M. In vitro reconstructed 3D corneal tissue models for ocular toxicology and ophthalmic drug development. In Vitro Cell Dev. Biol. Anim. 2021, 57, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Rauchman, S.H.; Locke, B.; Albert, J.; De Leon, J.; Peltier, M.R.; Reiss, A.B. Toxic External Exposure Leading to Ocular Surface Injury. Vision 2023, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lim, B.X.H.; Seah, I.; Xie, S.; Jaeger, J.E.; Symons, R.K.; Heffernan, A.L.; Curren, E.E.M.; Leong, S.C.Y.; Riau, A.K.; et al. Impact of Microplastics on the Ocular Surface. Int. J. Mol. Sci. 2023, 24, 3928. [Google Scholar] [CrossRef]
- Kitazawa, K.; Numa, K.; Patel, S.K.; King, C.D.; Matsumoto, A.; Sotozono, C.; Desprez, P.Y.; Schilling, B.; Campisi, J. Cellular senescence exacerbates features of aging in the eyes. Aging Biol. 2023, 1, 20230014. [Google Scholar] [CrossRef]
- Hub, E.S. Eye Irritation: In Vivo Rabbit Eye Test Template for Pre-Existing Data. Available online: https://joint-research-centre.ec.europa.eu/reference-measurement/european-union-reference-laboratories/eu-reference-laboratory-alternatives-animal-testing-eurl-ecvam/alternative-methods-toxicity-testing/validated-test-methods-health-effects/eye-irritationserious-eye-damage/eye-irritation-vivo_en (accessed on 17 January 2025).
- OECD. In Environment Directorate Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology; OECD: Paris, France, 2013.
- Yamamoto, N.; Hiramatsu, N.; Kato, Y.; Sato, A.; Kojima, H. Development of an Eye Irritation Test Method Using an In-House Fabrication of a Reconstructed Human Cornea-like Epithelium Model for Eye Hazard Identification. Bioengineering 2024, 11, 302. [Google Scholar] [CrossRef]
- OECD. Test No. 491: Short Time Exposure In Vitro Test Method for Identifying (i) Chemicals Inducing Serious Eye Damage and (ii) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage. Available online: https://www.oecd.org/en/publications/test-no-491-short-time-exposure-in-vitro-test-method-for-identifying-i-chemicals-inducing-serious-eye-damage-and-ii-chemicals-not-requiring-classification-for-eye-irritation-or-serious-eye-damage_9789264242432-en.html (accessed on 17 January 2025).
- Ma, J.; Chiu, Y.F.; Kao, C.C.; Chuang, C.N.; Chen, C.Y.; Lai, C.H.; Kuo, M.L. Fine particulate matter manipulates immune response to exacerbate microbial pathogenesis in the respiratory tract. Eur. Respir. Rev. 2024, 33, 230259. [Google Scholar] [CrossRef]
- Millen, A.E.; Dighe, S.; Kordas, K.; Aminigo, B.Z.; Zafron, M.L.; Mu, L. Air Pollution and Chronic Eye Disease in Adults: A Scoping Review. Ophthalmic Epidemiol. 2024, 31, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Jia, H.; Han, J.; Zhang, Z.; Yin, X.; Mu, N.; Zhu, Y.; Li, M. Correlation Between Air Quality Index and Tear Film Lipid Layer Thickness: Comparison Between Patients with Sjogren’s Syndrome and with Meibomian Gland Dysfunction. Curr. Eye Res. 2023, 48, 447–455. [Google Scholar] [CrossRef]
- Vitar, R.M.L.; Arana, A.G.H.; Marchini, T.O.; Evelson, P.A.; Ferreira, S.M. The Ocular Surface as a Target of Air Pollution. In Environmental Stressors and OxInflammatory Tissues Responses; CRC Press: Boca Raton, FL, USA, 2024; pp. 80–88. [Google Scholar]
- Lin, C.C.; Chiu, C.C.; Lee, P.Y.; Chen, K.J.; He, C.X.; Hsu, S.K.; Cheng, K.C. The Adverse Effects of Air Pollution on the Eye: A Review. Int. J. Environ. Res. Public Health 2022, 19, 1186. [Google Scholar] [CrossRef]
- Woo, S.H.; Kim, D.Y.; Choi, J.H. Roles of Vascular Smooth Muscle Cells in Atherosclerotic Calcification. J. Lipid Atheroscler. 2023, 12, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, S.; Zhang, T.; Ge, Y.; Chen, Z.; Zhang, J.; Pu, Y.; Liang, G. Organoids and organoids-on-a-chip as the new testing strategies for environmental toxicology-applications & advantages. Environ. Int. 2024, 184, 108415. [Google Scholar] [CrossRef] [PubMed]
- Anwar, K.N.; Soleimani, M.; Ashraf, M.J.; Moghtader, A.; Koganti, R.; Ghalibafan, S.; Baharnoori, M.; Arabpour, Z.; Cheraqpour, K.; Sebhat, A.M.; et al. Senescence and Stress Signaling Pathways in Corneal Cells After Nitrogen Mustard Injury. Cells 2024, 13, 2021. [Google Scholar] [CrossRef] [PubMed]
- Dammak, A.; Pastrana, C.; Martin-Gil, A.; Carpena-Torres, C.; Peral Cerda, A.; Simovart, M.; Alarma, P.; Huete-Toral, F.; Carracedo, G. Oxidative Stress in the Anterior Ocular Diseases: Diagnostic and Treatment. Biomedicines 2023, 11, 292. [Google Scholar] [CrossRef]
- Iqbal, S.; Ramini, A.; Kaja, S. Impact of particulate matter and air pollution on ocular surface disease: A systematic review of preclinical and clinical evidence. Ocul. Surf. 2025, 35, 100–116. [Google Scholar] [CrossRef]
- Alijagic, A.; Suljević, D.; Fočak, M.; Sulejmanović, J.; Šehović, E.; Särndahl, E.; Engwall, M. The triple exposure nexus of microplastic particles, plastic-associated chemicals, and environmental pollutants from a human health perspective. Environ. Int. 2024, 188, 108736. [Google Scholar] [CrossRef]
- Gruber, E.S.; Stadlbauer, V.; Pichler, V.; Resch-Fauster, K.; Todorovic, A.; Meisel, T.C.; Trawoeger, S.; Hollóczki, O.; Turner, S.D.; Wadsak, W.; et al. To Waste or Not to Waste: Questioning Potential Health Risks of Micro- and Nanoplastics with a Focus on Their Ingestion and Potential Carcinogenicity. Expo. Health 2023, 15, 33–51. [Google Scholar] [CrossRef]
- Yu, C.H.; Riker, C.D.; Lu, S.E.; Fan, Z.T. Biomonitoring of emerging contaminants, perfluoroalkyl and polyfluoroalkyl substances (PFAS), in New Jersey adults in 2016–2018. Int. J. Hyg. Environ. Health 2020, 223, 34–44. [Google Scholar] [CrossRef]
- Amato-Lourenco, L.F.; Dantas, K.C.; Junior, G.R.; Paes, V.R.; Ando, R.A.; de Oliveira Freitas, R.; da Costa, O.; Rabelo, R.S.; Soares Bispo, K.C.; Carvalho-Oliveira, R.; et al. Microplastics in the Olfactory Bulb of the Human Brain. JAMA Netw. Open 2024, 7, e2440018. [Google Scholar] [CrossRef]
- Qin, L.; Wu, J.; Sun, X.; Huang, X.; Huang, W.; Weng, C.; Cai, J. The regulatory role of metabolic organ-secreted factors in the nonalcoholic fatty liver disease and cardiovascular disease. Front. Cardiovasc. Med. 2023, 10, 1119005. [Google Scholar] [CrossRef]
- Wang, F.; So, K.F.; Xiao, J.; Wang, H. Organ-organ communication: The liver’s perspective. Theranostics 2021, 11, 3317–3330. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K.; Zikova-Kloas, A.; Marx-Stoelting, P.; Braeuning, A. Metabolism-Disrupting Chemicals Affecting the Liver: Screening, Testing, and Molecular Pathway Identification. Int. J. Mol. Sci. 2023, 24, 2686. [Google Scholar] [CrossRef] [PubMed]
- Milani, I.; Codini, M.; Guarisco, G.; Chinucci, M.; Gaita, C.; Leonetti, F.; Capoccia, D. Hepatokines and MASLD: The GLP1-Ras-FGF21-Fetuin-A Crosstalk as a Therapeutic Target. Int. J. Mol. Sci. 2024, 25, 10795. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Hong, D.G.; Yang, Y.M. Hepatokines and Non-Alcoholic Fatty Liver Disease: Linking Liver Pathophysiology to Metabolism. Biomedicines 2021, 9, 1903. [Google Scholar] [CrossRef]
- Orfanidou, M.; Polyzos, S.A. Retinopathy in Metabolic Dysfunction-Associated Steatotic Liver Disease. Medicina 2025, 61, 38. [Google Scholar] [CrossRef]
- Wu, J.; Duan, C.; Yang, Y.; Wang, Z.; Tan, C.; Han, C.; Hou, X. Insights into the liver-eyes connections, from epidemiological, mechanical studies to clinical translation. J. Transl. Med. 2023, 21, 712. [Google Scholar] [CrossRef]
- Mouliou, D.S. C-Reactive Protein: Pathophysiology, Diagnosis, False Test Results and a Novel Diagnostic Algorithm for Clinicians. Diseases 2023, 11, 132. [Google Scholar] [CrossRef]
- Chen, L.; Lu, J.; Hu, J.; Gong, X. Unveiling the multifaceted role of adropin in various diseases (Review). Int. J. Mol. Med. 2024, 54, 90. [Google Scholar] [CrossRef]
- Pennisi, G.; Maurotti, S.; Ciociola, E.; Jamialahmadi, O.; Bertolazzi, G.; Mirarchi, A.; Bergh, P.O.; Scionti, F.; Mancina, R.M.; Spagnuolo, R.; et al. ANGPTLownregulation Increases Intracellular Lipids by Reducing Energy Utilization. Arter. Thromb. Vasc. Biol. 2024, 44, 1086–1097. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, C.Y.; Chi, H.C.; Wu, M.H.; Lai, M.W.; Yeh, C.T. ANGPTL3 overcomes sorafenib resistance via suppression of SNAI1 and CPT1A in liver cancer. Transl. Oncol. 2025, 52, 102250. [Google Scholar] [CrossRef]
- Xu, J.; Wu, F.; Zhu, Y.; Wu, T.; Cao, T.; Gao, W.; Liu, M.; Qian, W.; Feng, G.; Xi, X.; et al. ANGPTL4 regulates ovarian cancer progression by activating the ERK1/2 pathway. Cancer Cell Int. 2024, 24, 54. [Google Scholar] [CrossRef] [PubMed]
- Baron-Menguy, C.; Freneau, M.; Mrad, M.-A.; Lebot, C.; Rio, M.; L’allinec, V.; Quillard, T.; Desal, H.; Redon, R.; Bourcier, R. ANGPTL6 variant induces cerebral vascular dysfunction and predisposes to intracranial aneurysm in mice. bioRxiv 2025. [Google Scholar] [CrossRef]
- Xu, F.; Wang, N.; Li, G.; Tian, D.; Shi, X. ANGPTL8/Betatrophin Improves Glucose Tolerance in Older Mice and Metabolomic Analysis Reveals Its Role in Insulin Resistance in HepG2 Cells. Diabetes Metab. Syndr. Obes. 2021, 14, 4209–4221. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yeung, C.L.S.; Yam, J.W.P.; Mao, X. An update on the role of complement in hepatocellular carcinoma. Front. Immunol. 2022, 13, 1007382. [Google Scholar] [CrossRef]
- Jensen-Cody, S.O.; Potthoff, M.J. Hepatokines and metabolism: Deciphering communication from the liver. Mol. Metab. 2021, 44, 101138. [Google Scholar] [CrossRef]
- Lee-Odegard, S.; Ueland, T.; Thorsby, P.M.; Aukrust, P.; Michelsen, A.E.; Halvorsen, B.; Drevon, C.A.; Birkeland, K.I. Fetuin-A mediates the difference in adipose tissue insulin resistance between young adult pakistani and norwegian patients with type 2 diabetes. BMC Endocr. Disord. 2022, 22, 208. [Google Scholar] [CrossRef]
- Dogru, T.; Kirik, A.; Gurel, H.; Rizvi, A.A.; Rizzo, M.; Sonmez, A. The Evolving Role of Fetuin-A in Nonalcoholic Fatty Liver Disease: An Overview from Liver to the Heart. Int. J. Mol. Sci. 2021, 22, 6627. [Google Scholar] [CrossRef]
- Yakout, S.M.; Hussein, S.; Al-Attas, O.S.; Hussain, S.D.; Saadawy, G.M.; Al-Daghri, N.M. Hepatokines fetuin A and fetuin B status in women with/without gestational diabetes mellitus. Am. J. Transl. Res. 2023, 15, 1291–1299. [Google Scholar]
- Pasmans, K.; Goossens, G.H.; Groenhuijzen, E.; Kemper, E.J.; Reijnders, D.; Most, J.; Blaak, E.E.; Watt, M.J.; Meex, R.C. Fetuin B in white adipose tissue induces inflammation and is associated with peripheral insulin resistance in mice and humans. Obesity 2024, 32, 517–527. [Google Scholar]
- De Sousa-Coelho, A.; Rodriguez-Rodriguez, R.; Softic, S.; Jonker, J.; Relat, J. FGF21 as a therapeutic target for obesity and insulin resistance: From rodent models to humans. Front. Endocrinol. 2023, 14, 1253675. [Google Scholar]
- Bielka, W.; Przezak, A.; Pawlik, A. Follistatin and follistatin-like 3 in metabolic disorders. Prostaglandins Other Lipid Mediat. 2023, 169, 106785. [Google Scholar] [CrossRef] [PubMed]
- Crisan, D.; Avram, L.; Morariu-Barb, A.; Grapa, C.; Hiriscau, I.; Craciun, R.; Donca, V.; Nemes, A. Sarcopenia in MASLD-Eat to Beat Steatosis, Move to Prove Strength. Nutrients 2025, 17, 178. [Google Scholar] [CrossRef] [PubMed]
- Elbasiony, E.; Cho, W.; Mittal, S.K.; Chauhan, S.K. Suppression of lipopolysaccharide-induced corneal opacity by hepatocyte growth factor. Sci. Rep. 2022, 12, 494. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.H.; Yue, Z.S.; Zhang, G.H.; Wang, L.; Dou, G.R. Beyond the Liver: Liver-Eye Communication in Clinical and Experimental Aspects. Front. Mol. Biosci. 2021, 8, 823277. [Google Scholar] [CrossRef]
- Takata, N.; Ishii, K.A.; Takayama, H.; Nagashimada, M.; Kamoshita, K.; Tanaka, T.; Kikuchi, A.; Takeshita, Y.; Matsumoto, Y.; Ota, T.; et al. LECT2 as a hepatokine links liver steatosis to inflammation via activating tissue macrophages in NASH. Sci. Rep. 2021, 11, 555. [Google Scholar] [CrossRef]
- Nono Nankam, P.A.; Bluher, M. Retinol-binding protein 4 in obesity and metabolic dysfunctions. Mol. Cell Endocrinol. 2021, 531, 111312. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Bayliss, J.; Devereux, C.; Bezawork-Geleta, A.; Roberts, D.; Huang, C.; Schittenhelm, R.B.; Ryan, A.; Townley, S.L.; Selth, L.A.; et al. SMOC1 is a glucose-responsive hepatokine and therapeutic target for glycemic control. Sci. Transl. Med. 2020, 12, eaaz8048. [Google Scholar] [CrossRef]
- Xi, X.; Han, L.; Ding, M.; Li, J.; Qiao, C.; Liu, Z.; Qie, S. Exploring the relationship between intestinal flora and the pathological mechanism of myopia in adolescents from the perspective of Chinese and Western medicine: A review. Medicine 2023, 102, e33393. [Google Scholar] [CrossRef]
- Wang, M.; Yu, X. A bibliographic study of “liver-eye” related research: A correlation function analytic research between age-related macular degeneration (AMD) and traditional chinese medicine (TCM) liver wind internal movement syndrome. Adv. Clin. Med. 2023, 13, 6342. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Shu, J.; Tu, J.-Y.; Zhang, C.-H.; Hong, J. Liver in the Chinese and Western Medicine. Integr. Med. Int. 2017, 4, 39–45. [Google Scholar] [CrossRef]
- Toragall, V.; Baskaran, V. Chitosan-sodium alginate-fatty acid nanocarrier system: Lutein bioavailability, absorption pharmacokinetics in diabetic rat and protection of retinal cells against H(2)O(2) induced oxidative stress in vitro. Carbohydr. Polym. 2021, 254, 117409. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Shin, D.; Ryu, I.H.; Kim, J.K.; Lee, I.S.; Koh, K.; Yoo, T.K. Association between cataract and fatty liver diseases from a nationwide cross-sectional study in South Korea. Sci. Rep. 2024, 14, 77. [Google Scholar] [CrossRef]
- Wilson, S.E.; Walker, J.W.; Chwang, E.L.; He, Y.G. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2544–2561. [Google Scholar]
- Pączek, S.; Zajkowska, M.; Mroczko, B. Pigment Epithelial-Derived Factor in Pancreatic and Liver Cancers—From Inflammation to Cancer. Biomedicines 2024, 12, 2260. [Google Scholar] [CrossRef]

| Plastic Debris Name | Particle Size |
|---|---|
| Macroplastics | >5 mm |
| Larger microplastics | 1–5 mm |
| Microplastics | 67–500 µm, 1–5000 µm, 20–5000 µm, or more broadly, as <5000 µm [definition supported by the National Oceanic and Atmospheric Administration (NOAA)] |
| Nanoplastics | <20 µm to as small as 1 nm |
| Hepatokines | Correlated with (Biological Functions) | Liver Is the Target Organ/Paracrine Mechanism? | Impacts the Liver–Eye Axis? Possible Key Factors for Liver–Eye Contact | References |
|---|---|---|---|---|
| Adropin | Macronutrient intake and estrogen. | Yes | Yes | [112,113,115] |
| ANGPTL3 | Increases lipid production in the liver and plasma lipid levels, promotes lipogenesis and the liver’s inflammatory response, and decreases glucose uptake. | Yes | Yes | [112,113,116,117] |
| ANGPTL4 | Inhibits lipoprotein lipase and activates cAMP-stimulated lipolysis in adipocytes. | Yes | [112,113,118] | |
| ANGPTL6 | Enhances insulin signaling in skeletal muscle and mitochondrial oxygen consumption in white adipose tissue. Inhibits gluconeogenesis in the liver. | Yes | Yes | [112,113,119] |
| ANGPTL8/betatrophin | Dubious action on beta-cell proliferation. | Yes | Yes | [112,113,120] |
| CFH | A complement inhibitor inhibits its excessive activation and is a key player in maintaining complement homeostasis. It is present as a soluble protein and is also attached to cell surfaces throughout the human body. | Yes | Yes | [110,111,121,122] |
| Fetuin-A | Increases inflammation and insulin resistance. | Yes | [123,124,125] | |
| Fetuin-B | Increases hepatic steatosis and mediates impaired insulin action and glucose intolerance. | Yes | [125,126] | |
| FGF-21 | An insulin-sensitizing hormone/metabolic actions. | Yes | Yes | [112,113,127] |
| Follistatin | Increases insulin resistance, promotes thermogenesis, and induces the differentiation of brown adipocytes. Enhances glucose levels and the uptake of free fatty acids after exercise training. Inhibits FSH production and suppresses skeletal muscle growth. | Yes | [128,129] | |
| GDF15 | Increases energy metabolism and lowers body weight; it is possibly implicated in the pathogenesis of anorexia. Stimulates thermogenic and lipolytic genes. Improves glucose tolerance and insulin sensitivity. Prevents liver steatosis. | Yes | [110,111,113,122] | |
| Hepassocin | Promotes insulin resistance and adipogenesis. | Yes | [110,111,122] | |
| HGF | Paracrine cellular growth, motility, and morphogenic factor. | Yes | Yes | [110,111,112,113,122,130,131] |
| LECT2 | Promotes the accumulation of lipids and inflammation in the liver and the development of insulin resistance in skeletal muscle. | Yes | [110,111,122,132] | |
| RBP4 | Depending on the source, the effect may be controversial. It increases lipolysis in adipocytes, is associated with insulin resistance and components of metabolic syndrome, promotes hepatic mitochondrial dysfunction and hepatic steatosis, and impairs insulin signaling. | ? | ? | [110,111,112,122,133] |
| Selenoprotein P | Inhibits hepatic gluconeogenesis and decreases glucose uptake in the skeletal muscle. | Yes | [122] | |
| SMOC1 | Improves glycemic control via inhibiting gluconeogenesis and glucose output from the liver. | Yes | [110,111,122,134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šoša, I.; Labinac, L.; Perković, M. Metabolic Dysfunction-Associated Steatotic Liver Disease Induced by Microplastics: An Endpoint in the Liver–Eye Axis. Int. J. Mol. Sci. 2025, 26, 2837. https://doi.org/10.3390/ijms26072837
Šoša I, Labinac L, Perković M. Metabolic Dysfunction-Associated Steatotic Liver Disease Induced by Microplastics: An Endpoint in the Liver–Eye Axis. International Journal of Molecular Sciences. 2025; 26(7):2837. https://doi.org/10.3390/ijms26072837
Chicago/Turabian StyleŠoša, Ivan, Loredana Labinac, and Manuela Perković. 2025. "Metabolic Dysfunction-Associated Steatotic Liver Disease Induced by Microplastics: An Endpoint in the Liver–Eye Axis" International Journal of Molecular Sciences 26, no. 7: 2837. https://doi.org/10.3390/ijms26072837
APA StyleŠoša, I., Labinac, L., & Perković, M. (2025). Metabolic Dysfunction-Associated Steatotic Liver Disease Induced by Microplastics: An Endpoint in the Liver–Eye Axis. International Journal of Molecular Sciences, 26(7), 2837. https://doi.org/10.3390/ijms26072837








