Impaired SERPIN–Protease Balance in the Peripheral Lungs of Stable COPD Patients
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of Subjects Providing Lung Parenchyma
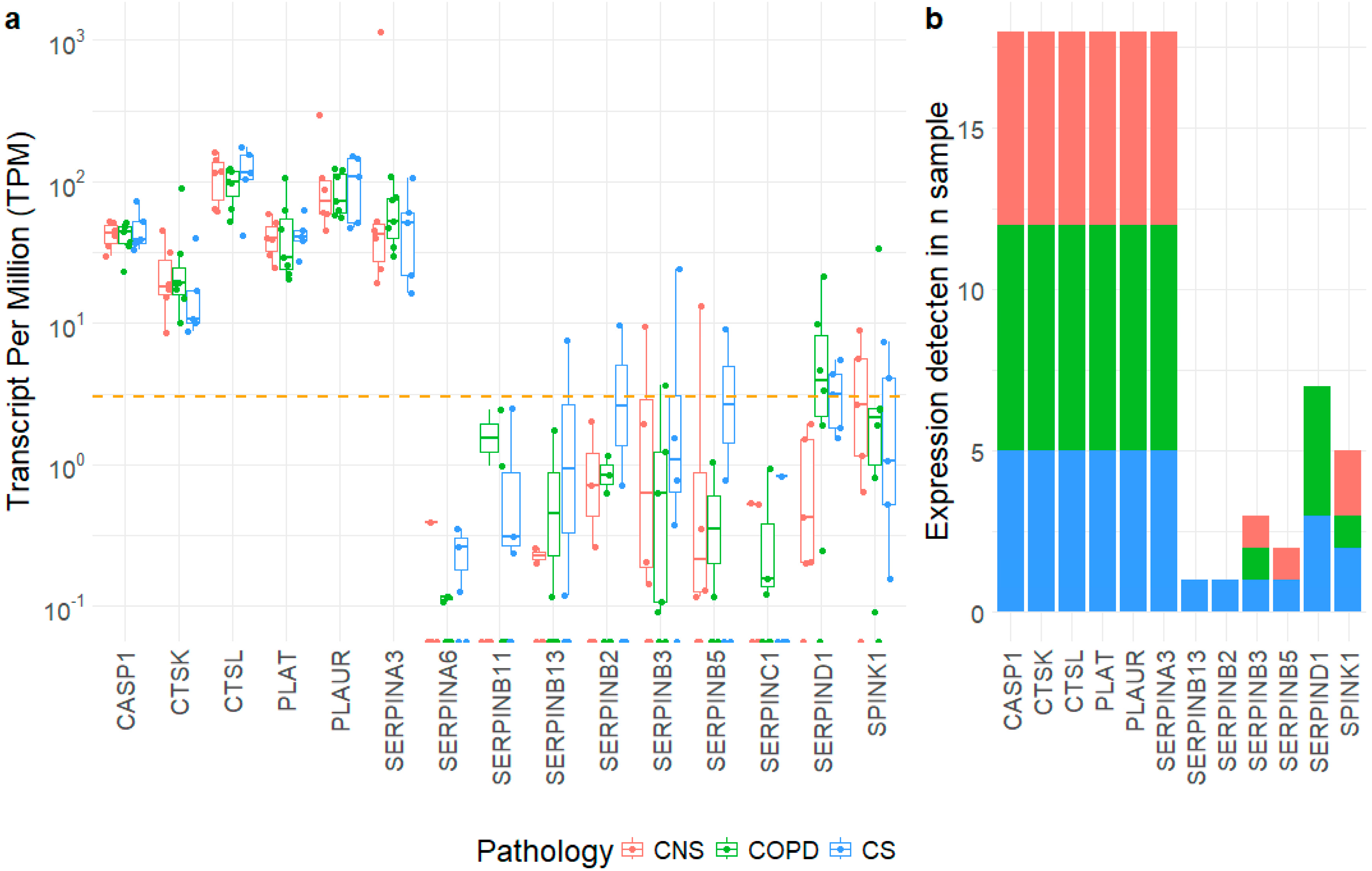
2.2. Gene Expression Level of SERPIN-Signaling Molecules in Lung Parenchyma
2.3. Immunohistochemistry for SERPIN-Signaling Proteins in the Peripheral Airways and Lung Parenchyma
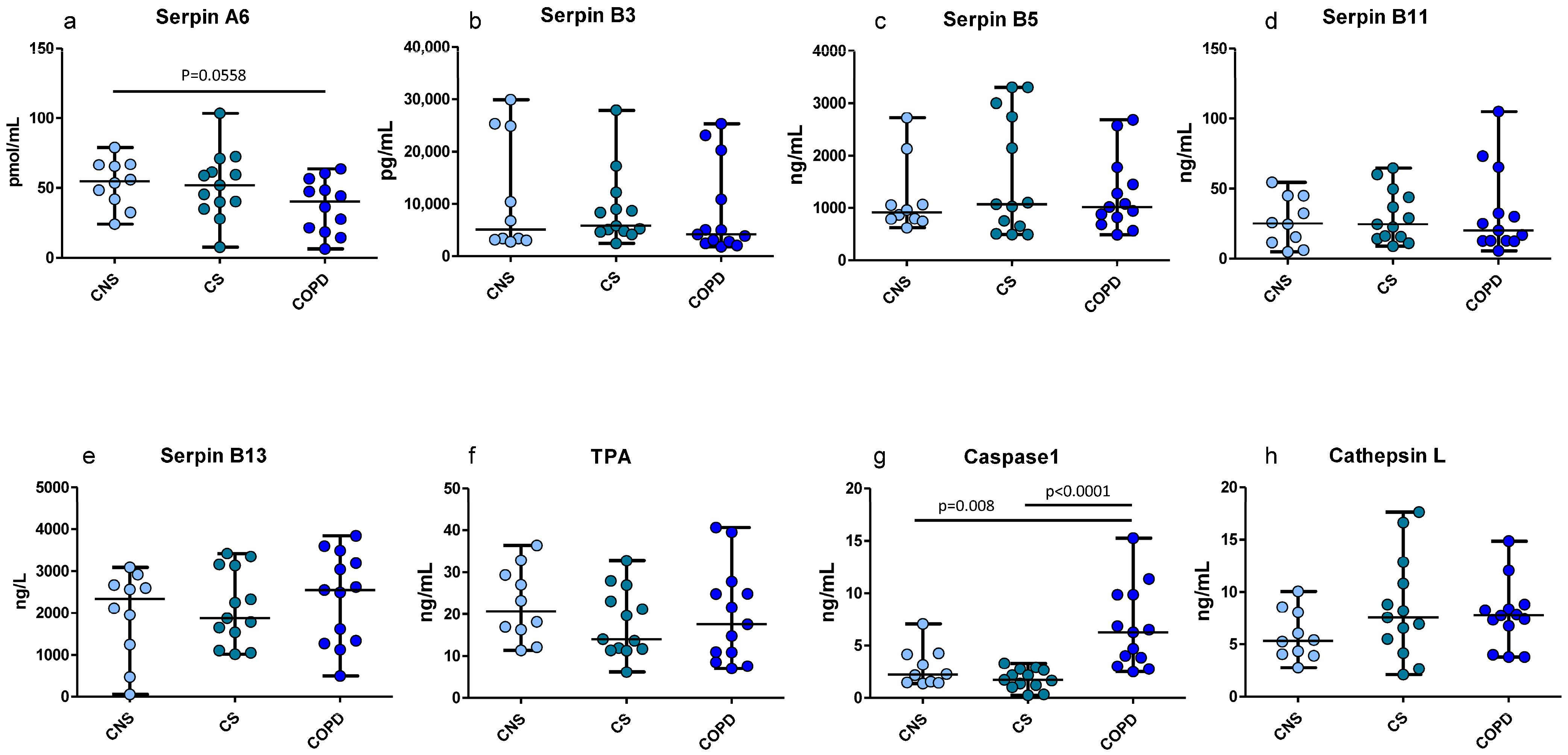
2.4. ELISA Tests for SERPIN-Signaling Proteins in Homogenized Peripheral Lung Tissue
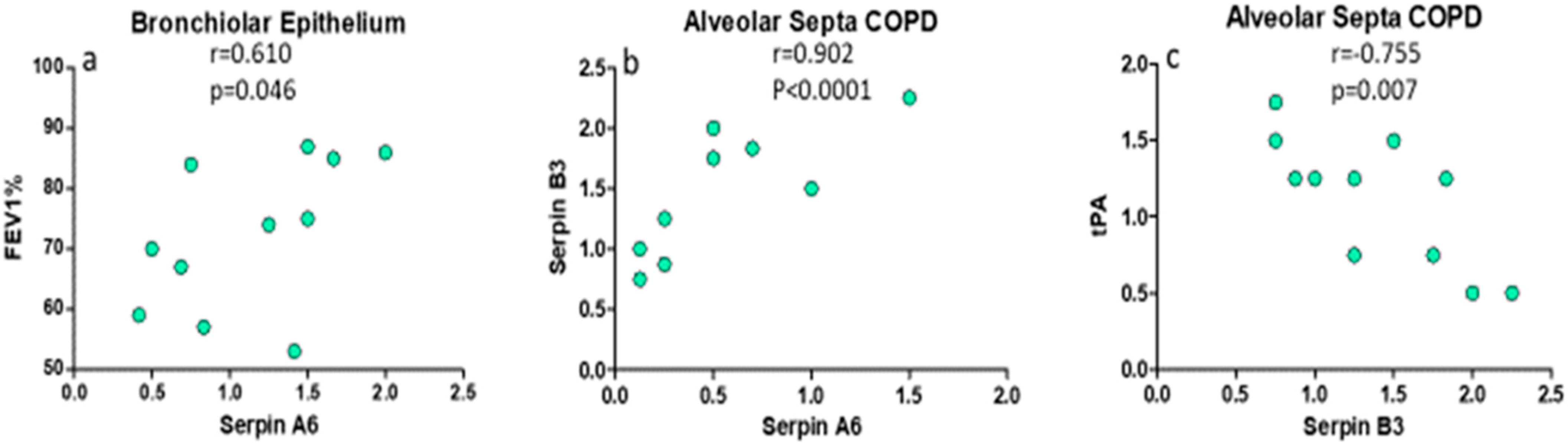
2.5. Principal Correlations Between Clinical Parameters, SERPINs and Proteases in Lung Parenchyma
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Lung Function Tests and Volumes
4.3. Collection and Processing of the Peripheral Lung Tissue
4.4. Immunohistochemistry in Human Peripheral Lung Tissue
4.5. Scoring System for Immunohistochemistry in the Peripheral Lung Tissue
4.6. ELISA Tests in the Peripheral Lung Tissue Homogenates
4.7. RNA Extraction and Sequencing from Lung Specimens
4.8. Data Analysis of RNA-Seq Data
4.9. Statistical Analysis Applied to Functional and Morphological Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kelly-Robinson, G.A.; Reihill, J.A.; Lundy, F.T.; McGarvey, L.P.; Lockhart, J.C.; Litherland, G.J.; Thornbury, K.D.; Martin, S.L. The Serpin Superfamily and Their Role in the Regulation and Dysfunction of Serine Protease Activity in COPD and Other Chronic Lung Diseases. Int. J. Mol. Sci. 2021, 22, 6351. [Google Scholar] [CrossRef]
- Carrell, R.W.; Whisstock, J.; Lomas, D.A. Conformational changes in serpins and the mechanism of alpha 1-antitrypsin deficiency. Am. J. Respir. Crit. Care Med. 1994, 150 Pt 2, S171. [Google Scholar] [CrossRef]
- Baker, C.; Belbin, O.; Kalsheker, N.; Morgan, K. SERPINA3 (aka alpha-1-antichymotrypsin). Front. Biosci. 2007, 12, 2821–2835. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.J.; Nenke, M.A.; Rankin, W.; Lewis, J.G.; Torpy, D.J. Corticosteroid-Binding Globulin: A Review of Basic and Clinical Advances. Horm. Metab. Res. 2016, 48, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Stasinopoulos, S.; Mariasegaram, M.; Gafforini, C.; Nagamine, Y.; Medcalf, R.L. The plasminogen activator inhibitor 2 transcript is destabilized via a multi-component 3’ UTR localized adenylate and uridylate-rich instability element in an analogous manner to cytokines and oncogenes. FEBS J. 2010, 277, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sheshadri, N.; Zong, W.X. SERPINB3 and B4: From biochemistry to biology. Semin. Cell Dev. Biol. 2017, 62, 170–177. [Google Scholar] [CrossRef]
- Schick, C.; Pemberton, P.A.; Shi, G.P.; Kamachi, Y.; Cataltepe, S.; Bartuski, A.J.; Gornstein, E.R.; Brömme, D.; Chapman, A.H.A.; Silverman, G.A. Cross-class inhibition of the cysteine proteinases cathepsins K, L, and S by the serpin squamous cell carcinoma antigen 1: A kinetic analysis. Biochemistry 1998, 37, 5258–5266. [Google Scholar] [CrossRef]
- Silverman, G.A.; Whisstock, J.C.; Askew, D.J.; Pak, S.C.; Luke, C.J.; Cataltepe, S.; Irving, J.; Bird, P. Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell Mol. Life Sci. 2004, 61, 301–325. [Google Scholar] [CrossRef]
- Seixas, S.; Ivanova, N.; Ferreira, Z.; Rocha, J.; Victor, B.L. Loss and gain of function in SERPINB11: An example of a gene under selection on standing variation, with implications for host-pathogen interactions. PLoS ONE 2012, 7, e32518. [Google Scholar] [CrossRef]
- Jayakumar, A.; Kang, Y.; Frederick, M.J.; Pak, S.C.; Henderson, Y.; Holton, P.R.; Mitsudo, K.; A Silverman, G.; El-Naggar, A.K.; Brömme, D.; et al. Inhibition of the cysteine proteinases cathepsins K and L by the serpin headpin (SERPINB13): A kinetic analysis. Arch. Biochem. Biophys. 2003, 409, 367–374. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Ma, H.; Zhu, M.; Zhou, Y.; Zhang, Q.; Peng, H. Relationship between Antithrombin III Activity and Mortality in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. COPD 2022, 19, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Sabit, R.; Thomas, P.; Shale, D.J.; Collins, P.; Linnane, S.J. The effects of hypoxia on markers of coagulation and systemic inflammation in patients with COPD. Chest 2010, 138, 47–51. [Google Scholar]
- Corral, J.; Aznar, J.; Gonzalez-Conejero, R.; Villa, P.; Minano, A.; Vaya, A.; Carrell, R.W.; Huntington, J.A.; Vicente, V. Homozygous deficiency of heparin cofactor II: Relevance of P17 glutamate residue in serpins, relationship with conformational diseases, and role in thrombosis. Circulation 2004, 110, 1303–1307. [Google Scholar]
- Chang, C.; Zhao, W.; Luo, Y.; Xi, L.; Chen, S.; Zhao, C.; Wang, G.; Guo, J.; Xu, C. Serine peptidase inhibitor Kazal type I (SPINK1) promotes BRL-3A cell proliferation via p38, ERK, and JNK pathways. Cell Biochem. Funct. 2017, 35, 339–348. [Google Scholar]
- Desmedt, S.; Desmedt, V.; Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. The intriguing role of soluble urokinase receptor in inflammatory diseases. Crit. Rev. Clin. Lab. Sci. 2017, 54, 117–133. [Google Scholar] [PubMed]
- Bocskei, R.M.; Benczur, B.; Losonczy, G.; Illyes, M.; Cziraki, A.; Muller, V.; Bohács, A.; Bikov, A. Soluble Urokinase-Type Plasminogen Activator Receptor and Arterial Stiffness in Patients with COPD. Lung 2019, 197, 189–197. [Google Scholar] [PubMed]
- Ismail, A.A.; Shaker, B.T.; Bajou, K. The Plasminogen-Activator Plasmin System in Physiological and Pathophysiological Angiogenesis. Int. J. Mol. Sci. 2021, 23, 337. [Google Scholar] [CrossRef]
- Bootle-Wilbraham, C.A.; Tazzyman, S.; Thompson, W.D.; Stirk, C.M.; Lewis, C.E. Fibrin fragment E stimulates the proliferation, migration and differentiation of human microvascular endothelial cells in vitro. Angiogenesis 2001, 4, 269–275. [Google Scholar] [CrossRef]
- Dickinson, D.P. Cysteine peptidases of mammals: Their biological roles and potential effects in the oral cavity and other tissues in health and disease. Crit. Rev. Oral Biol. Med. 2002, 13, 238–275. [Google Scholar]
- Novinec, M.; Grass, R.N.; Stark, W.J.; Turk, V.; Baici, A.; Lenarcic, B. Interaction between human cathepsins K, L, and S and elastins: Mechanism of elastinolysis and inhibition by macromolecular inhibitors. J. Biol. Chem. 2007, 282, 7893–7902. [Google Scholar] [CrossRef]
- Andrault, P.M.; Samsonov, S.A.; Weber, G.; Coquet, L.; Nazmi, K.; Bolscher, J.G.; Lalmanach, A.-C.; Jouenne, T.; Brömme, D.; Pisabarro, M.T.; et al. Antimicrobial Peptide LL-37 Is Both a Substrate of Cathepsins S and K and a Selective Inhibitor of Cathepsin L. Biochemistry 2015, 54, 2785–2798. [Google Scholar] [CrossRef] [PubMed]
- Taggart, C.C.; Lowe, G.J.; Greene, C.M.; Mulgrew, A.T.; O’Neill, S.J.; Levine, R.L.; McElvaney, N.G. Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor. J. Biol. Chem. 2001, 276, 33345–33352. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ishidoh, K.; Muno, D.; Ohwada, A.; Nukiwa, T.; Kominami, E.; Kira, S. Cathepsin L activity is increased in alveolar macrophages and bronchoalveolar lavage fluid of smokers. Am. Rev. Respir. Dis. 1993, 147 Pt 1, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Markelic, I.; Hlapcic, I.; Ceri, A.; Radic Antolic, M.; Samarzija, M.; Popovic-Grle, S.; Dugac, A.V.; Rumora, L. Activation of NLRP3 inflammasome in stable chronic obstructive pulmonary disease. Sci. Rep. 2022, 12, 7544. [Google Scholar] [CrossRef]
- Di Stefano, A.; Caramori, G.; Barczyk, A.; Vicari, C.; Brun, P.; Zanini, A.; Cappello, F.; Garofano, E.; Padovani, A.; Contoli, M.; et al. Innate immunity but not NLRP3 inflammasome activation correlates with severity of stable COPD. Thorax 2014, 69, 516–524. [Google Scholar] [CrossRef]
- Hogg, J.C.; McDonough, J.E.; Sanchez, P.G.; Cooper, J.D.; Coxson, H.O.; Elliott, W.M.; Naiman, D.; Pochettino, M.; Horng, D.; Gefter, W.B.; et al. Micro-computed tomography measurements of peripheral lung pathology in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009, 6, 546–549. [Google Scholar] [CrossRef]
- Vasilescu, D.M.; Hackett, T.L.; Martinez, F.J.; Curtis, J.L.; Hogg, J.C.; Han, M.K. Reply to Janssen and Wouters: Loss of Alveolar Attachments as a Pathomechanistic Link between Small Airway Disease and Emphysema. Am. J. Respir. Crit. Care Med. 2020, 201, 879–880. [Google Scholar] [CrossRef]
- Lee, W.H.; Larsson, S.C.; Wood, A.; Di Angelantonio, E.; Butterworth, A.S.; Burgess, S.; Allara, E. Genetically predicted plasma cortisol and common chronic diseases: A Mendelian randomization study. Clin. Endocrinol. 2024, 100, 238–244. [Google Scholar] [CrossRef]
- Bankier, S.; Wang, L.; Crawford, A.; Morgan, R.A.; Ruusalepp, A.; Andrew, R.; Björkegren, J.L.M.; Walker, B.R.; Michoel, T. Plasma cortisol-linked gene networks in hepatic and adipose tissues implicate corticosteroid-binding globulin in modulating tissue glucocorticoid action and cardiovascular risk. Front. Endocrinol. 2023, 14, 1186252. [Google Scholar] [CrossRef]
- Nenke, M.A.; Holmes, M.; Rankin, W.; Lewis, J.G.; Torpy, D.J. Corticosteroid-binding globulin cleavage is paradoxically reduced in alpha-1 antitrypsin deficiency: Implications for cortisol homeostasis. Clin. Chim. Acta 2016, 452, 27–31. [Google Scholar] [CrossRef]
- Nucera, F.; Mumby, S.; Paudel, K.R.; Dharwal, V.; DI Stefano, A.; Casolaro, V.; Hansbro, P.M.; Adcock, I.M.; Caramori, G. Role of oxidative stress in the pathogenesis of COPD. Minerva Med. 2022, 113, 370–404. [Google Scholar] [CrossRef]
- Janciauskiene, S.; Lechowicz, U.; Pelc, M.; Olejnicka, B.; Chorostowska-Wynimko, J. Diagnostic and therapeutic value of human serpin family proteins. Biomed. Pharmacother. 2024, 175, 116618. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Lu, L.; Zou, Q. Analysis of gene expression profiles of lung cancer subtypes with machine learning algorithms. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165822. [Google Scholar]
- Perrotta, F.; D’Agnano, V.; Scialo, F.; Komici, K.; Allocca, V.; Nucera, F.; Salvi, R.; Stella, G.M.; Bianco, A. Evolving concepts in COPD and lung cancer: A narrative review. Minerva Med. 2022, 113, 436–448. [Google Scholar] [CrossRef]
- Cagnin, S.; Pontisso, P.; Martini, A. SerpinB3: A Multifaceted Player in Health and Disease-Review and Future Perspectives. Cancers 2024, 16, 2579. [Google Scholar] [CrossRef] [PubMed]
- Nucera, F.; Di Stefano, A.; Ricciardolo, F.L.M.; Gnemmi, I.; Pizzimenti, C.; Monaco, F.; Tuccari, G.; Caramori, G.; Ieni, A. Role of ATG4 Autophagy-Related Protein Family in the Lower Airways of Patients with Stable COPD. Int. J. Mol. Sci. 2024, 25, 8182. [Google Scholar] [CrossRef]
- Park, S.J.; Lim, W.; Mun, J.; Paik, H.; Park, S.; Lim, H.; Kim, J.; Lee, E.J.; Yim, G.W.; Lee, N.; et al. SERPINB11 Expression Is Associated With Prognosis of High-grade Serous and Clear Cell Carcinoma of the Ovary. In Vivo 2021, 35, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- van Zoelen, M.A.D.; Schouten, M.; de Vos, A.F.; Florquin, S.; Meijers, J.C.M.; Nawroth, P.P.; Bierhaus, A.; van der Poll, T. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J. Immunol. 2009, 182, 4349–4356. [Google Scholar] [CrossRef]
- Vallese, D.; Ricciardolo, F.L.; Gnemmi, I.; Casolari, P.; Brun, P.; Sorbello, V.; Capelli, A.; Cappello, F.; Cavallesco, G.N.; Papi, A.; et al. Phospho-p38 MAPK expression in COPD patients and asthmatics and in challenged bronchial epithelium. Respiration 2015, 89, 329–342. [Google Scholar]
- Di Stefano, A.; Rosani, U.; Levra, S.; Gnemmi, I.; Brun, P.; Maniscalco, M.; D’anna, S.E.; Carriero, V.; Bertolini, F.; Ricciardolo, F.L.M. Bone Morphogenic Proteins and Their Antagonists in the Lower Airways of Stable COPD Patients. Biology 2023, 12, 1304. [Google Scholar] [CrossRef]
- Di Stefano, A.; Sangiorgi, C.; Gnemmi, I.; Casolari, P.; Brun, P.; Ricciardolo, F.L.M.; Contoli, M.; Papi, A.; Maniscalco, P.; Ruggeri, P.; et al. TGF-β Signaling Pathways in Different Compartments of the Lower Airways of Patients With Stable COPD. Chest 2018, 153, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [PubMed]

| Grups | Control Nonsmokers | Control Smokers | Patients with COPD |
|---|---|---|---|
| N° | 12 | 14 | 13 |
| Age (y) | 66 ± 4.6 | 67 ± 1.6 | 70 ± 1.7 |
| M/F | 5/7 | 9/5 | 11/2 |
| Ex/Current Smokers | --- | 9/5 | 10/3 |
| Pack Years | --- | 37 ± 4.8 | 54 ± 11 |
| FEV1 (% pred) pre-β2 | 113 ± 4.2 | 97 ± 2.9 | 73 ± 4.7 # |
| FEV1 (% pred) post-β2 | ND | ND | 82 ± 5.2 |
| FEV1/FVC (%) pre-β2 | 81 ± 1.4 | 73 ± 1.0 | 58 ± 2.4 # |
| TLC (%) | 105.2 ± 3.5 | 102.7 ± 2.1 | 114.6 ± 3.9 * |
| RV (%) | 109.0 ± 6.5 | 114.6 ± 3.7 | 150.7 ± 8.7 # |
| DLCO (%) | ND | 90.4 ± 3.6 | 80.6 ± 5.5 |
| Localization | Control Nonsmokers | Control Smokers | COPD Patients | Kruskal Wallis p-Value |
|---|---|---|---|---|
| Bronchiolar Epithelium (score 0–3) | ||||
| SERPINA3 | 1.0 (0.25–1.5) | 0.75 (0.5–1.5) | 0.75 (0.25–1.5) | 0.673 |
| SERINA6 | 1.58 (0.75–2.0) | 0.81 (0.25–1.36) * | 1.25 (0.41–2.0) | 0.025 |
| SERPINB2 (PAI-2) | 0.25 (0.0–0.625) | 0.125 (0.0–0.625) | 0.25 (0.0–0.5) | 0.968 |
| SERPINB3 | 2.56 (2.0–3.0) | 2.4 (1.0–2.5) | 2.56 (2.0–2.95) | 0.116 |
| SERPINB5 | 2.45 (1.25–3.0) | 3.0 (1.75–3.0) * | 2.75 (1.75–3.0) & | 0.016 |
| SERPINB11 | 2.75 (2.33–3.0) | 2.5 (1.37–2.87) | 2.87 (2.33–3.0) | 0.163 |
| SERPINB13 | 0.44 (0.0–0.62) | 0.77 (0.0–1.5) | 0.67 (0.0–2.0) | 0.130 |
| SERPINC1 | 3.0 (1.8–3.0) | 2.83 (1.5–3.0) | 3.0 (2.5–3.0) | 0.244 |
| SERPIND1 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.1) | 0.621 |
| SPINK1 | 2.96 (1.5–3.0) | 2.75 (1.83–3.0) | 2.95 (2.5–3.0) | 0.205 |
| PLAUR (UPAR) | 2.75 (1.25–3.0) | 2.95 (1.75–3.0) | 2.75 (1.5–3.0) | 0.199 |
| tPA | 1.75 (0.75–2.87) | 2.75 (0.75–3.0) * | 2.75 (0.5–3.0) * | 0.050 |
| Cathepsin K | 0.55 (0.0–1.75) | 0.75 (0.0–1.12) | 0.5 (0.15–1.16) | 0.772 |
| Cathepsin L | 2.25 (0.75–3.0) | 2.75 (1.5–3.0) | 2.87 (1.75–3.0) | 0.158 |
| Caspase1 | 2.92 (2.75–3.0) | 2.5 (1.5–3.0) | 2.85 (1.5–3.0) | 0.122 |
| Bronchiolar Lamina Propria (score 0–3) | ||||
| SERPINA3 | 1.0 (0.25–1.5) | 0.75 (0.5–1.5) | 0.75 (0.25–1.5) | 0.789 |
| SERPINA6 | 1.0 (0.5–1.5) | 0.5 (0.0–1.0) * | 0.5 (0.25–1.5) * | 0.029 |
| SERPINB2 (PAI-2) | 0.0 (0.0–0.5) | 0.5 (0.0–1.0) | 0.5 (0.0–0.5) | 0.590 |
| SERPINB3 | 1.0 (0.75–2.5) | 1.0 (0.5–1.0) | 1.0 (1.0–2.5) | 0.128 |
| SERPINB5 | 2.41 (1.25–3.0) | 2.87 (1.75–3.0) * | 2.75 (1.75–3.0) | 0.065 |
| SERPINB11 | 2.5 (2.0–2.92) | 2.25 (1.25–2.75) | 2.5 (2.0–2.5) | 0.252 |
| SERPINB13 | 0.12 (0.0–0.5) | 0.5 (0.0–1.0) * | 0.5 (0.0–2.0) * | 0.027 |
| SERPINC1 | 2.5 (1.5–2.87) | 2.5 (1.0–3.0) | 2.75 (2.0–3.0) | 0.099 |
| SERPIND1 | 0.0 (0.0–0.12) | 0.0 (0.0–0.0) | 0.0 (0.0–0.05) | 0.582 |
| SPINK1 | 2.5 (1.5–2.5) | 2.5 (1.5–2.75) | 2.5 (2.0–2.5) | 0.580 |
| PLAUR (UPAR) | 2.5 (1.25–2.75) | 2.5 (1.75–3.0) | 2.5 (1.5–2.75) | 0.700 |
| tPA | 1.0 (0.5–2.5) | 2.0 (0.5–2.5) | 2.25 (0.5–2.5) | 0.160 |
| Cathepsin K | 0.25 (0.0–0.5) | 0.25 (0.0–0.5) | 0.25 (0.0–0.5) | 0.917 |
| Cathepsin L | 2.0 (0.5–2.5) | 2.5 (1.5–3.0) | 2.5 (1.5–2.5) | 0.201 |
| Caspase1 | 2.75 (2.5–3.0) | 2.0 (1.0–3.0) * | 2.5 (1.5–3.0) | 0.021 |
| Alveolar Macrophages (score 0–3) | ||||
| SERPINA3 | 0.75 (0.25–1.36) | 1.0 (0.5–1.5) | 0.75 (0.5–1.75) | 0.319 |
| SERPINA6 | 1.75 (0.5–2.0) | 1.25 (0.5–2.0) | 1.0 (0.41–2.0) | 0.266 |
| SERPINB2 (PAI-2) | 0.5 (0.0–2.25) | 0.89 (0.1–2.0) | 0.5 (0.25–2.0) | 0.322 |
| SERPINB3 | 1.5 (0.75–2.5) | 1.25 (0.75–2.25) | 1.0 (0.5–2.25) | 0.482 |
| SERPINB5 | 2.0 (1.5–2.5) | 2.5 (1.75–3.0) * | 2.25 (1.83–3.0) | 0.017 |
| SERPINB11 | 2.25 (1.5–2.87) | 2.0 (1.5–2.5) | 2.25 (1.25–2.75) | 0.093 |
| SERPINB13 | 1.0 (0.12–2.0) | 2.0 (0.17–2.5) * | 2.0 (0.17–2.5) * | 0.012 |
| SERPINC1 | 3.0 (2.5–3.0) | 2.75 (2.0–3.0) | 2.87 (2.25–3.0) | 0.140 |
| SERPIND1 | 0.04 (0.0–0.57) | 0.06 (0.0–1.0) | 0.07 (0.0–0.25) | 0.708 |
| SPINK1 | 2.5 (2.0–2.92) | 2.5 (2.0–3.0) | 2.5 (1.0–3.0) | 0.778 |
| PLAUR (UPAR) | 2.5 (2.0–3.0) | 3.0 (2.0–3.0) * | 2.75 (1.83–3.0) & | 0.026 |
| tPA | 1.75 (0.75–2.5) | 2.0 (1.0–2.75) | 2.0 (0.75–2.75) | 0.215 |
| Cathepsin K | 1.5 (0.25–2.25) | 1.75 (0.5–2.5) | 1.37 (0.2–2.25) | 0.617 |
| Cathepsin L | 2.25 (1.25–2.75) | 2.75 (1.75–3.0) * | 2.75 (1.5–3.0) * | 0.063 |
| Caspase1 | 2.75 (2.25–3.0) | 2.5 (1.5–3.0) | 2.5 (2.25–3.0) | 0.111 |
| Alveolar Septa (score 0–3) | ||||
| SERPINA3 | 1.0 (0.5–1.7) | 0.83 (0.5–1.33) | 0.79 (0.5–125) | 0.385 |
| SERPINA6 | 0.75 (0.0–1.5) | 0.375 (0.0–1.5) | 0.25 (0.125–1.5) | 0.080 |
| SERPINB2 (PAI-2) | 1.0 (0.125–1.5) | 1.0 (0.08–1.5) | 1.25 (0.25–2.0) | 0.604 |
| SERPINB3 | 1.5 (1.0–2.5) | 1.25 (0.75–1.5) * | 1.25 (0.75–2.25) | 0.058 |
| SERPINB5 | 1.5 (1.0–2.5) | 2.25 (1.5–3.0) * | 2.0 (1.5–2.75) * | 0.029 |
| SERPINB11 | 1.87 (1.5–2.5) | 1.5 (1.0–2.0) * | 1.9 (1.25–2.5) & | 0.015 |
| SERPINB13 | 0.25 (0.08–0.83) | 1.0 (0.16–1.5) * | 0.75 (0.0–2.0) * | 0.022 |
| SERPINC1 | 1.91 (1.5–2.5) | 1.5 (1.0–2.5) | 1.75 (1.42–2.75) | 0.533 |
| SERPIND1 | 0.0 (0.0–0.29) | 0.0 (0.0–0.25) | 0.0 (0.0–0.25) | 0.576 |
| SPINK1 | 1.5 (1.0–2.0) | 1.5 (1.0–2.0) | 1.75 (1.0–2.5) | 0.466 |
| PLAUR (UPAR) | 2.0 (1.0–2.0) | 1.5 (1.0–2.0) | 1.5 (1.0–2.5) | 0.628 |
| tPA | 0.5 (0.25–1.5) | 1.0 (0.25–2.0) | 1.25 (0.5–1.75) | 0.112 |
| Cathepsin K | 0.5 (0.0–1.0) | 0.5 (0.25–1.0) | 0.37 (0.25–0.75) | 0.155 |
| Cathepsin L | 1.4 (0.5–2.25) | 2.0 (1.0–2.5) | 2.0 (1.25–2.5) * | 0.043 |
| Caspase1 | 2.5 (2.0–3.0) | 2.0 (1.0–3.0) * | 2.5 (2.0–2.75) | 0.046 |
| Lung Vessels (score 0–3) | ||||
| SERPINA3 | 1.12 (0.5–1.5) | 1.0 (0.5–1.5) | 1.0 (0.5–1.75) | 0.256 |
| SERPINA6 e | 0.5 (0.0–1.0) | 0.0 (0.0–1.0) * | 0.375 (0.0–1.0) | 0.063 |
| SERPINB2 e (PAI-2) | 0.5 (0.0–1.0) | 0.5 (0.0–1.5) | 0.5 (0.0–1.0) | 0.801 |
| SERPINB3 e | 0.75 (0.5–1.5) | 0.75 (0.5–1.0) | 0.75 (0.5–1.0) | 0.973 |
| SERPINB5 e | 1.5 (1.0–2.0) | 1.5 (1.0–2.5) | 1.75 (1.25–2.5) | 0.156 |
| SERPINB11 e | 1.5 (1.5–2.5) | 1.5 (0.75–2.0) * | 2.0 (1.0–2.5) & | 0.045 |
| SERPINB13 e | 0.5 (0.0–0.75) | 1.0 (0.0–1.25) * | 0.75 (0.0–1.5) * | 0.013 |
| SERPINC1 | 2.0 (2.0–2.5) | 2.0 (1.0–2.5) | 2.0 (1.0–2.5) | 0.139 |
| SERPIND1 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | NV |
| SPINK1 e | 1.5 (1.0–2.0) | 1.5 (1.0–1.5) | 1.5 (1.0–2.0) | 0.611 |
| PLAUR (UPAR) | 1.62 (1.0–2.0) | 1.5 (1.25–2.0) | 1.5 (1.0–2.5) | 0.676 |
| tPA | 0.87 (0.0–1.5) | 1.0 (0.25–1.75) * | 1.25 (0.5–2.0) * | 0.055 |
| Cathepsin K | 0.5 (0.0–1.5) | 0.75 (0.0–1.0) | 0.5 (0.0–1.0) | 0.374 |
| Cathepsin L e | 1.12 (0.25–2.0) | 1.25 (1.0–2.5) | 1.5 (0.5–2.0) | 0.399 |
| Caspase1 e | 2.25 (1.25–2.5) | 1.5 (1.0–2.5) * | 1.75 (1.5–2.5) * | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Stefano, A.; Nucera, F.; Rosani, U.; Brun, P.; Gnemmi, I.; Maniscalco, M.; D’Anna, S.E.; Leonardi, A.; Carriero, V.; Bertolini, F.; et al. Impaired SERPIN–Protease Balance in the Peripheral Lungs of Stable COPD Patients. Int. J. Mol. Sci. 2025, 26, 2832. https://doi.org/10.3390/ijms26072832
Di Stefano A, Nucera F, Rosani U, Brun P, Gnemmi I, Maniscalco M, D’Anna SE, Leonardi A, Carriero V, Bertolini F, et al. Impaired SERPIN–Protease Balance in the Peripheral Lungs of Stable COPD Patients. International Journal of Molecular Sciences. 2025; 26(7):2832. https://doi.org/10.3390/ijms26072832
Chicago/Turabian StyleDi Stefano, Antonino, Francesco Nucera, Umberto Rosani, Paola Brun, Isabella Gnemmi, Mauro Maniscalco, Silvestro Ennio D’Anna, Andrea Leonardi, Vitina Carriero, Francesca Bertolini, and et al. 2025. "Impaired SERPIN–Protease Balance in the Peripheral Lungs of Stable COPD Patients" International Journal of Molecular Sciences 26, no. 7: 2832. https://doi.org/10.3390/ijms26072832
APA StyleDi Stefano, A., Nucera, F., Rosani, U., Brun, P., Gnemmi, I., Maniscalco, M., D’Anna, S. E., Leonardi, A., Carriero, V., Bertolini, F., Freni, J., Ieni, A., Gangemi, S., Ruggeri, P., & Ricciardolo, F. L. M. (2025). Impaired SERPIN–Protease Balance in the Peripheral Lungs of Stable COPD Patients. International Journal of Molecular Sciences, 26(7), 2832. https://doi.org/10.3390/ijms26072832











