Effects of Different Intensities of Endurance Training on Neurotrophin Levels and Functional and Cognitive Outcomes in Post-Ischaemic Stroke Adults: A Randomised Clinical Trial
Abstract
1. Introduction
2. Results
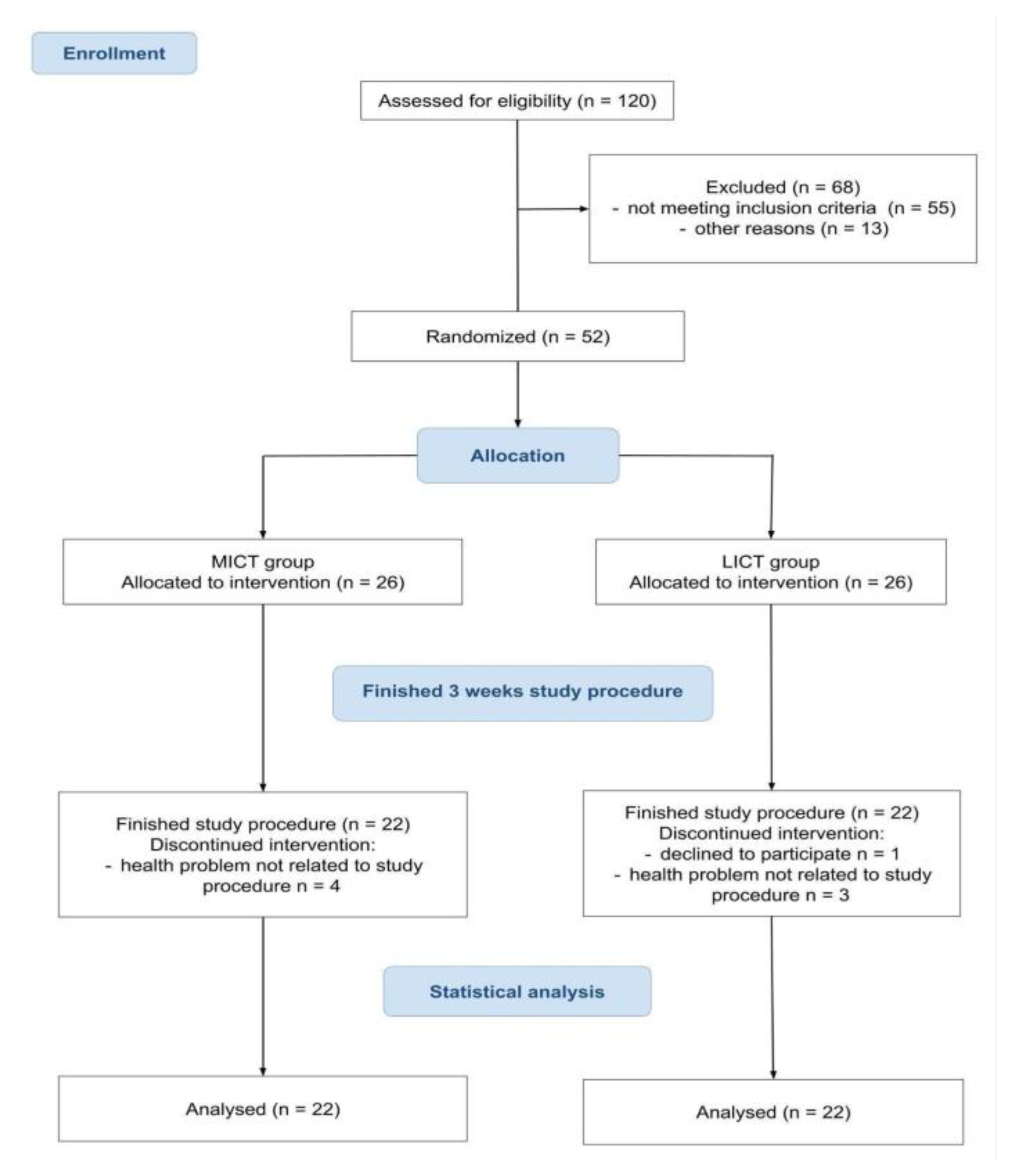
2.1. Flow of the Participants Through the Trial
2.2. Characteristics of the Participants
2.3. Effect of Endurance Exercise Intensity on the Motor Ability and Aerobic Capacity
2.4. Effect of Endurance Exercise Intensity on Cognitive Function
2.5. Effect of the Endurance Exercise Intensity on the Level of Selected Biomarkers
2.6. Correlation Between the Effect of Exercise and the Clinical Presentation
3. Discussion
Implications and Limitations of the Study
4. Materials and Methods
4.1. Study Participants
4.2. Physical Rehabilitation Programme
4.3. Outcome Measures
4.3.1. Cardiorespiratory Fitness
The Graded Cycling Test with Talk Test (GCT-TT)
Biomarkers
Body Mass Index (BMI)
BP
4.3.2. Physical Performance
VO2max
6-Minute Walk Test (6MWT)
Rivermead Motor Assessment (RMA)
Barthel Index (BI)
4.3.3. Cognitive Function
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE-III | Addenbrooke’s Cognitive Examination |
| ACE-III A | Addenbrooke’s Cognitive Examination-attention |
| ACE-III F | Addenbrooke’s Cognitive Examination-fluency |
| ACE-III L | Addenbrooke’s Cognitive Examination-language |
| ACE-III M | Addenbrooke’s Cognitive Examination-memory |
| ACE-III V | Addenbrooke’s Cognitive Examination-visuospatial |
| BDNF | brain-derived neurotrophic factor |
| BI | Barthel Index |
| GDNF | glial cell line–derived neurotrophic factor |
| IGF-1 | insulin-like growth factor-1 |
| LICT | low-intensity continuous training |
| M-ACE | Mini-Addenbrooke’s Cognitive Examination |
| MICT | moderate-intensity continuous training |
| RMA | Rivermead Motor Assessment |
| RMA-a | Rivermead Motor Assessment-arm |
| RMA-gf | Rivermead Motor Assessment-global function |
| RMA-lt | Rivermead Motor Assessment-lower trunk |
| VEGF-A | vascular endothelial growth factor A |
| VO2max | maximal oxygen uptake |
| 6MWT | 6-Minute Walking Test |
References
- Global Burden of Disease 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhang, W.; Kang, L.; Ma, Y.; Fu, L.; Jia, L.; Yu, H.; Chen, X.; Hou, L.; Wang, L.; et al. Clinical Evidence of Exercise Benefits for Stroke. Adv. Exp. Med. Biol. 2017, 1000, 131–151. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Chin, S.L.; Rangarajan, S.; Xavier, D.; Liu, L.; Zhang, H.; Rao-Melacini, P.; Zhang, X.; Pais, P.; Agapay, S.; et al. INTERSTROKE investigators. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case-control study. Lancet 2016, 388, 761–775. [Google Scholar] [PubMed]
- Nicholson, S.; Sniehotta, F.F.; van Wijck, F.; Greig, C.A.; Johnston, M.; McMurdo, M.E.; Dennis, M.; Mead, G.E. A systematic review of perceived barriers and motivators to physical activity after stroke. Int. J. Stroke 2013, 8, 357–364. [Google Scholar] [CrossRef]
- King, M.; Kelly, L.P.; Wallack, E.M.; Hasan, S.M.M.; Kirkland, M.C.; Curtis, M.E.; Chatterjee, T.; McCarthy, J.; Ploughman, M. Serum levels of insulin-like growth factor-1 and brain-derived neurotrophic factor as potential recovery biomarkers in stroke. Neurol. Res. 2019, 41, 354–363. [Google Scholar] [CrossRef]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef]
- Vanzella, C.; Neves, J.D.; Vizuete, A.F.; Aristimunha, D.; Kolling, J.; Longoni, A.; Gonçalves, C.A.S.; Wyse, A.T.S.; Netto, C.A. Treadmill running prevents age-related memory deficit and alters neurotrophic factors and oxidative damage in the hippocampus of Wistar rats. Behav. Brain Res. 2017, 334, 78–85. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Queme, L.F.; Weyler, A.A.; Cohen, E.R.; Hudgins, R.C.; Jankowski, M.P. A dual role for peripheral GDNF signaling in nociception and cardiovascular reflexes in the mouse. Proc. Natl. Acad. Sci. USA 2020, 117, 698–707. [Google Scholar] [CrossRef]
- Huberman, M.A.; d’Adesky, N.D.; Niazi, Q.B.; Perez-Pinzon, M.A.; Bramlett, H.M.; Raval, A.P. Irisin-associated neuroprotective and rehabilitative strategies for stroke. Neuromolecular. Med. 2022, 24, 62–73. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.I.; Oh, Y.; Kim, J.H.; et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol. Endocrinol. 2015, 29, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, A.; Nemet, D. Exercise training, physical fitness and the growth hormone-insulin-like growth factor-1 Axis and cytokine balance. Med. Sport Sci. 2010, 55, 128–140. [Google Scholar] [CrossRef]
- Kim, S.W.; Lajara, R.; Rotwein, P. Structure and function of a human insulin-like growth factor-I gene promoter. Mol. Endocrinol. 1991, 5, 1964–1972. [Google Scholar] [CrossRef]
- Åberg, N.D.; Gadd, G.; Åberg, D.; Hällgren, P.; Blomstrand, C.; Jood, K.; Nilsson, M.; Walker, F.R.; Svensson, J.; Jern, C.; et al. Relationship between Levels of Pre-Stroke Physical Activity and Post-Stroke Serum Insulin-Like Growth Factor I. Biomedicines 2020, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Mattlage, A.E.; Rippee, M.A.; Sandt, J.; Billinger, S.A. Decrease in insulin-like growth factor-1 and insulin-like growth factor-1 ratio in the first week of stroke is related to positive outcomes. J. Stroke Cereb. Dis. 2016, 25, 1800–1806. [Google Scholar] [CrossRef]
- Nindl, B.C.; Santtila, M.; Vaara, J.; Hakkinen, K.; Kyrolainen, H. Circulating IGF-I is associated with fitness and health outcomes in a population of 846 young healthy men. Growth Horm. IGF Res. 2011, 21, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Åberg, N.D.; Brywe, K.G.; Isgaard, J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Sci. World J. 2006, 6, 53–80. [Google Scholar] [CrossRef]
- Ruan, L.; Wang, B.; ZhuGe, Q.; Jin, K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015, 1623, 166–173. [Google Scholar] [CrossRef]
- Wang, Y.; Kilic, E.; Kilic, U.; Weber, B.; Bassetti, C.L.; Marti, H.H.; Hermann, D.M. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain 2005, 128 Pt 1, 52–63. [Google Scholar] [CrossRef]
- Geng, H.; Li, M.; Tang, J.; Lv, Q.; Li, R.; Wang, L. Early rehabilitation exercise after stroke improves neurological recovery through enhancing angiogenesis in patients and cerebral ischemia rat model. Int. J. Mol. Sci. 2022, 23, 10508. [Google Scholar] [CrossRef]
- Geiseler, S.J.; Morland, C. The Janus face of VEGF in stroke. Int. J. Mol. Sci. 2018, 19, 1362. [Google Scholar] [CrossRef]
- Reed, M.D.; Yim, Y.S.; Wimmer, R.D.; Kim, H.; Ryu, C.; Welch, G.M.; Andina, M.; King, H.O.; Waisman, A.; Halassa, M.M. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature 2020, 577, 249–253. [Google Scholar] [CrossRef]
- Luo, W.; Liu, T.; Li, S.; Wen, H.; Zhou, F.; Zafonte, R.; Luo, X.; Xu, M.; Black-Schaffer, R.; Wood, L.J.; et al. The Serum BDNF Level Offers Minimum Predictive Value for Motor Function Recovery After Stroke. Transl. Stroke Res. 2019, 10, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Billinger, S.A.; Arena, R.; Bernhardt, J.; Eng, J.J.; Franklin, B.A.; Johnson, C.M.; MacKay-Lyons, M.; Macko, R.F.; Mead, G.E.; Roth, E.J.; et al. Physical activity and exercise recommendations for stroke survivors: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 2532–2553. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.A.; Hallsworth, K.; Plötz, T.; Ford, G.A.; Rochester, L.; Trenell, M.I. Physical activity, sedentary behaviour and metabolic control following stroke: A cross-sectional and longitudinal study. PLoS ONE 2013, 8, e55263. [Google Scholar] [CrossRef]
- Luzum, G.; Gunnes, M.; Lydersen, S.; Saltvedt, I.; Tan, X.; Thingstad, P.; Thrane, G.; Askim, T. Physical Activity Behavior and Its Association With Global Cognitive Function Three Months After Stroke: The Nor-COAST Study. Phys. Ther. 2023, 103, pzad092. [Google Scholar] [CrossRef] [PubMed]
- Fini, N.A.; Holland, A.E.; Keating, J.; Simek, J.; Bernhardt, J. How physically active are people following stroke? Systematic review and quantitative synthesis. Phys. Ther. 2017, 97, 707–717. [Google Scholar] [CrossRef]
- Thilarajah, S.; Mentiplay, B.F.; Bower, K.J.; Tan, D.; Pua, Y.H.; Williams, G.; Koh, G.; Clark, R.A. Factors Associated With Post-Stroke Physical Activity: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 1876–1889. [Google Scholar] [CrossRef]
- Kobayashi, S.; Hasegawa, S.; Yamazaki, S.; Tsugane, T.; Takahashi, S.; Kaneko, M.; Usuda, S. Changes in physical activity level during hospitalization in patients with stroke and those with fracture: A prospective longitudinal study. J. Phys. Ther. Sci. 2024, 36, 33–43. [Google Scholar] [CrossRef]
- Barrett, M.; Snow, J.C.; Kirkland, M.C.; Kelly, L.P.; Gehue, M.; Downer, M.B.; McCarthy, J.; Ploughman, M. Excessive sedentary time during in-patient stroke rehabilitation. Top Stroke Rehabil. 2018, 25, 366–374. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Crozier, J.; Roig, M.; Eng, J.J.; MacKay-Lyons, M.; Fung, J.; Ploughman, M.; Bailey, D.M.; Sweet, S.N.; Giacomantonio, N.; Thiel, A.; et al. High-Intensity Interval Training After Stroke: An Opportunity to Promote Functional Recovery, Cardiovascular Health, and Neuroplasticity. Neurorehabil. Neural. Repair 2018, 32, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Gjellesvik, T.I.; Becker, F.; Tjønna, A.E.; Indredavik, B.; Lundgaard, E.; Solbakken, H.; Brurok, B.; Tørhaug, T.; Lydersen, S.; Askim, T. Effects of High-Intensity Interval Training After Stroke (The HIIT Stroke Study) on Physical and Cognitive Function: A Multicenter Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2021, 102, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, Y.; Zhang, L.; Fan, W.; Liu, Z.; Yong, M.; Wu, L. Comparative efficacy of different exercise methods to improve cardiopulmonary function in stroke patients: A network meta-analysis of randomized controlled trials. Front. Neurol. 2024, 15, 1288032. [Google Scholar] [CrossRef] [PubMed]
- Steen Krawcyk, R.; Vinther, A.; Petersen, N.C.; Faber, J.; Iversen, H.K.; Christensen, T.; Lambertsen, K.L.; Rehman, S.; Klausen, T.W.; Rostrup, E.; et al. Effect of Home-Based High-Intensity Interval Training in Patients With Lacunar Stroke: A Randomized Controlled Trial. Front. Neurol. 2019, 10, 664. [Google Scholar] [CrossRef]
- Kolmos, M.; Krawcyk, R.S.; Kruuse, C. Effect of high-intensity training on endothelial function in patients with cardiovascular and cerebrovascular disease: A systematic review. SAGE Open Med. 2016, 4, 2050312116682253. [Google Scholar] [CrossRef]
- Hussain, S.R.; Macaluso, A.; Pearson, S.J. High-intensity interval training versus moderate-intensity continuous training in the prevention/management of cardiovascular disease. Cardiol. Rev. 2016, 24, 273–281. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Yang, Y.L.; Li, Y.Q.; Chang, T.; Man, M.H.; Zhang, X.Y.; Guo, S.C.; Li, L.H. Low Serum Levels of Brain-Derived Neurotrophic Factor Were Associated with Poor Short-Term Functional Outcome and Mortality in Acute Ischemic Stroke. Mol. Neurobiol. 2017, 54, 7335–7342. [Google Scholar] [CrossRef]
- Stanne, T.M.; Åberg, N.D.; Nilsson, S.; Jood, K.; Blomstrand, C.; Andreasson, U.; Blennow, K.; Zetterberg, H.; Isgaard, J.; Svensson, J.; et al. Low Circulating Acute Brain-Derived Neurotrophic Factor Levels Are Associated With Poor Long-Term Functional Outcome After Ischemic Stroke. Stroke 2016, 47, 1943–1945. [Google Scholar] [CrossRef]
- Haavik, H.; Niazi, I.K.; Amjad, I.; Kumari, N.; Rashid, U.; Duehr, J.; Navid, M.S.; Trager, R.J.; Shafique, M.; Holt, K. The Effects of Four Weeks of Chiropractic Spinal Adjustments on Blood Biomarkers in Adults with Chronic Stroke: Secondary Outcomes of a Randomized Controlled. Trial. J. Clin. Med. 2022, 11, 7493. [Google Scholar] [CrossRef]
- Lukkahatai, N.; Ong, I.L.; Benjasirisan, C.; Saligan, L.N. Brain-Derived Neurotrophic Factor (BDNF) as a Marker of Physical Exercise or Activity Effectiveness in Fatigue, Pain, Depression, and Sleep Disturbances: A Scoping Review. Biomedicines 2025, 13, 332. [Google Scholar] [CrossRef]
- Tapia-Arancibia, L.; Rage, F.; Givalois, L.; Arancibia, S. Physiology of BDNF: Focus on hypothalamic function. Front Neuroendocrinol. 2004, 25, 77–107. [Google Scholar] [CrossRef]
- Brown, D.A.; Johnson, M.S.; Armstrong, C.J.; Lynch, J.M.; Caruso, N.M.; Ehlers, L.B.; Fleshner, M.; Spencer, R.L.; Moore, R.L. Short-term treadmill running in the rat: What kind of stressor is it? J. Appl. Physiol. 2007, 103, 1979–1985. [Google Scholar] [CrossRef]
- Alves, R.L.; Cardoso, B.R.L.; Ramos, I.P.R.; Oliveira, B.D.S.; Dos Santos, M.L.; de Miranda, A.S. Physical training improves exercise tolerance, cardiac function and promotes changes in neurotrophins levels in chagasic mice. Life Sci. 2019, 232, 116629. [Google Scholar] [CrossRef]
- Pedroso, V.S.P.; Vieira, É.L.M.; de Miranda, A.S.; Venna, V.R.; McCullough, L.D.; Teixeira, A.L. Early post-stroke depressive symptoms are associated with low peripheral levels of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) and glial cell-derived neurotrophic factor (GDNF). Curr. Neurovasc. Res. 2020, 17, 495–501. [Google Scholar] [CrossRef]
- Domaszewska, K.; Koper, M.; Wochna, K.; Czerniak, U.; Marciniak, K.; Wilski, M.; Bukowska, D. The Effects of Nordic Walking With Poles With an Integrated Resistance Shock Absorber on Cognitive Abilities and Cardiopulmonary Efficiency in Postmenopausal Women. Front. Aging Neurosci. 2020, 12, 586286. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Miu, L.; Yao, W.; Shi, Z. Effect of regular aerobic exercise on cognitive function, depression level and regulative role of neurotrophic factor: A prospective cohort study in the young and the middle-aged sample. Risk Manag. Healthc. Policy 2024, 17, 935–943. [Google Scholar] [CrossRef]
- Prodjohardjono, A.; Vidyanti, A.N.; Susianti, N.A.; Sudarmanta Sutarni, S.; Setyopranoto, I. Higher level of acute serum VEGF and larger infarct volume are more frequently associated with post-stroke cognitive impairment. PLoS ONE 2020, 15, e0239370. [Google Scholar] [CrossRef]
- Matsuo, R.; Ago, T.; Kamouchi, M.; Kuroda, J.; Kuwashiro, T.; Hata, J.; Sugimori, H.; Fukuda, K.; Gotoh, S.; Makihara, N.; et al. Clinical significance of plasma VEGF value in ischemic stroke—Research for biomarkers in ischemic stroke (REBIOS) study. BMC Neurol. 2013, 13, 32. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, H.; Yang, Y.M.; Wang, X.S. Exploration of the influence of early rehabilitation training on circulating endothelial progenitor cell mobilization in patients with acute ischemic stroke and its related mechanism under a lightweight artificial intelligence algorithm. Eur. Rev. Med. Pharmacol Sci. 2023, 27, 5338–5355. [Google Scholar] [CrossRef]
- Karakilic, A.; Yuksel, O.; Kizildag, S.; Hosgorler, F.; Topcugil, B.; Ilgin, R.; Gumus, H.; Guvendi, G.; Koc, B.; Kandis, S.; et al. Regular aerobic exercise increased VEGF levels in both soleus and gastrocnemius muscles correlated with hippocampal learning and VEGF levels. Acta Neurobiol. Exp. 2021, 81, 1–9. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, Y.; Xu, P.; Shen, X.; Fang, C.; Wu, X.; Qu, P.; Wu, T.; Wang, Q.M.; Luo, X.; et al. Mechanical digit sensory stimulation: A randomized control trial on neurological and motor recovery in acute stroke. Front. Neurosci. 2023, 17, 1134904. [Google Scholar] [CrossRef]
- Włodarczyk, L.; Cichoń, N.; Karbownik, M.S.; Saso, L.; Saluk, J.; Miller, E. Circulating serum VEGF, IGF-1 and MMP-9 and expression of their genes as potential prognostic markers of recovery in post-stroke rehabilitation-a prospective observational study. Brain Sci. 2023, 13, 846. [Google Scholar] [CrossRef]
- Ferreira, M.G.R.; Moro, C.H.C.; Franco, S.C. Cognitive performance after ischaemic stroke. Dement. Neuropsychol. 2015, 9, 165–175. [Google Scholar] [CrossRef]
- Viktorisson, A.; Andersson, E.M.; Lundström, E.; Sunnerhagen, K.S. Levels of physical activity before and after stroke in relation to early cognitive function. Sci. Rep. 2021, 11, 9078. [Google Scholar] [CrossRef]
- Barbay, M.; Taillia, H.; Nédélec-Ciceri, C.; Bompaire, F.; Bonnin, C.; Varvat, J.; Grangette, F.; Diouf, M.; Wiener, E.; Mas, J.L.; et al. Prevalence of Poststroke Neurocognitive Disorders Using National Institute of Neurological Disorders and Stroke-Canadian Stroke Network, VASCOG Criteria (Vascular Behavioral and Cognitive Disorders), and Optimized Criteria of Cognitive Deficit. Stroke 2018, 49, 1141–1147. [Google Scholar] [CrossRef]
- Parekh, N.; Roberts, C.B.; Vadiveloo, M.; Puvananayagam, T.; Albu, J.B.; Lu-Yao, G.L. Lifestyle, anthropometric, and obesity-related physiologic determinants of insulin-like growth factor-1 in the Third National Health and Nutrition Examination Survey (1988–1994). Ann. Epidemiol. 2010, 20, 182–193. [Google Scholar] [CrossRef]
- Åberg, D.; Jood, K.; Blomstrand, C.; Jern, C.; Nilsson, M.; Isgaard, J.; Aberg, N.D. Serum IGF-I levels correlate to improvement of functional outcome after ischemic stroke. J. Clin. Endocrinol. Metab. 2011, 96, E1055-64. [Google Scholar] [CrossRef]
- Reinholdsson, M.; Palstam, A.; Sunnerhagen, K.S. Prestroke physical activity could influence acute stroke severity (part of PAPSIGOT). Neurology 2018, 91, e1461–e1467. [Google Scholar] [CrossRef]
- Ursin, M.H.; Ihle-Hansen, H.; Fure, B.; Tveit, A.; Bergland, A. Effects of premorbid physical activity on stroke severity and post-stroke functioning. J. Rehabil. Med. 2015, 47, 612–617. [Google Scholar] [CrossRef]
- Åberg, N.D.; Åberg, D.; Jood, K.; Nilsson, M.; Blomstrand, C.; Kuhn, H.G.; Svensson, J.; Jern, C.; Isgaard, J. Altered levels of circulating insulin-like growth factor I (IGF-I) following ischemic stroke are associated with outcome—A prospective observational study. BMC Neurol. 2018, 18, 106. [Google Scholar] [CrossRef]
- Wu, H.; Guo, P.; Jin, Z.; Li, X.; Yang, X.; Tang, C.; Wang, Y.; Ke, J. Serum levels of irisin predict short-term outcomes in ischemic stroke. Cytokine 2019, 122, 154303. [Google Scholar] [CrossRef]
- Tu, W.J.; Qiu, H.C.; Cao, J.L.; Liu, Q.; Zeng, X.W.; Zhao, J.Z. Decreased concentration of irisin is associated with poor functional outcome in ischemic stroke. Neurotherapeutics 2018, 15, 1158–1167. [Google Scholar] [CrossRef]
- Kang, D.; Park, J.; Eun, S.D. The efficacy of community-based exercise programs on circulating irisin level, muscle strength, cardiorespiratory endurance, and body composition for ischemic stroke: A randomized controlled trial. Front. Neurol. 2023, 14, 1187666. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, X.; Liang, J.; Kirberger, M.; Chen, N. Irisin, an exercise-induced bioactive peptide beneficial for health promotion during aging process. Ageing Res. Rev. 2022, 80, 101680. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Xue, C.; Li, Y.; Lu, T. Effects of exercise dose based on the ACSM recommendations on patients with post-stroke cognitive impairment: A systematic review and meta-analyses. Front. Physiol. 2024, 15, 1364632. [Google Scholar] [CrossRef]
- Foster, C.; Porcari, J.P.; Anderson, J.; Paulson, M.; Smaczny, D.; Webber, H.; Doberstein, S.T.; Udermann, B. The talk test as a marker of exercise training intensity. J. Cardiopulm. Rehabil. Prev. 2008, 28, 24–30, quiz 31-2. [Google Scholar] [CrossRef]
- Steen Krawcyk, R.; Vinther, A.; Caesar Petersen, N.; Kruuse, C. Graded cycling test with talk test” is a reliable test to monitor cardiovascular fitness in patients with minor stroke. J. Stroke Cerebrovasc. Dis. 2017, 26, 494–499. [Google Scholar] [CrossRef]
- Åstrand, P.O.; Ryhming, I.A. Nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during submaximal work. J. Appl. Physiol. 1954, 7, 218–221. [Google Scholar] [CrossRef]
- Gillett, P.A. Senior Women’s Fitness Project. J. Women Aging 1993, 5, 49–66. [Google Scholar] [CrossRef]
- von Dôbeln, W.; Astrand, I.; Bergström, A. An analysis of age and other factors related to maximal oxygen uptake. J. Appl. Physiol. 1967, 22, 934–938. [Google Scholar] [CrossRef]
- Flansbjer, U.B.; Holmbäck, A.M.; Downham, D.; Patten, C.; Lexell, J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005, 37, 75–82. [Google Scholar] [CrossRef]
- Kurtaiş, Y.; Küçükdeveci, A.; Elhan, A.; Yilmaz, A.; Kalli, T.; Tur, B.S.; Tennant, A. Psychometric properties of the Rivermead Motor Assessment: Its utility in stroke. J. Rehabil. Med. 2009, 41, 1055–1061. [Google Scholar] [CrossRef]
- Sullivan, J.E.; Crowner, B.E.; Kluding, P.M.; Nichols, D.; Rose, D.K.; Yoshida, R.; Pinto Zipp, G. Outcome measures for individuals with stroke: Process and recommendations from the American Physical Therapy Association neurology section task force. Phys. Ther. 2013, 93, 1383–1396. [Google Scholar] [CrossRef]
- Duffy, L.; Gajree, S.; Langhorne, P.; Stott, D.J.; Quinn, T.J. Reliability (inter-rater agreement) of the Barthel index for assessment of stroke survivors. Stroke 2013, 44, 462–468. [Google Scholar] [CrossRef]
- Hsieh, S.; Schubert, S.; Hoon, C.; Mioshi, E.; Hodges, J.R. Validation of the Addenbrooke’s Cognitive Examination III in frontotemporal dementia and Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2013, 36, 242–250. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Ilkowska, Z.; Kropinska, S.; Tobis, S.; Krzyminska-Siemaszko, R.; Kaluzniak-Szymanowska, A.; Wieczorowska-Tobis, K. Applying ACE-III, M-ACE and MMSE to Diagnostic Screening Assessment of Cognitive Functions within the Polish Population. Int. J. Environ. Res. Public Health 2022, 19, 12257. [Google Scholar] [CrossRef]
| Characteristic | Group | |
|---|---|---|
| Low-Intensity Continuous Training | Moderate-Intensity Continuous Training | |
| n = 22 | n = 22 | |
| Age (years), mean (SD) | 68.48 (5.51) | 63.68 (9.38) |
| Gender, male (%) | 11 (50.0) | 10 (45.5) |
| Post-stroke period in weeks, mean (SD) | 2.48 (1.21) | 3.45 (1.63) |
| Hemispheric localisation of stroke, right hemisphere (%) | 9 (40.9) | 11 (50.0) |
| Height (cm), mean (SD) | 166.31 (9.32) | 168.45 (9.14) |
| Body weight (kg), mean (SD) | 79.99 (17.74) | 76.82 (20.38) |
| Body mass index, mean (SD) | 28.86 (5.51) | 26.89 (5.90) |
| Blood pressure | ||
| Systolic (mmHg), mean (SD) | 131.22 (18.41) | 127.73 (16.82) |
| Diastolic (mmHg), mean (SD) | 75.18 (11.41) | 78.18 (8.68) |
| Risk factors, n (%) | ||
| Obesity, yes (%) | 10 (45.5) | 8 (36.4) |
| Hypertension, yes (%) | 20 (90.9) | 17 (77.3) |
| Alcoholism, yes (%) | 2 (9.1) | 4 (18.2) |
| Diabetes, yes (%) | 12 (54.5) | 9 (40.9) |
| Tobacco smoking, yes (%) | 6 (27.3) | 9 (40.9) |
| Epilepsy, yes (%) | 2 (9.1) | 2 (9.1) |
| Dyslipidaemia, yes (%) | 10 (45.5) | 4 (18.2) |
| Medication, n (%) | ||
| Antihypertensive/diuretic, yes (%) | 20 (90.9) | 19 (86.4) |
| Lipid-lowering, yes (%) | 19 (86.4) | 20 (90.9) |
| Antiplatelet/anticoagulant, yes (%) | 21 (95.5) | 21 (95.5) |
| Antidepressant, yes (%) | 6 (27.3) | 11 (50.0) |
| Antianxiety/sedative, yes (%) | 2 (9.1) | 5 (22.7) |
| Diabetic, yes (%) | 8 (36.4) | 3 (13.6) |
| Antipsychotic, yes (%) | 3 (13.6) | 2 (9.1) |
| Analgesic, no (%) | 22 (100.0) | 22 (100.0) |
| Insomnia, yes (%) | 2 (9.1) | 2 (9.1) |
| Outcome | Groups | Within-Group Differences | Between-Group Differences | ||||
|---|---|---|---|---|---|---|---|
| Baseline | After 3 Weeks | 3 Weeks Minus Baseline | 3 Weeks Minus Baseline | ||||
| MICT | LICT | MICT | LICT | MICT | LICT | MICT Minus LICT | |
| n = 22 | n = 22 | n = 22 | n = 22 | n = 22 | n = 22 | ||
| Total ACE-III (points) | 63 (45 to 81) | 65 (46 to 84) | 73 (54 to 92) | 66 (44 to 89) | 4 (0.4 to 7.6) | 0.5 (−1.1 to 2.1) | 6.5 (−5.1 to 18.1) |
| ACE-III A (points) | 13 (11 to 15) | 12 (8 to 15) | 15 (12 to 18) | 12 (8 to 16) | 2 (1 to 3) | 0 (−0.5 to 0.5) | 3.5 (1.5 to 5.5) |
| ACE-III M (points) | 14 (6 to 21) | 12 (8 to 16) | 16 (9 to 24) | 15 (11 to 19) | 1.5 (0 to 3) | 0 (0 to 0) | 1.5 (−2 to 5) |
| ACE-III F (points) | 6 (2 to 10) | 5 (2 to 8) | 7 (4 to 10) | 5 (2 to 8) | 0 (−0.5 to 0.5) | 0 (0 to 0) | 2 (0.3 to 3.7) |
| ACE-III L (points) | 23 (17 to 29) | 23 (16 to 30) | 26 (19 to 32) | 24 (17 to 30) | 0 (−0.5 to 0.5) | 0 (0 to 0) | 2 (−1.6 to 5.6) |
| ACE-III V (points) | 13 (10 to 16) | 11 (8 to 14) | 14 (11 to 17) | 12 (8 to 14) | 0 (−0.5 to 0.5) | 0 (0 to 0) | 2.5 (0.7 to 4.3) |
| M-ACE (points) | 15 (7 to 23) | 16 (10 to 23) | 18 (11 to 25) | 16 (10 to 23) | 1 (−0.5 to 2.5) | 0 (−0.5 to 0.5) | 1.5 (−2.1 to 5.1) |
| 6MWT (m) | 241 (106 to 377) | 148 (85 to 210) | 316 (190 to 442) | 152 (63 to 242) | 31.2 (−6.1 to 68.5) | 10 (−4.5 to 24.5) | 163.8 (100 to 227.5) |
| RMA total (points) | 28 (22 to 35) | 26 (16 to 36) | 36 (33 to 38) | 34 (28 to 40) | 6 (3 to 9) | 5 (2 to 8) | 1.5 (−1.8 to 4.8) |
| RMA-gf (points) | 8 (5 to 11) | 7 (4 to 10) | 11 (10 to 12) | 11 (9 to 13) | 2 (0 to 4) | 2.5 (1 to 4) | 0 (−1.1 to 1.1) |
| RMA-lt (points) | 10 (9 to 11) | 8 (6 to 11) | 10 (10 to 10) | 10 (9 to 11) | 0 (−1 to 1) | 1 (0 to 2) | 0 (−0.5 to 0.5) |
| RMA-a (points) | 12 (9 to 15) | 10 (7 to 14) | 15 (14 to 16) | 13 (9 to 17) | 2 (1 to 3) | 2 (1 to 3) | 2 (0 to 4) |
| BI (points) | 72 (50 to 95) | 55 (32 to 78) | 100 (100 to 100) | 90 (75 to 105) | 22.5 (4.6 to 40.4) | 25 (11.9 to 38.1) | 10 (−0.1 to 20.1) |
| VO2max (mL/min/kg) | 25 (19 to 32) | 20 (15 to 24) | 29 (21 to 36) | 22 (17 to 27) | 1.1 (−0.8 to 3) | 0.9 (−0.7 to 2.5) | 6.8 (3.7 to 9.9) |
| Outcome | Groups | Within-Group Differences | Between-Group Differences | ||||
|---|---|---|---|---|---|---|---|
| Baseline | After 3 Weeks | 3 Weeks Minus Baseline | 3 Weeks Minus Baseline | ||||
| MICT | LICT | MICT | LICT | MICT | LICT | MICT Minus LICT | |
| n = 22 | n = 22 | n = 22 | n = 22 | n = 22 | n = 22 | ||
| BDNF (ng/mL) | 9 (7 to 10) | 9 (7 to 11) | 8 (6 to 10) | 10 (8 to 12) | 0.6 (−1.5 to 2.7) | 1.8 (−1 to 4.6) | −2.2 (−3.7 to −0.7) |
| GDNF (ng/mL) | 2 (1 to 2) | 1 (1 to 2) | 1 (1 to 2) | 1 (1 to 2) | −0.2 (−0.5 to 0.1) | 0 (−0.5 to 0.5) | 0 (−0.3 to 0.4) |
| IGF-1 (ng/mL) | 86 (67 to 104) | 85 (53 to 118) | 96 (58 to 135) | 94 (55 to 133) | −0.2 (−39.5 to 39.1) | −4.1 (−31.9 to 23.7) | 2.8 (−19.1 to 24.6) |
| Irisin (ng/mL) | 509 (293 to 724) | 386 (243 to 529) | 478 (377 to 578) | 437 (307 to 567) | −18.2 (−258.3 to 221.9) | 30.5 (−148.9 to 209.9) | 40.7 (−62.6 to 143.9) |
| VEGF-A (ng/mL) | 16 (12 to 21) | 18 (13 to 23) | 24 (19 to 28) | 18 (11 to 25) | 6.9 (−0.4 to 14.2) | −1.7 (−9.7 to 6.3) | 6.1 (−5 to 17.2) |
| Variables | BDNF (ng/mL) | GDNF (ng/mL) | IGF-1 (ng/mL) | Irisin (ng/mL) | VEGF-A (ng/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson’s r | 95% Upper CI | 95% Lower CI | Pearson’s r | 95% Upper CI | 95% Lower CI | Pearson’s r | 95% Upper CI | 95% Lower CI | Pearson’s r | 95% Upper CI | 95% Lower CI | Pearson’s r | 95% Upper CI | 95% Lower CI | |
| Age (years) | −0.121 | 0.183 | −0.403 | −0.080 | 0.222 | −0.369 | −0.016 | 0.222 | −0.369 | −0.316 | −0.021 | −0.561 | 0.113 | 0.397 | −0.190 |
| Total ACE-III (points) | −0.029 | 0.270 | −0.324 | −0.050 | 0.250 | −0.342 | −0.018 | 0.250 | −0.342 | 0.073 | 0.362 | −0.228 | 0.116 | 0.399 | −0.187 |
| ACE-III A (points) | −0.006 | 0.291 | −0.303 | −0.140 | 0.164 | −0.419 | 0.005 | 0.164 | −0.419 | −0.040 | 0.260 | −0.333 | 0.157 | 0.434 | −0.147 |
| ACE-III M (points) | −0.003 | 0.294 | −0.300 | −0.037 | 0.263 | −0.330 | −0.023 | 0.263 | −0.330 | 0.132 | 0.413 | −0.172 | −0.007 | 0.291 | −0.303 |
| ACE-III F (points) | −0.124 | 0.180 | −0.406 | −0.052 | 0.248 | −0.344 | −0.069 | 0.248 | −0.344 | 0.038 | 0.331 | −0.262 | 0.006 | 0.302 | −0.292 |
| ACE-III L (points) | −0.046 | 0.255 | −0.338 | −0.017 | 0.282 | −0.312 | −0.046 | 0.282 | −0.312 | 0.159 | 0.435 | −0.145 | 0.165 | 0.440 | −0.139 |
| ACE-III V (points) | 0.029 | 0.323 | −0.270 | 0.004 | 0.300 | −0.294 | 0.061 | 0.300 | −0.294 | −0.073 | 0.229 | −0.362 | 0.191 | 0.461 | −0.113 |
| M-ACE (points) | −0.073 | 0.229 | −0.362 | −0.105 | 0.198 | −0.390 | 0.007 | 0.198 | −0.390 | 0.103 | 0.388 | −0.200 | 0.029 | 0.323 | −0.270 |
| 6MWT (m) | 0.010 | 0.306 | −0.288 | −0.062 | 0.239 | −0.353 | 0.161 | 0.239 | −0.353 | 0.221 | 0.486 | −0.081 | −0.030 | 0.269 | −0.324 |
| RMA (points) | 0.046 | 0.338 | −0.254 | 0.025 | 0.319 | −0.274 | 0.090 | 0.319 | −0.274 | 0.045 | 0.338 | −0.255 | −0.072 | 0.230 | −0.361 |
| RMA-gf (points) | 0.099 | 0.384 | −0.204 | −0.043 | 0.257 | −0.336 | 0.111 | 0.257 | −0.336 | 0.022 | 0.317 | −0.277 | −0.036 | 0.264 | −0.329 |
| RMA-lt (points) | 0.041 | 0.334 | −0.259 | 0.014 | 0.310 | −0.284 | 0.091 | 0.310 | −0.284 | 0.085 | 0.372 | −0.218 | 0.078 | 0.367 | −0.224 |
| RMA-a (points) | −0.027 | 0.272 | −0.321 | 0.055 | 0.346 | −0.246 | 0.074 | 0.346 | −0.246 | 0.033 | 0.327 | −0.266 | −0.153 | 0.151 | −0.430 |
| BI (points) | 0.205 | 0.473 | −0.097 | −0.156 | 0.148 | −0.432 | 0.065 | 0.148 | −0.432 | −0.134 | 0.169 | −0.415 | 0.171 | 0.445 | −0.133 |
| Variables | BDNF (ng/mL) | GDNF (ng/mL) | IGF-1 (ng/mL) | Irisin (ng/mL) | VEGF-A (ng/mL) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson’s r | 95% Upper CI | 95% Lower CI | Pearson’s r | 95% Upper CI | 95% Lower CI | Pearson’s r | 95% Upper CI | 95% Lower CI | Pearson’s r | 95% Upper CI | 95% Lower CI | Pearson’s r | 95% Upper CI | 95% Lower CI | |
| Age (years) | −0.08 | −0.37 | 0.22 | −0.10 | −0.38 | 0.20 | −0.29 | −0.55 | 0.02 | 0.06 | −0.24 | 0.35 | −0.22 | −0.49 | 0.08 |
| Total ACE-III (points) | 0.04 | −0.26 | 0.33 | 0.07 | −0.23 | 0.36 | −0.23 | −0.50 | 0.08 | −0.03 | −0.33 | 0.27 | −0.15 | −0.43 | 0.15 |
| ACE-III A (points) | −0.05 | −0.34 | 0.26 | −0.05 | −0.34 | 0.25 | −0.17 | −0.45 | 0.14 | 0.03 | −0.27 | 0.32 | −0.20 | −0.47 | 0.11 |
| ACE-III M (points) | 0.09 | −0.21 | 0.38 | 0.04 | −0.26 | 0.34 | −0.26 | −0.53 | 0.05 | −0.05 | −0.35 | 0.25 | −0.15 | −0.43 | 0.15 |
| ACE-III F (points) | −0.02 | −0.32 | 0.28 | 0.03 | −0.27 | 0.32 | −0.20 | −0.48 | 0.11 | 0.02 | −0.28 | 0.31 | −0.13 | −0.41 | 0.18 |
| ACE-III L (points) | 0.05 | −0.25 | 0.35 | 0.16 | −0.15 | 0.43 | −0.26 | −0.53 | 0.05 | −0.09 | −0.38 | 0.21 | −0.15 | −0.43 | 0.15 |
| ACE-III V (points) | 0.04 | −0.26 | 0.34 | 0.08 | −0.23 | 0.37 | −0.06 | −0.36 | 0.25 | 0.06 | −0.24 | 0.35 | −0.06 | −0.35 | 0.24 |
| M-ACE (points) | 0.04 | −0.26 | 0.34 | −0.03 | −0.32 | 0.27 | −0.28 | −0.54 | 0.03 | −0.03 | −0.33 | 0.27 | −0.16 | −0.44 | 0.14 |
| 6MWT (m) | 0.18 | −0.13 | 0.45 | −0.16 | −0.43 | 0.15 | 0.26 | −0.05 | 0.53 | 0.08 | −0.22 | 0.37 | 0.22 | −0.08 | 0.49 |
| RMA (points) | 0.15 | −0.16 | 0.42 | −0.20 | −0.47 | 0.10 | 0.15 | −0.16 | 0.44 | −0.10 | −0.38 | 0.21 | −0.06 | −0.35 | 0.24 |
| RMA-gf (points) | 0.13 | −0.18 | 0.41 | −0.29 | −0.54 | 0.01 | 0.17 | −0.14 | 0.46 | −0.09 | −0.38 | 0.21 | 0.02 | −0.27 | 0.32 |
| RMA-lt (points) | 0.06 | −0.24 | 0.35 | −0.23 | −0.49 | 0.07 | 0.09 | −0.23 | 0.38 | −0.11 | −0.39 | 0.20 | −0.07 | −0.36 | 0.23 |
| RMA-a (points) | 0.16 | −0.14 | 0.44 | −0.12 | −0.40 | 0.19 | 0.13 | −0.18 | 0.42 | −0.08 | −0.37 | 0.22 | −0.10 | −0.38 | 0.20 |
| BI (points) | 0.16 | −0.15 | 0.43 | −0.12 | −0.40 | 0.18 | 0.19 | −0.13 | 0.47 | 0.07 | −0.23 | 0.36 | 0.13 | −0.17 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górna, S.; Podgórski, T.; Kleka, P.; Domaszewska, K. Effects of Different Intensities of Endurance Training on Neurotrophin Levels and Functional and Cognitive Outcomes in Post-Ischaemic Stroke Adults: A Randomised Clinical Trial. Int. J. Mol. Sci. 2025, 26, 2810. https://doi.org/10.3390/ijms26062810
Górna S, Podgórski T, Kleka P, Domaszewska K. Effects of Different Intensities of Endurance Training on Neurotrophin Levels and Functional and Cognitive Outcomes in Post-Ischaemic Stroke Adults: A Randomised Clinical Trial. International Journal of Molecular Sciences. 2025; 26(6):2810. https://doi.org/10.3390/ijms26062810
Chicago/Turabian StyleGórna, Sara, Tomasz Podgórski, Paweł Kleka, and Katarzyna Domaszewska. 2025. "Effects of Different Intensities of Endurance Training on Neurotrophin Levels and Functional and Cognitive Outcomes in Post-Ischaemic Stroke Adults: A Randomised Clinical Trial" International Journal of Molecular Sciences 26, no. 6: 2810. https://doi.org/10.3390/ijms26062810
APA StyleGórna, S., Podgórski, T., Kleka, P., & Domaszewska, K. (2025). Effects of Different Intensities of Endurance Training on Neurotrophin Levels and Functional and Cognitive Outcomes in Post-Ischaemic Stroke Adults: A Randomised Clinical Trial. International Journal of Molecular Sciences, 26(6), 2810. https://doi.org/10.3390/ijms26062810






