The Relationship between the Brain-Derived Neurotrophic Factor Gene Polymorphism (Val66Met) and Substance Use Disorder and Relapse
Abstract
1. Introduction
2. Results
2.1. Patients in Addiction Treatment for the First Time vs. the Control Group
2.2. Patients with Addiction Relapse vs. the Control Group
2.3. Patients in Addiction Treatment for the First Time vs. Patients with Addiction Relapse
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Psychometric Tests
4.3. Genotyping
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldstein, R.Z.; Volkow, N.D. Drug Addiction and Its Underlying Neurobiological Basis: Neuroimaging Evidence for the Involvement of the Frontal Cortex. Am. J. Psychiatry 2002, 159, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Wang, H.; d’Oleire Uquillas, F.; Wang, X.; Ding, J.; Chen, H. Definition of Substance and Non-Substance Addiction. Adv. Exp. Med. Biol. 2017, 1010, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Camí, J.; Farré, M. Drug Addiction. N. Engl. J. Med. 2003, 349, 975–986. [Google Scholar] [CrossRef] [PubMed]
- Dackis, C.A.; O’Brien, C.P. Cocaine Dependence: A Disease of the Brain’s Reward Centers. J. Subst. Abuse Treat. 2001, 21, 111–117. [Google Scholar] [CrossRef]
- Wagner, F.A.; Anthony, J.C. From First Drug Use to Drug Dependence; Developmental Periods of Risk for Dependence upon Marijuana, Cocaine, and Alcohol. Neuropsychopharmacology 2002, 26, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, C.A.; Nestler, E.J. Neurotrophic Mechanisms in Drug Addiction. Neuromol. Med. 2004, 5, 69–83. [Google Scholar] [CrossRef]
- Li, X.; Wolf, M.E. Multiple Faces of BDNF in Cocaine Addiction. Behav. Brain Res. 2015, 279, 240–254. [Google Scholar] [CrossRef]
- Fox, H.C.; Hong, K.I.A.; Siedlarz, K.; Sinha, R. Enhanced Sensitivity to Stress and Drug/Alcohol Craving in Abstinent Cocaine-Dependent Individuals Compared to Social Drinkers. Neuropsychopharmacology 2008, 33, 796–805. [Google Scholar] [CrossRef]
- McKee, S.A.; Sinha, R.; Weinberger, A.H.; Sofuoglu, M.; Harrison, E.L.; Lavery, M.; Wanzer, J. Stress Decreases the Ability to Resist Smoking and Potentiates Smoking Intensity and Reward. J. Psychopharmacol. 2011, 25, 490–502. [Google Scholar] [CrossRef]
- Sinha, R.; Fox, H.C.; Hong, K.I.A.; Hansen, J.; Tuit, K.; Kreek, M.J. Effects of Adrenal Sensitivity, Stress- and Cue-Induced Craving, and Anxiety on Subsequent Alcohol Relapse and Treatment Outcomes. Arch. Gen. Psychiatry 2011, 68, 942–952. [Google Scholar] [CrossRef]
- Wrase, J.; Makris, N.; Braus, D.F.; Mann, K.; Smolka, M.N.; Kennedy, D.N.; Caviness, V.S.; Hodge, S.M.; Tang, L.; Albaugh, M.; et al. Amygdala Volume Associated with Alcohol Abuse Relapse and Craving. Am. J. Psychiatry 2008, 165, 1179–1184. [Google Scholar] [CrossRef]
- Greenfield, S.F.; Weiss, R.D.; Muenz, L.R.; Vagge, L.M.; Kelly, J.F.; Bello, L.R.; Michael, J. The Effect of Depression on Return to Drinking: A Prospective Study. Arch. Gen. Psychiatry 1998, 55, 259–265. [Google Scholar] [CrossRef]
- Dodge, R.; Sindelar, J.; Sinha, R. The Role of Depression Symptoms in Predicting Drug Abstinence in Outpatient Substance Abuse Treatment. J. Subst. Abuse Treat. 2005, 28, 189–196. [Google Scholar] [CrossRef] [PubMed]
- D’Sa, C.; Fox, H.C.; Hong, A.K.; Dileone, R.J.; Sinha, R. Increased Serum Brain-Derived Neurotrophic Factor Is Predictive of Cocaine Relapse Outcomes: A Prospective Study. Biol. Psychiatry 2011, 70, 706–711. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF Val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Lang, U.E.; Hellweg, R.; Sander, T.; Gallinat, J. The Met Allele of the BDNF Val66Met Polymorphism Is Associated with Increased BDNF Serum Concentrations. Mol. Psychiatry 2009, 14, 120–122. [Google Scholar] [CrossRef] [PubMed]
- McCrae, R.R.; John, O.P. An Introduction to the Five-Factor Model and Its Applications. J. Pers. 1992, 60, 175–215. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Jing, D.; Bath, K.G.; Ieraci, A.; Khan, T.; Siao, C.J.; Herrera, D.G.; Toth, M.; Yang, C.; McEwen, B.S.; et al. Genetic Variant BDNF (Val66Met) Polymorphism Alters Anxiety-Related Behavior. Science 2006, 314, 140. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Patel, P.D.; Sant, G.; Meng, C.X.; Teng, K.K.; Hempstead, B.L.; Lee, F.S. Variant Brain-Derived Neurotrophic Factor (BDNF) (Met66) Alters the Intracellular Trafficking and Activity-Dependent Secretion of Wild-Type BDNF in Neurosecretory Cells and Cortical Neurons. J. Neurosci. 2004, 24, 4401–4411. [Google Scholar] [CrossRef]
- Bueller, J.A.; Aftab, M.; Sen, S.; Gomez-Hassan, D.; Burmeister, M.; Zubieta, J.K. BDNF Val66Met Allele Is Associated with Reduced Hippocampal Volume in Healthy Subjects. Biol. Psychiatry 2006, 59, 812–815. [Google Scholar] [CrossRef]
- Pezawas, L.; Verchinski, B.A.; Mattay, V.S.; Callicott, J.H.; Kolachana, B.S.; Straub, R.E.; Egan, M.F.; Meyer-Lindenberg, A.; Weinberger, D.R. The Brain-Derived Neurotrophic Factor Val66met Polymorphism and Variation in Human Cortical Morphology. J. Neurosci. 2004, 24, 10099–10102. [Google Scholar] [CrossRef] [PubMed]
- Enoch, M.A.; White, K.V.; Waheed, J.; Goldman, D. Neurophysiological and Genetic Distinctions between Pure and Comorbid Anxiety Disorders. Depress. Anxiety 2008, 25, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Shimizu, E.; Iyo, M. Critical Role of Brain-Derived Neurotrophic Factor in Mood Disorders. Brain Res. Rev. 2004, 45, 104–114. [Google Scholar] [CrossRef]
- Hwang, J.P.; Tsai, S.J.; Hong, C.J.; Yang, C.H.; Lirng, J.F.; Yang, Y.M. The Val66Met Polymorphism of the Brain-Derived Neurotrophic-Factor Gene Is Associated with Geriatric Depression. Neurobiol. Aging 2006, 27, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Iga, J.I.; Ueno, S.I.; Yamauchi, K.; Numata, S.; Tayoshi-Shibuya, S.; Kinouchi, S.; Nakataki, M.; Song, H.; Hokoishi, K.; Tanabe, H.; et al. The Val66Met Polymorphism of the Brain-Derived Neurotrophic Factor Gene Is Associated with Psychotic Feature and Suicidal Behavior in Japanese Major Depressive Patients. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Hong, C.J.; Yu, Y.W.Y.; Chen, T.J.; Wu, H.C.; Tsai, S.J. Brain-Derived Neurotrophic Factor (Val66Met) Genetic Polymorphism Is Associated with Substance Abuse in Males. Brain Res. Mol. Brain Res. 2005, 140, 86–90. [Google Scholar] [CrossRef]
- Jia, W.; Shi, J.G.; Wu, B.; Ao, L.; Zhang, R.; Zhu, Y.S. Polymorphisms of Brain-Derived Neurotrophic Factor Associated with Heroin Dependence. Neurosci. Lett. 2011, 495, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Lan, J.; Wang, Y.; Song, M.; Gao, X.; Ran, L.; Moira, S.; Wang, W. Influence of Brain-Derived Neurotrophic Factor Genetic Polymorphisms on the Ages of Onset for Heroin Dependence in a Chinese Population. Genet. Test. Mol. Biomark. 2012, 16, 1044–1050. [Google Scholar] [CrossRef]
- Su, H.; Tao, J.; Zhang, J.; Xie, Y.; Sun, Y.; Li, L.; Xu, K.; Han, B.; Lu, Y.; Sun, H.; et al. An Association between BDNF Val66Met Polymorphism and Impulsivity in Methamphetamine Abusers. Neurosci. Lett. 2014, 582, 16–20. [Google Scholar] [CrossRef]
- Hill, P.L.; Roberts, B.W. Personality and Health: Reviewing Recent Research and Setting a Directive for the Future. In Handbook of the Psychology of Aging, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 205–218. [Google Scholar] [CrossRef]
- Fiske, D.W. Consistency of the Factorial Structures of Personality Ratings from Different Sources. J. Abnorm. Soc. Psychol. 1949, 44, 329–344. [Google Scholar] [CrossRef]
- Smith, G.M. Usefulness of Peer Ratings of Personality in Educational Research. Educ. Psychol. Meas. 1967, 27, 967–984. [Google Scholar] [CrossRef]
- Norman, W.T. 2800 Personality Trait Descriptors—Normative Operating Characteristics for a University Population; University of Michigan: Ann Arbor, MI, USA, 1967. [Google Scholar]
- Goldberg, L.R. Language and Individual Differences: The Search for Universals in Personality Lexicons. Rev. Personal. Soc. Psychol. 1981, 2, 141–156. [Google Scholar]
- McCrae, R.R.; Costa, P.T. Validation of the Five-Factor Model of Personality Across Instruments and Observers. J. Pers. Soc. Psychol. 1987, 52, 81–90. [Google Scholar] [CrossRef] [PubMed]
- John, O.P.; Naumann, L.P.; Soto, C.J. Paradigm Shift to the Integrative Big Five Trait Taxonomy. In Handbook of Personality: Theory and Research; Guilford Press: New York, NY, USA, 2008; Volume 3, pp. 114–158. [Google Scholar]
- Yang, J.; McCrae, R.R.; Costa, P.T.; Dai, X.; Yao, S.; Cai, T.; Gao, B. Cross-Cultural Personality Assessment in Psychiatric Populations: The NEO-PI-R in the People’s Republic of China. Psychol. Assess. 1999, 11, 359–368. [Google Scholar] [CrossRef]
- Kang, W. Big Five Personality Traits Predict Illegal Drug Use in Young People. Acta Psychol. 2022, 231, 103794. [Google Scholar] [CrossRef]
- Kulkarni, P.; Parkar, S.; Kate, N.; Ninawe, K.; Limbachiya, R. Role of Personality in Tobacco Smoking Behavior in Corporate Sector: A Cross-Sectional Study. Ind. Psychiatry J. 2018, 27, 103. [Google Scholar] [CrossRef]
- Dash, G.F.; Slutske, W.S.; Martin, N.G.; Statham, D.J.; Agrawal, A. Big Five Personality Traits and Alcohol, Nicotine, Cannabis, and Gambling Disorder Comorbidity. Psychol. Addict. Behav. 2019, 33, 420–429. [Google Scholar] [CrossRef]
- Terracciano, A.; Löckenhoff, C.E.; Crum, R.M.; Bienvenu, O.J.; Costa, P.T. Five-Factor Model Personality Profiles of Drug Users. BMC Psychiatry 2008, 8, 22. [Google Scholar] [CrossRef]
- Sutin, A.R.; Evans, M.K.; Zonderman, A.B. Personality Traits and Illicit Substances: The Moderating Role of Poverty. Drug Alcohol. Depend. 2013, 131, 247–251. [Google Scholar] [CrossRef]
- Szeszko, P.R.; Lipsky, R.; Mentschel, C.; Robinson, D.; Gunduz-Bruce, H.; Sevy, S.; Ashtari, M.; Napolitano, B.; Bilder, R.M.; Kane, J.M.; et al. Brain-Derived Neurotrophic Factor Val66met Polymorphism and Volume of the Hippocampal Formation. Mol. Psychiatry 2005, 10, 631–636. [Google Scholar] [CrossRef]
- Hariri, A.R.; Goldberg, T.E.; Mattay, V.S.; Kolachana, B.S.; Callicott, J.H.; Egan, M.F.; Weinberger, D.R. Brain-Derived Neurotrophic Factor Val66met Polymorphism Affects Human Memory-Related Hippocampal Activity and Predicts Memory Performance. J. Neurosci. 2003, 23, 6690. [Google Scholar] [CrossRef]
- Merikangas, K.R.; Swendsen, J.D.; Preisig, M.A.; Chazan, R.Z. Psychopathology and Temperament in Parents and Offspring: Results of a Family Study. J. Affect. Disord. 1998, 51, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Pietras, T.; Witusik, A.; Panek, M.; Szemraj, J.; Górski, P. Anxiety, Depression and Methods of Stress Coping in Patients with Nicotine Dependence Syndrome. Med. Sci. Monit. 2011, 17, 272–276. [Google Scholar] [CrossRef][Green Version]
- Costa, P.T.; McCrae, R.R. The Revised NEO Personality Inventory (NEO-PI-R). In The SAGE Handbook of Personality Theory and Assessment: Volume 2—Personality Measurement and Testing; Sage Publications Ltd.: Thousand Oaks, CA, USA, 2008; pp. 179–198. [Google Scholar] [CrossRef]

| Hardy–Weinberg Equilibrium, Including Analysis for Estimation Bias | Observed (Expected) | Allele Freq | χ2 (p-Value) | |
|---|---|---|---|---|
| rs6265 BDNF patients in addiction treatment for the first time n = 143 | G/G | 101 (101.5) | p (G) = 0.84 q (A) = 0.16 | 0.1161 (0.7333) |
| A/A | 3 (3.5) | |||
| A/G | 39 (37.9) | |||
| rs6265 BDNF patients with addiction relapse n = 148 | G/G | 107 (107.3) | p (G) = 0.85 q (A) = 0.15 | 0.0308 (0.8606) |
| A/A | 3 (3.3) | |||
| A/G | 38 (37.5) | |||
| rs6265 BDNF control n = 242 | G/G | 143 (142.2) | p (G) = 0.77 q (A) = 0.23 | 0.084 (0.7714) |
| A/A | 14 (13.2) | |||
| A/G | 85 (86.6) | |||
| rs6265 BDNF | |||||
|---|---|---|---|---|---|
| Genotypes | Alleles | ||||
| G/G n (%) | A/A n (%) | A/G n (%) | G n (%) | A n (%) | |
| First-time treatment n = 143 | 101 (70.63%) | 3 (2.10%) | 39 (27.27%) | 241 (84.27%) | 45 (15.73%) |
| Control n = 242 | 143 (59.09%) | 14 (5.79%) | 85 (35.12%) | 371 (76.65%) | 113 (23.35%) |
| χ2 (p-value) | 6.376 (0.0412) * | 6.390 (0.0115) * | |||
| Relapse addiction n = 148 | 107 (72.30%) | 3 (2.03%) | 38 (25.68%) | 252 (85.14%) | 296 (14.86%) |
| Control n = 242 | 143 (59.09%) | 14 (5.79%) | 85 (35.12%) | 371 (76.65%) | 113 (23.35%) |
| χ2 (p-value) | 8.074 (0.01765) * | 8.220 (0.0041) * | |||
| STAI/NEO Five-Factor Inventory | First-Time Treatment (n = 143) | Control (n = 242) | Z | (p-Value) |
| STAI trait scale | 6.94 ± 2.28 | 5.18 ± 2.29 | 6.622 | 0.0000 * |
| STAI state scale | 5.58 ± 2.46 | 4.72 ± 2.20 | 3.549 | 0.0003 * |
| Neuroticism scale | 6.42 ± 2.16 | 4.71 ± 2.06 | 6.806 | 0.0000 * |
| Extraversion scale | 5.88 ± 2.15 | 6.39 ± 1.99 | −2.266 | 0.0234 * |
| Openness scale | 5.13 ± 2.03 | 4.59 ± 1.61 | 2.341 | 0.0192 * |
| Agreeability scale | 4.53 ± 1.98 | 5.43 ± 2.09 | −3.995 | 0.0001 * |
| Conscientiousness scale | 5.84 ± 2.13 | 5.92 ± 2.17 | −0.270 | 0.7872 |
| STAI/NEO Five-Factor Inventory | Relapse of addiction (n = 148) | Control (n = 242) | Z | (p-Value) |
| STAI trait scale | 7.30 ± 2.29 | 5.19 ± 2.29 | 8.081 | 0.0000 * |
| STAI state scale | 6.16 ± 2.37 | 4.72 ± 2.20 | 5.840 | 0.0000 * |
| Neuroticism scale | 7.01 ± 2.12 | 4.71 ± 2.06 | 9.489 | 0.0000 * |
| Extraversion scale | 5.62 ± 2.17 | 6.39 ± 1.99 | −3.294 | 0.0009 * |
| Openness scale | 5.24 ± 2.18 | 4.58 ± 1.61 | 3.170 | 0.0015 * |
| Agreeability scale | 4.06 ± 1.84 | 5.43 ± 2.09 | −6.305 | 0.0000 * |
| Conscientiousness scale | 5.43 ± 2.31 | 5.93 ± 2.17 | −2.125 | 0.0335 * |
| STAI/NEO Five-Factor Inventory | First-time treatment (n = 143) | Relapse of addiction (n = 148) | Z | (p-Value) |
| STAI trait scale | 6.94 ± 2.28 | 7.30 ± 2.29 | −1.959 | 0.0500 * |
| STAI state scale | 5.58 ± 2.46 | 6.16 ± 2.37 | −1.421 | 0.1553 |
| Neuroticism scale | 6.42 ± 2.16 | 7.01 ± 2.12 | −2.527 | 0.0115 * |
| Extraversion scale | 5.88 ± 2.15 | 5.62 ± 2.17 | 0.842 | 0.3995 |
| Openness scale | 5.13 ± 2.03 | 5.24 ± 2.18 | −0.674 | 0.5000 |
| Agreeability scale | 4.53 ± 1.98 | 4.06 ± 1.84 | 2.060 | 0.0393 * |
| Conscientiousness scale | 5.84 ± 2.13 | 5.43 ± 2.31 | 1.628 | 0.1035 |
| STAI/NEO Five-Factor Inventory | Group | rs6265 BDNF | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| G/G n = 243 M ± SD | A/A n = 17 M ± SD | A/G n = 123 M ± SD | Factor | F (p-Value) | η2 | Power (Alfa = 0.05) | ||
| STAI trait scale | First time in addiction treatment (FTT); n = 143 | 5.50 ± 2.53 | 7.33 ± 2.08 | 5.66 ± 2.30 | Intercept FTT/control rs6265 FTT/control × rs6265 | F1.378 = 435.92 (p < 0.0001) F1.378 = 7.64 (p = 0.0059) F2.378 = 0.76 (p = 0.4641) F2.378 = 0.93 (p = 0.3950) | 0.536 0.020 0.004 0.005 | 1.000 0.788 0.181 0.211 |
| Control; n = 242 | 4.80 ± 2.25 | 4.79 ± 2.07 | 4.57 ± 2.18 | |||||
| STAI state scale | First time in addiction treatment (FTT); n = 143 | 6.78 ± 2.37 | 8.67 ± 1.15 | 7.24 ± 2.03 | Intercept FTT/control rs6265 FTT/control × rs6265 | F1.378 = 653.12 (p < 0.0001) F1.378 = 14.93 (p = 0.0001) F2.378 = 2.76 (p = 0.0646) F2.378 = 0.31 (p = 0.7348) | 0.634 0.038 0.014 0.002 | 1.000 0.971 0.543 0.099 |
| C: Control; n = 242 | 5.08 ± 2.22 | 6.50 ± 2.74 | 5.14 ± 2.29 | |||||
| Neuroticism scale | First time in addiction treatment (FTT); n = 143 | 6.43 ± 2.24 | 8.33 ± 1.15 | 6.26 ± 1.94 | Intercept FTT/control rs6265 FTT/control × rs6265 | F1.378 = 620.51 (p < 0.0001) F1.378 = 21.66 (p = 0.0000) F2.378 = 1.73 (p = 0.1789) F2.378 = 0.68 (p = 0.5048) | 0.621 0.054 0.009 0.004 | 1.000 0.996 0.362 0.165 |
| Control; n = 242 | 4.76 ± 2.14 | 5.07 ± 2.05 | 4.57 ± 1.92 | |||||
| Extraversion scale | First time in addiction treatment (FTT); n = 143 | 5.95 ± 2.18 | 5.33 ± 0.58 | 5.73 ± 2.18 | Intercept FTT/control rs6265 FTT/control × rs6265 | F1.378 = 651.73 (p < 0.0001) F1.378 = 1.30 (p = 0.2557) F2.378 = 0.54 (p = 0.5847) F2.378 = 0.70 (p = 0.4979) | 0.633 0.003 0.003 0.004 | 1.000 0.206 0.138 0.168 |
| Control; n = 242 | 6.30 ± 2.10 | 5.64 ± 1.94 | 6.66 ± 1.80 | |||||
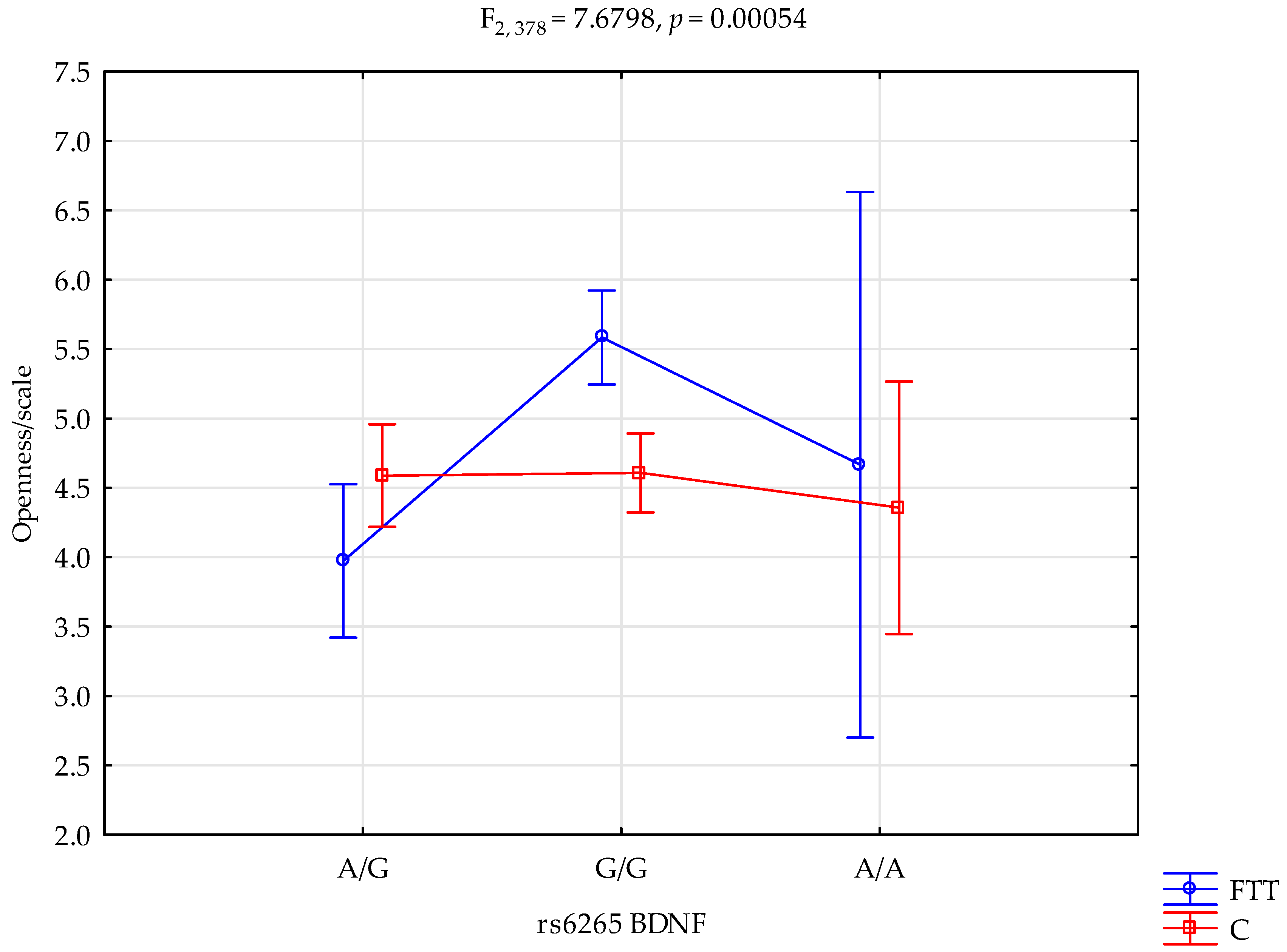
| Openness scale | First time in addiction treatment (FTT); n = 143 | 5.58 ± 1.99 | 4.67 ± 1.53 | 3.97 ± 1.68 | Intercept FTT/control rs6265 FTT/control × rs6265 | F1.378 = 559.45 (p < 0.0001) F1.378 = 0.32 (p = 0.5682) F2.378 = 8.29 (p = 0.0003) F2.378 = 7.68 (p = 0.0005 *) | 0.596 0.001 0.042 0.039 | 1.000 0.088 0.960 0.947 |
| Control; n = 242 | 4.60 ± 1.59 | 4.36 ± 1.86 | 4.59 ± 1.63 | |||||
| Agreeability scale | First time in addiction treatment (FTT); n = 1431 | 4.64 ± 2.06 | 6.00 ± 2.65 | 4.11 ± 1.67 | Intercept FTT/control rs6265 FTT/control × rs6265 | F1.378 = 504.71 (p < 0.0001) F1.378 = 1.61 (p = 0.2046) F2.378 = 1.69 (p = 0.1847) F2.378 = 0.94 (p = 0.3923) | 0.572 0.004 0.009 0.005 | 1.000 0.244 0.356 0.212 |
| Control; n = 242 | 5.42 ± 2.15 | 5.71 ± 2.33 | 5.38 ± 1.96 | |||||
| Conscientiousness scale | First time in addiction treatment (FTT); n = 143 | 5.76 ± 2.34 | 4.33 ± 0.58 | 6.16 ± 2.25 | Intercept FTT/control rs6265 FTT/control × rs6265 | F1.378 = 524.49 (p < 0.0001) F1.378 = 1.56 (p = 0.2128) F2.378 = 2.34 (p = 0.0974) F2.378 = 0.91 (p = 0.4021) | 0.581 0.004 0.012 0.005 | 1.000 0.238 0.473 0.208 |
| Control; n = 242 | 5.67 ± 2.28 | 6.14 ± 2.51 | 6.31 ± 1.86 | |||||
| STAI/NEO Five-Factor Inventory | Group | rs6265 | ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| G/G n = 250 M ± SD | A/A n = 17 M ± SD | A/G n = 123 M ± SD | Factor | F (p-Value) | η2 | Power (Alfa = 0.05) | ||
| STAI trait scale | Addiction relapses (AR); n = 148 | 7.65 ± 2.09 | 5.67 ± 1.53 | 6.44 ± 2.63 | Intercept AR/control rs6265 AR/control × rs6265 | F1.384 = 568.49 (p < 0.0001) F1.384 = 3.95 (p = 0.0474) F2.384 = 2.39 (p = 0.0928) F2.384 = 5.03 (p = 0.0070) * | 0.597 0.010 0.012 0.026 | 1.000 0.509 0.482 0.815 |
| Control; n = 242 | 5.08 ± 2.22 | 6.50 ± 2.74 | 5.14 ± 2.28 | |||||
| STAI state scale | Addiction relapses (AR); n = 148 | 6.27 ± 2.24 | 6.33 ± 1.53 | 5.84 ± 2.76 | Intercept AR/control rs6265 AR/control × rs6265 | F1.384 = 447.75 (p < 0.0001) F1.384 = 7.71 (p = 0.0057) F2.384 = 0.78 (p = 0.4594) F2.384 = 0.08 (p = 0.9263) | 0.538 0.020 0.004 0.0004 | 1.000 0.791 0.183 0.061 |
| C: Control; n = 242 | 2.76 ± 2.24 | 4.78 ± 2.01 | 4.57 ± 2.18 | |||||
| Neuroticism scale | Addiction relapses (AR); n = 148 | 7.13 ± 2.01 | 6.33 ± 1.15 | 6.71 ± 2.46 | Intercept AR/control rs6265 AR/control × rs6265 | F1.384 = 597.44 (p < 0.0001) F1.384 = 16.59 (p = 0.0001) F2.384 = 0.79 (p = 0.4500) F2.384 = 0.41 (p = 0.6627) | 0.608 0.041 0.004 0.002 | 1.000 0.982 0.186 0.116 |
| Control; n = 242 | 4.76 ± 2.14 | 5.07 ± 2.06 | 4.57 ± 1.92 | |||||
| Extraversion scale | Addiction relapses (AR); n = 148 | 5.57 ± 2.21 | 6.67 ± 2.31 | 5.68 ± 2.11 | Intercept RT/control rs6265 AR/control × rs6265 | F1.384 = 679.69 (p < 0.0001) F1.384 = 0.24 (p = 0.6235) F2.384 = 0.49 (p = 0.6136) F2.384 = 1.06 (p = 0.3460) | 0.638 0.0006 0.002 0.005 | 1.000 0.077 0.130 0.236 |
| Control; n = 242 | 6.31 ± 2.10 | 5.64 ± 1.95 | 6.66 ± 1.80 | |||||
| Openness scale | Addiction relapses (AR); n = 148 | 5.19 ± 2.19 | 5.67 ± 1.53 | 5.37 ± 2.21 | Intercept RT/control rs6265 AR/control × rs6265 | F1.384 = 560.99 (p < 0.0001) F1.384 = 4.50 (p = 0.0344) F2.384 = 0.08 (p = 0.9224) F2.384 = 0.26 (p = 0.7670) | 0.594 0.011 0.0004 0.001 | 1.000 0.562 0.062 0.092 |
| Control; n = 242 | 4.61 ± 1.59 | 4.35 ± 1.86 | 4.59 ± 1.63 | |||||
| Agreeability scale | Addiction relapses (AR); n = 148 | 4.01 ± 1.73 | 4.66 ± 2.31 | 4.16 ± 2.12 | Intercept AR/control rs6265 AR/control × rs6265 | F1.384 = 465.25 (p < 0.0001) F1.384 = 7.33 (p = 0.0071) F2.384 = 0.27 (p = 0.7622) F2.384 = 0.12 (p = 0.8882) | 0.548 0.019 0.001 0.0006 | 1.000 0.770 0.093 0.068 |
| Control; n = 242 | 5.43 ± 2.15 | 5.71 ± 2.33 | 5.38 ± 1.96 | |||||
| Conscientiousness scale | Addiction relapses (AR); n = 148 | 5.43 ± 2.26 | 5.67 ± 1.15 | 5.39 ± 2.29 | Intercept AR/control rs6265 AR/control × rs6265 | F1.384 = 540.2 (p < 0.0001) F1.384 = 1.21 (p = 0.2728) F2.384 = 0.73 (p = 0.4798) F2.384 = 0.84 (p = 0.4343) | 0.584 0.003 0.004 0.004 | 1.000 0.195 0.175 0.193 |
| Control; n = 242 | 5.67 ± 2.28 | 6.14 ± 2.51 | 6.31 ± 1.86 | |||||
| rs6265 BDNF and Openness Scale | ||||||
| {1} M = 3.97 | {2} M = 5.58 | {3} M = 4.67 | {4} M = 4.59 | {5} M = 4.61 | {6} M = 4.36 | |
| First time in addiction treatment G/G {1} | 0.0000 * | 0.5050 | 0.0698 | 0.0454 * | 0.4793 | |
| First time in addiction treatment A/A {2} | 0.3665 | 0.0001 * | 0.0000 * | 0.0134 * | ||
| First time in addiction treatment A/G {3} | 0.9386 | 0.9540 | 0.7789 | |||
| Control G/G {4} | 0.9323 | 0.6439 | ||||
| Control A/A {5} | 0.6048 | |||||
| Control A/G {6} | ||||||
| rs6265 BDNF and STAI trait scale | ||||||
| {1} M = 6.45 | {2} M = 7.65 | {3} M = 5.67 | {4} M = 5.14 | {5} M = 5.08 | {6} M = 6.50 | |
| Addiction relapses G/G {1} | 0.0049 * | 0.5647 | 0.0032 * | 0.0010 * | 0.9406 | |
| Addiction relapses A/A {2} | 0.1336 | 0.0000 * | 0.0000 * | 0.0729 | ||
| Addiction relapses A/G {3} | 0.6923 | 0.6585 | 0.5623 | |||
| Control G/G {4} | 0.8532 | 0.0377 * | ||||
| Control A/A {5} | 0.0257 * | |||||
| Control A/G {6} | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strońska-Pluta, A.; Suchanecka, A.; Chmielowiec, K.; Chmielowiec, J.; Boroń, A.; Masiak, J.; Sipak-Szmigiel, O.; Recław, R.; Grzywacz, A. The Relationship between the Brain-Derived Neurotrophic Factor Gene Polymorphism (Val66Met) and Substance Use Disorder and Relapse. Int. J. Mol. Sci. 2024, 25, 788. https://doi.org/10.3390/ijms25020788
Strońska-Pluta A, Suchanecka A, Chmielowiec K, Chmielowiec J, Boroń A, Masiak J, Sipak-Szmigiel O, Recław R, Grzywacz A. The Relationship between the Brain-Derived Neurotrophic Factor Gene Polymorphism (Val66Met) and Substance Use Disorder and Relapse. International Journal of Molecular Sciences. 2024; 25(2):788. https://doi.org/10.3390/ijms25020788
Chicago/Turabian StyleStrońska-Pluta, Aleksandra, Aleksandra Suchanecka, Krzysztof Chmielowiec, Jolanta Chmielowiec, Agnieszka Boroń, Jolanta Masiak, Olimpia Sipak-Szmigiel, Remigiusz Recław, and Anna Grzywacz. 2024. "The Relationship between the Brain-Derived Neurotrophic Factor Gene Polymorphism (Val66Met) and Substance Use Disorder and Relapse" International Journal of Molecular Sciences 25, no. 2: 788. https://doi.org/10.3390/ijms25020788
APA StyleStrońska-Pluta, A., Suchanecka, A., Chmielowiec, K., Chmielowiec, J., Boroń, A., Masiak, J., Sipak-Szmigiel, O., Recław, R., & Grzywacz, A. (2024). The Relationship between the Brain-Derived Neurotrophic Factor Gene Polymorphism (Val66Met) and Substance Use Disorder and Relapse. International Journal of Molecular Sciences, 25(2), 788. https://doi.org/10.3390/ijms25020788







