Identification of a Novel NPC1L1 Inhibitor from Danshen and Its Role in Nonalcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Results
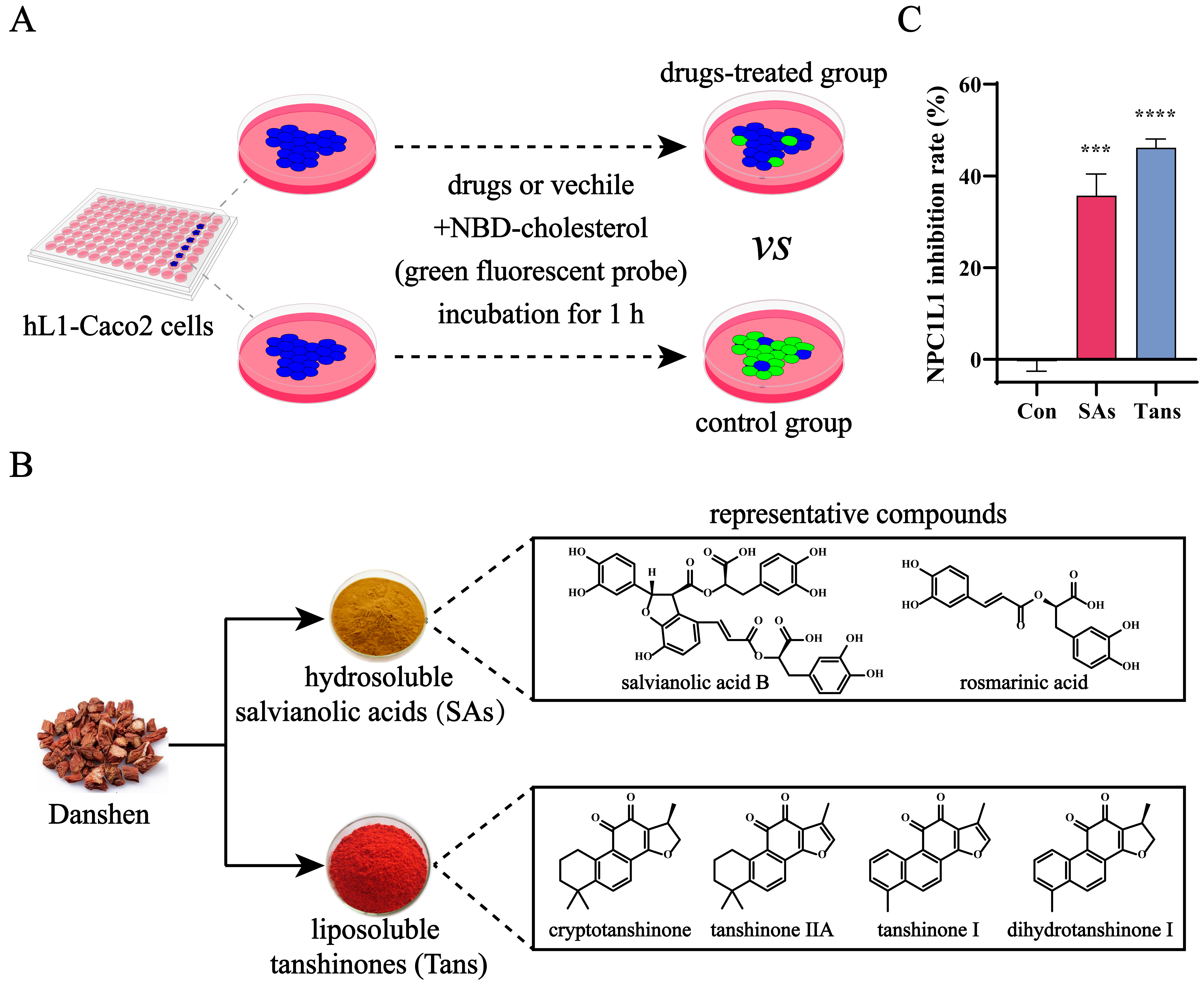
2.1. Tans Inhibits NPC1L1-Mediated Intestinal Cholesterol Absorption
2.2. Tans Exhibits a Protective Effect on NAFLD in HFD-Fed Mice
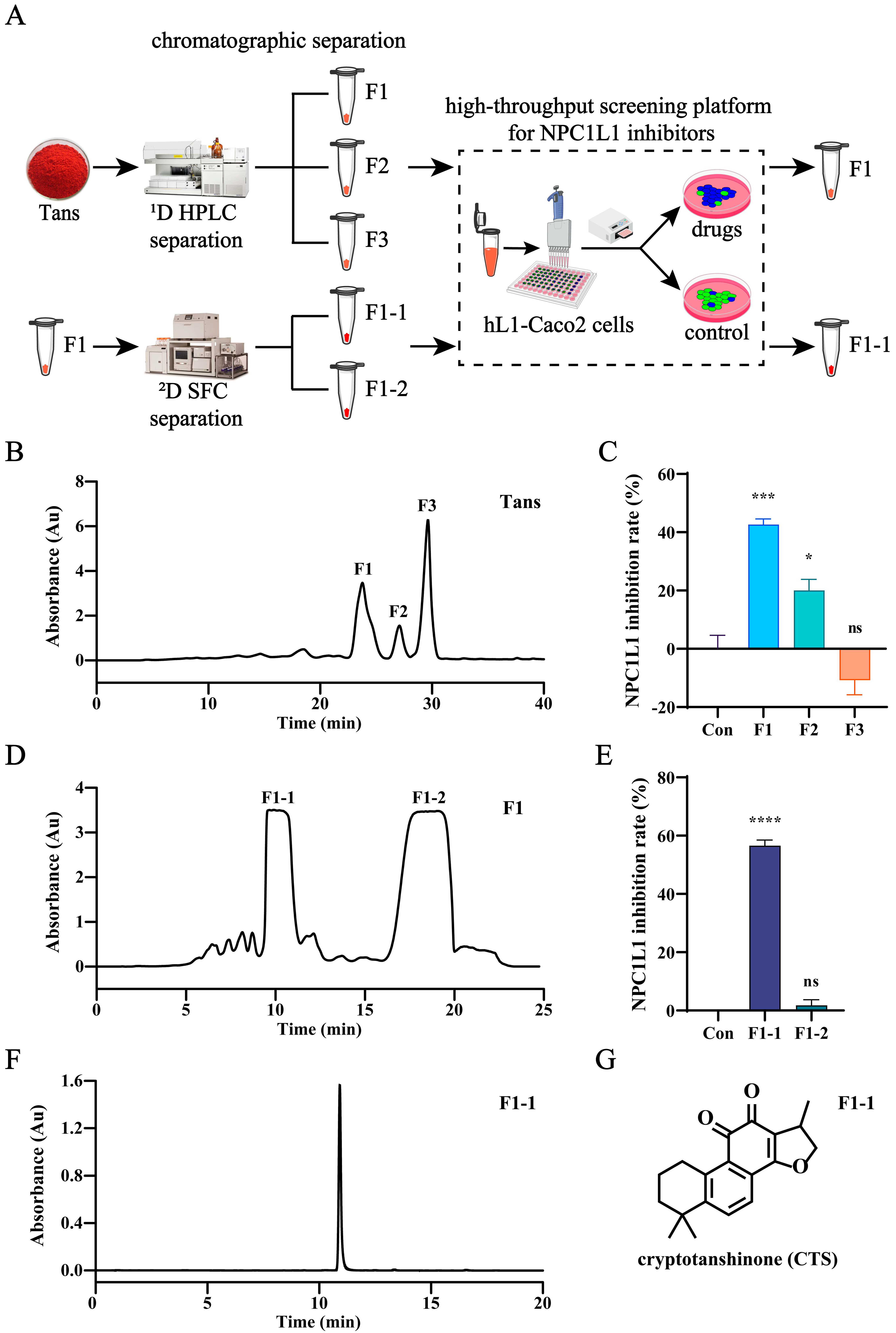
2.3. CTS Is the Active Compound in Tans That Inhibits NPC1L1-Mediated Intestinal Cholesterol Absorption
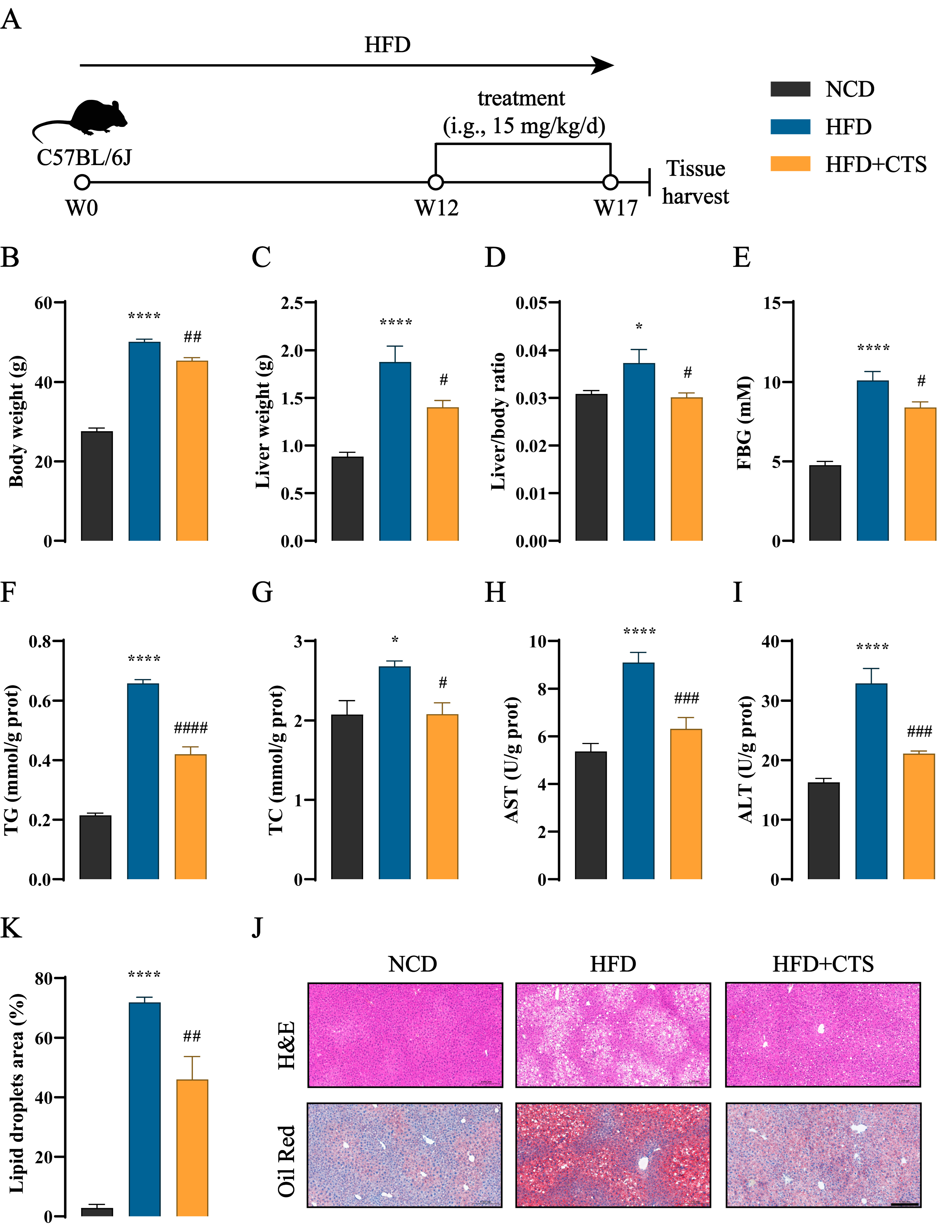
2.4. CTS Alleviates NAFLD in HFD-Fed Mice
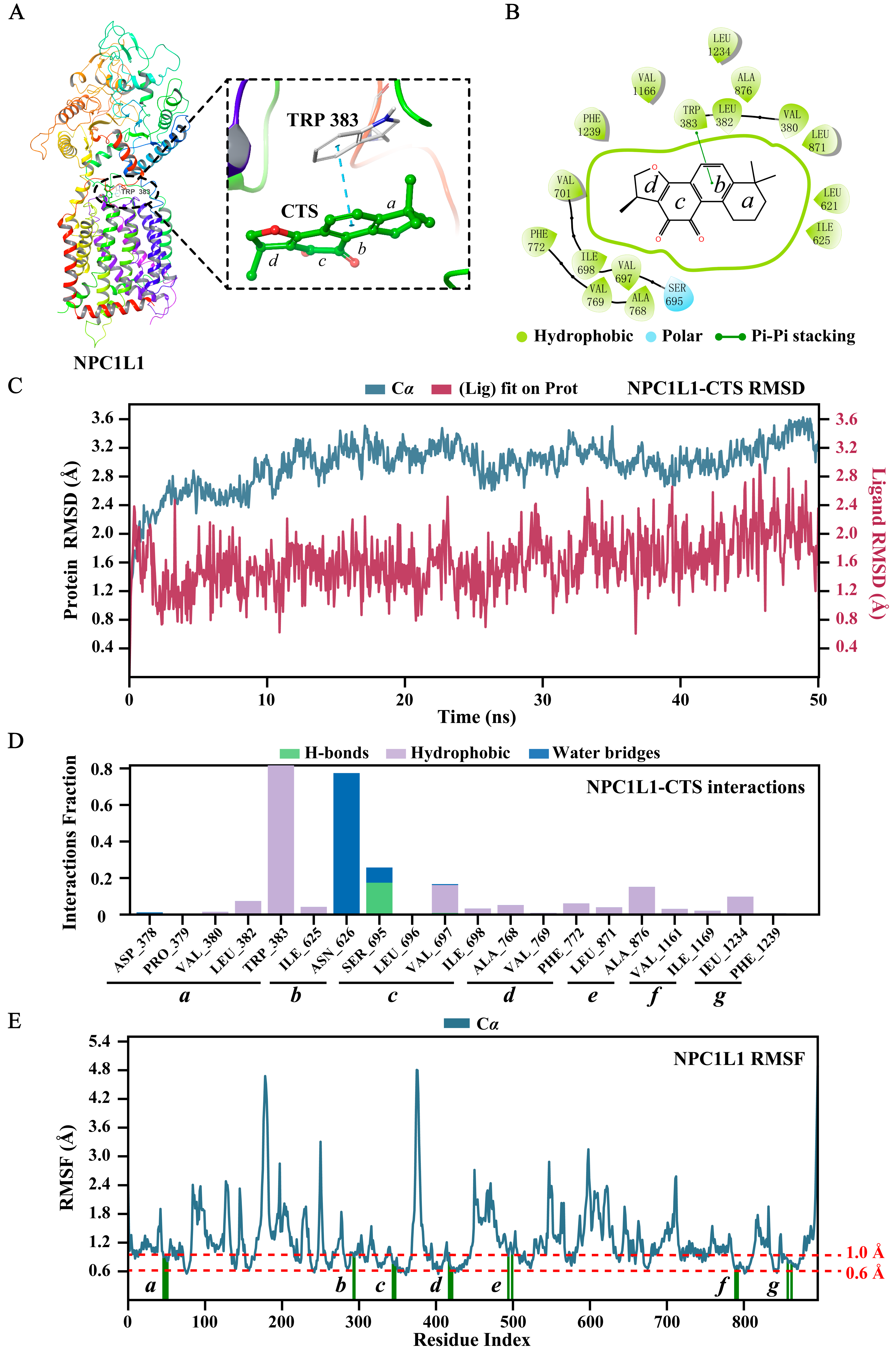
2.5. Molecular Docking and Dynamics Simulation
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Animal Experiments
4.3. Establishment of Stable Caco2 Cell Lines Expressing Human-NPC1L1 (hL1-Caco2) and Cell Culture
4.4. Biochemical Analysis
4.5. Pathological Analysis
4.6. High-Throughput Screening of NPC1L1 Inhibitors
4.7. Separation and Analysis of Tans and Related Fractions
4.8. Molecular Docking and Molecular Dynamics (MD) Studies
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powell, E.E.; Wong, V.W.S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Carr, R.M.M. A new perspective on NAFLD: Focusing on lipid droplets. J. Hepatol. 2022, 76, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Friedman, S.L.; Shulman, G.I. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- Petroni, M.L.; Brodosi, L.; Bugianesi, E.; Marchesini, G. Management of non-alcoholic fatty liver disease. BMJ 2021, 372, m4747. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Singal, A.G.; Kono, Y.; Tan, D.J.H.; El-Serag, H.B.; Loomba, R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022, 34, 969–977. [Google Scholar] [CrossRef]
- Wong, V.W.S.; Ekstedt, M.; Wong, G.L.H.; Hagström, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef]
- Song, Y.F.; Liu, J.J.; Zhao, K.; Gao, L.; Zhao, J.J. Cholesterol-induced toxicity: An integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021, 33, 1911–1925. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.L.; Lau, H.C.H.; Wang, Y.X.; Chan, A.W.H.; Wei, H.; Yang, X.Y.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef]
- Li, J.B.; Wang, Y.F.; Matye, D.J.; Chavan, H.; Krishnamurthy, P.; Li, F.; Li, T.G. Sortilin 1 Modulates Hepatic Cholesterol Lipotoxicity in Mice via Functional Interaction with Liver Carboxylesterase 1. J. Biol. Chem. 2017, 292, 146–160. [Google Scholar] [CrossRef]
- Gan, L.T.; Van Rooyen, D.M.; Koina, M.E.; McCuskey, R.S.; Teoh, N.C.; Farrell, G.C. Hepatocyte free cholesterol lipotoxicity results from JNK1-mediated mitochondrial injury and is HMGB1 and TLR4-dependent. J. Hepatol. 2014, 61, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, H.Y.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Betters, J.L.; Yu, L.Q. Niemann-Pick C1-Like 1 (NPC1L1) Protein in Intestinal and Hepatic Cholesterol Transport. Annu. Rev. Physiol. 2011, 73, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Ma, Y.Y.; Rong, S.X.; Betters, J.L.; Xie, P.; Chung, S.; Wang, N.P.; Tang, W.Q.; Yu, L.Q. Niemann-Pick C1-Like 1 deletion in mice prevents high-fat diet-induced fatty liver by reducing lipogenesis. J. Lipid Res. 2010, 51, 3135–3144. [Google Scholar] [CrossRef]
- Ramirez, R.; Cohen, J.C.; Hobbs, H.H.; Browning, J.D. Hepatic Triglyceride Content in Individuals with Reduced Intestinal Cholesterol Absorption Due to Variants in Nieman Pick C1-Like 1. Hepatology 2011, 54, 736–737. [Google Scholar] [CrossRef]
- Lee, D.H.; Han, D.H.; Nam, K.T.; Park, J.S.; Kim, S.H.; Lee, M.; Kim, G.; Min, B.S.; Cha, B.S.; Lee, Y.S.; et al. Ezetimibe, an NPC1L1 inhibitor, is a potent Nrf2 activator that protects mice from diet-induced nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2016, 99, 520–532. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.H.; Ou, X.; Ouyang, X.P.; Tang, C.K. Hepatic cholesterol transport and its role in non-alcoholic fatty liver disease and atherosclerosis. Prog. Lipid Res. 2021, 83, 101109. [Google Scholar] [CrossRef]
- Yang, J.; Zou, J.; Mai, H.Y.; Hong, T.; Liu, H.; Feng, D. Curcumin protects against high-fat diet-induced nonalcoholic simple fatty liver by inhibiting intestinal and hepatic NPC1L1 expression via down-regulation of SREBP-2/HNF1α pathway in hamsters. J. Nutr. Biochem. 2023, 119, 109403. [Google Scholar] [CrossRef]
- Yan, M.Y.; Zhao, Y.X.; Man, S.L.; Dai, Y.J.; Ma, L.; Gao, W.Y. Diosgenin as a substitute for cholesterol alleviates NAFLD by affecting CYP7A1 and NPC1L1-related pathway. Phytomedicine 2024, 125, 155299. [Google Scholar] [CrossRef]
- Peng, H.; He, Y.; Zheng, G.; Zhang, W.; Yao, Z.; Xie, W. Meta-analysis of traditional herbal medicine in the treatment of nonalcoholic fatty liver disease. Cell Mol. Biol. 2016, 62, 88–95. [Google Scholar]
- Zhu, J.; Guo, J.N.; Liu, Z.J.; Liu, J.; Yuan, A.N.; Chen, H.; Qiu, J.N.; Dou, X.B.; Lu, D.Z.; Le, Y.F. Salvianolic acid A attenuates non-alcoholic fatty liver disease by regulating the AMPK-IGFBP1 pathway. Chem. Biol. Interact. 2024, 400, 111162. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, R.; Zhu, R.Y.; Chen, B.B.; Tian, Y.M.; Zhang, H.; Xia, B.K.; Jia, Q.Q.; Wang, L.L.; Zhao, D.D.; et al. Salvianolic acid B prevents body weight gain and regulates gut microbiota and LPS/TLR4 signaling pathway in high-fat diet-induced obese mice. Food Funct. 2020, 11, 8743–8756. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.C.; Zheng, J.Y.; Qiu, Y.H.; Zheng, L.; Zheng, J.Y.; Liu, Y.Q.; Miao, X.L.; Lu, X.Y. Salvianolic acid B ameliorates non-alcoholic fatty liver disease by inhibiting hepatic lipid accumulation and NLRP3 inflammasome in ob/ob mice. Int. Immunopharmacol. 2022, 111, 109099. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Lu, X.Y.; Zhang, S.J.; Chiu, A.P.; Lo, L.H.; Largaespada, D.A.; Chen, Q.B.; Keng, V.W. Sodium tanshinone IIA sulfonate ameliorates hepatic steatosis by inhibiting lipogenesis and inflammation. Biomed. Pharmacother. 2019, 111, 68–75. [Google Scholar] [CrossRef]
- Zheng, L.L.; Li, B.Y.; Yuan, A.L.; Bi, S.J.; Puscher, H.; Liu, C.Q.; Qiao, L.S.; Qiao, Y.J.; Wang, S.F.; Zhang, Y.L. TFEB activator tanshinone IIA and derivatives derived from Salvia miltiorrhiza Bge. Attenuate hepatic steatosis and insulin resistance. J. Ethnopharmacol. 2024, 335, 118662. [Google Scholar] [CrossRef]
- Wang, W.; Kong, Y.L.; Wang, X.; Wang, Z.; Tang, C.L.; Li, J.Y.; Yang, Q.; Chen, Y.Q.; Zhu, S.L. Identification of novel SCD1 inhibitor alleviates nonalcoholic fatty liver disease: Critical role of liver-adipose axis. Cell Commun. Signal. 2023, 21, 268. [Google Scholar] [CrossRef]
- Zhang, R.S.; Liu, W.J.; Zeng, J.; Meng, J.S.; Jiang, H.F.; Wang, J.; Xing, D.M. Niemann-Pick C1-Like 1 inhibitors for reducing cholesterol absorption. Eur. J. Med. Chem. 2022, 230, 114111. [Google Scholar] [CrossRef]
- Huang, C.S.; Yu, X.C.; Fordstrom, P.; Choi, K.; Chung, B.C.; Roh, S.H.; Chiu, W.; Zhou, M.Y.; Min, X.S.; Wang, Z.L. Cryo-EM structures of NPC1L1 reveal mechanisms of cholesterol transport and ezetimibe inhibition. Sci. Adv. 2020, 6, eabb1989. [Google Scholar] [CrossRef]
- Jia, A.; Jiang, H.F.; Liu, W.J.; Chen, P.W.; Xu, Q.; Zhang, R.S.; Sun, J.F. Novel application potential of cinaciguat in the treatment of mixed hyperlipidemia through targeting PTL/NPC1L1 and alleviating intestinal microbiota dysbiosis and metabolic disorders. Pharmacol. Res. 2023, 194, 106854. [Google Scholar] [CrossRef]
- Langhi, C.; Otero, Y.F.; Le Joubioux, F.; Guigas, B.; Peltier, S.; Sirvent, P. TOTUM-070 Hypocholesterolemic Effect Linked to Intestinal Cholesterol Absorption Inhibition. An in vivo and in vitro Study. Circulation 2022, 146, A9575. [Google Scholar] [CrossRef]
- Zhou, W.J.; Liu, Y.M.; Wang, J.X.; Guo, Z.M.; Shen, A.J.; Liu, Y.F.; Liang, X.M. Application of two-dimensional liquid chromatography in the separation of traditional Chinese medicine. J. Sep. Sci. 2020, 43, 87–104. [Google Scholar] [CrossRef]
- Li, H.Y.; Gao, C.D.; Liu, C.; Liu, L.J.; Zhuang, J.; Yang, J.; Zhou, C.; Feng, F.B.; Sun, C.G.; Wu, J.B. A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Biomed. Pharmacother. 2021, 137, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.B.; Zhan, X.Y.; Xu, G.; Wang, Z.L.; Li, R.S.; Wang, Y.; Qin, Q.; Shi, W.; Hou, X.R.; Yang, R.C.; et al. Cryptotanshinone specifically suppresses NLRP3 inflammasome activation and protects against inflammasome-mediated diseases. Pharmacol. Res. 2021, 164, 11. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, J.; Qi, W.; Miao, H.H.; Cao, J.; Qu, Y.X.; Li, B.L.; Song, B.L. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008, 7, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Altmann, S.W.; Davis, H.R.; Zhu, L.J.; Yao, X.R.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.N.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef]
- Li, X.D.; Liu, Y.W.; Zhang, H.; Ren, L.M.; Li, Q.Y.; Li, N. Animal models for the atherosclerosis research: A review. Protein Cell 2011, 2, 189–201. [Google Scholar]
- Hu, M.Q.; Yang, F.; Huang, Y.W.; You, X.; Liu, D.S.; Sun, S.; Sui, S.F. Structural insights into the mechanism of human NPC1L1-mediated cholesterol uptake. Sci. Adv. 2021, 7, 15. [Google Scholar] [CrossRef]
- Xiao, J.; Dong, L.W.; Liu, S.; Meng, F.H.; Xie, C.; Lu, X.Y.; Zhang, W.P.J.; Luo, J.; Song, B.L. Bile acids-mediated intracellular cholesterol transport promotes intestinal cholesterol absorption and NPC1L1 recycling. Nat. Commun. 2023, 14, 16. [Google Scholar] [CrossRef]
- Liu, Z.P.; Xu, S.W.; Huang, X.Y.; Wang, J.J.; Gao, S.; Li, H.; Zhou, C.H.; Ye, J.T.; Chen, S.R.; Jin, Z.G.; et al. Cryptotanshinone, an orally bioactive herbal compound from Danshen, attenuates atherosclerosis in apolipoprotein E-deficient mice: Role of lectin-like oxidized LDL receptor-1 (LOX-1). Br. J. Pharmacol. 2015, 172, 5661–5675. [Google Scholar] [CrossRef]
- Wang, P.; Hou, T.; Xu, F.F.; Luo, F.B.; Zhou, H.; Liu, F.; Xie, X.M.; Liu, Y.F.; Wang, J.X.; Guo, Z.M.; et al. Discovery of Flavonoids as Novel Inhibitors of ATP Citrate Lyase: Structure-Activity Relationship and Inhibition Profiles. Int. J. Mol. Sci. 2022, 23, 10747. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, D.; Jiang, X.; Xie, X.; Zhou, H.; Yu, D.; Jin, G.; Ye, X.; Zhu, S.; Guo, Z.; Liang, X. Identification of a Novel NPC1L1 Inhibitor from Danshen and Its Role in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2025, 26, 2793. https://doi.org/10.3390/ijms26062793
Xia D, Jiang X, Xie X, Zhou H, Yu D, Jin G, Ye X, Zhu S, Guo Z, Liang X. Identification of a Novel NPC1L1 Inhibitor from Danshen and Its Role in Nonalcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2025; 26(6):2793. https://doi.org/10.3390/ijms26062793
Chicago/Turabian StyleXia, Donghai, Xuan Jiang, Xiaomin Xie, Han Zhou, Dongping Yu, Gaowa Jin, Xianlong Ye, Shenglong Zhu, Zhimou Guo, and Xinmiao Liang. 2025. "Identification of a Novel NPC1L1 Inhibitor from Danshen and Its Role in Nonalcoholic Fatty Liver Disease" International Journal of Molecular Sciences 26, no. 6: 2793. https://doi.org/10.3390/ijms26062793
APA StyleXia, D., Jiang, X., Xie, X., Zhou, H., Yu, D., Jin, G., Ye, X., Zhu, S., Guo, Z., & Liang, X. (2025). Identification of a Novel NPC1L1 Inhibitor from Danshen and Its Role in Nonalcoholic Fatty Liver Disease. International Journal of Molecular Sciences, 26(6), 2793. https://doi.org/10.3390/ijms26062793






