5G Radiofrequency Exposure Reduces PRDM16 and C/EBP β mRNA Expression, Two Key Biomarkers for Brown Adipogenesis
Abstract
1. Introduction
2. Results
2.1. UCP3 mRNA Expression Is Downregulated in 5G-Exposed Rats Compared to Those Exposed to 2G
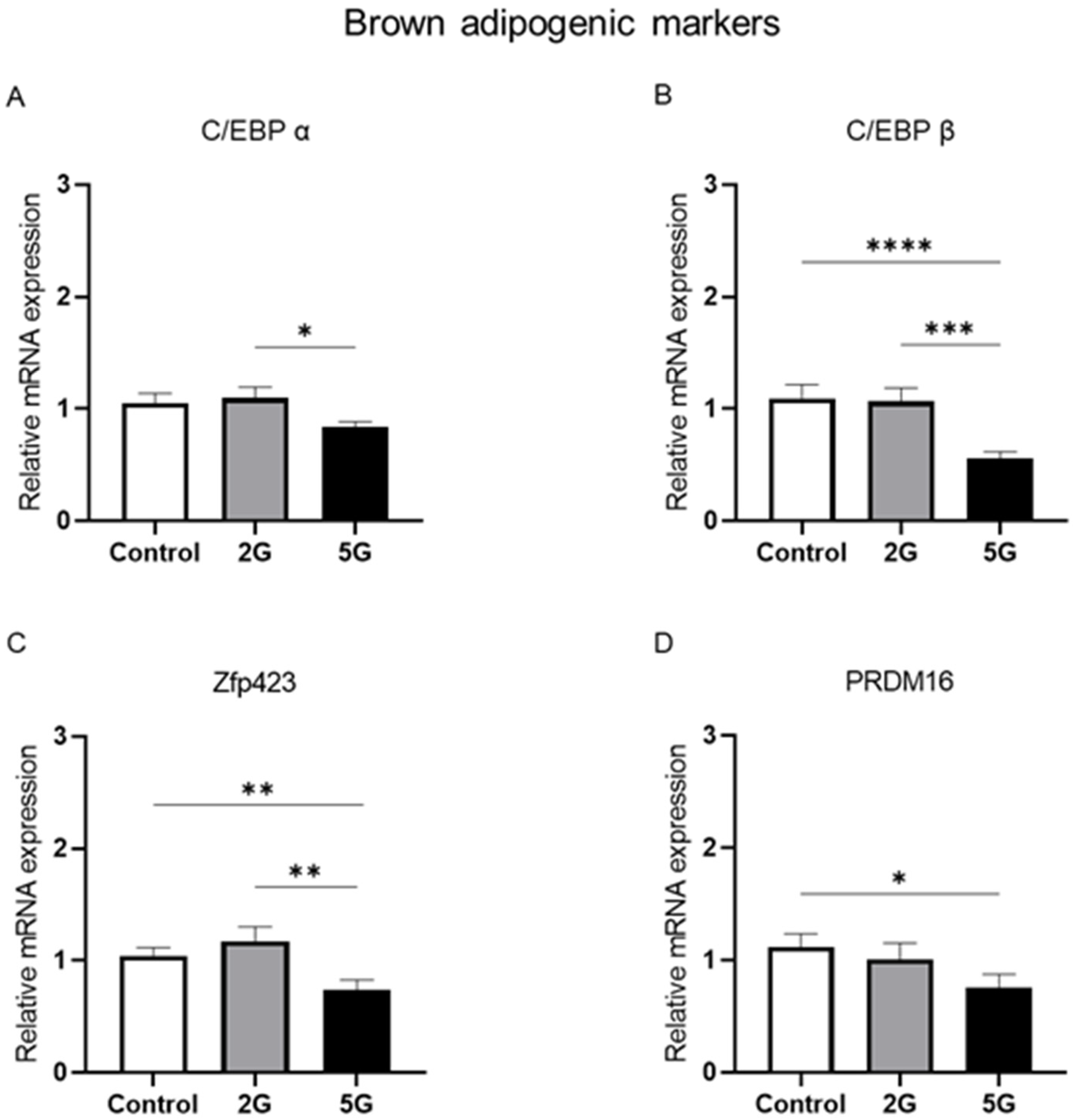
2.2. Fifth-Generation Radiofrequency Exposure Reduced the mRNA Levels of Adipogenic C/EBP β, Zfp423, and PRDM16 Markers in BAT
2.3. Age-Dependent Modulation of PPAR α mRNA Expression After 2G Exposure
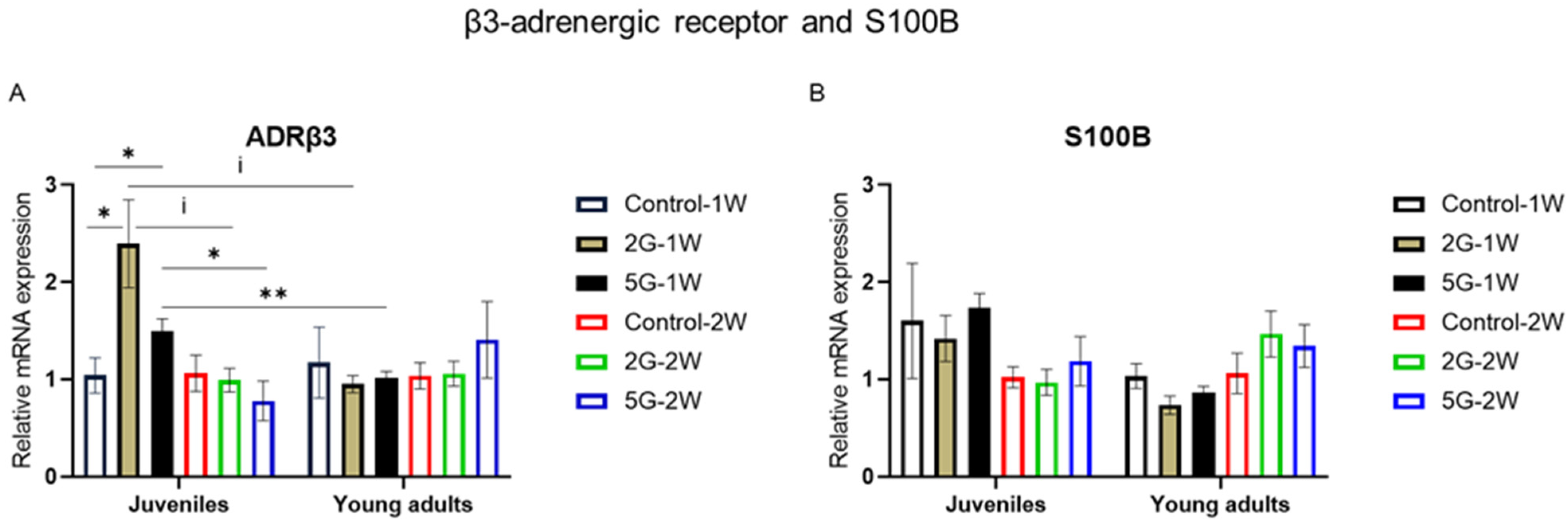
2.4. ADRβ3 mRNA Expression Was Modulated by Age, Exposure Duration, and RF Exposure
3. Discussion
3.1. The Core Transcriptional Pathway of UCP1 Thermogenesis Is Not Impacted by RF Exposure
3.2. Does Short-Term 5G Exposure Reduce Brown Adipogenesis?
3.3. RF Exposure Amplifies Age-Related PPAR α Downregulation
3.4. Age-Related Effect of RF on ADRβ3 After 1-Week Exposure
3.5. Limitations of This Study
4. Materials and Methods
4.1. Ethics Statement
4.2. Animal Housing
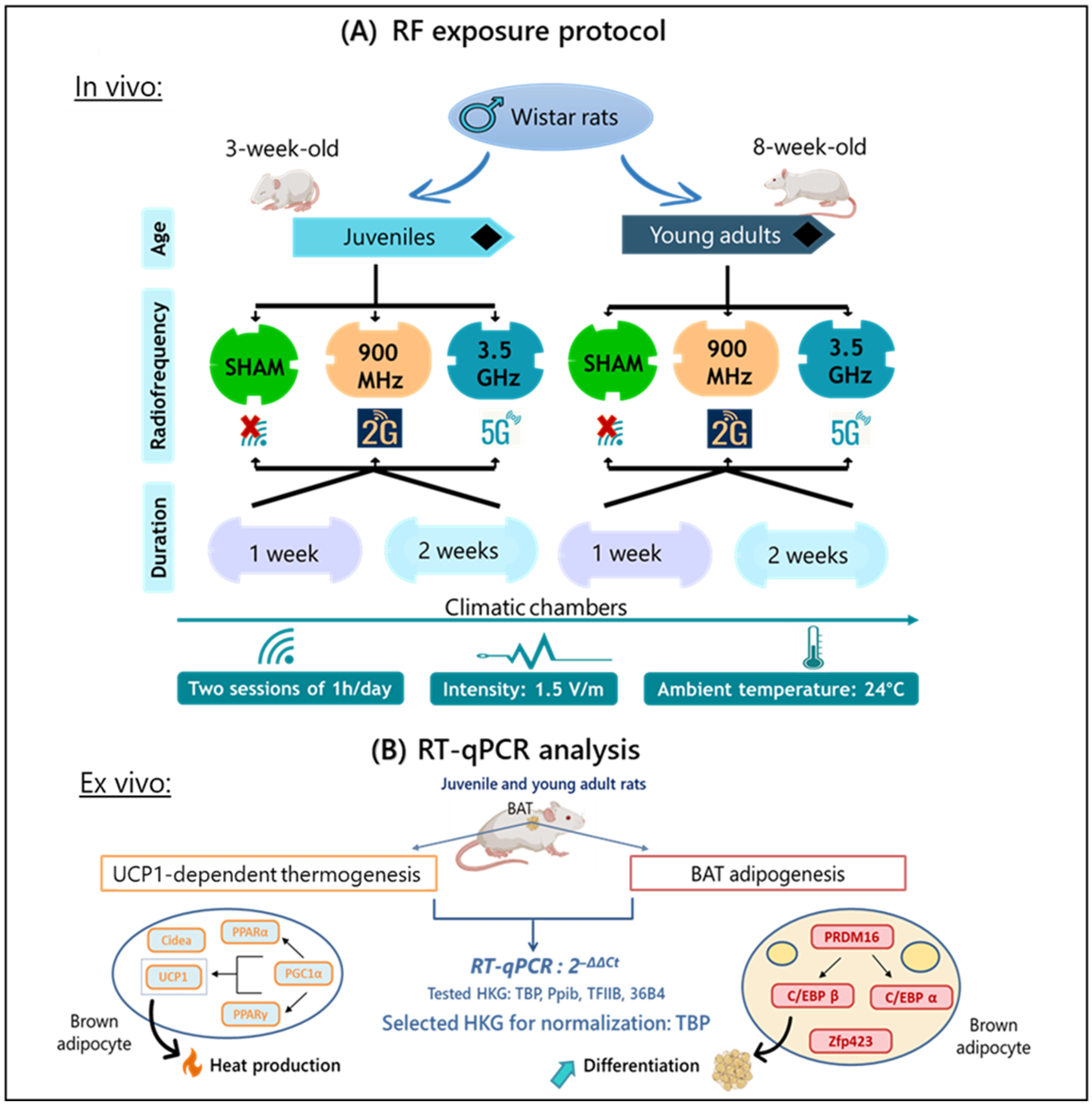
4.3. Experimental Design
4.4. RF Exposure System
4.5. Organ Collection
4.6. Relative Gene Expression: RT-qPCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arendash, G.W.; Mori, T.; Dorsey, M.; Gonzalez, R.; Tajiri, N.; Borlongan, C. Electromagnetic Treatment to Old Alzheimer’s Mice Reverses β-Amyloid Deposition, Modifies Cerebral Blood Flow, and Provides Selected Cognitive Benefit. PLoS ONE 2012, 7, e35751. [Google Scholar] [CrossRef]
- Arendash, G.W.; Sanchez-Ramos, J.; Mori, T.; Mamcarz, M.; Lin, X.; Runfeldt, M.; Wang, L.; Zhang, G.; Sava, V.; Tan, J.; et al. Electromagnetic Field Treatment Protects against and Reverses Cognitive Impairment in Alzheimer’s Disease Mice. J. Alzheimers Dis. 2010, 19, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.C.; Braun, A.; Bach, V.; Pelletier, A.; de Seze, R. Low-Level Radiofrequency Exposure Induces Vasoconstriction in Rats. Bioelectromagnetics 2021, 42, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.C.; Delanaud, S.; Bach, V.; Braun, A.; Pelletier, A.; de Seze, R. Effect of Non-Thermal Radiofrequency on Body Temperature in Mice. Sci. Rep. 2020, 10, 5724. [Google Scholar] [CrossRef]
- Pelletier, A.; Delanaud, S.; de Seze, R.; Bach, V.; Libert, J.-P.; Loos, N. Does Exposure to a Radiofrequency Electromagnetic Field Modify Thermal Preference in Juvenile Rats? PLoS ONE 2014, 9, e99007. [Google Scholar] [CrossRef]
- Pelletier, A.; Delanaud, S.; Décima, P.; Thuroczy, G.; de Seze, R.; Cerri, M.; Bach, V.; Libert, J.-P.; Loos, N. Effects of Chronic Exposure to Radiofrequency Electromagnetic Fields on Energy Balance in Developing Rats. Environ. Sci. Pollut. Res. Int. 2013, 20, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Bortkiewicz, A.; Gadzicka, E.; Szymczak, W.; Zmyślony, M. Changes in Tympanic Temperature during the Exposure to Electromagnetic Fields Emitted by Mobile Phone. Int. J. Occup. Med. Environ. Health 2012, 25, 145–150. [Google Scholar] [CrossRef]
- Loughran, S.P.; Verrender, A.; Dalecki, A.; Burdon, C.A.; Tagami, K.; Park, J.; Taylor, N.A.S.; Croft, R.J. Radiofrequency Electromagnetic Field Exposure and the Resting EEG: Exploring the Thermal Mechanism Hypothesis. Int. J. Environ. Res. Public Health 2019, 16, 1505. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Jeong, J.H.; Chang, J.S.; Jo, Y.-H. Intracellular Glycolysis in Brown Adipose Tissue Is Essential for Optogenetically Induced Nonshivering Thermogenesis in Mice. Sci. Rep. 2018, 8, 6672. [Google Scholar] [CrossRef]
- Gill, J.A.; La Merrill, M.A. An Emerging Role for Epigenetic Regulation of Pgc-1α Expression in Environmentally Stimulated Brown Adipose Thermogenesis. Environ. Epigenetics 2017, 3, dvx009. [Google Scholar] [CrossRef]
- Reilly, S.M.; Saltiel, A.R. A Futile Approach to Fighting Obesity? Cell 2015, 163, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.L.; Boutet, E.; Puri, V.; Chawla, A.; Czech, M.P. Identification of the Lipid Droplet Targeting Domain of the Cidea Protein. J. Lipid Res. 2010, 51, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Ranjit, S.; Konda, S.; Nicoloro, S.M.C.; Straubhaar, J.; Chawla, A.; Chouinard, M.; Lin, C.; Burkart, A.; Corvera, S.; et al. Cidea Is Associated with Lipid Droplets and Insulin Sensitivity in Humans. Proc. Natl. Acad. Sci. USA 2008, 105, 7833–7838. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Lei, J.; He, X.; Hao, J.; Zhang, F.; Huang, X.; Gu, W.; Yang, X.; Yu, J. Keys to the Switch of Fat Burning: Stimuli That Trigger the Uncoupling Protein 1 (UCP1) Activation in Adipose Tissue. Lipids Health Dis. 2024, 23, 322. [Google Scholar] [CrossRef]
- Villarroya, F.; Iglesias, R.; Giralt, M. PPARs in the Control of Uncoupling Proteins Gene Expression. PPAR Res. 2007, 2007, 74364. [Google Scholar] [CrossRef]
- Harms, M.J.; Lim, H.-W.; Ho, Y.; Shapira, S.N.; Ishibashi, J.; Rajakumari, S.; Steger, D.J.; Lazar, M.A.; Won, K.-J.; Seale, P. PRDM16 Binds MED1 and Controls Chromatin Architecture to Determine a Brown Fat Transcriptional Program. Genes. Dev. 2015, 29, 298–307. [Google Scholar] [CrossRef]
- Jiang, N.; Yang, M.; Han, Y.; Zhao, H.; Sun, L. PRDM16 Regulating Adipocyte Transformation and Thermogenesis: A Promising Therapeutic Target for Obesity and Diabetes. Front. Pharmacol. 2022, 13, 870250. [Google Scholar] [CrossRef]
- Gupta, R.K.; Mepani, R.J.; Kleiner, S.; Lo, J.C.; Khandekar, M.J.; Cohen, P.; Frontini, A.; Bhowmick, D.C.; Ye, L.; Cinti, S.; et al. Zfp423 Expression Identifies Committed Preadipocytes and Localizes to Adipose Endothelial and Perivascular Cells. Cell Metab. 2012, 15, 230–239. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-Tissue Plasticity in Health and Disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Fukano, K.; Okamatsu-Ogura, Y.; Tsubota, A.; Nio-Kobayashi, J.; Kimura, K. Cold Exposure Induces Proliferation of Mature Brown Adipocyte in a SS3-Adrenergic Receptor-Mediated Pathway. PLoS ONE 2016, 11, e0166579. [Google Scholar] [CrossRef]
- Zeng, X.; Ye, M.; Resch, J.M.; Jedrychowski, M.P.; Hu, B.; Lowell, B.B.; Ginty, D.D.; Spiegelman, B.M. Innervation of Thermogenic Adipose Tissue via a Calsyntenin 3β-S100b Axis. Nature 2019, 569, 229–235. [Google Scholar] [CrossRef]
- Atlı Şekeroğlu, Z.; Akar, A.; Şekeroğlu, V. Evaluation of the Cytogenotoxic Damage in Immature and Mature Rats Exposed to 900 MHz Radiofrequency Electromagnetic Fields. Int. J. Radiat. Biol. 2013, 89, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Wiart, J.; Hadjem, A.; Wong, M.F.; Bloch, I. Analysis of RF Exposure in the Head Tissues of Children and Adults. Phys. Med. Biol. 2008, 53, 3681–3695. [Google Scholar] [CrossRef] [PubMed]
- Sangün, Ö.; Dündar, B.; Çömlekçi, S.; Büyükgebiz, A. The Effects of Electromagnetic Field on the Endocrine System in Children and Adolescents. Pediatr. Endocrinol. Rev. 2015, 13, 531–545. [Google Scholar] [PubMed]
- Cypess, A.M.; Kahn, C.R. The Role and Importance of Brown Adipose Tissue in Energy Homeostasis. Curr. Opin. Pediatr. 2010, 22, 478–484. [Google Scholar] [CrossRef]
- Anses Exposition de La Population Aux Champs Électromagnétiques Liée Au Déploiement de La Technologie de Communication «5G» et Effets Sanitaires Associés: Réponses Aux Commentaires Reçus Par l’intermédiaire Du Formulaire Internet Lors de La Consultation Publique (Saisine N° 2019-SA-0006) 2022. Available online: https://www.anses.fr/fr/system/files/AP2019SA0006Ra.pdf (accessed on 7 March 2025).
- Inoue, S.-I.; Emmett, M.J.; Lim, H.-W.; Midha, M.; Richter, H.J.; Celwyn, I.J.; Mehmood, R.; Chondronikola, M.; Klein, S.; Hauck, A.K.; et al. Short-Term Cold Exposure Induces Persistent Epigenomic Memory in Brown Fat. Cell Metab. 2024, 36, 1764–1778.e9. [Google Scholar] [CrossRef]
- Shore, A.M.; Karamitri, A.; Kemp, P.; Speakman, J.R.; Graham, N.S.; Lomax, M.A. Cold-Induced Changes in Gene Expression in Brown Adipose Tissue, White Adipose Tissue and Liver. PLoS ONE 2013, 8, e68933. [Google Scholar] [CrossRef]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms Controlling Mitochondrial Biogenesis and Respiration through the Thermogenic Coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1 (PGC-1) Family in Physiological and Pathophysiological Process and Diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Hondares, E.; Rosell, M.; Díaz-Delfín, J.; Olmos, Y.; Monsalve, M.; Iglesias, R.; Villarroya, F.; Giralt, M. Peroxisome Proliferator-Activated Receptor α (PPARα) Induces PPARγ Coactivator 1α (PGC-1α) Gene Expression and Contributes to Thermogenic Activation of Brown Fat: Involvement of PRDM16. J. Biol. Chem. 2011, 286, 43112–43122. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T.H.; Banerjee, S.; Sharma, V.M.; Donohue, J.; Couldwell, S.; Sosinsky, A.; Frulla, A.; Robinson, A.; Puri, V. Effects of a High Fat Diet and Voluntary Wheel Running Exercise on Cidea and Cidec Expression in Liver and Adipose Tissue of Mice. PLoS ONE 2015, 10, e0130259. [Google Scholar] [CrossRef]
- Abreu-Vieira, G.; Fischer, A.W.; Mattsson, C.; de Jong, J.M.A.; Shabalina, I.G.; Rydén, M.; Laurencikiene, J.; Arner, P.; Cannon, B.; Nedergaard, J.; et al. Cidea Improves the Metabolic Profile through Expansion of Adipose Tissue. Nat. Commun. 2015, 6, 7433. [Google Scholar] [CrossRef]
- Barbatelli, G.; Murano, I.; Madsen, L.; Hao, Q.; Jimenez, M.; Kristiansen, K.; Giacobino, J.P.; De Matteis, R.; Cinti, S. The Emergence of Cold-Induced Brown Adipocytes in Mouse White Fat Depots Is Determined Predominantly by White to Brown Adipocyte Transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1244–E1253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yon Toh, S.; Chen, Z.; Guo, K.; Ng, C.P.; Ponniah, S.; Lin, S.-C.; Hong, W.; Li, P. Cidea-Deficient Mice Have Lean Phenotype and Are Resistant to Obesity. Nat. Genet. 2003, 35, 49–56. [Google Scholar] [CrossRef]
- Hiraike, Y.; Waki, H.; Yu, J.; Nakamura, M.; Miyake, K.; Nagano, G.; Nakaki, R.; Suzuki, K.; Kobayashi, H.; Yamamoto, S.; et al. NFIA Co-Localizes with PPARγ and Transcriptionally Controls the Brown Fat Gene Program. Nat. Cell Biol. 2017, 19, 1081–1092. [Google Scholar] [CrossRef]
- Wang, H.; Liu, L.; Lin, J.Z.; Aprahamian, T.R.; Farmer, S.R. Browning of White Adipose Tissue with Roscovitine Induces a Distinct Population of UCP1+ Adipocytes. Cell Metab. 2016, 24, 835–847. [Google Scholar] [CrossRef]
- Maalouf, J.; Pelletier, A.; Corona, A.; Gay-Quéheillard, J.; Bach, V.; de Seze, R.; Selmaoui, B. Dose- and Time-Dependent Effects of Radiofrequency Electromagnetic Field on Adipose Tissue: Implications of Thermoregulation and Mitochondrial Signaling. Int. J. Mol. Sci. 2023, 24, 10628. [Google Scholar] [CrossRef]
- Gong, D.W.; He, Y.; Karas, M.; Reitman, M. Uncoupling Protein-3 Is a Mediator of Thermogenesis Regulated by Thyroid Hormone, Beta3-Adrenergic Agonists, and Leptin. J. Biol. Chem. 1997, 272, 24129–24132. [Google Scholar] [CrossRef]
- Hilse, K.E.; Kalinovich, A.V.; Rupprecht, A.; Smorodchenko, A.; Zeitz, U.; Staniek, K.; Erben, R.G.; Pohl, E.E. The Expression of UCP3 Directly Correlates to UCP1 Abundance in Brown Adipose Tissue. Biochim. Biophys. Acta 2016, 1857, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, E.; Senese, R.; De Matteis, R.; Cioffi, F.; Moreno, M.; Lanni, A.; Gentile, A.; Busiello, R.A.; Salzano, A.M.; Scaloni, A.; et al. Absence of Uncoupling Protein 3 at Thermoneutrality Influences Brown Adipose Tissue Mitochondrial Functionality in Mice. FASEB J. 2020, 34, 15146–15163. [Google Scholar] [CrossRef]
- Cohen, P.; Kajimura, S. The Cellular and Functional Complexity of Thermogenic Fat. Nat. Rev. Mol. Cell Biol. 2021, 22, 393–409. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Clegg, D.J. Non-Shivering Thermogenesis as a Mechanism to Facilitate Sustainable Weight Loss. Obes. Rev. 2017, 18, 819–831. [Google Scholar] [CrossRef]
- Nedergaard, J.; Cannon, B. UCP1 mRNA Does Not Produce Heat. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2013, 1831, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Keipert, S.; Kutschke, M.; Ost, M.; Schwarzmayr, T.; van Schothorst, E.M.; Lamp, D.; Brachthäuser, L.; Hamp, I.; Mazibuko, S.E.; Hartwig, S.; et al. Long-Term Cold Adaptation Does Not Require FGF21 or UCP1. Cell Metab. 2017, 26, 437–446.e5. [Google Scholar] [CrossRef]
- Romanovsky, A.A.; Ivanov, A.I.; Shimansky, Y.P. Selected Contribution: Ambient Temperature for Experiments in Rats: A New Method for Determining the Zone of Thermal Neutrality. J. Appl. Physiol. 2002, 92, 2667–2679. [Google Scholar] [CrossRef]
- Klingenspor, M. Cold-Induced Recruitment of Brown Adipose Tissue Thermogenesis. Exp. Physiol. 2003, 88, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yoneshiro, T.; Matsushita, M. Activation and Recruitment of Brown Adipose Tissue by Cold Exposure and Food Ingredients in Humans. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 537–547. [Google Scholar] [CrossRef]
- Harms, M.J.; Ishibashi, J.; Wang, W.; Lim, H.-W.; Goyama, S.; Sato, T.; Kurokawa, M.; Won, K.-J.; Seale, P. Prdm16 Is Required for the Maintenance of Brown Adipocyte Identity and Function in Adult Mice. Cell Metab. 2014, 19, 593–604. [Google Scholar] [CrossRef]
- Jung, S.M.; Sanchez-Gurmaches, J.; Guertin, D.A. Brown Adipose Tissue Development and Metabolism. Handb. Exp. Pharmacol. 2019, 251, 3–36. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Hu, W. Molecular and Cellular Regulation of Thermogenic Fat. Front. Endocrinol. 2023, 14, 1215772. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S. Promoting Brown and Beige Adipocyte Biogenesis through the PRDM16 Pathway. Int. J. Obes. Suppl. 2015, 5, S11–S14. [Google Scholar] [CrossRef]
- Kajimura, S.; Seale, P.; Kubota, K.; Lunsford, E.; Frangioni, J.V.; Gygi, S.P.; Spiegelman, B.M. Initiation of Myoblast to Brown Fat Switch by a PRDM16-C/EBP-Beta Transcriptional Complex. Nature 2009, 460, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.E.; Dennis, R.J.; Obi, U.; O’Rahilly, S.; Rochford, J.J. Investigating the Involvement of the ATF6α Pathway of the Unfolded Protein Response in Adipogenesis. Int. J. Obes. 2012, 36, 1248–1251. [Google Scholar] [CrossRef]
- Darlington, G.J.; Ross, S.E.; MacDougald, O.A. The Role of C/EBP Genes in Adipocyte Differentiation. J. Biol. Chem. 1998, 273, 30057–30060. [Google Scholar] [CrossRef]
- Gupta, R.K.; Arany, Z.; Seale, P.; Mepani, R.J.; Ye, L.; Conroe, H.M.; Roby, Y.A.; Kulaga, H.; Reed, R.R.; Spiegelman, B.M. Transcriptional Control of Preadipocyte Determination by Zfp423. Nature 2010, 464, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Janesick, A.; Blumberg, B. Endocrine Disrupting Chemicals and the Developmental Programming of Adipogenesis and Obesity. Birth Defects Res. C Embryo Today 2011, 93, 34–50. [Google Scholar] [CrossRef]
- Cotgreave, I.A. Biological Stress Responses to Radio Frequency Electromagnetic Radiation: Are Mobile Phones Really so (Heat) Shocking? Arch. Biochem. Biophys. 2005, 435, 227–240. [Google Scholar] [CrossRef]
- Adair, E.R.; Black, D.R. Thermoregulatory Responses to RF Energy Absorption. Bioelectromagnetics 2003, 24 (Suppl. S6), S17–S38. [Google Scholar] [CrossRef]
- Nirengi, S.; Stanford, K. Brown Adipose Tissue and Aging: A Potential Role for Exercise. Exp. Gerontol. 2023, 178, 112218. [Google Scholar] [CrossRef] [PubMed]
- Darcy, J.; Tseng, Y.-H. ComBATing Aging-Does Increased Brown Adipose Tissue Activity Confer Longevity? Geroscience 2019, 41, 285–296. [Google Scholar] [CrossRef]
- Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 Is Essential for Adaptive Adrenergic Nonshivering Thermogenesis. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E350–E357. [Google Scholar] [CrossRef]
- Prewit, E.B.; Porter, C.; Rosa, M.L.; Bhattarai, N.; Yin, H.; Gamble, P.; Kechichian, T.; Sidossis, L.S. Adipose Tissue Uncoupling Protein 1 Levels and Function Are Increased in a Mouse Model of Developmental Obesity Induced by Maternal Exposure to High Fat Diet. J. Dev. Orig. Health Dis. 2018, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Coulter, A.; Rim, J.S.; Koza, R.A.; Kozak, L.P. Transcriptional Synergy and the Regulation of Ucp1 during Brown Adipocyte Induction in White Fat Depots. Mol. Cell. Biol. 2005, 25, 8311–8322. [Google Scholar] [CrossRef]
- Marques-Oliveira, G.H.; Silva, T.M.; Valadares, H.M.S.; Raposo, H.F.; Carolino, R.d.O.G.; Garófalo, M.A.R.; Anselmo-Franci, J.A.; do Carmo Kettelhut, I.; de Oliveira, H.C.F.; Chaves, V.E. Identification of Suitable Reference Genes for Quantitative Gene Expression Analysis in Innervated and Denervated Adipose Tissue from Cafeteria Diet-Fed Rats. Lipids 2019, 54, 231–244. [Google Scholar] [CrossRef]
- Harazaki, T.; Inoue, S.; Imai, C.; Mochizuki, K.; Goda, T. Resistant Starch Improves Insulin Resistance and Reduces Adipose Tissue Weight and CD11c Expression in Rat OLETF Adipose Tissue. Nutrition 2014, 30, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Pizzinat, N.; Ong-Meang, V.; Bourgailh-Tortosa, F.; Blanzat, M.; Perquis, L.; Cussac, D.; Parini, A.; Poinsot, V. Extracellular Vesicles of MSCs and Cardiomyoblasts Are Vehicles for Lipid Mediators. Biochimie 2020, 178, 69–80. [Google Scholar] [CrossRef]
- Zhang, W.-X.; Fan, J.; Ma, J.; Rao, Y.-S.; Zhang, L.; Yan, Y.-E. Selection of Suitable Reference Genes for Quantitative Real-Time PCR Normalization in Three Types of Rat Adipose Tissue. Int. J. Mol. Sci. 2016, 17, 968. [Google Scholar] [CrossRef]
- Golmohamadi, E.; Mahmazi, S.; Rahnema, M. Effect of Cinnamon Hydro Alcoholic Extract on Ucp3 Expression in Adipose Tissue of High Fat Dietary Obese Male Wistar Rats. J. Food Sci. Nutr. 2023, 9, 167. [Google Scholar] [CrossRef]
- Ghanbari-Niaki, A.; Ghanbari-Abarghooi, S.; Rahbarizadeh, F.; Zare-Kookandeh, N.; Gholizadeh, M.; Roudbari, F.; Zare-Kookandeh, A. Heart ABCA1 and PPAR- α Genes Expression Responses in Male Rats: Effects of High Intensity Treadmill Running Training and Aqueous Extraction of Black Crataegus-Pentaegyna. Res. Cardiovasc. Med. 2013, 2, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Mousovich-Neto, F.; Matos, M.S.; Costa, A.C.R.; de Melo Reis, R.A.; Atella, G.C.; Miranda-Alves, L.; Carvalho, D.P.; Ketzer, L.A.; Corrêa da Costa, V.M. Brown Adipose Tissue Remodelling Induced by Corticosterone in Male Wistar Rats. Exp. Physiol. 2019, 104, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, Y.; Ning, C.; Zhang, M.; Fan, P.; Lei, D.; Du, J.; Gale, M.; Ma, Y.; Yang, Y. Ellagic Acid Promotes Browning of White Adipose Tissues in High-Fat Diet-Induced Obesity in Rats through Suppressing White Adipocyte Maintaining Genes. Endocr. J. 2019, 66, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Khakisahneh, S.; Zhang, X.-Y.; Han, S.-Y.; Song, E.-J.; Nam, Y.-D.; Kim, H. Yijung-Tang Improves Thermogenesis and Reduces Inflammation Associated with Gut Microbiota in Hypothyroid Rats. NPJ Biofilms Microbiomes 2023, 9, 32. [Google Scholar] [CrossRef]
- Karadedeli, M.S.; Schreckenberg, R.; Kutsche, H.S.; Schlüter, K.-D. Effects of Voluntary Exercise on the Expression of Browning Markers in Visceral and Subcutaneous Fat Tissue of Normotensive and Spontaneously Hypertensive Rats. Pflug. Arch. Eur. J. Physiol. 2022, 474, 205–215. [Google Scholar] [CrossRef]
- Meyer, F.; Bitsch, A.; Forman, H.J.; Fragoulis, A.; Ghezzi, P.; Henschenmacher, B.; Kellner, R.; Kuhne, J.; Ludwig, T.; Sachno, D.; et al. The Effects of Radiofrequency Electromagnetic Field Exposure on Biomarkers of Oxidative Stress In Vivo and In Vitro: A Systematic Review of Experimental Studies. Environ. Int. 2024, 194, 108940. [Google Scholar] [CrossRef]


| Function | Gene | Sequences (5′ to 3′) | Accession Number | Sequence Source |
|---|---|---|---|---|
| Housekeeping genes | TBP | F: CACCGTGAATCTTGGCTGTAAAC | NM_001004198.1 | [67] |
| R: CGCAGTTGTTCGTGGCTCTC | ||||
| TFIIB | F: CCTGGCAGGAGTCCTATCTCT | NM_031041.2 | [68] | |
| R: ACCAGCAATATCCCCGATTT | ||||
| Ppib | F: CAGTCAAGACCTCCTGGCTAGA | NM_022536.2 | Designed | |
| R: CCGTCTTGGTGTTCTCCACCTT | ||||
| 36B4 | F: GGAACGTGGGCTTTGTGTTC | NM_022402.2 | [69] | |
| R: GTACTGTGACCTCACACGGG | ||||
| Uncoupling proteins | UCP1 | F: GCCTCTACGATACGGTCCAA | NM_012682.2 | [70] |
| R: TGCATTCTGACCTTCACCAC | ||||
| UCP3 | F: GACTCACAGGCAGCAAAGGAA | NM_013167.2 | [71] | |
| R: GAGGAGATCAGCAAAACAGGC | ||||
| PPAR signaling pathway | PPAR α | F: ACTCGCAGGAAAGACTAGCA | NM_013196.2 | [72] |
| R: AGCAGTGGAAGAATCGGACC | ||||
| PGC1 α | F: ATGGATATACTTTACGCAGGTCG | NM_031347.1 | [73] | |
| R: TGGAAGCAGGGTCAAAATCG | ||||
| PPAR γ | F: GTTGACACAGAGATGCCATTC | NM_013124.3 | [74] | |
| R: CGCACTTTGGTATTCTTGGAG | ||||
| Brown adipogenic markers | C/EBP α | F: CGCTGTTGCTGAAGGAACTTGA | NM_001287577.1 | Designed |
| R: TTAGCATAGACGCGCACACTGA | ||||
| C/EBP β | F: ACTTGATGCAATCCGGATCAAACG | NM_001301715.1 | Designed | |
| R: CAGTTACACGTGTGTTGCGTCAGT | ||||
| Zfp423 | F: GCCAGATGACCTTCGAGAACGA | NM_001393718.1 | Designed | |
| R: CGAACATCTGGTTGCACAGCTT | ||||
| PRDM16 | F: ACTTCGAGCTGCGAGAGTCC | NM_001427303.2 | [75] | |
| R: GCAGCTCTCCTGGGATGACA | ||||
| β3-Adrenergic Signaling | ADRβ3 | F: CTCACCGCTCAACAGGTTTGAT | NM_013108.2 | Designed |
| R: TTCTCCAGAAGTCAGGCTCCTT | ||||
| CLSTN3β pathway | S100B | F: TCAACAACGAGCTCTCTCACTT | NM_013191.2 | Designed |
| R: AGGCCATAAACTCCTGGAAGTC | ||||
| Thermogenic marker | Cidea | F: AGAAATGGACACCGGGCAAT | NM_001170467.1 | [76] |
| R: TGAAGCTTGTGCAGCGGATA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seewooruttun, C.; Bouguila, B.; Corona, A.; Delanaud, S.; Bodin, R.; Bach, V.; Desailloud, R.; Pelletier, A. 5G Radiofrequency Exposure Reduces PRDM16 and C/EBP β mRNA Expression, Two Key Biomarkers for Brown Adipogenesis. Int. J. Mol. Sci. 2025, 26, 2792. https://doi.org/10.3390/ijms26062792
Seewooruttun C, Bouguila B, Corona A, Delanaud S, Bodin R, Bach V, Desailloud R, Pelletier A. 5G Radiofrequency Exposure Reduces PRDM16 and C/EBP β mRNA Expression, Two Key Biomarkers for Brown Adipogenesis. International Journal of Molecular Sciences. 2025; 26(6):2792. https://doi.org/10.3390/ijms26062792
Chicago/Turabian StyleSeewooruttun, Chandreshwar, Bélir Bouguila, Aurélie Corona, Stéphane Delanaud, Raphaël Bodin, Véronique Bach, Rachel Desailloud, and Amandine Pelletier. 2025. "5G Radiofrequency Exposure Reduces PRDM16 and C/EBP β mRNA Expression, Two Key Biomarkers for Brown Adipogenesis" International Journal of Molecular Sciences 26, no. 6: 2792. https://doi.org/10.3390/ijms26062792
APA StyleSeewooruttun, C., Bouguila, B., Corona, A., Delanaud, S., Bodin, R., Bach, V., Desailloud, R., & Pelletier, A. (2025). 5G Radiofrequency Exposure Reduces PRDM16 and C/EBP β mRNA Expression, Two Key Biomarkers for Brown Adipogenesis. International Journal of Molecular Sciences, 26(6), 2792. https://doi.org/10.3390/ijms26062792







