Headspace Extraction onto a 3D-Printed Device for GC-MS Quantification of Polychlorinated Biphenyls in Newborn Urine
Abstract
1. Introduction
2. Results and Discussion
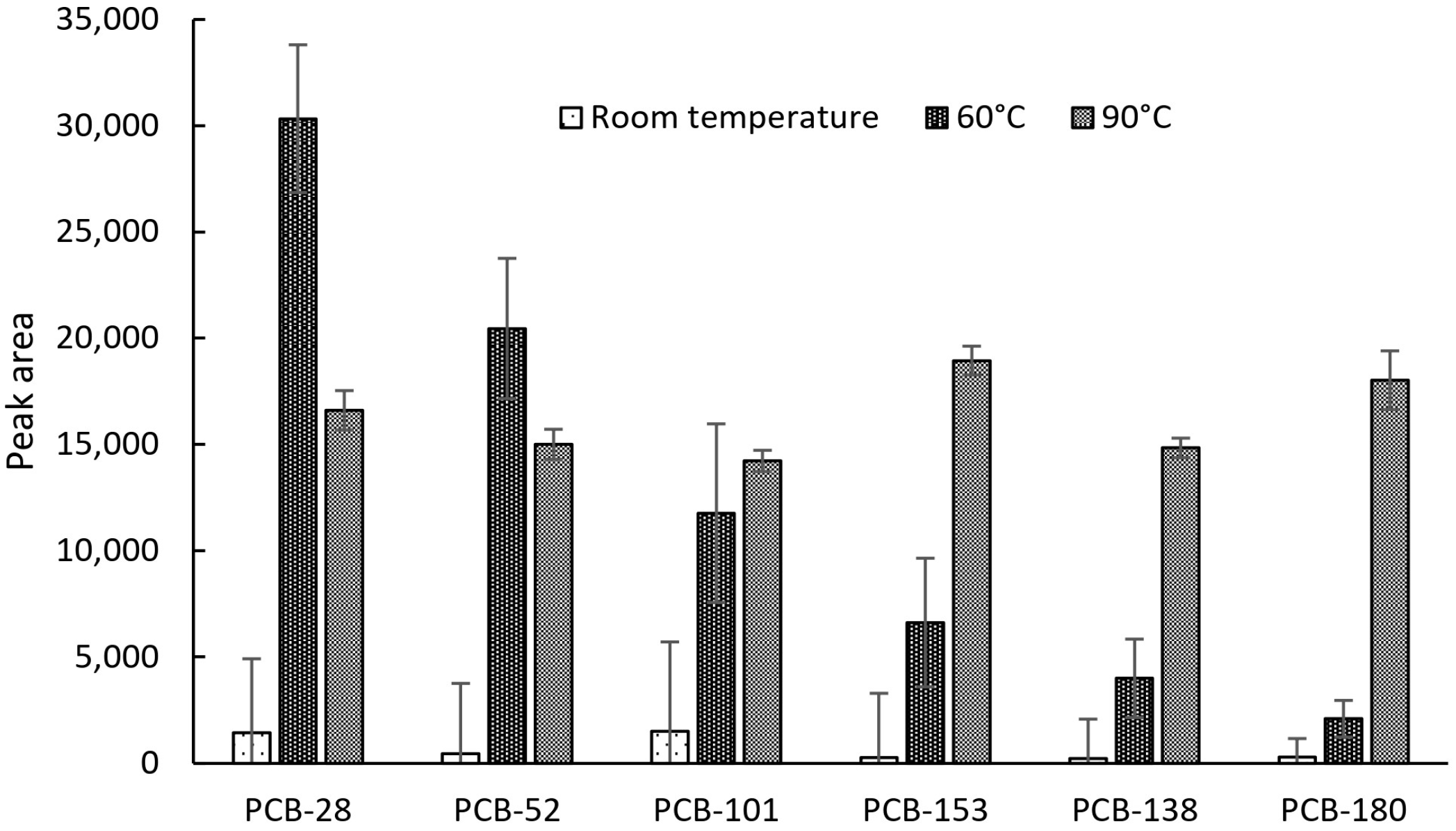
2.1. Primary Assessment of Device Performance and Temperature Effects
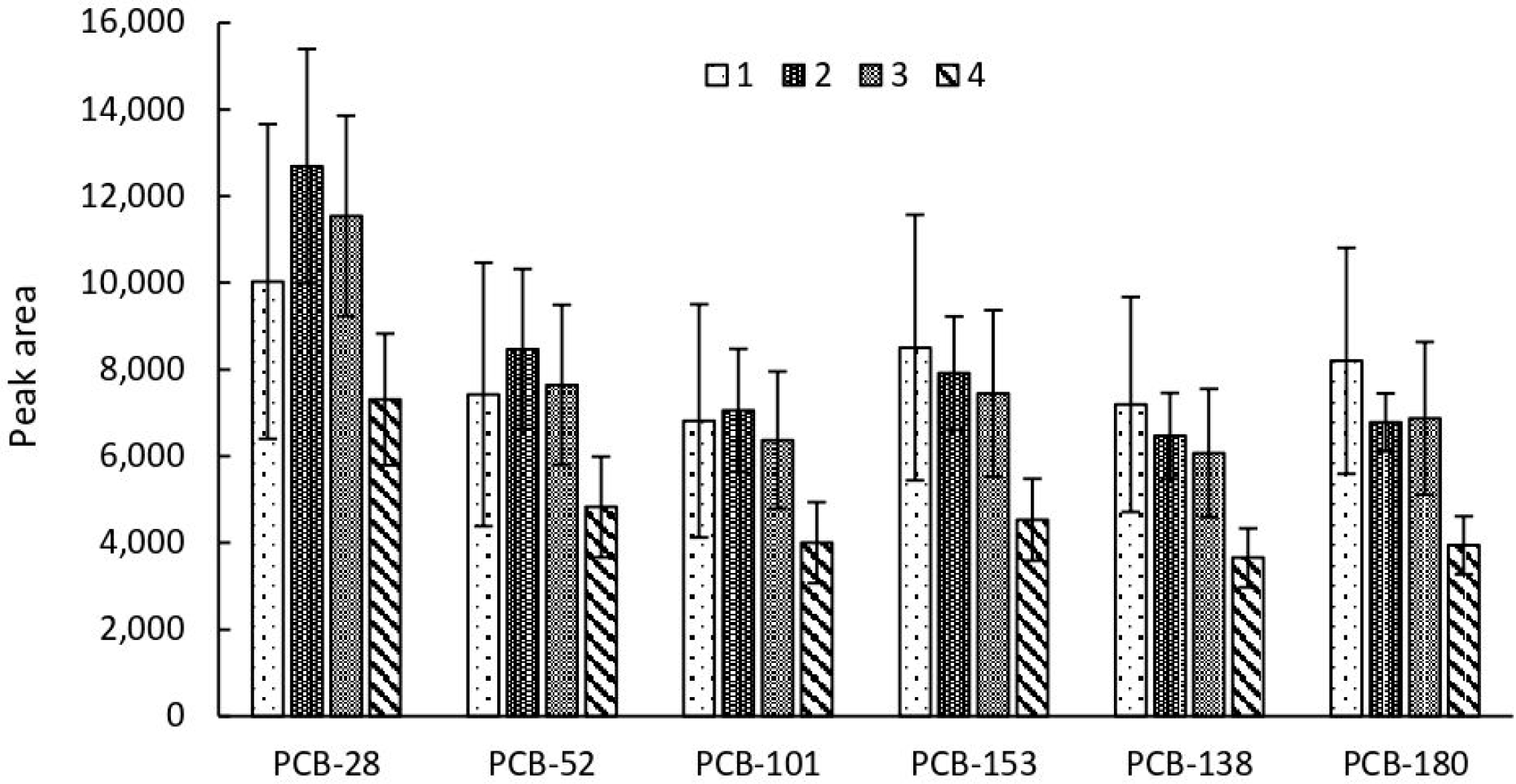
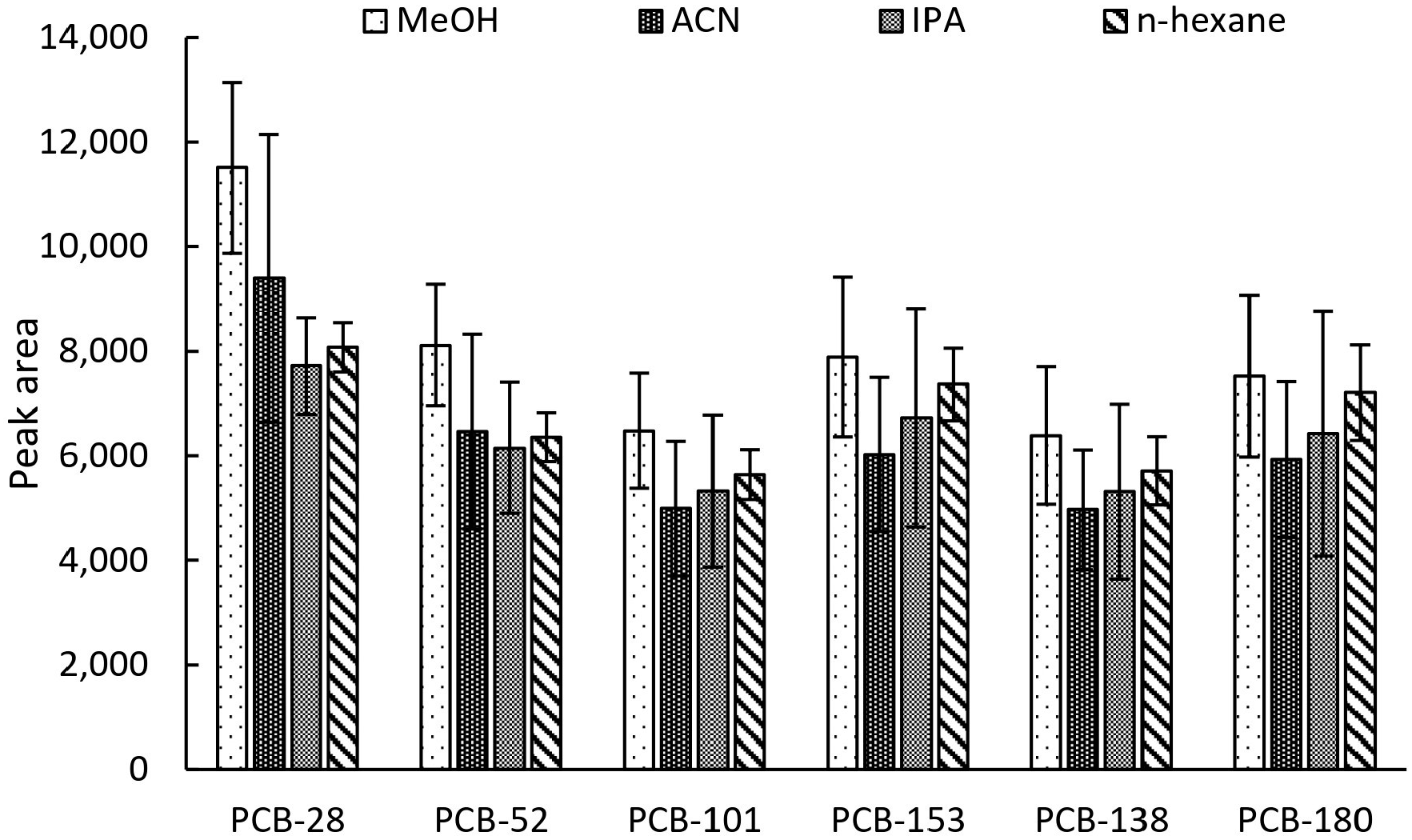
2.2. Optimization of the Extraction Procedure
2.3. Impact of C18 Functionalization on Extraction Performance
2.4. Validation Study
2.5. Real Sample Analysis
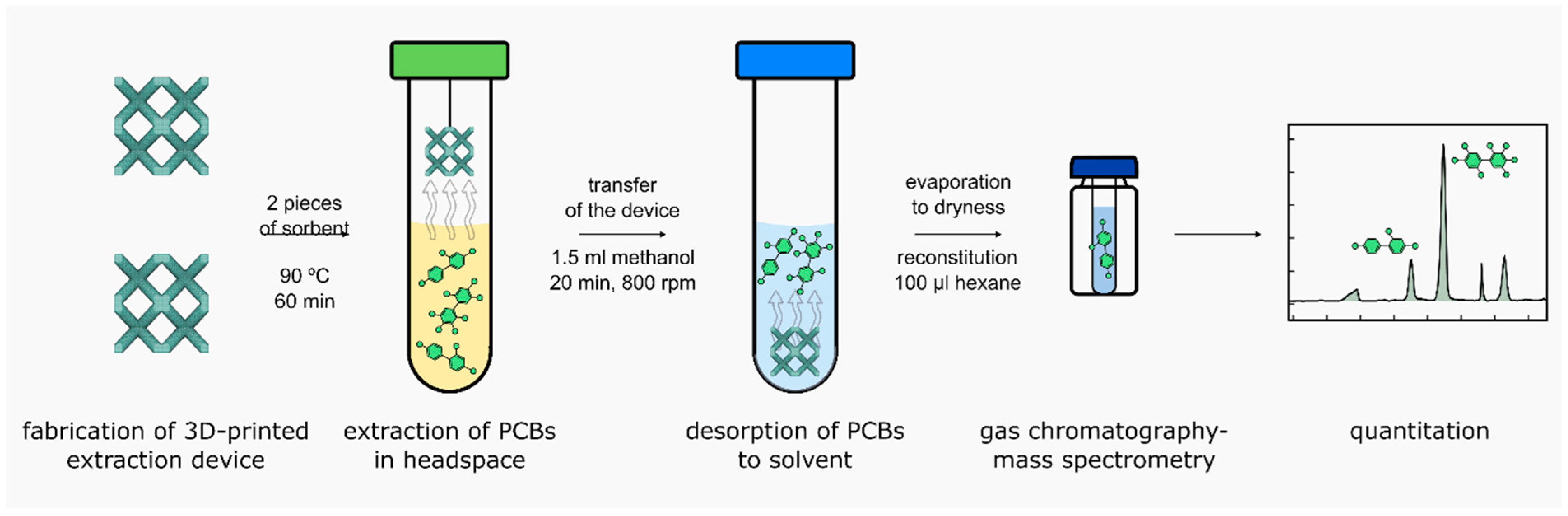
3. Materials and Methods
3.1. Chemicals
3.2. Samples
3.3. 3D Printing Procedure—Fabrication of a Sorbent Device
3.4. Primary Investigation of Sorption Properties Under Different Conditions
3.5. Sample Preparation Procedure—Final Conditions
3.6. Optimization of the Sample Preparation Procedure
3.7. Gas Chromatography–Mass Spectrometry
3.8. Validation Study
3.8.1. Calibration Curve and Range
3.8.2. The Lower Limit of Quantitation
3.8.3. Precision and Accuracy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Markowitz, G. From Industrial Toxins to Worldwide Pollutants: A Brief History of Polychlorinated Biphenyls. Public Health Rep. 2018, 133, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Melymuk, L.; Blumenthal, J.; Sáňka, O.; Shu-Yin, A.; Singla, V.; Šebková, K.; Fedinick, K.P.; Diamond, M.L. Persistent Problem: Global Challenges to Managing PCBs. Environ. Sci. Technol. 2022, 56, 9029–9040. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, G.; Rosner, D. Monsanto, PCBs, and the creation of a “world-wide ecological problem”. J. Public Health Policy 2018, 39, 463–540. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.; Snyder, R. PCBs in Construction Materials: Old Chemical, New Context. Environ. Claims J. 2015, 27, 244–263. [Google Scholar] [CrossRef]
- Borlakoglu, J.T.; Haegele, K.D. Comparative aspects on the bioaccumulation, metabolism and toxicity with PCBs. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991, 100, 327–338. [Google Scholar] [CrossRef]
- Beyer, A.; Biziuk, M. Environmental fate and global distribution of polychlorinated biphenyls. Rev. Environ. Contam. Toxicol. 2009, 201, 137–158. [Google Scholar] [CrossRef]
- Zhu, M.; Yuan, Y.; Yin, H.; Guo, Z.; Wei, X.; Qi, X.; Liu, H.; Dang, Z. Environmental contamination and human exposure of polychlorinated biphenyls (PCBs) in China: A review. Sci. Total Environ. 2022, 805, 150270. [Google Scholar] [CrossRef]
- Berntssen, M.H.G.; Maage, A.; Lundebye, A.K. Chemical Contamination of Finfish with Organic Pollutants and Metals. In Chemical Contaminants and Residues in Food, 2nd ed.; Elsevier: Cambridge, UK, 2017; pp. 517–551. [Google Scholar] [CrossRef]
- Elangovan, S.; Babu Soundra Pandian, G.S.J.; Joshi, S.J.; Elangovan, S.; Pandian, S.B.S.; Joshi, S.J. Polychlorinated Biphenyls (PCBs): Environmental Fate, Challenges and Bioremediation; Springer: Singapore, 2019; pp. 165–188. [Google Scholar] [CrossRef]
- Learn About Polychlorinated Biphenyls|US EPA, (n.d.). Available online: https://www.epa.gov/pcbs/learn-about-polychlorinated-biphenyls (accessed on 12 June 2024).
- Safe, S.; Hutzinger, O. Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): Biochemistry, toxicology, and mechanism of action. Crit. Rev. Toxicol. 1984, 13, 319–395. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Loomis, D.; Grosse, Y.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. Lancet Oncol. 2013, 14, 287–288. [Google Scholar] [CrossRef]
- McFarland, V.A.; Clarke, J.U. Environmental occurrence, abundance, and potential toxicity of polychlorinated biphenyl congeners: Considerations for a congener-specific analysis. Environ. Health Perspect. 1989, 81, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Sobek, A.; McLachlan, M.S.; Borgå, K.; Asplund, L.; Lundstedt-Enkel, K.; Polder, A.; Gustafsson, Ö. A comparison of PCB bioaccumulation factors between an arctic and a temperate marine food web. Sci. Total Environ. 2010, 408, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Adamcakova-Dodd, A.; Lehmler, H.J.; Hu, D.; Kania-Korwel, I.; Hornbuckle, K.C.; Thorne, P.S. Time course of congener uptake and elimination in rats after short-term inhalation exposure to an airborne polychlorinated biphenyl (PCB) mixture. Environ. Sci. Technol. 2010, 44, 6893–6900. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, N.F.; Ching-López, A.; de Labry Lima, A.O.; Salamanca-Fernández, E.; Pérez-Gómez, B.; Jiménez-Moleón, J.J.; Sánchez, M.J.; Rodríguez-Barranco, M. Relationship between exposure to mixtures of persistent, bioaccumulative, and toxic chemicals and cancer risk: A systematic review. Environ. Res. 2020, 188, 109787. [Google Scholar] [CrossRef]
- Chan-Hon-Tong, A.; Charles, M.A.; Forhan, A.; Heude, B.; Sirot, V. Exposure to food contaminants during pregnancy. Sci. Total Environ. 2013, 458–460, 27–35. [Google Scholar] [CrossRef]
- Genuis, S.J.; Beesoon, S.; Birkholz, D. Biomonitoring and Elimination of Perfluorinated Compounds and Polychlorinated Biphenyls through Perspiration: Blood, Urine, and Sweat Study. Int. Sch. Res. Not. 2013, 2013, 483832. [Google Scholar] [CrossRef]
- Poli, D.; Caglieri, A.; Goldoni, M.; Castoldi, A.F.; Coccini, T.; Roda, E.; Vitalone, A.; Ceccatelli, S.; Mutti, A. Single step determination of PCB 126 and 153 in rat tissues by using solid phase microextraction/gas chromatography–mass spectrometry: Comparison with solid phase extraction and liquid/liquid extraction. J. Chromatogr. B 2009, 877, 773–783. [Google Scholar] [CrossRef]
- Zhao, A.; Wang, X.; Ma, M.; Wang, W.; Sun, H.; Yan, Z.; Xu, Z.; Wang, H. Temperature-assisted ionic liquid dispersive liquid-liquid microextraction combined with high performance liquid chromatography for the determination of PCBs and PBDEs in water and urine samples. Microchim. Acta 2012, 177, 229–236. [Google Scholar] [CrossRef]
- Barri, T.; Bergström, S.; Norberg, J.; Jönsson, J.Å. Miniaturized and Automated Sample Pretreatment for Determination of PCBs in Environmental Aqueous Samples Using an On-Line Microporous Membrane Liquid-Liquid Extraction-Gas Chromatography System. Anal. Chem. 2004, 76, 1928–1934. [Google Scholar] [CrossRef]
- Koel, M.; Kaljurand, M. Application of the principles of green chemistry in analytical chemistry. Pure Appl. Chem. 2006, 78, 1993–2002. [Google Scholar] [CrossRef]
- Westbom, R.; Thörneby, L.; Zorita, S.; Mathiasson, L.; Björklund, E. Development of a solid-phase extraction method for the determination of polychlorinated biphenyls in water. J. Chromatogr. A 2004, 1033, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Quinete, N.; Esser, A.; Kraus, T.; Schettgen, T. Determination of hydroxylated polychlorinated biphenyls (OH-PCBs) in human urine in a highly occupationally exposed German cohort: New prospects for urinary biomarkers of PCB exposure. Environ. Int. 2016, 97, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.E.; Pyo, H.; Park, S.J.; Lee, W. Solid-phase microextraction with on-fiber derivatization for the determination of hydroxy-polychlorinated biphenyl compounds in urine. Anal. Chim. Acta 2005, 539, 55–60. [Google Scholar] [CrossRef]
- Simsek, I.; Kuzukiran, O.; Yurdakok-Dikmen, B.; Snoj, T.; Filazi, A. Determination of Persistent Organic Pollutants (POPs) in Propolis by Solid-Phase Extraction (SPE) and Gas Chromatography—Mass Spectrometry (GC-MS). Anal. Lett. 2020, 54, 1668–1682. [Google Scholar] [CrossRef]
- Ridgway, K.; Lalljie, S.P.D.; Smith, R.M. Sample preparation techniques for the determination of trace residues and contaminants in foods. J. Chromatogr. A 2007, 1153, 36–53. [Google Scholar] [CrossRef]
- Szynkiewicz, D.; Ulenberg, S.; Georgiev, P.; Hejna, A.; Mikolaszek, B.; Bączek, T.; Baron, G.V.; Denayer, J.F.M.; Desmet, G.; Belka, M. Development of a 3D-Printable, Porous, and Chemically Active Material Filled with Silica Particles and its Application to the Fabrication of a Microextraction Device. Anal. Chem. 2023, 95, 11632–11640. [Google Scholar] [CrossRef]
- Belka, M.; Bączek, T. Additive manufacturing and related technologies—The source of chemically active materials in separation science. TrAC Trends Anal. Chem. 2021, 142, 116322. [Google Scholar] [CrossRef]
- Capel, A.J.; Rimington, R.P.; Lewis, M.P.; Christie, S.D.R. 3D printing for chemical, pharmaceutical and biological applications. Nat. Rev. Chem. 2018, 2, 422–436. [Google Scholar] [CrossRef]
- Georgiev, P.; Belka, M.; Kroll, D.; Bączek, T.; Opiełka, M.; Rutkowska, A.; Ulenberg, S. 3D-printed extraction devices fabricated from silica particles suspended in acrylate resin. J. Chromatogr. A 2024, 1717, 464671. [Google Scholar] [CrossRef]
- Jorissen, J. Literature review: Outcomes associated with postnatal exposure to polychlorinated biphenyls (PCBs) via breast milk. Adv. Neonatal Care 2007, 7, 230–237. [Google Scholar] [CrossRef]
- Ma, J.; Qiu, X.; Ren, A.; Jin, L.; Zhu, T. Using placenta to evaluate the polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) exposure of fetus in a region with high prevalence of neural tube defects. Ecotoxicol. Environ. Saf. 2012, 86, 141–146. [Google Scholar] [CrossRef]
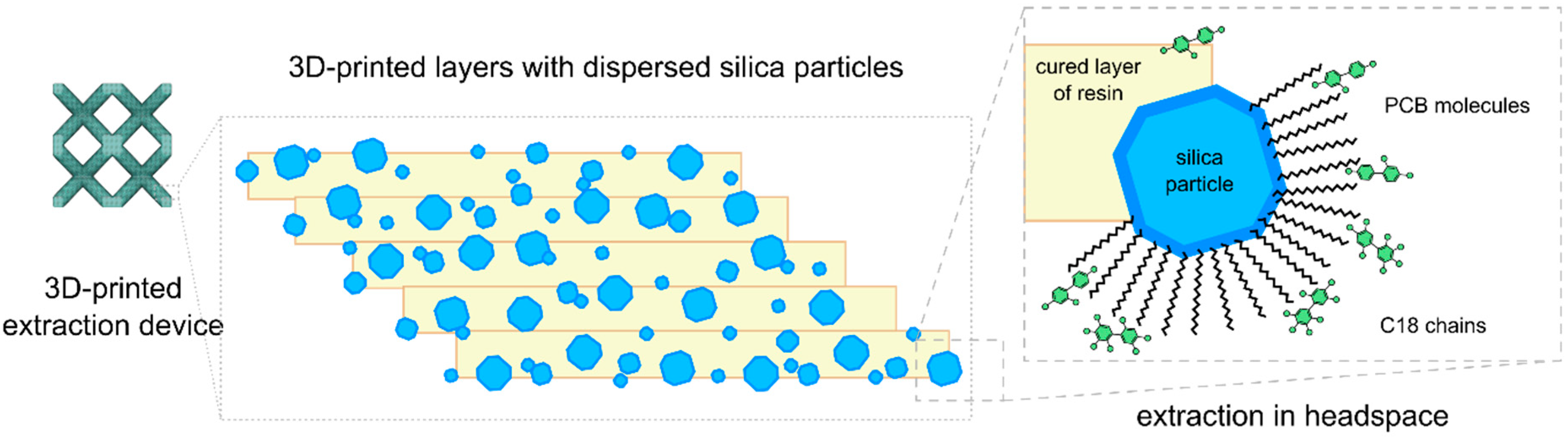

| Extraction Devices Without C18 Particles | Extraction Devices with C18 Particles | |||
|---|---|---|---|---|
| Analyte | Extraction Efficiency [%] | RSD [%] | Extraction Efficiency [%] | RSD [%] |
| PCB-28 | 24 | 12 | 31 | 5 |
| PCB-52 | 30 | 12 | 35 | 3 |
| PCB-101 | 36 | 13 | 40 | 2 |
| PCB-138 | 31 | 32 | 43 | 8 |
| PCB-153 | 32 | 27 | 41 | 8 |
| PCB-180 | 28 | 55 | 43 | 10 |
| Analyte | Calibration Curve Regression Parameters | ||
|---|---|---|---|
| a | B | R2 | |
| PCB-28 | 0.0368 | 0.00212 | 0.969 |
| PCB-52 | 0.2130 | 0.0429 | 0.989 |
| PCB-101 | 0.0179 | 6.769 × 10−4 | 0.961 |
| PCB-138 | 0.0118 | 4.943 × 10−4 | 0.975 |
| PCB-153 | 0.0138 | 7.003 × 10−4 | 0.957 |
| PCB-180 | 0.0704 | 0.00692 | 0.989 |
| Accuracy | Precision | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-Day [%] (n = 3) | Inter-Day [%] (n = 9) | Intra-Day [%] (n = 3) | Inter-Day [%] (n = 9) | |||||||||
| Concentration [pg/mL] | 15 | 60 | 90 | 15 | 60 | 90 | 15 | 60 | 90 | 15 | 60 | 90 |
| PCB-28 | 105 | 98 | 103 | 109 | 104 | 104 | 11 | 7 | 5 | 14 | 9 | 7 |
| PCB-52 | 97 | 102 | 107 | 103 | 103 | 109 | 8 | 5 | 1 | 11 | 9 | 2 |
| PCB-101 | 110 | 105 | 105 | 114 | 108 | 106 | 10 | 7 | 4 | 12 | 8 | 5 |
| PCB-138 | 107 | 103 | 102 | 112 | 107 | 106 | 7 | 7 | 3 | 9 | 9 | 5 |
| PCB-153 | 109 | 104 | 102 | 115 | 108 | 105 | 11 | 8 | 5 | 14 | 8 | 6 |
| PCB-180 | 109 | 100 | 97 | 111 | 104 | 96 | 8 | 8 | 5 | 14 | 9 | 7 |
| Patient Number | PCB-28 [pg/mL] | PCB-52 [pg/mL] | PCB-101 [pg/mL] | PCB-138 [pg/mL] | PCB-153 [pg/mLl] | PCB-180 [pg/mL] |
|---|---|---|---|---|---|---|
| 1 | 43.01 | <LLOQ | 50.36 | 73.15 | 65.71 | <LLOQ |
| 2 | 29.90 | 10.81 | 33.31 | 41.39 | 40.51 | <LLOQ |
| 3 | 31.16 | <LLOQ | 35.88 | 52.78 | 46.79 | <LLOQ |
| 4 | 33.35 | <LLOQ | 36.94 | 51.01 | 53.07 | <LLOQ |
| 5 | 29.62 | <LLOQ | 30.79 | 41.35 | 37.37 | <LLOQ |
| Analyte | Abbreviation | Retention Time [min] | Quantifier Ion [m/z] | Qualifier Ions [m/z] | Boiling Point [°C] |
|---|---|---|---|---|---|
| 2,4,4′-trichlorobiphenyl | PCB-28 | 12.14 | 256 | 186; 150 | 334.36 |
| 2,3,3′,5′-tetrachlorobiphenyl | PCB-58 | 12.73 | 292 | 186; 150 | 374.95 |
| 2,2′,4,5,5′-pentachlorobiphenyl | PCB-101 | 14.95 | 326 | 128; 254 | 412.3 |
| 2,2′,4,4′,5,5′-hexachlorobiphenyl | PCB-153 | 16.93 | 360 | 145; 290 | 446.99 |
| 2,2′,3,4,4′,5′-hexachlorobiphenyl | PCB-138 | 17.23 | 360 | 290; 145 | 446 |
| 2,2′,3,4,4′,5,5′-heptachlorobiphenyl | PCB-180 | 22.39 | 394 | 252; 324 | 479 |
| 2′-chloro-2,3,4,5,6-pentadeuterio-1,1′-biphenyl (internal standard) | PCB-1 | 6.48 | 193 | 195; 157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev, P.; Belka, M.; Ulenberg, S.; Kroll, D.; Marciniak, B.; Drążkowska, I.; Bączek, T.; Płotka-Wasylka, J. Headspace Extraction onto a 3D-Printed Device for GC-MS Quantification of Polychlorinated Biphenyls in Newborn Urine. Int. J. Mol. Sci. 2025, 26, 2755. https://doi.org/10.3390/ijms26062755
Georgiev P, Belka M, Ulenberg S, Kroll D, Marciniak B, Drążkowska I, Bączek T, Płotka-Wasylka J. Headspace Extraction onto a 3D-Printed Device for GC-MS Quantification of Polychlorinated Biphenyls in Newborn Urine. International Journal of Molecular Sciences. 2025; 26(6):2755. https://doi.org/10.3390/ijms26062755
Chicago/Turabian StyleGeorgiev, Paweł, Mariusz Belka, Szymon Ulenberg, Dagmara Kroll, Bartosz Marciniak, Izabela Drążkowska, Tomasz Bączek, and Justyna Płotka-Wasylka. 2025. "Headspace Extraction onto a 3D-Printed Device for GC-MS Quantification of Polychlorinated Biphenyls in Newborn Urine" International Journal of Molecular Sciences 26, no. 6: 2755. https://doi.org/10.3390/ijms26062755
APA StyleGeorgiev, P., Belka, M., Ulenberg, S., Kroll, D., Marciniak, B., Drążkowska, I., Bączek, T., & Płotka-Wasylka, J. (2025). Headspace Extraction onto a 3D-Printed Device for GC-MS Quantification of Polychlorinated Biphenyls in Newborn Urine. International Journal of Molecular Sciences, 26(6), 2755. https://doi.org/10.3390/ijms26062755






