Spermidine as a Potential Protective Agents Against Poly(I:C)-Induced Immune Response, Oxidative Stress, Apoptosis, and Testosterone Decrease in Yak Leydig Cells
Abstract
1. Introduction
2. Results
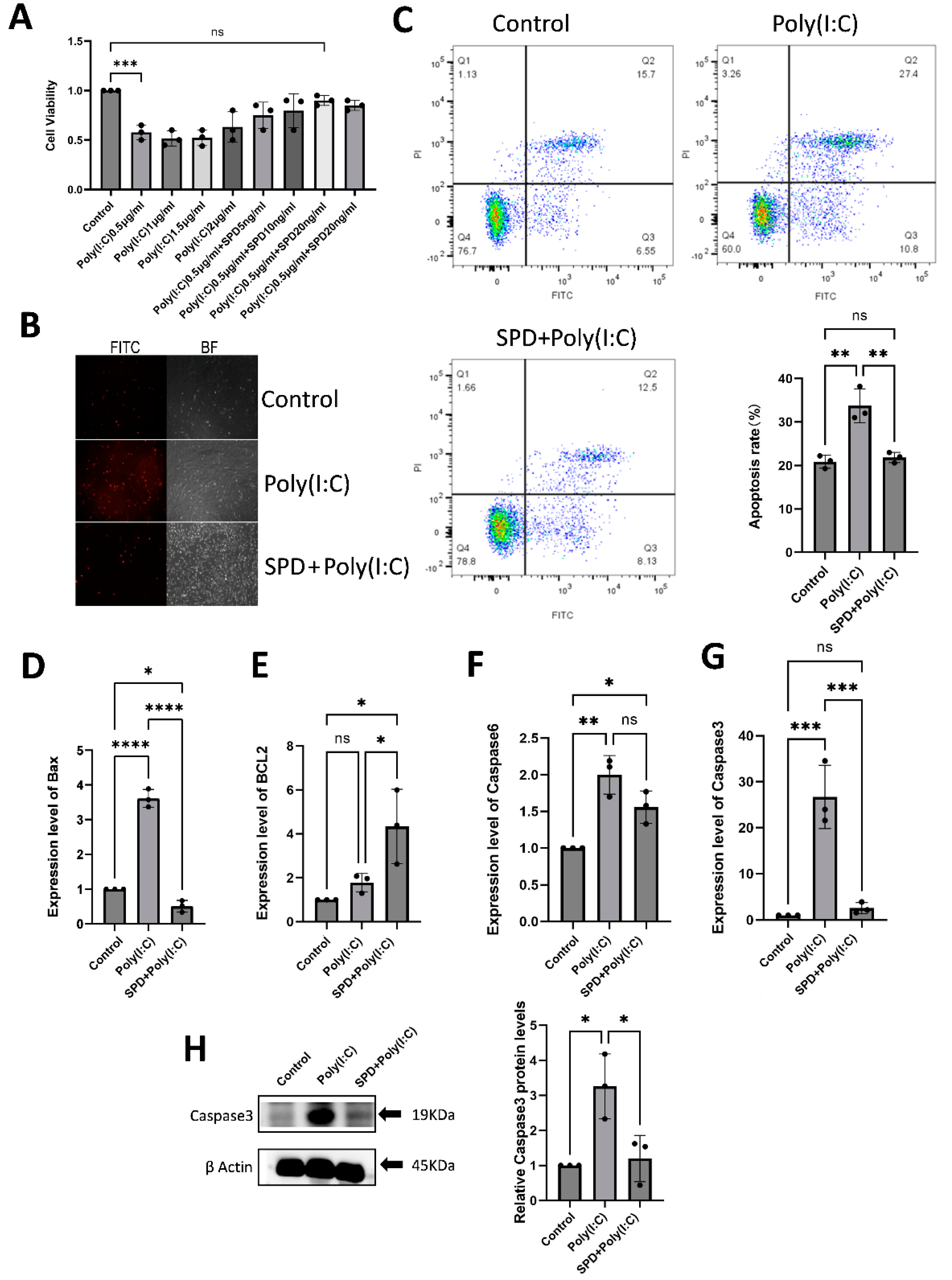
2.1. In Vitro Toxicity Validation of SPD
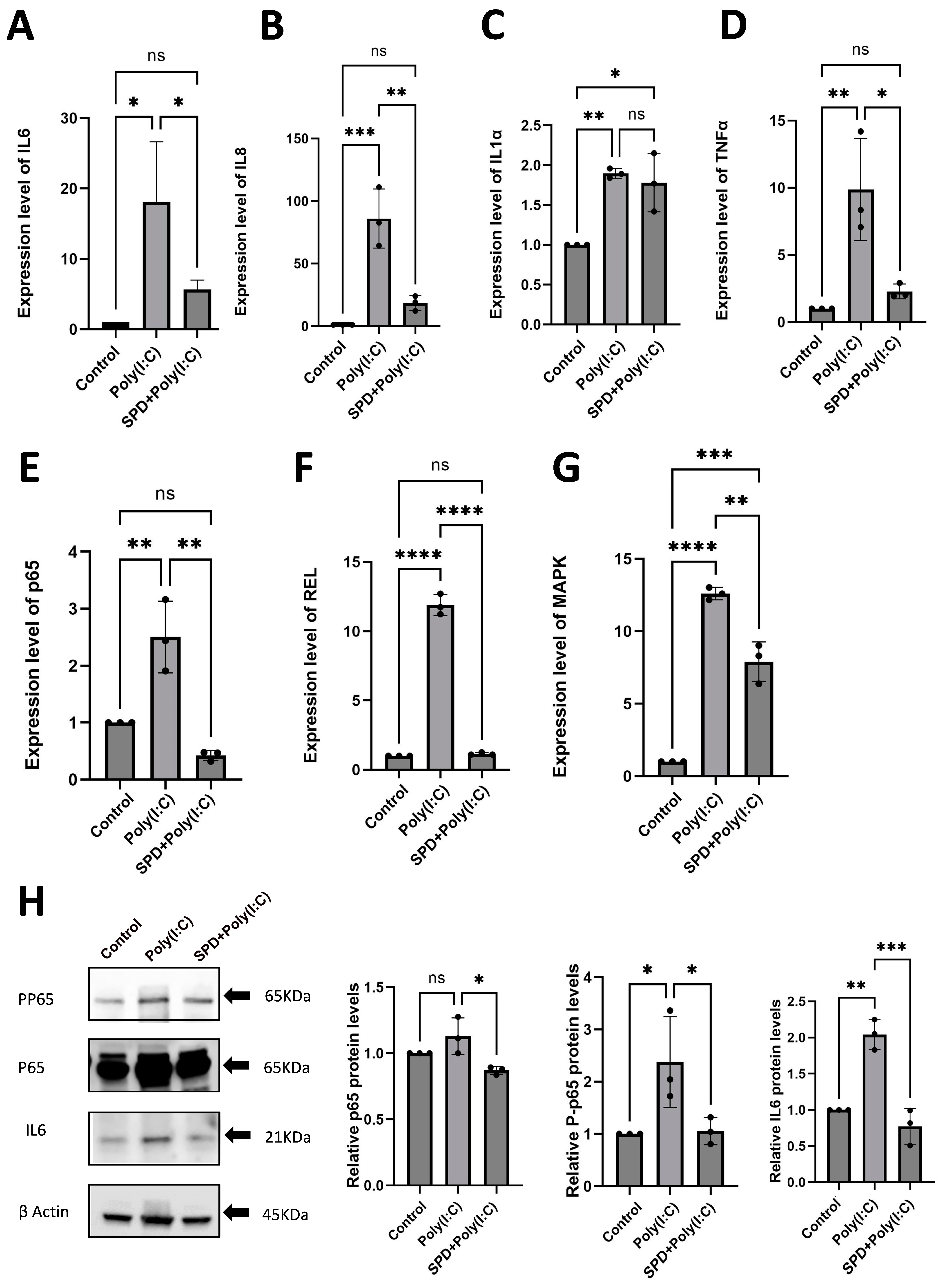
2.2. Poly(I:C) Induces Immune Response, Apoptosis, and Functional Damage in LCs
2.3. SPD Attenuates Poly(I:C)-Induced Immune Response in Leydig Cell
2.4. SPD Recuses Poly(I:C)-Induced LC Apoptosis
2.5. SPD Alleviated Poly(I:C)-Induced Oxidative Stress of LCs
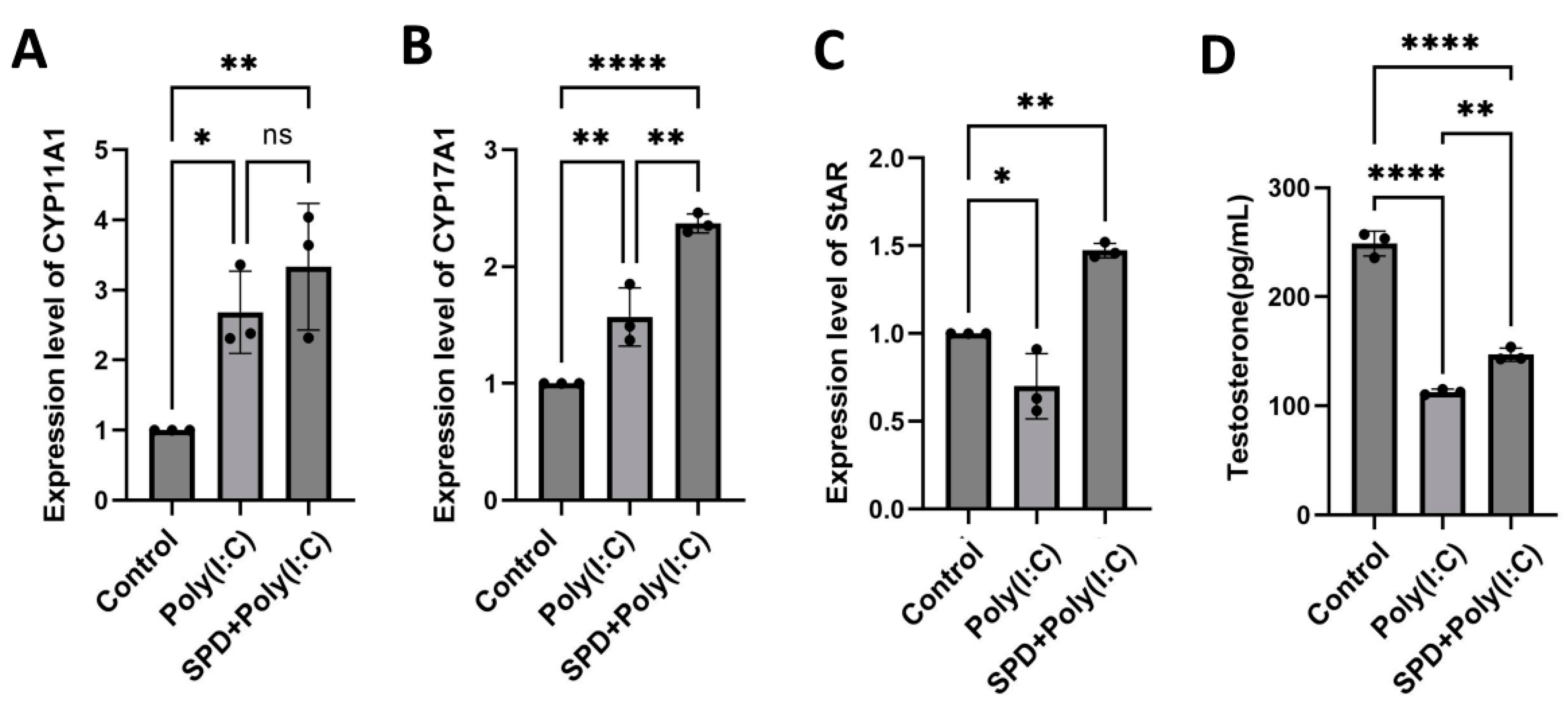
2.6. SPD Alleviated the Decrease in Testosterone Synthesis Caused by Poly(I:C) in LCs
2.7. Expression Trends and Pathway Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Isolation and Identification of LCs
4.2. Identification and Preservation of Testicular LCs
4.3. Experiment Design
4.4. Safety Assessment of Spermidine
4.5. Cell Viability Analysis
4.6. Measurement of Cell Apoptosis
4.7. RT-qPCR
4.8. ELISA for the Detection of SOD, CAT, GSH, MDA and Testosterone Contents in LCs
4.9. Western Immunoblotting
4.10. RNA-Seq and Data Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IL-8 | Interleukin-8 |
| TNF | Tumor necrosis factor |
| NF-κB | Nuclear factor kappa B |
| RELA | V-rel avian reticuloendotheliosis viral oncogene homolog A (part of NF-κB) |
| REL | v-Rel avian reticuloendotheliosis viral oncogene homolog |
| BAX | Bcl-2-associated X protein |
| BCL-2 | B-cell lymphoma 2 |
| Caspase-3 | Caspase-3 |
| Caspase-6 | Caspase-6 |
| CYP11A1 | Cytochrome P450 family 11 subfamily A member 1 |
| CYP17A1 | Cytochrome P450 family 17 subfamily A member 1 |
| StAR | Steroidogenic acute regulatory protein |
| SPD | Spermidine |
| GSH | Glutathione |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| CAT | Catalase |
| CCK-8 | Cell Counting Kit-8 |
| cAMP | Cyclic adenosine monophosphate |
| PARP | Poly ADP-ribose polymerase |
| TLR3 | Toll-like receptor 3 |
| MAPK | Mitogen-activated protein kinase |
| DMEM | Dulbecco’s Modified Eagle Medium |
| Poly(I:C) | Polyinosinic-Polycytidylic Acid |
| p-p65 | Phosphorylated p65 |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| RBC | Red blood cell count |
| HGB | Hemoglobin |
| HCT | Hematocrit |
| MCV | Mean corpuscular volume |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
References
- Leisegang, K.; Henkel, R. The in vitro modulation of steroidogenesis by inflammatory cytokines and insulin in TM3 Leydig cells. Reprod. Biol. Endocrinol. 2018, 16, 26. [Google Scholar] [CrossRef]
- Riris, A.A.I.D.C.; I’Tishom, R.; Khaerunnisa, S. Role of antioxidant to protect Leydig cells induced by reactive oxygen species: A literature review. Qanun Med. Med. J. Fac. Med. Muhammadiyah Surabaya 2021, 5, 49. [Google Scholar] [CrossRef]
- Xia, K.; Chen, H.; Wang, J.; Feng, X.; Gao, Y.; Wang, Y.; Deng, R.; Wu, C.; Luo, P.; Zhang, M.; et al. Restorative functions of Autologous Stem Leydig Cell transplantation in a Testosterone-deficient non-human primate model. Theranostics 2020, 10, 8705–8720. [Google Scholar] [CrossRef] [PubMed]
- Midzak, A.S.; Chen, H.; Papadopoulos, V.; Zirkin, B.R. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol. Cell. Endocrinol. 2009, 299, 23–31. [Google Scholar] [CrossRef]
- Miller, W.L.; Bose, H.S. Early steps in steroidogenesis: Intracellular cholesterol trafficking. J. Lipid Res. 2011, 52, 2111–2135. [Google Scholar] [CrossRef]
- Rone, M.B.; Fan, J.; Papadopoulos, V. Cholesterol transport in steroid biosynthesis: Role of protein–protein interactions and implications in disease states. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2009, 1791, 646–658. [Google Scholar] [CrossRef]
- Chung, J.-Y.; Brown, S.; Chen, H.; Liu, J.; Papadopoulos, V.; Zirkin, B. Effects of pharmacologically induced Leydig cell testosterone production on intratesticular testosterone and spermatogenesis†. Biol. Reprod. 2020, 102, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Pretto, S.; Tagliabue, E.; Balsari, A.; Sfondrini, L. Exploiting poly(I:C) to induce cancer cell apoptosis. Cancer Biol. Ther. 2017, 18, 747–756. [Google Scholar] [CrossRef]
- Liao, C.-Y.; Kummert, O.M.P.; Bair, A.M.; Alavi, N.; Alavi, J.; Miller, D.M.; Bagga, I.; Schempf, A.M.; Hsu, Y.-M.; Woods, B.D.; et al. The Autophagy Inducer Spermidine Protects Against Metabolic Dysfunction During Overnutrition. J. Gerontol. Ser. A 2021, 76, 1714–1725. [Google Scholar] [CrossRef]
- Sano, H.; Kratz, A.; Nishino, T.; Imamura, H.; Yoshida, Y.; Shimizu, N.; Kitano, H.; Yachie, A. Nicotinamide mononucleotide (NMN) alleviates the poly(I:C)-induced inflammatory response in human primary cell cultures. Sci. Rep. 2023, 13, 11765. [Google Scholar] [CrossRef]
- Chai, N.; Zhang, H.; Li, L.; Yu, X.; Liu, Y.; Lin, Y.; Wang, L.; Yan, J.; Nikolaevna, S.E.; Zhao, Y. Spermidine Prevents Heart Injury in Neonatal Rats Exposed to Intrauterine Hypoxia by Inhibiting Oxidative Stress and Mitochondrial Fragmentation. Biol. Chem. 2019, 2019, 5406468. [Google Scholar]
- Jiang, D.; Wang, X.; Zhou, X.; Wang, Z.; Li, S.; Sun, Q.; Jiang, Y.; Ji, C.; Ling, W.; An, X.; et al. Spermidine alleviating oxidative stress and apoptosis by inducing autophagy of granulosa cells in Sichuan white geese. Poult. Sci. 2023, 102, 102879. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Cha, H.J.; Han, M.H.; Hwang, S.J.; Lee, D.S.; Yoo, J.S.; Choi, I.W.; Kim, S.; Kim, H.S.; Kim, G.Y.; et al. Spermidine Protects Against Oxidative Stress in Inflammation Models Using Mac-rophages and Zebrafish. Cell Physiol. Biochem. 2018, 26, 146–156. [Google Scholar]
- Liu, R.; Li, X.; Ma, H.; Yang, Q.; Shang, Q.; Song, L.; Zheng, Z.; Zhang, S.; Pan, Y.; Huang, P.; et al. Spermidine endows macrophages anti-inflammatory properties by inducing mitochondrial superox-ide-dependent AMPK activation, Hif-1α upregulation and autophagy. Free Rad. Biol. Med. 2020, 161, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Niechcial, A.; Schwarzfischer, M.; Wawrzyniak, M.; Atrott, K.; Laimbacher, A.; Morsy, Y.; Katkeviciute, E.; Häfliger, J.; Westermann, P.; Akdis, C.A.; et al. Spermidine Ameliorates Colitis via Induction of Anti-Inflammatory Macrophages and Prevention of Intestinal Dysbiosis. J. Crohn’s Colitis 2023, 17, 1489–1503. [Google Scholar] [CrossRef]
- Yu, L.; Pan, J.; Guo, M.; Duan, H.; Zhang, H.; Narbad, A.; Zhai, Q.; Tian, F.; Chen, W. Gut microbiota and anti-aging: Focusing on spermidine. Crit. Rev. Food Sci. Nutr. 2023, 64, 10419–10437. [Google Scholar] [CrossRef]
- Mandal, S.; Mandal, A.; Park, M.H. Depletion of the polyamines spermidine and spermine by overexpression of spermidine/spermine N1-acetyltransferase 1 (SAT1) leads to mitochondria-mediated apoptosis in mammalian cells. Biochem. J. 2015, 468, 435–447. [Google Scholar] [CrossRef]
- Jiang, D.; Sun, Q.; Jiang, Y.; Zhou, X.; Kang, L.; Wang, Z.; Wang, X.; An, X.; Ji, C.; Ling, W.; et al. Effects of exogenous spermidine on autophagy and antioxidant capacity in ovaries and granulosa cells of Sichuan white geese. J. Anim. Sci. 2023, 101, skad301. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; Liu, J.; Ye, J.; Xu, Y.; Wang, Z.; Yu, J.; Ye, D.; Zhao, M.; Feng, Y.; et al. The role of interleukin-10 family members in cardiovascular diseases. Int. Immunopharmacol. 2021, 94, 107475. [Google Scholar] [CrossRef]
- Mori, H.; Murakami, M.; Tsuda, T.; Kameda, K.; Utsunomiya, R.; Masuda, K.; Shiraishi, K.; Dai, X.; Tohyama, M.; Nakaoka, H.; et al. Reduced-HMGB1 suppresses poly(I:C)-induced inflammation in keratinocytes. J. Dermatol. Sci. 2018, 90, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-H.; Luo, M.-Y.; Liang, N.; Gong, S.-X.; Chen, W.; Huang, W.-Q.; Tian, Y.; Wang, A.-P. Interleukin-6: A Novel Target for Cardio-Cerebrovascular Diseases. Front. Pharmacol. 2021, 12, 745061. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Meng, L.; Chen, L.; Liang, Y.; Wang, B.; Shao, Q.; Wang, H.; Yang, X. IL-6 Expression Promoted by Poly(I:C) in Cervical Cancer Cells Regulates Cytokine Expression and Recruitment of Macrophages. J. Cell Mol. Med. 2020, 24, 101–110. [Google Scholar] [CrossRef]
- Meng, X.; Cui, X.; Shao, X.; Liu, Y.; Xing, Y.; Smith, V.; Xiong, S.; Macip, S.; Chen, Y. poly(I:C) synergizes with proteasome inhibitors to induce apoptosis in cervical cancer cells. Transl. Oncol. 2022, 18, 101362. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Reports, L.J.M.M. Poly (I:C) Transfection Induces Mitochondrial-Mediated Apoptosis in Cervical Cancer. Cancer Lett. 2016, 13, 223–230. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, Y.; Lv, Q.; Liu, B.; Jin, M.; Zhang, W.; He, Q.; Deng, M.; Liu, X.; Li, G.; et al. Toll-like Receptor 3 (TLR3) Induces Apoptosis via Death Receptors and Mitochondria by Up-regulating the Transactivating p63 Isoform α (TAP63α). J. Biol. Chem. 2011, 286, 15918–15928. [Google Scholar] [CrossRef]
- Chamoto, K.; Zhang, B.; Tajima, M.; Honjo, T.; Fagarasan, S. Spermidine—An old molecule with a new age-defying immune function. Trends Cell Biol. 2023, 34, 363–370. [Google Scholar] [CrossRef]
- Puleston, D.J.; Buck, M.D.; Geltink, R.I.K.; Kyle, R.L.; Caputa, G.; O’sullivan, D.; Cameron, A.M.; Castoldi, A.; Musa, Y.; Kabat, A.M.; et al. Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metab. 2019, 30, 352–363.e8. [Google Scholar] [CrossRef]
- Yang, Q.; Zheng, C.; Cao, J.; Cao, G.; Shou, P.; Lin, L.; Velletri, T.; Jiang, M.; Chen, Q.; Han, Y.; et al. Spermidine Alleviates Experimental Autoimmune Encephalomyelitis by Inducing Inhibitory Macrophages. Cell Death Differ. 2016, 23, 1850–1861. [Google Scholar] [CrossRef]
- Li, X.; Zhou, X.; Liu, X.; Li, X.; Jiang, X.; Shi, B.; Wang, S. Spermidine protects against acute kidney injury by modulating macrophage NLRP3 inflammasome activation and mitochondrial respiration in an eIF5A hypusination-related pathway. Mol. Med. 2022, 28, 103. [Google Scholar] [CrossRef]
- Puleston, D.J.; Zhang, H.; Powell, T.J.; Lipina, E.; Sims, S.; Panse, I.; Watson, A.S.; Cerundolo, V.; Townsend, A.R.; Klenerman, P.; et al. Autophagy is a critical regulator of memory CD8+ T cell formation. eLife 2014, 3, e03706. [Google Scholar] [CrossRef] [PubMed]
- Carriche, G.M.; Almeida, L.; Stüve, P.; Velasquez, L.; Dhillon-LaBrooy, A.; Roy, U.; Lindenberg, M.; Strowig, T.; Plaza-Sirvent, C.; Schmitz, I.; et al. R[M1] egulating T-cell differentiation through the polyamine spermidine. J. Allergy Clin. Immunol. 2021, 147, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Alsaleh, G.; Feltham, J.; Sun, Y.; Napolitano, G.; Riffelmacher, T.; Charles, P.; Frau, L.; Hublitz, P.; Yu, Z.; et al. Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell 2019, 76, 110–125.e9. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.L.; Assmann, N.; O’Connor, E.; Keane, C.; Walls, J.; Choi, C.; Oefner, P.J.; Gardiner, C.M.; Dettmer, K.; Finlay, D.K. De novo polyamine synthesis supports metabolic and functional responses in activated murine NK cells. Eur. J. Immunol. 2020, 51, 91–102. [Google Scholar] [CrossRef]
- Hansen, M. Regulation of Autophagy in Aging and Disease. Autophagy 2020, 16, 809–810. [Google Scholar]
- He, R.; Wang, Z.; Cui, M.; Liu, S.; Wu, W.; Chen, M.; Wu, Y.; Qu, Y.; Lin, H.; Chen, S.; et al. HIF1A Alleviates compression-induced apoptosis of nucleus pulposus derived stem cells via upregulating autophagy. Autophagy 2021, 17, 3338–3360. [Google Scholar] [CrossRef]
- Yuan, X.; Tian, G.G.; Pei, X.; Hu, X.; Wu, J. Spermidine Induces Cytoprotective Autophagy of Female Germline Stem Cells in Vitro and Ame-liorates Aging Caused by Oxidative Stress through Upregulated Sequestosome-1/p62 Expression. Aging Cell 2021, 11, 107. [Google Scholar]
- Yan, J.; Yan, J.Y.; Wang, Y.X.; Ling, Y.-N.; Song, X.-D.; Wang, S.-Y.; Liu, H.-Q.; Liu, Q.-C.; Zhang, Y.; Yang, P.-Z.; et al. Spermidine-enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting the AMPK/mTOR signalling pathway. Br. J. Pharmacol. 2019, 176, 3126–3142. [Google Scholar] [CrossRef]
- Niu, C.; Jiang, D.; Guo, Y.; Wang, Z.; Sun, Q.; Wang, X.; Ling, W.; An, X.; Ji, C.; Li, S.; et al. Spermidine suppresses oxidative stress and ferroptosis by Nrf2/HO-1/GPX4 and Akt/FHC/ACSL4 pathway to alleviate ovarian damage. Life Sci. 2023, 332, 122109. [Google Scholar] [CrossRef]
- Gillies, L.A.; Kuwana, T. Apoptosis Regulation at the Mitochondrial Outer Membrane. J. Cell. Biochem. 2014, 115, 632–640. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.-H.; Hu, W.; Zhang, X.-B.; Wang, W.; Zhang, L.-J. Protective Effect of Adrenomedullin on Rat Leydig Cells from Lipopolysaccharide-Induced Inflammation and Apoptosis via the PI3K/Akt Signaling Pathway ADM on Rat Leydig Cells from Inflammation and Apoptosis. Mediat. Inflamm. 2016, 2016, 7201549. [Google Scholar] [CrossRef] [PubMed]
- Elena, T.; Rosanna, D.P.; Emanuela, M.; Esposito, E.; Virginia, M.; Salvatore, C. Anti-Inflammatory Effects of Adrenomedullin on Acute Lung Injury Induced by Carrageenan in Mice. Mediat. Inflamm. 2012, 2012, 717851. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Aslani, B.A.; Ghobadi, S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016, 146, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Spermidine Rescues Proximal Tubular Cells from Oxidative Stress and Necrosis After Ischemic Acute Kidney Injury. Kidney Int. 2017, 92, 123–134. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Silvestri, Y.; Minguzzi, M.; Santi, S.; Cattini, L.; Filardo, G.; Flamigni, F.; Borzì, R.M. Spermidine rescues the deregulated autophagic response to oxidative stress of osteoarthritic chondrocytes. Free. Radic. Biol. Med. 2020, 153, 159–172. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative Stress in Oocyte Aging and Female Reproduction. J. Cell Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Jiang, D.; Jiang, Y.; Long, S.; Chen, Z.; Li, Y.; Mo, G.; Bai, L.; Hao, X.; Yan, Y.; Li, L.; et al. Spermidine at supraphysiological doses induces oxidative stress and granulosa cell apoptosis in mouse ovaries. Theriogenology 2021, 168, 25–32. [Google Scholar] [CrossRef]
- Das, A.; Roychoudhury, S. Reactive oxygen species in the reproductive system: Sources and physiological roles. Adv. Exp. Med. Biol. 2022, 1358, 9–40. [Google Scholar] [PubMed]
- Kopalli, S.R.; Hwang, S.-Y.; Won, Y.-J.; Kim, S.-W.; Cha, K.-M.; Han, C.-K.; Hong, J.-Y.; Kim, S.-K. Korean red ginseng extract rejuvenates testicular ineffectiveness and sperm maturation process in aged rats by regulating redox proteins and oxidative defense mechanisms. Exp. Gerontol. 2015, 69, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.H.; Abdelkader, N.F.; El-Raouf, O.M.A.; Fawzy, H.M.; El-Denshary, E.-E.S. Carvedilol alleviates testicular and spermatological damage induced by cisplatin in rats via modulation of oxidative stress and inflammation. Arch. Pharmacal Res. 2016, 39, 1693–1702. [Google Scholar] [CrossRef]
- Hu, W.; Shi, L.; Li, M.-Y.; Zhou, P.-H.; Qiu, B.; Yin, K.; Zhang, H.-H.; Gao, Y.; Kang, R.; Qin, S.-L.; et al. Adrenomedullin protects Leydig cells against lipopolysaccharide-induced oxidative stress and inflammatory reaction via MAPK/NF-κB signalling pathways. Sci. Rep. 2017, 7, 16479. [Google Scholar] [CrossRef]
- Tremblay, J.J. Molecular regulation of steroidogenesis in endocrine Leydig cells. Steroids 2015, 103, 3–10. [Google Scholar] [CrossRef]
- Martinot, E.; Boerboom, D. Slit/Robo signaling regulates Leydig cell steroidogenesis. Cell Commun. Signal. 2021, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Manoli, I.; Alesci, S.; Blackman, M.R.; Su, Y.A.; Rennert, O.M.; Chrousos, G.P. Mitochondria as key components of the stress response. Trends Endocrinol. Metab. 2007, 18, 190–198. [Google Scholar] [CrossRef]
- Pegg, A.E. The function of spermine. IUBMB Life 2014, 66, 8–18. [Google Scholar] [CrossRef]
- Ge, R.-S.; Dong, Q.; Sottas, C.M.; Papadopoulos, V.; Zirkin, B.R.; Hardy, M.P. In search of rat stem Leydig cells: Identification, isolation, and lineage-specific development. Proc. Natl. Acad. Sci. USA 2006, 103, 2719–2724. [Google Scholar] [CrossRef] [PubMed]




| Gene Symbol | Primer Sequence (5′-3′) | Annealing Temperature (°C) | Product Size (bp) | Accession Number |
|---|---|---|---|---|
| IL6 | F: CTCGTATGCCAATGCCCTCA R: CCCAGATTGGAAGCATCCGT | 60 °C | 195 | XM_005901249.2 |
| TNFα | F: CTCGTATGCCAATGCCCTCA R: TGGTAGGAGACTGCAATGCG | 60 °C | 174 | NM_173966.3 |
| REL | F: GTAAAGATGCAGTTGCGGCG R: CTCCACAATCCTGCCACAGT | 60 °C | 151 | XM_005889034.1 |
| P65 | F: GCCAGGTTCCAGACCTCTTC R: ATAGTGGGGTGGGTCTTGGT | 60 °C | 187 | XM_005894097.1 |
| IL8 | F: ACCCCAAGGAAAAGTGGGTG R: CCCACACAGTACATGAGGCA | 60 °C | 183 | XM_005891246.2 |
| MAPK | F: TATTCGAGCACCGACCATCG R: GCAGCAGGTTGGAAGGTTTG | 60 °C | 203 | NM_175793.2 |
| CYP11A1 | F: TTCAACCTCATCCTGACGCC R: GTGCAAGAGGTGTGGACTGA | 60 °C | 204 | NM_176644.2 |
| CYP17A1 | F: GATCGTGGCCTACCTGCTAC R: CCACAACGTCTGTGCCTTTG | 60 °C | 242 | NM_174304.3 |
| StAR | F: CTGCCCTGCTCTTGAAGCTA R: GAAAACGTGCCACCACCTTG | 60 °C | 160 | NM_174189.3 |
| Caspase-6 | F: GCTAAGCTCTCCGCTACGAT R: CCTGTTCGGCAGGGTTAAGT | 60 °C | 210 | XM_005896232.2 |
| Caspase-3 | F: CGTCGTAGCTGAACCGTGA R: TTACTGCATCCTGTCTCCTCCT | 60 °C | 164 | XM_010820245.4 |
| BAX | F: CTCTGAGCAGATCATGAAGACAG R: CAGAAAACATTTCAGCCGCCA | 60 °C | 260 | NM_173894.1 |
| BCL-2 | F: CGGAGCAGCCTGTTTAGGAA R: ACAAAAGCGGTTTCTCACGC | 60 °C | 127 | XM_005224105.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Li, H.; Zeng, Y.; Yang, C.; Zhang, R.; Lund, A.K.; Zhang, M. Spermidine as a Potential Protective Agents Against Poly(I:C)-Induced Immune Response, Oxidative Stress, Apoptosis, and Testosterone Decrease in Yak Leydig Cells. Int. J. Mol. Sci. 2025, 26, 2753. https://doi.org/10.3390/ijms26062753
Tang Y, Li H, Zeng Y, Yang C, Zhang R, Lund AK, Zhang M. Spermidine as a Potential Protective Agents Against Poly(I:C)-Induced Immune Response, Oxidative Stress, Apoptosis, and Testosterone Decrease in Yak Leydig Cells. International Journal of Molecular Sciences. 2025; 26(6):2753. https://doi.org/10.3390/ijms26062753
Chicago/Turabian StyleTang, Yujun, Hao Li, Yutian Zeng, Cuiting Yang, Run Zhang, Arab Khan Lund, and Ming Zhang. 2025. "Spermidine as a Potential Protective Agents Against Poly(I:C)-Induced Immune Response, Oxidative Stress, Apoptosis, and Testosterone Decrease in Yak Leydig Cells" International Journal of Molecular Sciences 26, no. 6: 2753. https://doi.org/10.3390/ijms26062753
APA StyleTang, Y., Li, H., Zeng, Y., Yang, C., Zhang, R., Lund, A. K., & Zhang, M. (2025). Spermidine as a Potential Protective Agents Against Poly(I:C)-Induced Immune Response, Oxidative Stress, Apoptosis, and Testosterone Decrease in Yak Leydig Cells. International Journal of Molecular Sciences, 26(6), 2753. https://doi.org/10.3390/ijms26062753







