Functional Characterization of the Hephaestin Variant D568H Provides Novel Mechanistic Insights on Iron-Dependent Asbestos-Induced Carcinogenesis
Abstract
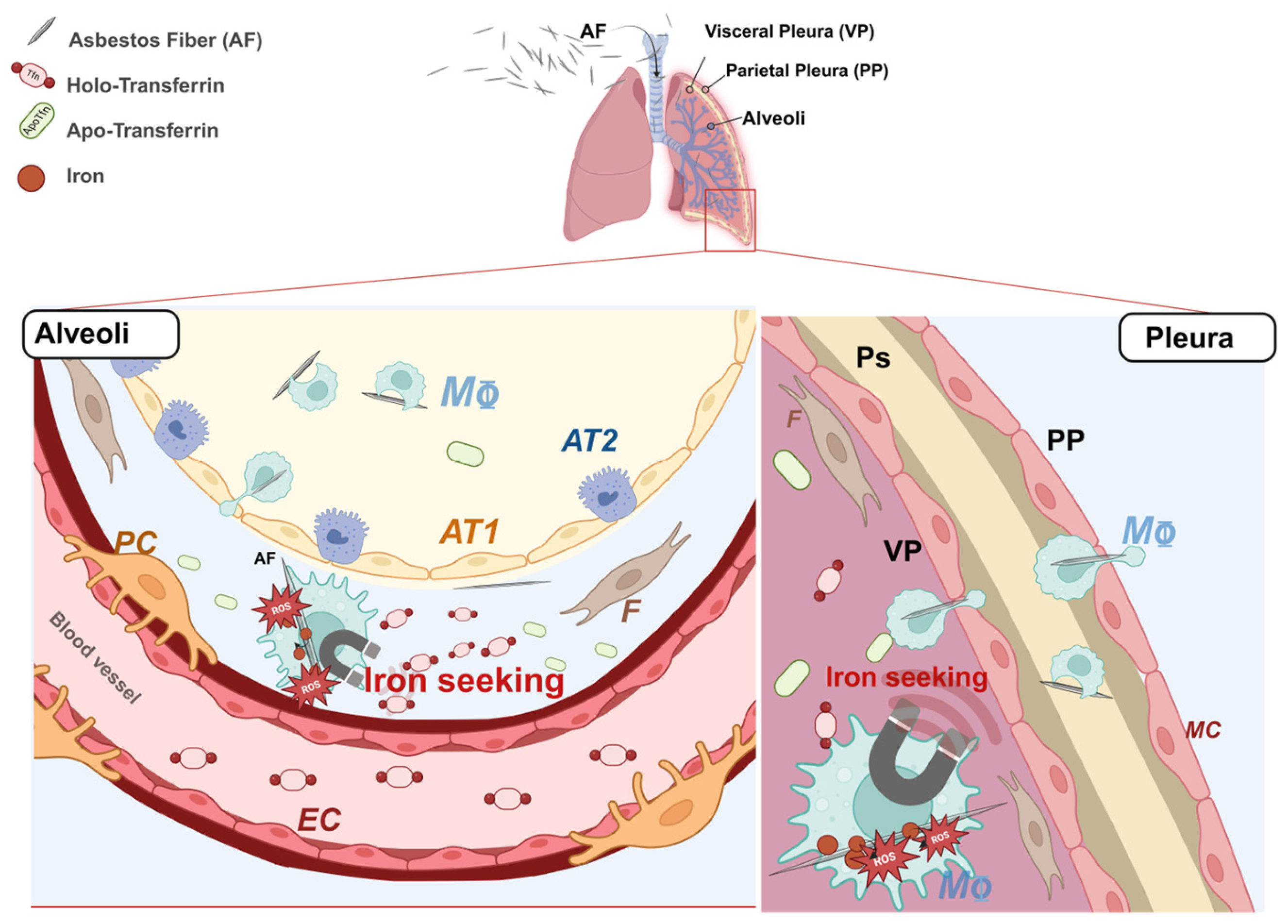
1. Introduction
2. Results
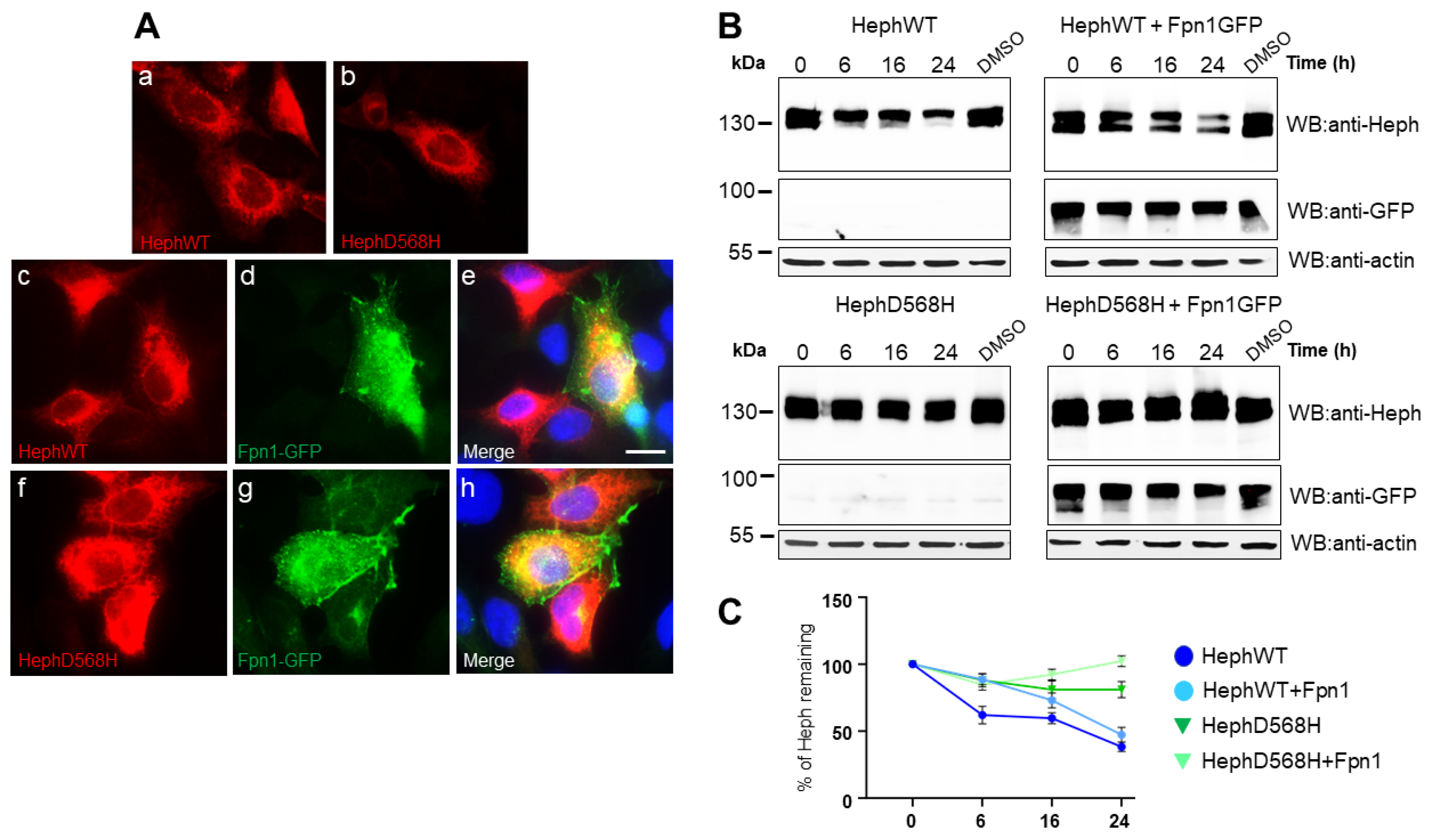
2.1. HephD568H Distributes like HephWT When Ectopically Expressed in HEK293T Cells but Has a Prolonged Half-Life
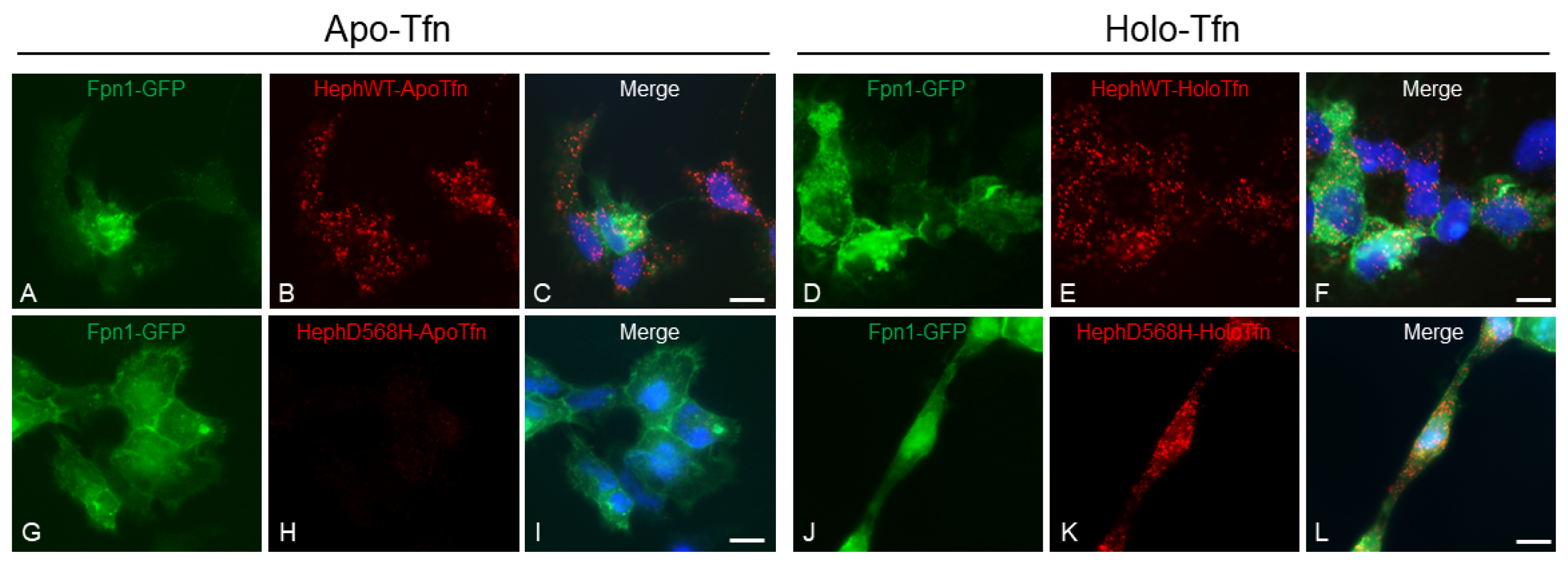
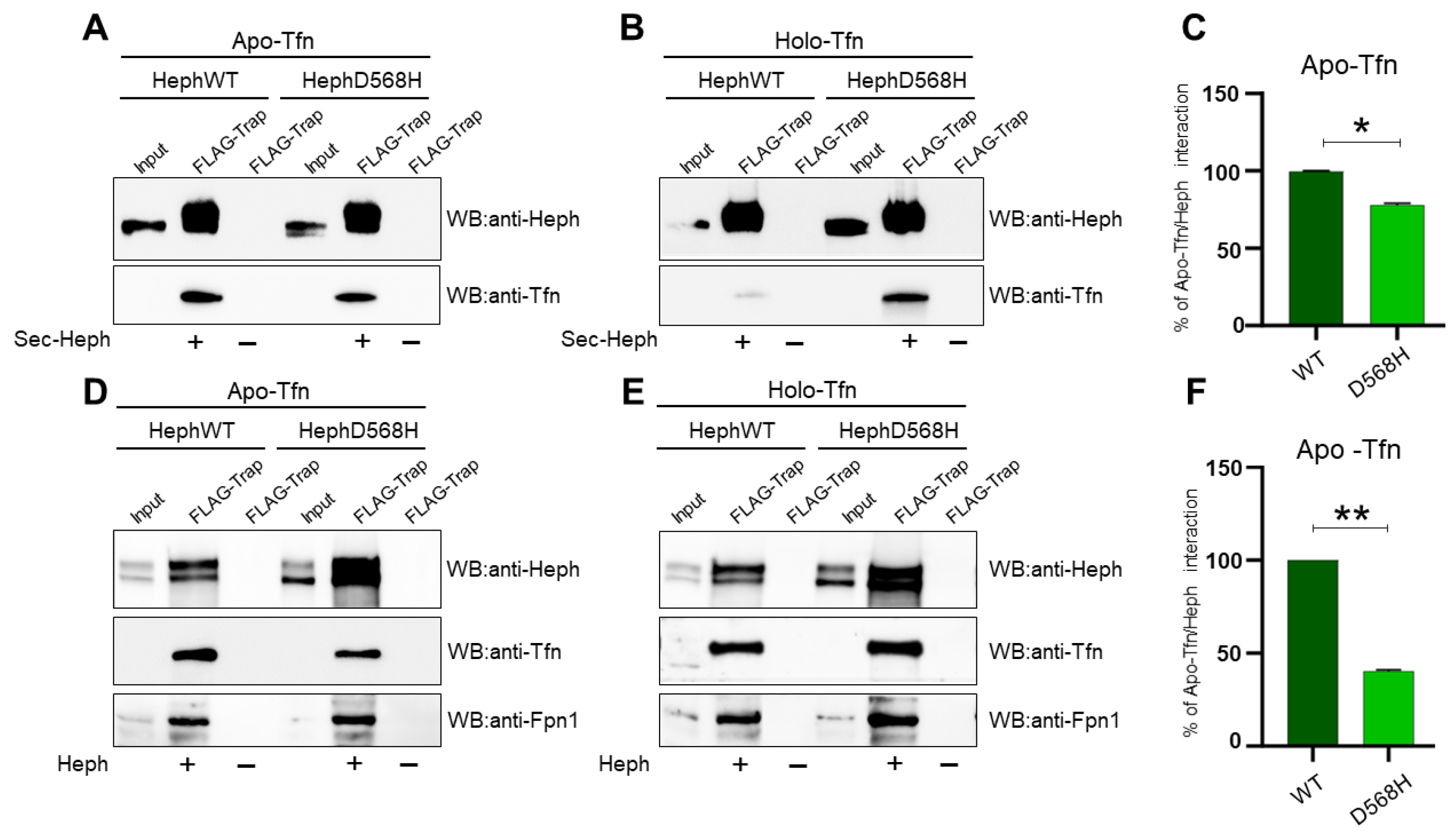
2.2. HephD568H Interacts with the Permease Fpn1 but the Complex Is More Enriched at the Cell Surface with Respect to HephWT
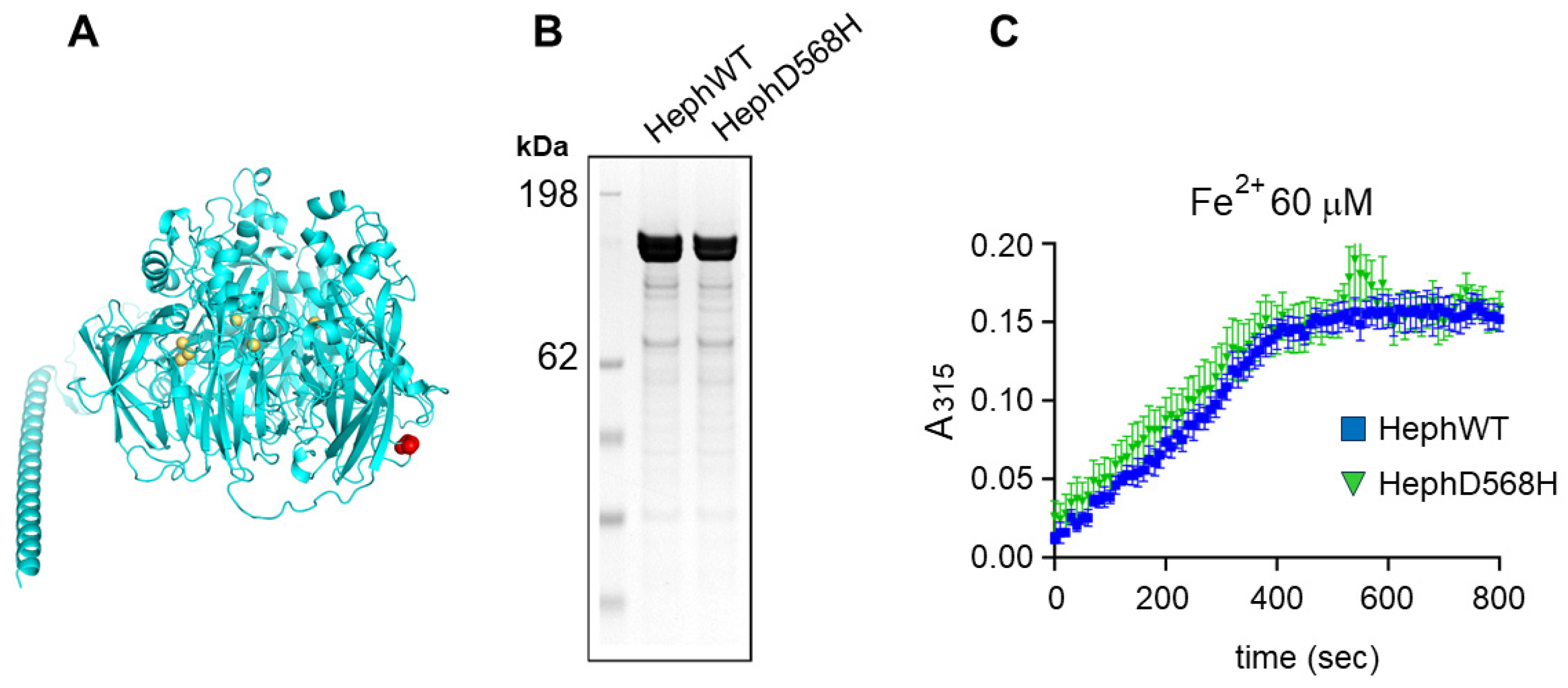
2.3. HephD568H Is Not Impaired in the Ferroxidase Activity
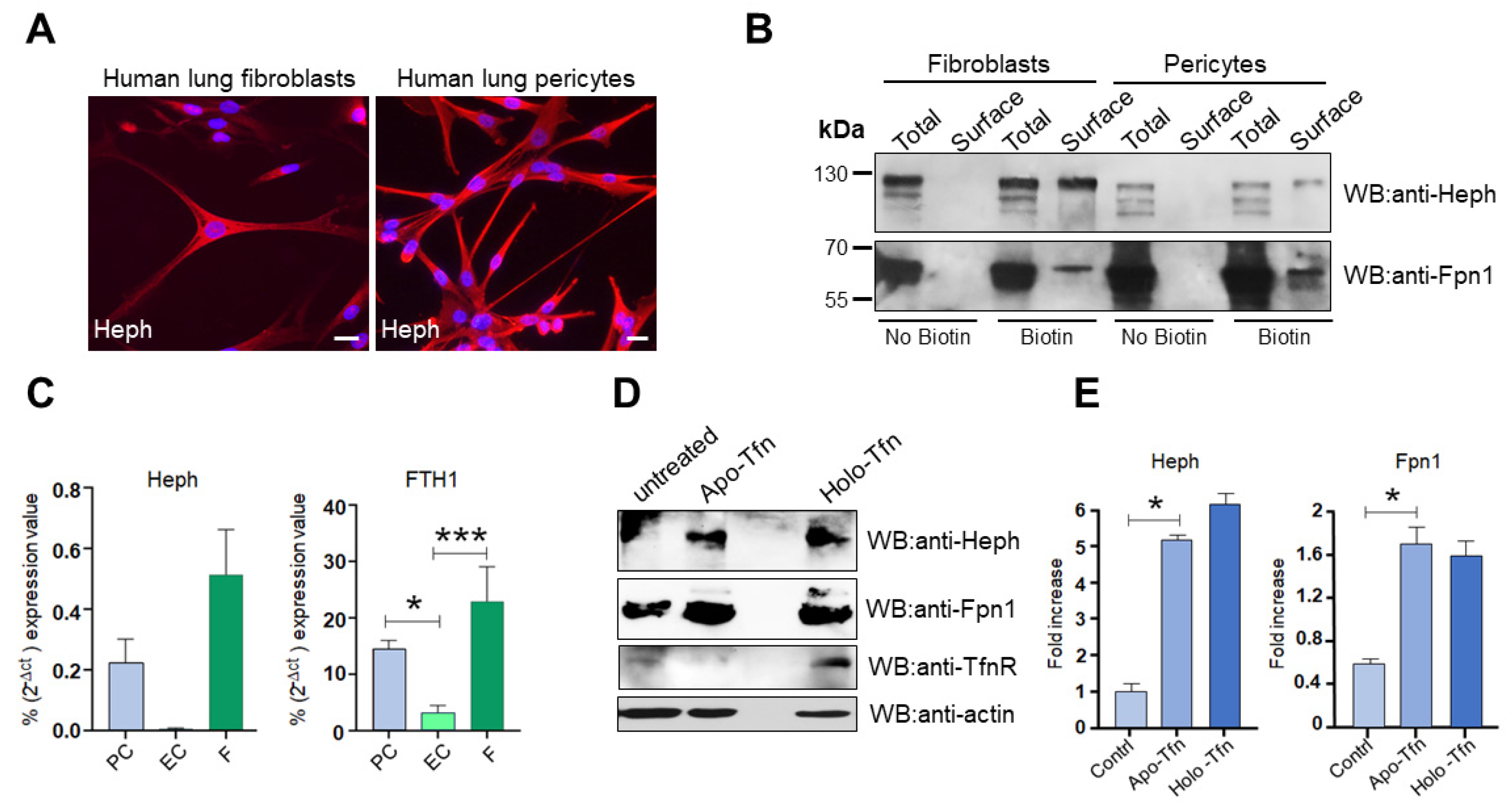
2.4. HephD568H Affects Iron Sensing
2.5. Heph Is Expressed by Human Lung Pericytes and Fibroblasts
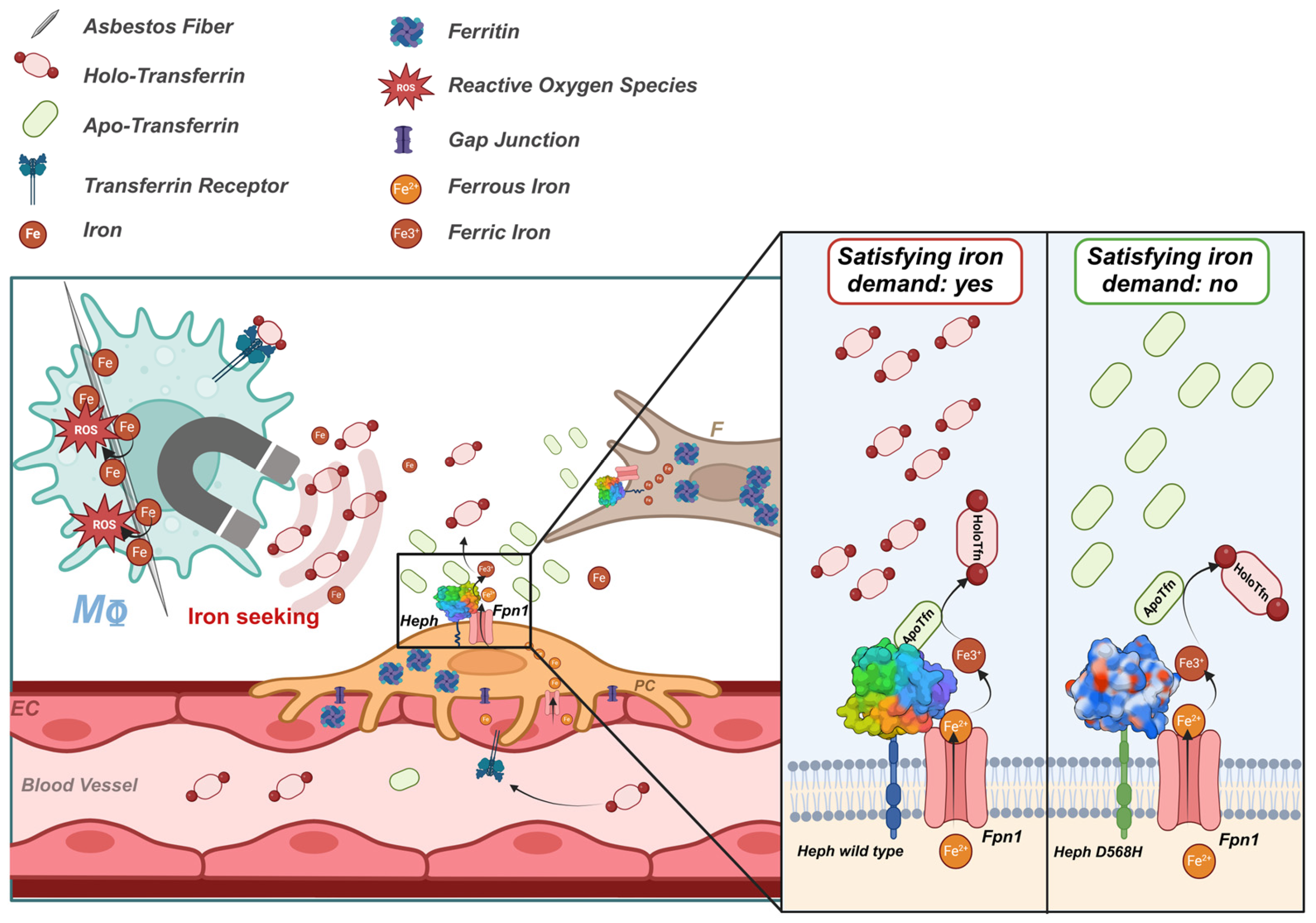
3. Discussion
4. Materials and Methods
4.1. Plasmids and Mutagenesis
4.2. Chemicals and Antibodies
4.3. Cell Culture and Transfections
4.4. Recombinant Protein Expression and Purification
4.5. Ferroxidase Activity Assay
4.6. Immunocytochemistry and Proximity Ligation Assay (PLA)
4.7. Surface Biotinylation, Immunoprecipitation and Western Blot Analysis
4.8. Gene Expression Analysis
4.9. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Apo-Tfn | Transferrin (protein), Iron-Depleted Form |
| BBB | Blood–Brain Barrier |
| CP | Ceruloplasmin |
| CT | Threshold Cycle |
| FBS | Fetal Bovine Serum |
| Fpn1 | Ferroportin |
| FTH1 | Ferritin Heavy Chain |
| GFP | Green Fluorescent Protein |
| GPI | Glycosylphosphatidylinositol |
| HEK293T | Human Embryonic Kidney Cell |
| HEPH | Hephaestin (gene) |
| Heph | Hephaestin (protein) |
| HMC | Human Mesothelial primary cell |
| Holo-Tfn | Transferrin (protein), iron-loaded form |
| HPMEC | Human Pulmonary Microvascular Endothelial Cells |
| Huvec | Human Umbilical Vein Cell |
| LC | Lung Cancer |
| MeT-5A | Human Mesothelial Cells |
| MPM | Malignant Pleural Mesothelioma |
| PCR | Polymerase Chain Reaction |
| PLA | Proximity Ligation Assay |
| qRT-PCR | Quantitative Real-Time PCR |
| ROS | Reactive Oxygen Species |
| SNP | Single Nucleotide Polymorphism |
| TF | Transferrin (gene) |
| TfnR | Transferrin Receptor |
| TT1 | Immortal Alveolar Type-1-Like Cells |
References
- Jamrozik, E.; de Klerk, N.; Musk, A.W. Asbestos-related disease. Intern. Med. J. 2011, 41, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Andujar, P.; Lacourt, A.; Brochard, P.; Pairon, J.C.; Jaurand, M.C.; Jean, D. Five years update on relationships between malignant pleural mesothelioma and exposure to asbestos and other elongated mineral particles. J. Toxicol. Environ. Health B Crit. Rev. 2016, 19, 151–172. [Google Scholar] [CrossRef]
- Gilham, C.; Rake, C.; Burdett, G.; Nicholson, A.G.; Davison, L.; Franchini, A.; Carpenter, J.; Hodgson, J.; Darnton, A.; Peto, J. Pleural mesothelioma and lung cancer risks in relation to occupational history and asbestos lung burden. Occup. Environ. Med. 2016, 73, 290–299. [Google Scholar] [CrossRef]
- Lemen, R.A. Mesothelioma from asbestos exposures: Epidemiologic patterns and impact in the United States. J. Toxicol. Environ. Health B Crit. Rev. 2016, 19, 250–265. [Google Scholar] [CrossRef]
- Dostert, C.; Pétrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef]
- Ghio, A.J.; Hilborn, E.D.; Stonehuerner, J.G.; Dailey, L.A.; Carter, J.D.; Richards, J.H.; Crissman, K.M.; Foronjy, R.F.; Uyeminami, D.L.; Pinkerton, K.E. Particulate matter in cigarette smoke alters iron homeostasis to produce a biological effect. Am. J. Respir. Crit. Care Med. 2008, 178, 1130–1138. [Google Scholar] [CrossRef]
- Ghio, A.J.; Pavlisko, E.N.; Roggli, V.L. Iron and Iron-Related Proteins in Asbestosis. J. Environ. Pathol. Toxicol. Oncol. 2015, 34, 277–285. [Google Scholar] [CrossRef]
- Jiang, L.; Akatsuka, S.; Nagai, H.; Chew, S.; Ohara, H.; Okazaki, Y.; Yamashita, Y.; Yoshikawa, Y.; Yasui, H.; Ikuta, K.; et al. Iron overload signature in chrysotile-induced malignant mesothelioma. J. Pathol. 2012, 228, 366–377. [Google Scholar] [CrossRef]
- Mossman, B.T. In vitro studies on the biologic effects of fibers: Correlation with in vivo bioassays. Environ. Health Perspect. 1990, 88, 319–322. [Google Scholar] [CrossRef]
- Mossman, B.T.; Churg, A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 1998, 157, 1666–1680. [Google Scholar] [CrossRef]
- Mossman, B.T.; Shukla, A.; Heintz, N.H.; Verschraegen, C.F.; Thomas, A.; Hassan, R. New insights into understanding the mechanisms, pathogenesis, and management of malignant mesotheliomas. Am. J. Pathol. 2013, 182, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S. Iron overload as a major targetable pathogenesis of asbestos-induced mesothelial carcinogenesis. Redox Rep. 2014, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shannahan, J.H.; Ghio, A.J.; Schladweiler, M.C.; McGee, J.K.; Richards, J.H.; Gavett, S.H.; Kodavanti, U.P. The role of iron in Libby amphibole-induced acute lung injury and inflammation. Inhal. Toxicol. 2011, 23, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.H.; Toyokuni, S. Malignant mesothelioma as an oxidative stress-induced cancer: An update. Free Radic. Biol. Med. 2015, 86, 166–178. [Google Scholar] [CrossRef]
- Aust, E.A.; Lund, L.G.; Chao, C.C.; Park, S.H.; Fang, R. Role of Iron in the Cellular Effects of Asbestos. Inhal. Toxicol. 2000, 12 (Suppl. S3), 75–80. [Google Scholar] [CrossRef]
- Aust, A.E.; Cook, P.M.; Dodson, R.F. Morphological and chemical mechanisms of elongated mineral particle toxicities. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 40–75. [Google Scholar] [CrossRef]
- Ather, J.L.; Martin, R.A.; Ckless, K.; Poynter, M.E. Inflammasome Activity in Non-Microbial Lung Inflammation. J. Environ. Immunol. Toxicol. 2014, 1, 108–117. [Google Scholar] [CrossRef][Green Version]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Ying, J.F.; Lu, Z.B.; Fu, L.Q.; Tong, Y.; Wang, Z.; Li, W.F.; Mou, X.Z. The role of iron homeostasis and iron-mediated ROS in cancer. Am. J. Cancer Res. 2021, 11, 1895–1912. [Google Scholar]
- Harington, J.S.; Miller, K.; Macnab, G. Hemolysis by asbestos. Environ. Res. 1971, 4, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.G.; Aust, A.E. Iron-catalyzed reactions may be responsible for the biochemical and biological effects of asbestos. Biofactors 1991, 3, 83–89. [Google Scholar] [PubMed]
- Hardy, J.A.; Aust, A.E. Iron in Asbestos Chemistry and Carcinogenicity. Chem. Rev. 1995, 95, 97–118. [Google Scholar] [CrossRef]
- Ghio, A.J.; Stewart, M.; Sangani, R.G.; Pavlisko, E.N.; Roggli, V.L. Asbestos and Iron. Int. J. Mol. Sci. 2023, 24, 12390. [Google Scholar] [CrossRef]
- Zangari, M.; Borelli, V.; Bernareggi, A.; Zabucchi, G. Asbestos fibers promote iron oxidation and compete with apoferritin enzymatic activity. J. Toxicol. Environ. Health A 2023, 86, 69–73. [Google Scholar] [CrossRef]
- Mossman, B.T.; Lippmann, M.; Hesterberg, T.W.; Kelsey, K.T.; Barchowsky, A.; Bonner, J.C. Pulmonary endpoints (lung carcinomas and asbestosis) following inhalation exposure to asbestos. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 76–121. [Google Scholar] [CrossRef]
- Neri, M.; Ugolini, D.; Dianzani, I.; Gemignani, F.; Landi, S.; Cesario, A.; Magnani, C.; Mutti, L.; Puntoni, R.; Bonassi, S. Genetic susceptibility to malignant pleural mesothelioma and other asbestos-associated diseases. Mutat. Res. 2008, 659, 126–136. [Google Scholar] [CrossRef]
- Carbone, M.; Yang, H. Molecular pathways: Targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin. Cancer Res. 2012, 18, 598–604. [Google Scholar] [CrossRef]
- Ascoli, V.; Cavone, D.; Merler, E.; Barbieri, P.G.; Romeo, L.; Nardi, F.; Musti, M. Mesothelioma in blood related subjects: Report of 11 clusters among 1954 Italy cases and review of the literature. Am. J. Ind. Med. 2007, 50, 357–369. [Google Scholar] [CrossRef]
- Ugolini, D.; Neri, M.; Ceppi, M.; Cesario, A.; Dianzani, I.; Filiberti, R.; Gemignani, F.; Landi, S.; Magnani, C.; Mutti, L.; et al. Genetic susceptibility to malignant mesothelioma and exposure to asbestos: The influence of the familial factor. Mutat. Res. 2008, 658, 162–171. [Google Scholar] [CrossRef]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat. Genet. 2011, 43, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- de Klerk, N.; Alfonso, H.; Olsen, N.; Reid, A.; Sleith, J.; Palmer, L.; Berry, G.; Musk, A.B. Familial aggregation of malignant mesothelioma in former workers and residents of Wittenoom, Western Australia. Int. J. Cancer 2013, 132, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Betti, M.; Casalone, E.; Ferrante, D.; Aspesi, A.; Morleo, G.; Biasi, A.; Sculco, M.; Mancuso, G.; Guarrera, S.; Righi, L.; et al. Germline mutations in DNA repair genes predispose asbestos-exposed patients to malignant pleural mesothelioma. Cancer Lett. 2017, 405, 38–45. [Google Scholar] [CrossRef]
- Betti, M.; Aspesi, A.; Ferrante, D.; Sculco, M.; Righi, L.; Mirabelli, D.; Napoli, F.; Rondón-Lagos, M.; Casalone, E.; Vignolo Lutati, F.; et al. Sensitivity to asbestos is increased in patients with mesothelioma and pathogenic germline variants in BAP1 or other DNA repair genes. Genes Chromosomes Cancer 2018, 57, 573–583. [Google Scholar] [CrossRef]
- Cadby, G.; Mukherjee, S.; Musk, A.W.; Reid, A.; Garlepp, M.; Dick, I.; Robinson, C.; Hui, J.; Fiorito, G.; Guarrera, S.; et al. A genome-wide association study for malignant mesothelioma risk. Lung Cancer 2013, 82, 1–8. [Google Scholar] [CrossRef]
- Bianchi, C.; Brollo, A.; Ramani, L.; Zuch, C. Asbestos-related mesothelioma in Monfalcone, Italy. Am. J. Ind. Med. 1993, 24, 149–160. [Google Scholar] [CrossRef]
- Ohar, J.A.; Cheung, M.; Talarchek, J.; Howard, S.E.; Howard, T.D.; Hesdorffer, M.; Peng, H.; Rauscher, F.J.; Testa, J.R. Germline BAP1 Mutational Landscape of Asbestos-Exposed Malignant Mesothelioma Patients with Family History of Cancer. Cancer Res. 2016, 76, 206–215. [Google Scholar] [CrossRef]
- Liu, C.Y.; Stücker, I.; Chen, C.; Goodman, G.; McHugh, M.K.; D’Amelio, A.M., Jr.; Etzel, C.J.; Li, S.; Lin, X.; Christiani, D.C. Genome-wide Gene-Asbestos Exposure Interaction Association Study Identifies a Common Susceptibility Variant on 22q13.31 Associated with Lung Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1564–1573. [Google Scholar] [CrossRef]
- Wei, S.; Wang, L.E.; McHugh, M.K.; Han, Y.; Xiong, M.; Amos, C.I.; Spitz, M.R.; Wei, Q.W. Genome-wide gene-environment interaction analysis for asbestos exposure in lung cancer susceptibility. Carcinogenesis 2012, 33, 1531–1537. [Google Scholar] [CrossRef]
- Kettunen, E.; Hernandez-Vargas, H.; Cros, M.P.; Durand, G.; Le Calvez-Kelm, F.; Stuopelyte, K.; Jarmalaite, S.; Salmenkivi, K.; Anttila, S.; Wolff, H.; et al. Asbestos-associated genome-wide DNA methylation changes in lung cancer. Int. J. Cancer 2017, 141, 2014–2029. [Google Scholar] [CrossRef]
- Crovella, S.; Bianco, A.M.; Vuch, J.; Zupin, L.; Moura, R.R.; Trevisan, E.; Schneider, M.; Brollo, A.; Nicastro, E.M.; Cosenzi, A.; et al. Iron signature in asbestos-induced malignant pleural mesothelioma: A population-based autopsy study. J. Toxicol. Environ. Health A 2016, 79, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Celsi, F.; Crovella, S.; Moura, R.R.; Schneider, M.; Vita, F.; Finotto, L.; Zabucchi, G.; Zacchi, P.; Borelli, V. Pleural mesothelioma and lung cancer: The role of asbestos exposure and genetic variants in selected iron metabolism and inflammation genes. J. Toxicol. Environ. Health A 2019, 82, 1088–1102. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.; Brownlie, A.; Zhou, Y.; Shepard, J.; Pratt, S.J.; Moynihan, J.; Paw, B.H.; Drejer, A.; Barut, B.; Zapata, A.; et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 2000, 403, 776–781. [Google Scholar] [CrossRef] [PubMed]
- McKie, A.T.; Marciani, P.; Rolfs, A.; Brennan, K.; Wehr, K.; Barrow, D.; Miret, S.; Bomford, A.; Peters, T.J.; Farzaneh, F.; et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 2000, 5, 299–309. [Google Scholar] [CrossRef]
- Helman, S.L.; Zhou, J.; Fuqua, B.K.; Lu, Y.; Collins, J.F.; Chen, H.; Vulpe, C.D.; Anderson, G.J.; Frazer, D.M. The biology of mammalian multi-copper ferroxidases. Biometals 2023, 36, 263–281. [Google Scholar] [CrossRef]
- Vashchenko, G.; MacGillivray, R.T. Multi-copper oxidases and human iron metabolism. Nutrients 2013, 5, 2289–2313. [Google Scholar] [CrossRef]
- Anderson, G.J.; Vulpe, C.D. Mammalian iron transport. Cell Mol. Life Sci. 2009, 66, 3241–3261. [Google Scholar] [CrossRef]
- Vulpe, C.D.; Kuo, Y.M.; Murphy, T.L.; Cowley, L.; Askwith, C.; Libina, N.; Gitschier, J.; Anderson, G.J. Hephaestin, a ceruloplasmin homologue implicated in intestinal iron transport, is defective in the sla mouse. Nat. Genet. 1999, 21, 195–199. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M.; McKie, A.T.; Vulpe, C.D. The ceruloplasmin homolog hephaestin and the control of intestinal iron absorption. Blood Cells Mol. Dis. 2002, 29, 367–375. [Google Scholar] [CrossRef]
- McCarthy, R.C.; Kosman, D.J. Ferroportin and exocytoplasmic ferroxidase activity are required for brain microvascular endothelial cell iron efflux. J. Biol. Chem. 2013, 288, 17932–17940. [Google Scholar] [CrossRef]
- McCarthy, R.C.; Kosman, D.J. Mechanisms and regulation of iron trafficking across the capillary endothelial cells of the blood-brain barrier. Front. Mol. Neurosci. 2015, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Baringer, S.L.; Palsa, K.; Spiegelman, V.S.; Simpson, I.A.; Connor, J.R. Apo- and holo-transferrin differentially interact with hephaestin and ferroportin in a novel mechanism of cellular iron release regulation. J. Biomed. Sci. 2023, 30, 36. [Google Scholar] [CrossRef]
- Zacchi, P.; Belmonte, B.; Mangogna, A.; Morello, G.; Scola, L.; Martorana, A.; Borelli, V. The Ferroxidase Hephaestin in Lung Cancer: Pathological Significance and Prognostic Value. Front. Oncol. 2021, 11, 638856. [Google Scholar] [CrossRef]
- Schnell, S.; Mendoza, C. Closed Form Solution for Time-dependent Enzyme Kinetics. J. Theor. Biol. 1997, 187, 207–212. [Google Scholar] [CrossRef]
- Petrič, B.; Goličnik, M.; Bavec, A. The Removal of Time-Concentration Data Points from Progress Curves Improves the Determination of Km: The Example of Paraoxonase 1. Molecules 2022, 27, 1306. [Google Scholar] [CrossRef]
- Vashchenko, G.; Macgillivray, R.T. Functional role of the putative iron ligands in the ferroxidase activity of recombinant human hephaestin. J. Biol. Inorg. Chem. 2012, 17, 1187–1195. [Google Scholar] [CrossRef]
- Burkhart, A.; Skjørringe, T.; Johnsen, K.B.; Siupka, P.; Thomsen, L.B.; Nielsen, M.S.; Thomsen, L.L.; Moos, T. Expression of Iron-Related Proteins at the Neurovascular Unit Supports Reduction and Reoxidation of Iron for Transport Through the Blood-Brain Barrier. Mol. Neurobiol. 2016, 53, 7237–7253. [Google Scholar] [CrossRef]
- Li, P.; Wu, Y.; Goodwin, A.J.; Halushka, P.V.; Wilson, C.L.; Schnapp, L.M.; Fan, H. Generation of a new immortalized human lung pericyte cell line: A promising tool for human lung pericyte studies. Lab. Investig. 2021, 101, 625–635. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Betsholtz, C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell 2011, 21, 193–215. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Neal, E.H.; Marinelli, N.A.; Shi, Y.; McClatchey, P.M.; Balotin, K.M.; Gullett, D.R.; Hagerla, K.A.; Bowman, A.B.; Ess, K.C.; Wikswo, J.P.; et al. A Simplified, Fully Defined Differentiation Scheme for Producing Blood-Brain Barrier Endothelial Cells from Human iPSCs. Stem Cell Rep. 2019, 12, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Sasson, E.; Anzi, S.; Bell, B.; Yakovian, O.; Zorsky, M.; Deutsch, U.; Engelhardt, B.; Sherman, E.; Vatine, G.; Dzikowski, R.; et al. Nano-scale architecture of blood-brain barrier tight-junctions. eLife 2021, 10, e63253. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, R.L.; Buck, L.D.; Vajrala, K.P.; Quick, R.E.; Card, O.A. Historical and current perspectives on blood endothelial cell heterogeneity in the brain. Cell Mol. Life Sci. 2022, 79, 372. [Google Scholar] [CrossRef] [PubMed]
- Chiou, B.; Neal, E.H.; Bowman, A.B.; Lippmann, E.S.; Simpson, I.A.; Connor, J.R. Endothelial cells are critical regulators of iron transport in a model of the human blood-brain barrier. J. Cereb. Blood Flow. Metab. 2019, 39, 2117–2131. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef]
- Benarroch, E. What Are the Roles of Pericytes in the Neurovascular Unit and Its Disorders? Neurology 2023, 100, 970–977. [Google Scholar] [CrossRef]
- Kemp, S.J.; Thorley, A.J.; Gorelik, J.; Seckl, M.J.; O’Hare, M.J.; Arcaro, A.; Korchev, Y.; Goldstraw, P.; Tetley, T.D. Immortalization of human alveolar epithelial cells to investigate nanoparticle uptake. Am. J. Respir. Cell Mol. Biol. 2008, 39, 591–597. [Google Scholar] [CrossRef]
- Yuan, K.; Agarwal, S.; Chakraborty, A.; Condon, D.F.; Patel, H.; Zhang, S.; Huang, F.; Mello, S.A.; Kirk, O.I.; Vasquez, R.; et al. Lung Pericytes in Pulmonary Vascular Physiology and Pathophysiology. Compr. Physiol. 2021, 11, 2227–2247. [Google Scholar] [CrossRef]
- Hare, D.J.; Double, K.L. Iron and dopamine: A toxic couple. Brain 2016, 139 Pt 4, 1026–1035. [Google Scholar] [CrossRef]
- Santiago González, D.A.; Cheli, V.T.; Wan, R.; Paez, P.M. Iron Metabolism in the Peripheral Nervous System: The Role of DMT1, Ferritin, and Transferrin Receptor in Schwann Cell Maturation and Myelination. J. Neurosci. 2019, 39, 9940–9953. [Google Scholar] [CrossRef]
- Rogers, L.K.; Cismowski, M.J. Oxidative Stress in the Lung—The Essential Paradox. Curr. Opin. Toxicol. 2018, 7, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Khiroya, H.; Turner, A.M. The role of iron in pulmonary pathology. Multidiscip. Respir. Med. 2015, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Haider, T.; Gassmann, M.; Muckenthaler, M.U. Iron Homeostasis in the Lungs—A Balance between Health and Disease. Pharmaceuticals 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chang, F.C.; Wu, C.F.; Chou, Y.H.; Hsu, H.L.; Chiang, W.C.; Shen, J.; Chen, Y.M.; Wu, K.D.; Tsai, T.J.; et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int. 2011, 80, 1170–1181. [Google Scholar] [CrossRef]
- Yao, L.; Hou, J.; Wu, X.; Lu, Y.; Jin, Z.; Yu, Z.; Yu, B.; Li, J.; Yang, Z.; Li, C.; et al. Cancer-associated fibroblasts impair the cytotoxic function of NK cells in gastric cancer by inducing ferroptosis via iron regulation. Redox Biol. 2023, 67, 102923. [Google Scholar] [CrossRef]
- Barron, L.; Gharib, S.A.; Duffield, J.S. Lung Pericytes and Resident Fibroblasts: Busy Multitaskers. Am. J. Pathol. 2016, 186, 2519–2531. [Google Scholar] [CrossRef]
- Palsa, K.; Baringer, S.L.; Shenoy, G.; Spiegelman, V.S.; Simpson, I.A.; Connor, J.R. Exosomes are involved in iron transport from human blood-brain barrier endothelial cells and are modified by endothelial cell iron status. J. Biol. Chem. 2023, 299, 102868. [Google Scholar] [CrossRef]
- Munson, P.; Lam, Y.W.; MacPherson, M.; Beuschel, S.; Shukla, A. Mouse serum exosomal proteomic signature in response to asbestos exposure. J. Cell Biochem. 2018, 119, 6266–6273. [Google Scholar] [CrossRef]
- Okamoto, T.; Kawamoto, E.; Takagi, Y.; Akita, N.; Hayashi, T.; Park, E.J.; Suzuki, K.; Shimaoka, M. Gap junction-mediated regulation of endothelial cellular stiffness. Sci. Rep. 2017, 7, 6134. [Google Scholar] [CrossRef]
- Bonaccorsi di Patti, M.C.; Polticelli, F.; Cece, G.; Cutone, A.; Felici, F.; Persichini, T.; Musci, G. A structural model of human ferroportin and of its iron binding site. FEBS J. 2014, 281, 2851–2860. [Google Scholar] [CrossRef]

| Hephaestin | Km | SEM | Vmax (µM/min) | SEM |
|---|---|---|---|---|
| WT | 7.71 | 1.55 | 10.60 | 0.80 |
| D568H | 5.38 | 0.97 | 10.30 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacchi, P.; Longo, F.; Marconato, A.; Amadei, M.; Bonaccorsi di Patti, M.C.; Avolio, E.; Li, P.; Fan, H.; Tetley, T.D.; Zabucchi, G.; et al. Functional Characterization of the Hephaestin Variant D568H Provides Novel Mechanistic Insights on Iron-Dependent Asbestos-Induced Carcinogenesis. Int. J. Mol. Sci. 2025, 26, 2607. https://doi.org/10.3390/ijms26062607
Zacchi P, Longo F, Marconato A, Amadei M, Bonaccorsi di Patti MC, Avolio E, Li P, Fan H, Tetley TD, Zabucchi G, et al. Functional Characterization of the Hephaestin Variant D568H Provides Novel Mechanistic Insights on Iron-Dependent Asbestos-Induced Carcinogenesis. International Journal of Molecular Sciences. 2025; 26(6):2607. https://doi.org/10.3390/ijms26062607
Chicago/Turabian StyleZacchi, Paola, Francesco Longo, Alice Marconato, Matteo Amadei, Maria Carmela Bonaccorsi di Patti, Elisa Avolio, Pengfei Li, Hongkuan Fan, Teresa D. Tetley, Giuliano Zabucchi, and et al. 2025. "Functional Characterization of the Hephaestin Variant D568H Provides Novel Mechanistic Insights on Iron-Dependent Asbestos-Induced Carcinogenesis" International Journal of Molecular Sciences 26, no. 6: 2607. https://doi.org/10.3390/ijms26062607
APA StyleZacchi, P., Longo, F., Marconato, A., Amadei, M., Bonaccorsi di Patti, M. C., Avolio, E., Li, P., Fan, H., Tetley, T. D., Zabucchi, G., & Borelli, V. (2025). Functional Characterization of the Hephaestin Variant D568H Provides Novel Mechanistic Insights on Iron-Dependent Asbestos-Induced Carcinogenesis. International Journal of Molecular Sciences, 26(6), 2607. https://doi.org/10.3390/ijms26062607










