Th1/Th2 Immune Imbalance in the Spleen of Mice Induced by Hypobaric Hypoxia Stimulation and Therapeutic Intervention of Astragaloside IV
Abstract
1. Introduction
2. Results
2.1. Establishment of Hypobaric Hypoxia Immune Injury Model
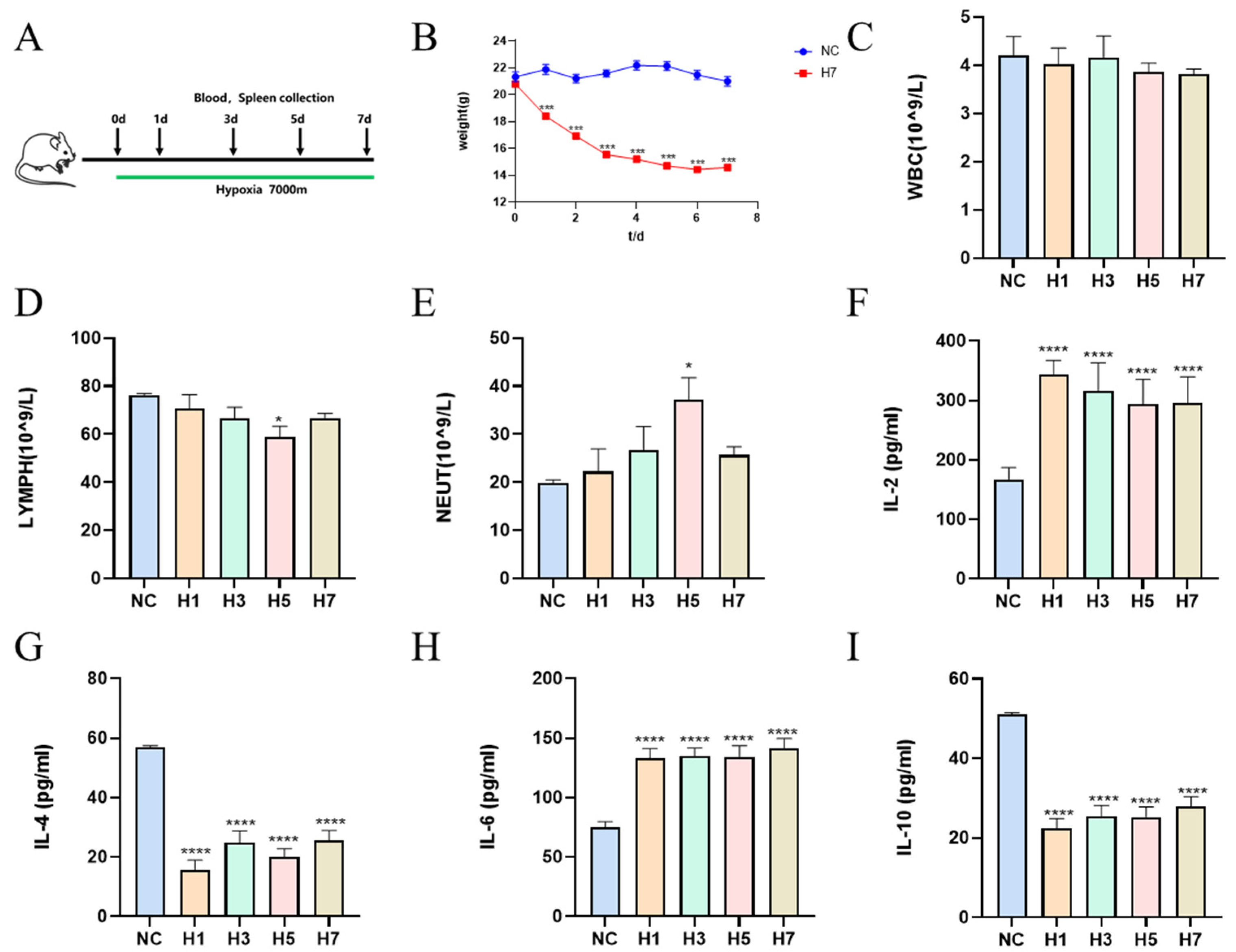
2.1.1. Effects of Different Times of Hypobaric Hypoxia on the Physiological Indices in Mice
2.1.2. Effects of Different Hypobaric Hypoxia Times on Splenic Index and Pathological Changes of Spleen in Mice
2.1.3. Effects of Different Hypobaric Hypoxia Times on Mouse Spleen Immune Cells
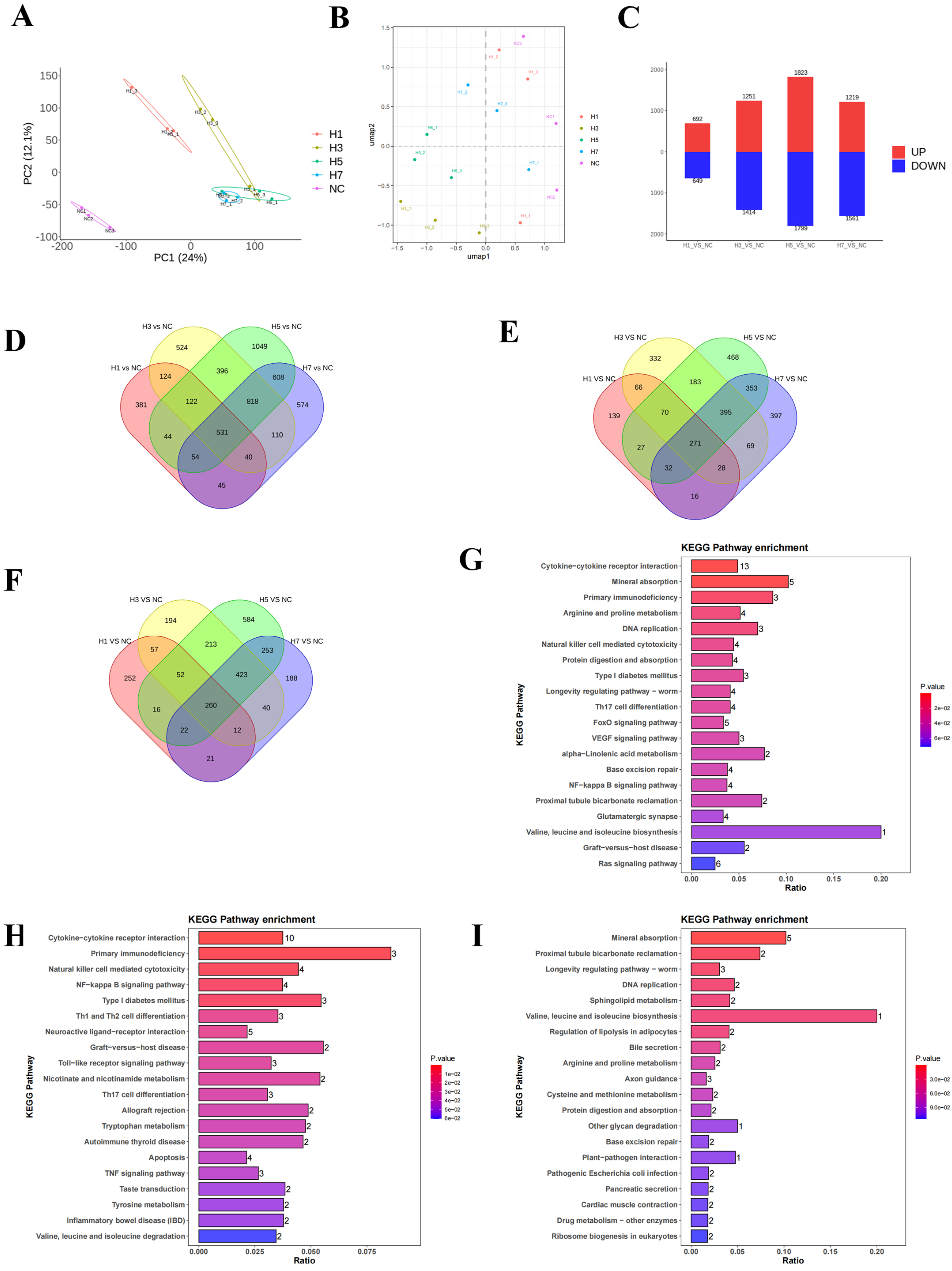
2.1.4. Effects of Different Hypobaric Hypoxia Times on the Transcriptome and Bioinformatics of Mouse Spleen
2.2. Regulatory Effects of AS-IV on the Hypobaric Hypoxia Immune Injury Model
2.2.1. Network Pharmacology of AS-IV on Immune Impairment
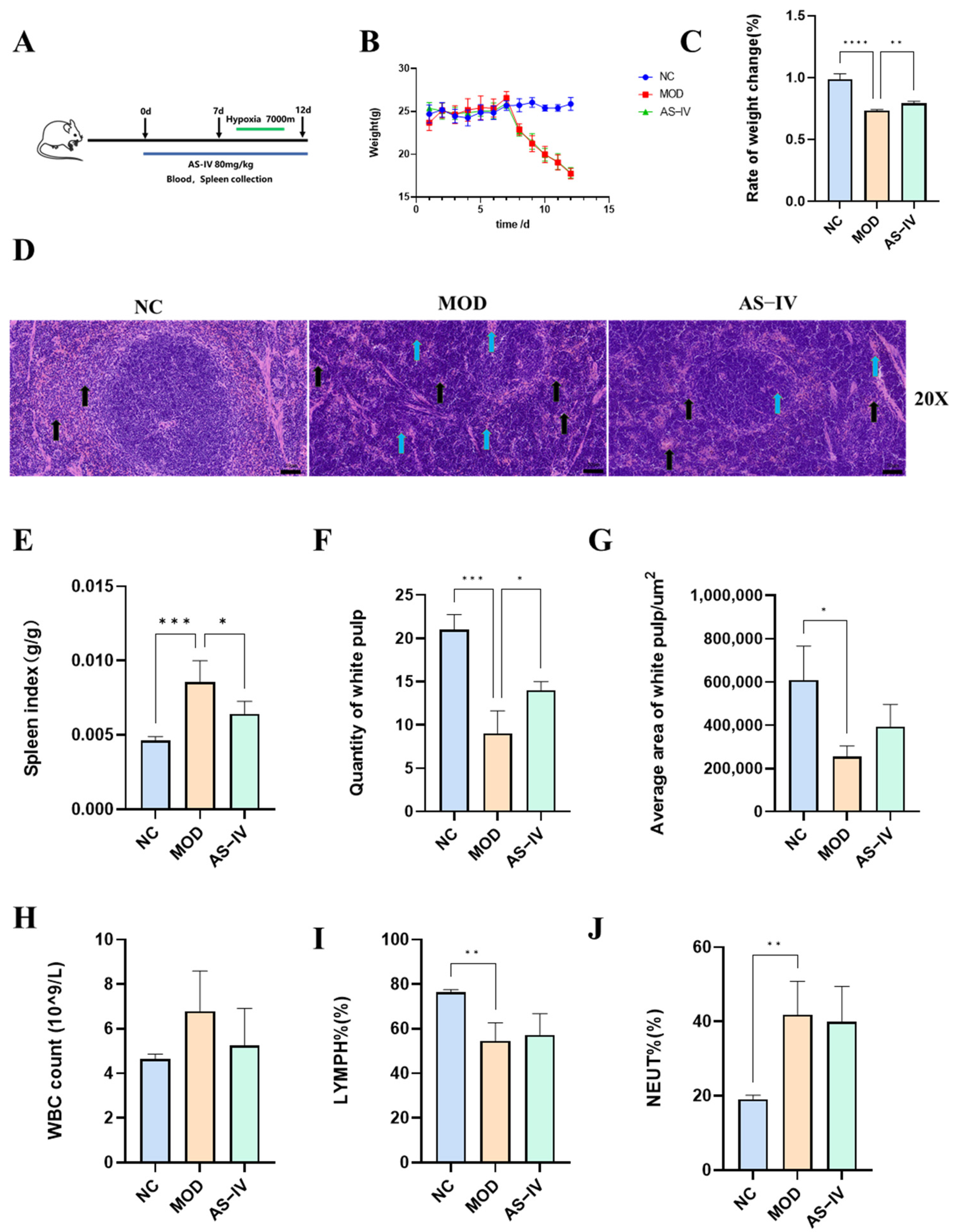
2.2.2. Effects of AS-IV on Splenic Indices and Pathology in a Plateau Hypoxic Immune Injury Model
2.2.3. Effects of AS-IV on Physiological Indexes in Plateau Hypoxic Immune Injury Model
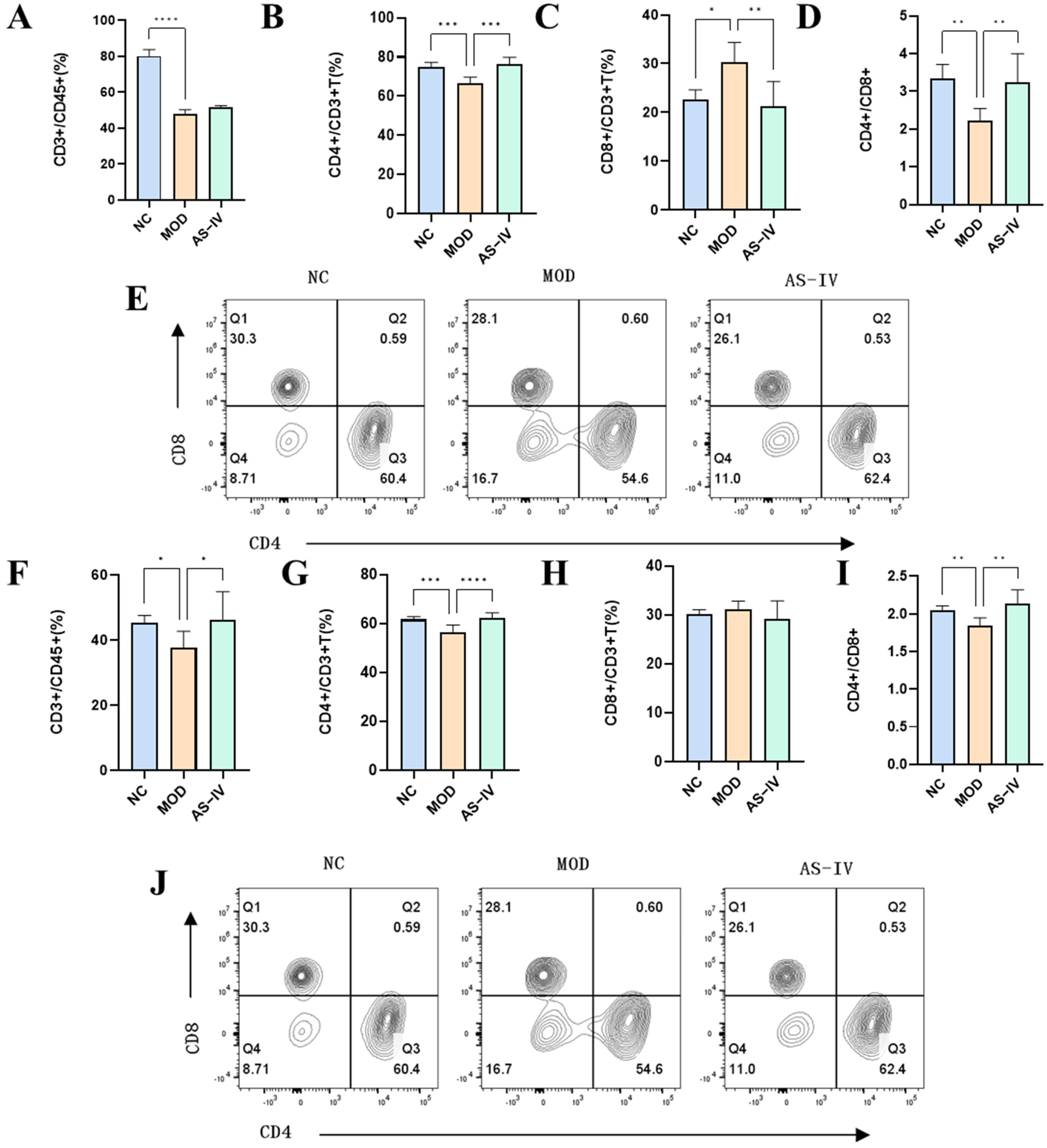
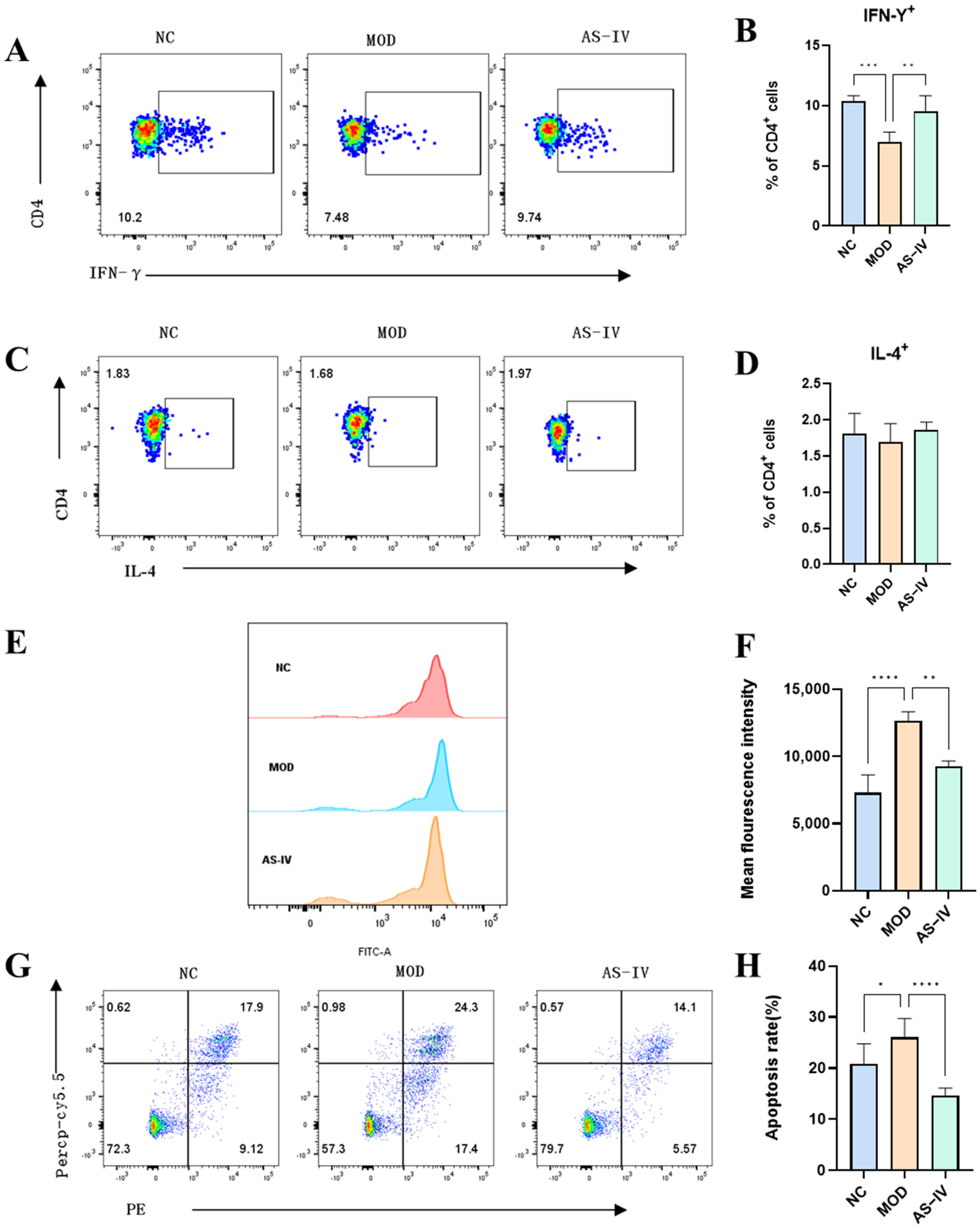
2.2.4. Effects of AS-IV on Peripheral Blood and Spleen Lymphocytes in a Plateau Hypoxic Immune Injury Model
2.2.5. Effects of AS-IV on Reactive Oxygen Species and Apoptotic Cells of Spleen Cells in a Plateau Hypoxic Immune Injury Model
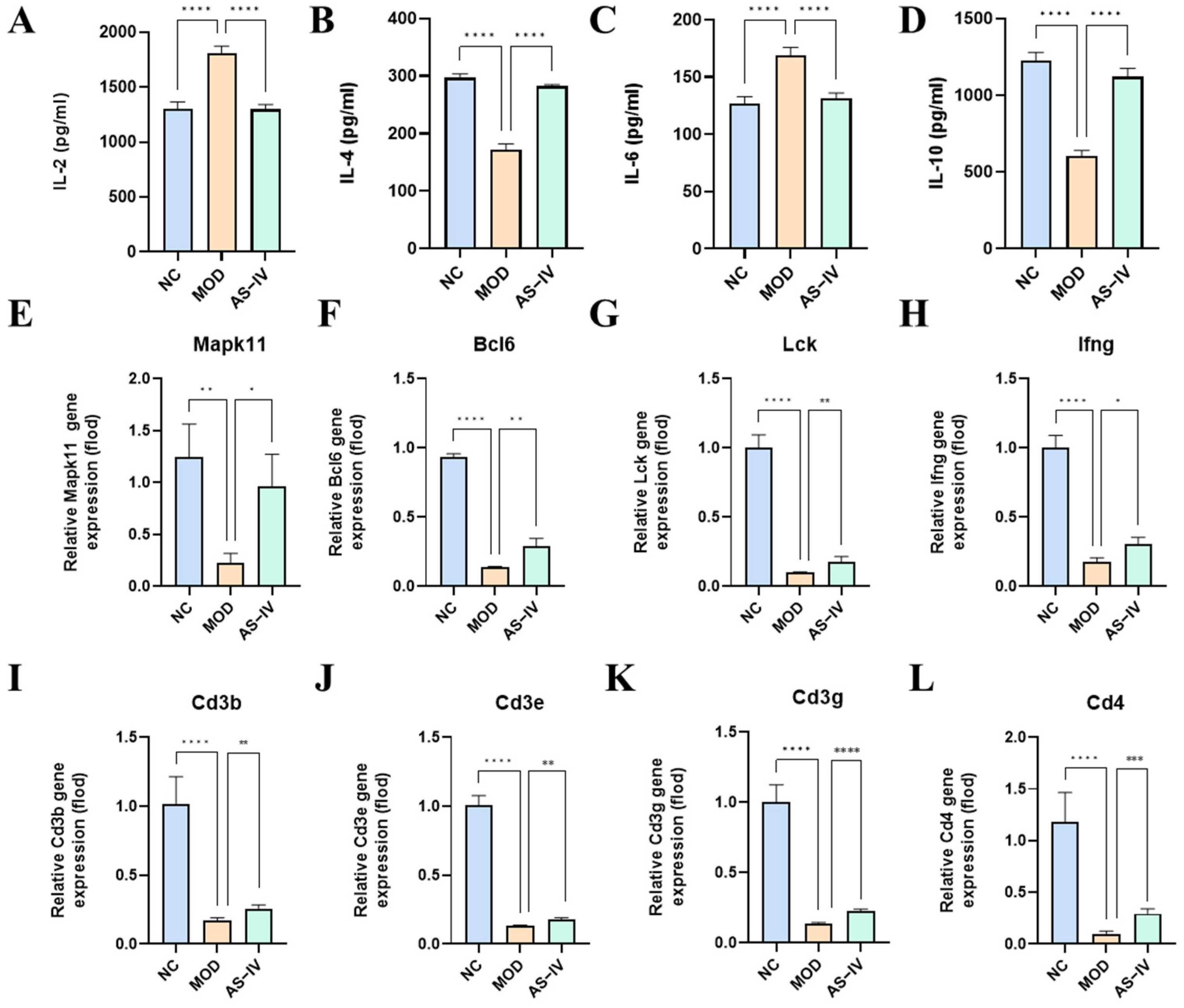
2.2.6. Effect of AS-IV on Peripheral Blood Serum Inflammatory Factors in a Plateau Hypoxic Immune Injury Model
2.2.7. Effects of AS-IV on Spleen mRNA in a Plateau Hypoxic Immune Injury Model
3. Discussion
4. Materials and Methods
4.1. Mice Model
4.2. Chemical Reagents and Instruments
4.3. Sample Collection
4.4. Blood Routine Tests
4.5. Spleen Index
4.6. Flow Cytometry
4.7. ROS Detection
4.8. Pharmingen PE Annexin V Apoptosis Detection
4.9. Histopathological Analysis
4.10. RNA Preparation and Sequencing
4.11. Enzyme-Linked Immunosorbent Assay (ELISA)
4.12. Quantitative Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
4.13. Network Pharmacology Analysis
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Yan, M.; Jiang, H.; Wang, Q.; Guan, X.; Chen, J.; Wang, C. Protective effects of puerarin on acute lung and cerebrum injury induced by hypobaric hypoxia via the regulation of aquaporin (AQP) via NF-κB signaling pathway. Int. Immunopharmacol. 2016, 40, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Colgan, S.P.; Furuta, G.T.; Taylor, C.T. Hypoxia and innate immunity: Keeping up with the HIFsters. Annu. Rev. Immunol. 2020, 38, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, R.S.; Child, A.; Butterfield, G.E.; Braun, B.; Rock, P.B.; Wolfel, E.E.; Zamudio, S.; Moore, L.G. Sympathoadrenal responses to submaximal exercise in women after acclimatization to 4300 meters. Metabolism 2000, 49, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Caris, A.V.; Lira, F.S.; de Mello, M.T.; Oyama, L.M.; dos Santos, R.V.T. Carbohydrate and glutamine supplementation modulates the th1/th2 balance after exercise performed at a simulated altitude of 4500 m. Nutrition 2014, 30, 1331–1336. [Google Scholar] [CrossRef]
- Ashraf, M.V.; Khan, S.; Misri, S.; Gaira, K.S.; Rawat, S.; Rawat, B.; Khan MA, H.; Shah, A.A.; Asgher, M.; Ahmad, S. High-altitude medicinal plants as promising source of phytochemical antioxidants to combat lifestyle-associated oxidative stress-induced disorders. Pharmaceuticals 2024, 17, 975. [Google Scholar] [CrossRef]
- Han, R.; Yang, X.; Ji, X.; Zhou, B. Remote ischemic preconditioning prevents high-altitude cerebral edema by enhancing glucose metabolic reprogramming. CNS Neurosci. Ther. 2024, 30, e70026. [Google Scholar] [CrossRef]
- McNamee, E.N.; Korns Johnson, D.; Homann, D.; Clambey, E.T. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol. Res. 2013, 55, 58–70. [Google Scholar] [CrossRef]
- Yao, J.; Liu, J.; He, Y.; Liu, L.; Xu, Z.; Lin, X.; Liu, N.; Kai, G. Systems pharmacology reveals the mechanism of astragaloside IV in improving immune activity on cyclophosphamide-induced immunosuppressed mice. J. Ethnopharmacol. 2023, 313, 116533. [Google Scholar] [CrossRef]
- McGarry, T.; Biniecka, M.; Veale, D.J.; Fearon, U. Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 2018, 125, 15–24. [Google Scholar] [CrossRef]
- Wang, J.; Pei, L.; Zhao, T.; Liu, X.; Wang, Q.; Zhang, S.; Li, J.; Wu, H.; Niu, D. CD4+ T cells related to disease severity in elderly and frailty community-acquired pneumonia patients: A retrospective cohort study. Immun. Inflamm. Dis. 2023, 11, e1009. [Google Scholar] [CrossRef]
- Krzywinska, E.; Stockmann, C. Hypoxia, metabolism and immune cell function. Biomedicines 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, M.; Chai, Z.; Zhang, J.; Cao, K.; Deng, L.; Liu, Y.; Jiao, C.; Zou, G.M.; Wu, J.; et al. Anticancer effects and mechanisms of astragaloside-IV. Oncol. Rep. 2023, 49, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Han, B.; Zhao, H.; Xu, C.; Xu, D.; Sieniawska, E.; Lin, X.; Kai, G. Biological active ingredients of astragali radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine 2022, 98, 153918. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, S. Effects of astragaloside IV on inflammation and immunity in rats with experimental periodontitis. Braz. Oral. Res. 2019, 33, e032. [Google Scholar] [CrossRef]
- Chen, T.; Yang, P.; Jia, Y. Molecular mechanisms of astragaloside-IV in cancer therapy (review). Int. J. Mol. Med. 2021, 47, 13. [Google Scholar] [CrossRef]
- Liu, J.; Nile, S.; Xu, G.; Wang, Y.; Kai, G. Corrigendum to systematic exploration of astragalus membranaceus and panax ginseng as immune regulators: Insights from the comparative biological and computational analysis phytomedicine. Phytomedicine 2021, 91, 153730. [Google Scholar] [CrossRef]
- Liu, J.; Nile, S.; Xu, G.; Wang, Y.; Kai, G. Alleviative effects of astragaloside IV on cyclophosphamide-induced oxidative damage and immunosuppression in tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 264, 109503. [Google Scholar]
- Weng, S.; Huang, L.; Cai, B.; He, L.; Wen, S.; Li, J.; Zhong, Z.; Zhang, H.; Huang, C.; Yang, Y.; et al. Astragaloside IV ameliorates experimental autoimmune myasthenia gravis by regulating CD4 + T cells and altering gut microbiota. Chin. Med. 2023, 18, 97. [Google Scholar] [CrossRef]
- Pham, K.; Vargas, A.; Frost, S.; Shah, S.; Heinrich, E.C. Changes in immune cell populations during acclimatization to high altitude. Physiol. Rep. 2024, 12, e70024. [Google Scholar] [CrossRef]
- Purdy, G.M.; James, M.A.; Rees, J.L.; Ondrus, P.; Keess, J.L.; Day, T.A.; Steinback, C.D. Spleen reactivity during incremental ascent to altitude. J. Appl. Physiol. 2019, 126, 152–159. [Google Scholar] [CrossRef]
- Feuerecker, M.; Crucian, B.E.; Quintens, R.; Buchheim, J.I.; Salam, A.P.; Rybka, A.; Moreels, M.; Strewe, C.; Stowe, R.; Mehta, S.; et al. Immune sensitization during 1 year in the antarctic high-altitude concordia environment. Allergy 2019, 74, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, L.; Li, Y.; Zhang, L. Genetic and immune changes in tibetan high-altitude populations contribute to biological adaptation to hypoxia. Environ. Health Prev. Med. 2022, 27, 39. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yong, S.; Xu, Y.; Hu, Y.; Li, J.; Long, Q.; Wang, X.; Gu, C.; Miao, Z. Integrative analysis of proteomics and metabolism reveals the potential roles of arachidonic acid metabolism in hypoxia response in mouse spleen. Molecules 2022, 27, 8102. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2023, 56, 1152. [Google Scholar] [CrossRef]
- Lin, Z.-L.; Xu, X.; Yang, J.-J.; Lu, L.-Q.; Huang, H.; Hua, X.-Z.; Lu, L.-D. Efficacy of budesonide/formoterol inhalation powder in treating viral pneumonia in children. World J. Clin. Cases 2024, 12, 4469–4475. [Google Scholar] [CrossRef]
- Diederichsen, A.C.; Hjelmborg Jv Christensen, P.B.; Zeuthen, J.; Fenger, C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol. Immunother. 2003, 52, 423–428. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, J.; Feng, L.; Wang, S.; Xiang, K.; Huang, Y.; Li, H.; Li, X.; Hu, X.; Che, L.; et al. Cognitive impairment mechanisms in high-altitude exposure: Proteomic and metabolomic insights. J. Proteome Res. 2024, 23, 5586–5599. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, X.; Gong, X.; Yang, Y.; Chen, C.; Shan, G.; Yao, Q. Astragaloside IV from Astragalus membranaceus ameliorates renal interstitial fibrosis by inhibiting inflammation via TLR4/NF-кB in vivo and in vitro. Int. Immunopharmacol. 2017, 42, 18–24. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Xu, M.; Fu, C.; Huang, Y.; Wang, T.-H.; Zuo, Z.-F.; Liu, X.-Z. A mechanistic exploratory study on the therapeutic efficacy of astragaloside IV against diabetic retinopathy revealed by network pharmacology. Front. Pharmacol. 2022, 13, 903485. [Google Scholar] [CrossRef]
- Wang, X.; Tan, P.; Kong, W. Astragaloside IV reduces renal injury in mice with IgA nephropathy by inhibiting TIM-1 signaling pathway and increasing th1/th2 cell ratio. Xi Bao Yu Fen. Zi Mian Yi Xue Za Zhi. 2024, 40, 501–507. [Google Scholar]
- Jin, L.; Hu, Q.; Hu, Y.; Chen, Z.; Liao, W. Respiratory syncytial virus infection reduces kynurenic acid production and reverses th17/treg balance by modulating indoleamine 2,3-dioxygenase (IDO) molecules in plasmacytoid dendritic cells. Med. Sci. Monit. 2020, 26, e926763. [Google Scholar] [CrossRef] [PubMed]
- Lundeberg, J.; Feiner, J.R.; Schober, A.; Sall Jeffery, W.; Eilers, H.; Bickler, P.E. Increased cytokines at high altitude: Lack of ef-fect of ibuprofen on acute mountain sickness, physiological variables, or cytokine levels. High. Alt. Med. Biol. 2018, 19, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-K.; Guo, M.-K.; Bai, X.-H.; Chen, L.-Q.; Su, S.-M.; Li, L.; Li, J.-Q. Astragaloside IV ameliorates intermittent hypoxia-induced inflammatory dysfunction by suppressing MAPK/NF-κB signalling pathways in beas-2B cells. Sleep. Breath. 2020, 24, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yu, S.; Zhao, F.; Liu, Y.; Wang, Y.; Fan, S.; Su, Y.; Lu, M.; Wang, H. Astragaloside IV alleviates brain injury induced by hypoxia via the calpain-1 signaling pathway. Neural Plast. 2022, 2022, 6509981. [Google Scholar] [CrossRef]
- Li, H.; Yao, C.; Shi, K.; Zhao, Y.; Du, J.; Hu, D.; Liu, Z. Astragaloside IV attenuates hypoxia/reoxygenation injury-induced apoptosis of type II alveolar epithelial cells through miR-21-5p. Bioengineered 2021, 12, 7747–7754. [Google Scholar] [CrossRef]
- Zhou, S.F.; Ma, J.; Qu, H.T.; Liu, Z.T.; He, W.D.; Wang, J.D.; Dou, A.X.; Zhang, N.; Liu, J.L.; Guo, C.S.; et al. Characterization of Th1- and Th2-associated Chemokine Receptor Expression in Spleens of Patients with Immune Thrombocytopenia. J. Clin. Immunol. 2013, 33, 938–946. [Google Scholar] [CrossRef]
- Wang, X.; Ali, W.; Zhang, K.; Ma, Y.; Zou, H.; Tong, X.; Zhu, J.; Song, R.; Zhao, H.; Liu, Z.; et al. The attenuating effects of serine against cadmium induced immunotoxicity through regulating M1/M2 and Th1/Th2 balance in spleen of C57BL/6 mice. Ecotoxicol. Environ. Saf. 2024, 286, 117216. [Google Scholar] [CrossRef]


| Gene | 5′-3′ | 3′-5′ |
|---|---|---|
| Mapk11 | GCGGGATTCTACCGGCAAG | GAGCAGACTGAGCCGTAGG |
| Bcl6 | TAGAGCCCATAAGACAGTGCT | CACCGCCATGATATTGCCTTC |
| Lck | TGGAGAACATTGACGTGTGTG | ATCCCTCATAGGTGACCAGTG |
| Ifng | GCAACAGCAAGGCGAAAAAG | CGCTTCCTGAGGCTGGATTC |
| Cd3d | AGCGGGATTCTGGCTAGTCT | CGCTGGTATTGCAGGTCACAA |
| Cd3e | ATGCGGTGGAACACTTTCTGG | GCACGTCAACTCTACACTGGT |
| Cd3g | ACTGTAGCCCAGACAAATAAAGC | TGCCCAGATTCCATGTGTTTT |
| Actb | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Wu, Z.; Dang, W.; Yang, T.; Chen, J.; Cheng, H.; Cui, J.; Lin, L.; Shen, X.; Li, F.; et al. Th1/Th2 Immune Imbalance in the Spleen of Mice Induced by Hypobaric Hypoxia Stimulation and Therapeutic Intervention of Astragaloside IV. Int. J. Mol. Sci. 2025, 26, 2584. https://doi.org/10.3390/ijms26062584
Gao R, Wu Z, Dang W, Yang T, Chen J, Cheng H, Cui J, Lin L, Shen X, Li F, et al. Th1/Th2 Immune Imbalance in the Spleen of Mice Induced by Hypobaric Hypoxia Stimulation and Therapeutic Intervention of Astragaloside IV. International Journal of Molecular Sciences. 2025; 26(6):2584. https://doi.org/10.3390/ijms26062584
Chicago/Turabian StyleGao, Rong, Zhenhui Wu, Wanyun Dang, Tingyu Yang, Junru Chen, Hongbo Cheng, Jialu Cui, Lin Lin, Xin Shen, Fangyang Li, and et al. 2025. "Th1/Th2 Immune Imbalance in the Spleen of Mice Induced by Hypobaric Hypoxia Stimulation and Therapeutic Intervention of Astragaloside IV" International Journal of Molecular Sciences 26, no. 6: 2584. https://doi.org/10.3390/ijms26062584
APA StyleGao, R., Wu, Z., Dang, W., Yang, T., Chen, J., Cheng, H., Cui, J., Lin, L., Shen, X., Li, F., Yan, J., Gao, Y., Gao, Y., & Ma, Z. (2025). Th1/Th2 Immune Imbalance in the Spleen of Mice Induced by Hypobaric Hypoxia Stimulation and Therapeutic Intervention of Astragaloside IV. International Journal of Molecular Sciences, 26(6), 2584. https://doi.org/10.3390/ijms26062584






