Mode of Action of Brassinosteroids: Seed Germination and Seedling Growth and Development—One Hypothesis
Abstract
1. Introduction
2. Observations Regarding the Applied Research Methodology
3. Germination of Seeds of Different Plant Species—Early Phases of Seedling Growth and Development and Some Processes Affecting Seedling Growth and Development Under the Influence of Different Brassinosteroids
4. Some Chemical Changes During Seed Germination and the Early Development and Growth of Seedlings of Different Plant Species Under the Influence of Different Brassinosteroids
5. Changes in Thermodynamic and Energy Parameters During Seed Germination and the Early Stages of Growth and Development of Corn Seedlings—Implications for the Vigor of Young Corn Plants Influenced by BRs
6. Conclusions and Further Directions Regarding the Mode of Action of BR Compounds
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kranner, I.; Minibayeva, F.V.; Beckett, R.P.; Seal, C.E. What is stress? Concepts, definitions and applications in seed science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, V.; Sredojevic, S. Thermodynamics of Seed and Plant Growth. In Thermodynamics—Systems in Equilibrium and Non-Equilibrium; Moreno-Piraján, J.C., Ed.; InTech: Rijeka, Croatia, 2011; Volume 1, pp. 1–20. ISBN 978-953-307-283-8. [Google Scholar] [CrossRef]
- Fincher, G.B. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 305–346. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C.; Smith, S.M. Starch degradation. Annu. Rev. Plant Biol. 2005, 56, 73–98. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.R.; Rodriguez, R.L. Metabolite Signals Regulate Gene Expression and Source/Sink Relations in Cereal Seedlings. Plant Physiol. 1994, 106, 1235–1239. [Google Scholar] [CrossRef]
- Bradford, K.J.; Benech-Arnold, R.L.; Come, D.; Corbineau, F. Quantifying the sensitivity of barley seed germination to oxygen, abscisic acid, and giberellin using a population-based treshold model. J. Exp. Bot. 2008, 59, 335–347. [Google Scholar] [CrossRef]
- Liu, X.; Hu, P.; Huang, M.; Tang, Y.; Li, Y.; Li, L.; Hou, X. The NF-YC–RGL2 module integrates GA and ABA signalling to regulate seed germination in Arabidopsis. Nat. Commun. 2016, 7, 12768. [Google Scholar] [CrossRef]
- Liu, X.; Ho, X. Antagonistic Regulation of ABA and GA in Metabolism and Signaling Pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef]
- Perata, P.; Matsukura, C.; Vernieri, P.; Yamaguchi, J. Sugar Repression of a Gibberellin-Dependent Signaling Pathway in Barley Embryos. Plant Cell 1997, 9, 2197–2208. [Google Scholar] [CrossRef]
- Leubner-Metzger, G. Brassinosteroids and gibberelins promote tobacco seed germination by distinct pathways. Planta 2001, 213, 758–763. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Xue, L.-W.; Dua, J.-B.; Yang, H.; Xua, F.; Yuan, S.; Lin, H.-H. Brassinosteroids Counteract Abscisic Acid in Germination and Growth of Arabidopsis. Zeit. Für Naturforsch. 2009, 64, 225–230. [Google Scholar] [CrossRef] [PubMed]
- El-Maarouf-Bouteau, H.; Sajjad, Y.; Bazin, J.; Langlade, N.; Cristescu, S.M.; Balzergue, S.; Baudouin, E.; Baily, C. Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower germination. Plant Cell Environ. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Q. Methods for the Study of Water Relations Under Desiccation Stress. In Desiccation and Survival in Plants. Drying Without Dying; Black, M., Pritchard, H.W., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 47–91. ISBN 978-0-85199-534-2. [Google Scholar] [CrossRef]
- Buitink, J.; Hoekstra, F.A.; Leprince, O. Biochemistry and Biophysics of Tolerance system. In Desiccation and Survival in Plants. Drying Without Dying; Black, M., Pritchard, H.W., Eds.; CABI Publishing: Wallingford, UK, 2002; pp. 293–318. ISBN 978-0-85199-534-2. [Google Scholar]
- Flock, T.; Weatheritt, R.J.; Latysheva, N.S.; Babu, M.M. Controlling entropy to tune the functions of intrinsically disordered regions. Curr. Opin. Struct. Biol. 2014, 26, 62–72. [Google Scholar] [CrossRef]
- Ashby, W.R. An Introduction to Cybernetics; Chapman & Hall Ltd.: London, UK, 1957; Available online: https://philpapers.org/archive/ASHAIT.pdf (accessed on 5 March 2024).
- Moreland, D.E. Mechanism of action of herbicides. Annu. Rev. Plant Physiol. 1980, 31, 597–638. Available online: https://www.annualreviews.org/content/journals/10.1146/annurev.pp.31.060180.003121 (accessed on 5 March 2024). [CrossRef]
- Waisi, H.; Nikolić, B.; Dragićević, V.; Šaponjić, B.; Jovanović, V.; Trifković, J.; Milojković-Opsenica, D. Different aspects of mode of action of brassinosteroids in maize. In Proceedings of the “AGROSYM 2015”—6th International Scientific Agricultural Symposium, Jahorina Mountain (near Sarajevo), Bosnia and Herzegovina, 15–18 October 2015; pp. 332–339, ISBN 978-99976-632-2-1. Available online: https://agrosym.ues.rs.ba/article/showpdf/BOOK_OF_PROCEEDINGS_2015.pdf (accessed on 5 March 2024).
- Symons, G.M.; Ross, J.J.; Jager, C.E.; Reid, J.B. Brassinosteroid transport. J. Exp. Bot. 2008, 59, 17–24. [Google Scholar] [CrossRef]
- Božilović, B.; Nikolić, B.; Waisi, H.; Trifković, J.; Dodevski, V.; Janković, B.; Krstić, S.; Mojović, M. Influence of 24-Epibrassinolide on the Energetic Parameters and Early Stages of Growth and Development in Seedlings of Two Maize (Zea mays L.) Genotypes. Agronomy 2023, 13, 1673. [Google Scholar] [CrossRef]
- Waisi, H.; Petković, A.; Nikolić, B.; Janković, B.; Raičević, V.; Lalević, B.; Giba, Z. Influence of 24-epibrassinolide on seedling growth and distribution of mineral elements in two maize hybrids. Chem. Ind. 2017, 71, 201–209. [Google Scholar] [CrossRef]
- Derevyanchuk, M.V.; Kretynin, S.; Kolesnikov, Y.; Litvinovskay, R.P.; Martinec, J.; Khripach, V.A.; Kravets, V.S. Seed germination, respiratory processes and phosphatidic acid accumulation in Arabidopsis diacylglycerol kinase knockouts—The effect of brassinosteroid, brassinazole and salinity. Steroids 2019, 147, 28–36. [Google Scholar] [CrossRef]
- Steber, C.M.; McCourt, P. A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 2001, 125, 763–769. [Google Scholar] [CrossRef]
- Sirhindi, G.; Kumar, S.; Bhardwaj, R.; Kumar, M. Effects of 24-epibrassinolide and 28-homobrassinolide on the growth and antioxidant enzyme activities in the seedlings of Brassica juncea L. Physiol. Mol. Biol. Plants 2009, 15, 335–341. [Google Scholar] [CrossRef]
- Kumar, M.; Sirhindi, G.; Bhardwaj, R.; Kumar, S.; Jain, G. Effect of exogenous H2O2 on antioxidant enzymes of Brassica juncea L. seedlings in relation to 24-epibrassinolide under chilling stress. Ind. J. Biochem. Biophys. 2010, 47, 378–382. [Google Scholar]
- Mahesh, K.; Balaraju, P.; Ramakrishna, B.; Rao, S.S.R. Effect of Brassinosteroids on Germination and Seedling Growth of Radish (Raphanus sativus L.) under PEG-6000 Induced Water Stress. Am. J. Plant Sci. 2013, 4, 2305–2313. [Google Scholar] [CrossRef]
- Kumar, S.; Sirhindi, G.; Bhardwaj, R.; Kumar, M.; Arora, P. Role of 24-Epibrassinolide in Amelioration of High Temperature Stress through Antioxidant Defense System in Brassica juncea L. Plant Stress 2012, 6, 55–58. [Google Scholar]
- Singh, I.; Kumar, U.; Singh, S.K.; Gupta, C.; Singh, M.; Kushwaha, S.R. Physiological and biochemical effect of 24-epibrassinoslide on cold tolerance in maize seedlings. Physiol. Mol. Biol. Plants 2012, 18, 229–236. [Google Scholar] [CrossRef]
- Sharma, I.; Bhardwaj, R.; Pati, P.K. Mitigation of adverse effects of chlorpyrifos by 24-epibrassinolide and analysis of stress markers in a rice variety Pusa Basmati-1. Ecotox. Environ. Saf. 2012, 85, 72–81. [Google Scholar] [CrossRef]
- Kaur, H.; Sirhindi, G.; Bhardwaj, R. Influence of 28-Homobrassinolide on Photochemical Efficiency in Brassica juncea Under Dual Stress of Extreme Temperatures and Salt. Can. J. Pure App. Sci. 2017, 11, 4205–4213. [Google Scholar] [CrossRef]
- Sharma, P.; Bhardwaj, R.; Arora, N.; Arora, H.K. Effect of 28-homobrassinolide on growth, zinc metal uptake and antioxidative enzyme activities in Brassica juncea L. seedlings. Braz. J. Plant Physiol. 2007, 19, 203–210. [Google Scholar] [CrossRef]
- Thussagunpanit, J.; Jutamanee, K.; Kaveeta, L.; Chai-arree, W.; Pankean, P.; Homvisasevongsa, S.; Suksamrarn, A. Comparative Effects of Brassinosteroid and Brassinosteroid Mimic on Improving Photosynthesis, Lipid Peroxidation, and Rice Seed Set under Heat Stress. J. Plant Growth Regul. 2015, 34, 320–331. [Google Scholar] [CrossRef]
- Yadav, P.; Kaur, R.; Kanwar, M.K.; Sharma, A.; Verma, V.; Sirhindi, G.; Bhardwaj, R. Castasterone confers copper stress tolerance by regulating antioxidant enzyme responses, antioxidants, and amino acid balance in B. juncea seedlings. Ecotox. Environ. Saf. 2018, 147, 725–734. [Google Scholar] [CrossRef]
- Chmur, M.; Bajguz, A. Brassinolide Enhances the Level of Brassinosteroids, Protein, Pigments, and Monosaccharides in Wolffia arrhiza Treated with Brassinazole. Plants 2021, 10, 1311. [Google Scholar] [CrossRef]
- Yi, S.-A.; Cho, Y.J.; Park, W.J. An exception of synergistic interaction between brassinosteroids and auxin. J. Nano Biotech. 2004, 1, 108–110. [Google Scholar]
- Cao, F.; Liu, L.; Ibrahim, W.; Cai, Y.; Wu, F. Alleviating Effects of Exogenous Glutathione, Glycinebetaine, Brassinosteroids and Salicylic Acid on Cadmium Toxicity in Rice Seedlings (Oryza sativa). Agrotechnolog 2013, 2, 107. [Google Scholar] [CrossRef]
- Yusuf, M.; Fariduddin, Q.; Ahmad, A. 24-Epibrassinolide modulates growth, nodulation, antioxidant system, and osmolyte in tolerant and sensitive varieties of Vigna radiata under different levels of nickel: A shotgun approach. Plant Physiol. Biochem. 2012, 57, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Van Esse, G.W.; van Mourik, S.; Stigler, H.; ten Hove, C.A.; Molenaar, J.; de Vries, S.C. A Mathematical Model for BRASSINOSTEROID INSENSITIVE1-Mediated Signaling in Root Growth and Hypocotil Elongation. Plant Physiol. 2012, 160, 523–532. [Google Scholar] [CrossRef][Green Version]
- Waisi, H. The Influence of Brassinosteroid 24-Epibrassinolide on Germination and Early Stages of Growth and Development of Different Maize Hybrids (Zea mays L.). Ph.D. Thesis, Faculty of Biology, University of Belgrade, Belgrade, Serbia, 2016. (In Serbian with summary in English). Available online: http://nardus.mpn.gov.rs/handle/123456789/6772 (accessed on 27 December 2024).
- Carpita, N. Structure and Biogenesis of the Cell Walls of Grasses. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 445–476. [Google Scholar] [CrossRef]
- Wolf, S.; Mravec, J.; Greiner, S.; Mouille, G.; Höfte, H. Plant Cell Wall Homeostasis Is Mediated by Brassinosteroid Feedback Signaling. Curr. Biol. 2012, 22, 1732–1737. [Google Scholar] [CrossRef]
- Brosa, C. Biological Effects of Brassinosteroids. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 339–358. [Google Scholar] [CrossRef]
- Waisi, H.; Janković, B.; Janković, M.; Nikolić, B.; Dimkić, I.; Lalević, B.; Raičević, V. New insights in dehydration stress behavior of two maize hybrids using advanced distributed reactivity model (DRM). Responses to the impact of 24-epibrassinolide. PLoS ONE 2017, 12, e0179650. [Google Scholar] [CrossRef]
- Waisi, H.; Janković, B.; Nikolić, B.; Dragičević, V.; Panić, I.; Tosti, T.; Trifković, J. Influence of various concentrations of 24-epibrassinolide on the kinetic parameters during isothermal dehydration of two maize hybrids. S. Afr. J. Bot. 2018, 119, 69–79. [Google Scholar] [CrossRef]
- Sharma, P.; Bhardwaj, R. Effect of 24-Epibrassinolide on Seed Germination, Seedling Growth and Heavy Metal Uptake in Brassica juncea L. Gen. Appl. Plant Physiol. 2007, 33, 59–73. [Google Scholar]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars—Metabolism, sensing and abiotic stress. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Fincher, G.B.; Stone, B.A.; Clarke, A.E. Arabinogalactan-Proteins: Structure, Biosynthesis, and Function. Ann. Rev. Plant Physiol. 1983, 34, 47–70. [Google Scholar] [CrossRef]
- Neto, J.-R.C.F.; de Melo Souza, A.C.; da Silva, M.D.; Benko-Iseppon, A.M.; Pandolfi, V.; da Costa, A.F.; Kido, E.K. The Transcriptional Modulation of Inositol and Raffinose Family Oligosaccharides Pathways in Plants—An (A)biotic Stress, Perspective. In Abiotic and Biotic Stress in Plants: Recent Advances and Future Perspectives; Arun, K., Shanker, C., Eds.; IntechOpen: London, UK, 2016; pp. 81–99. ISBN 978-953-307-394-1. [Google Scholar] [CrossRef]
- Roland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef]
- Iordachescu, M.; Imai, R. Trehalose and Abiotic Stress in Biological Systems. In Abiotic Stress in Plants—Mechanims and Adaptation; Shanker, A., Ed.; InTech: London, UK, 2011; pp. 215–234. ISBN 978-953-307-394-1. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and Gluthathione: Keeping Active Oxygen Under Control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Møller, I.M. Plant Mitochondria and Oxidative Stress: Electron Transport, NADPH Turnover, and Metabolism of Reactive Oxygen Species. Annu. Rev. Plant Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef]
- Waisi, H.; Kosović, A.; Krstić, Đ.; Milojković-Opsenica, D.; Nikolić, B.; Dragićević, V.; Trifković, J. Polyphenolic profile of maize seedlings treated with 24-epibrassinolide. J. Chem. 2015, 2015, 976971. [Google Scholar] [CrossRef]
- Ho, L.C. Metabolism and Compartmentation of Imported Sugars in Sink Organs in Relation to Sink Strength. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 355–378. [Google Scholar] [CrossRef]
- Turgeon, R. The Sink-Source Transition in Leaves. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 119–138. [Google Scholar] [CrossRef]
- Sonnewald, U.; Fernie, A.R. Next-generation strategies for understanding and influencing source sink relations in crop plants. Curr. Opin. Plant Biol. 2018, 43, 63–70. [Google Scholar] [CrossRef]
- Komor, E. Source physiology and assimilate transport: The interaction of sucrose metabolism, starch storage and phloem export in source leaves and the effects on sugar status in plants. Aust. J. Plant Physiol. 2000, 27, 497–505. [Google Scholar] [CrossRef]
- Derevyanchuk, M.V.; Grabelnyh, O.I.; Litvinovskaya, R.P.; Voinikov, V.K.; Sauchuk, A.L.; Khripach, V.; Kravets, V. Influence of brassinosteroids on plant cell alternative respiration pathway and antioxidant systems activity under abiotic stress conditions. Biopolym. Cell 2014, 30, 436–442. [Google Scholar] [CrossRef]
- Derevyanchuk, M.V.; Kretynin, S.; Iakovenko, O.; Litvinovskaya, R.P.; Zhabinskii, V.; Martinec, J.; Blume, Y.; Khripach, V.A.; Kravets, V.S. Effect of 24-epibrassinolide on Brassica napus alternative respiratory pathway, guard cells movement and phospholipid signaling under salt stress. Steroids 2017, 117, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Quoc, B.; Krivitzky, M.; Huber, S.C.; Lecharny, A. Sucrose Synthase in Developing Maize Leaves. Regulation of Activity by Protein Level during the Import to Export Transition. Plant Physiol. 1990, 94, 516–523. [Google Scholar] [CrossRef]
- Godt, D.E.; Roitsch, T. Regulation and Tissue-Specific Distribution of m RNAs for Three Extracellular Invertase Isoenzymes of Tomato Suggest an Important Function in Establishing and Maintaining Sink Metabolism. Plant Physiol. 1997, 115, 273–282. [Google Scholar] [CrossRef]
- Ren, H.; Qi, H.; Zhao, M.; Zhou, W.; Wang, X.; Gong, X.; Jiang, Y.; Li, C. Characterization of source–sink traits and carbon translocation in maize hybrids under high plant density. Agronomy 2022, 12, 961. [Google Scholar] [CrossRef]
- Minchin, P.E.H.; Thorpe, M.R.; Farrar, J.F.; Koroleva, O.A. Source-sink coupling in young barley plants and control of phloem loading. J. Exp. Bot. 2002, 53, 1671–1676. [Google Scholar] [CrossRef]
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential Regulators of Plant Growth and Development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef]
- Kumar, R.; Bishop, E.; Bridges, W.C.; Tharayil, N.; Sekhon, R.S. Sugar partitioning and source–sink interaction are key determinants of leaf senescence in maize. Plant Cell Environ. 2019, 42, 2597–2611. [Google Scholar] [CrossRef]
- Leyser, O. Auxin, Self-Organisation, and the Colonial Nature of Plants. Curr. Biol. 2011, 21, R331–R337. [Google Scholar] [CrossRef]
- Reinhardt, D.; Pesce, E.R.; Stieger, P.; Mandel, T.; Baltensperger, K.; Bennet, M.; Traas, J.; Friml, J.; Kuhlmeier, C. Regulation of phyllotaxis by polar auxin transport. Nature 2003, 426, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S.; Guyomarc’h, S.; Mandel, T.; Reinhardt, D.; Kuhlmeier, C.; Prusinkiewic, P. A plausible model of phyllotaxis. Proc. Natl. Acad. Sci. USA 2006, 103, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.; Shinohara, N.; Sieberer, T.; Williamson, L.; George, G.; Hepworth, J.; Müller, D.; Domagalska, M.A.; Leyser, O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 2010, 137, 2905–2913. [Google Scholar] [CrossRef]
- Sankar, M.; Osmont, K.S.; Rolcik, J.; Gujas, B.; Tarkowska, D.; Strnad, M.; Xenarios, I.; Hardtke, C.S. A qualitative continuous model of cellular auxin and brassinosteroid signaling and their crosstalk. Bioinformatics 2011, 27, 1404–1412. [Google Scholar] [CrossRef]
- Hartwig, T.; Wang, Z.-Y. The molecular circuit of steroid signalling in plants. Essays Biochem. 2015, 58, 71–82. [Google Scholar] [CrossRef]
- Hong, Z.; Ueguchi-Tanaka, M.; Matsuoka, M. Brassinosteroids and Rice Architecture. J. Pestic. Sci. 2004, 29, 184–188. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, Y.; Lu, Y.; Wang, X. Mechanisms of brassinosteroids interacting with multiple hormones. Plant Signal Behav. 2009, 4, 1117–1120. [Google Scholar] [CrossRef]
- Gururani, M.A.; Mohanta, T.K.; Bae, H. Current Understanding of the Interplay between Phytohormones and Photosynthesis under Environmental Stress. Inter. J. Mol. Sci. 2015, 16, 19055–19085. [Google Scholar] [CrossRef]
- Marková, H.; Tarkowská, D.; Čečetka, P.; Kočová, M.; Rothová, O.; Holá, D. Contents of endogenous brassinosteroids and the response to drought and/or exogenously applied 24-epibrassinolide in two different maize leaves. Front. Plant Sci. 2023, 14, 1139162. [Google Scholar] [CrossRef]
- Kutschera, U.; Wang, Z.Y. Brassinosteroiod action in flowering plants: A Darwinian perspective. J. Exp. Bot. 2012, 63, 3511–3522. [Google Scholar] [CrossRef]
- Vriet, C.; Russinova, E.; Reuzeau, C. From squalene to brassinolide: The steroid metabolic and signaling pathways across the plant kingdom. Mol. Plant 2013, 6, 1738–1757. [Google Scholar] [CrossRef] [PubMed]
- Waisi, H.; Nikolić, B.; Janković, B. Transformation of Matter and Energy in Crops Under the Influence of Brassinosteroids. In Brassinosteroids: Plant Growth and Development; Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A., Eds.; Springer Nature: Singapore, 2019. [Google Scholar] [CrossRef]
- Popović, M.; Minceva, M. Standard Thermodynamic Properties, Biosynthesis rates, and the Driving Force of Growth of Five Agricultural Plants. Front. Plant Sci. 2021, 12, 671868. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.G.; Stoll, M.; Santos, T.; de Sousa, C.; Chaves, M.M.; Grant, O.M. Use of infrared thermography for monitoring stomatal closure in the field: Application to grapevine. J. Exp. Bot. 2002, 53, 2249–2260. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Subhash, C.; Yan, J.; Song, C.; Zhao, J.; Li, J. Maize leaf temperature responses to drought: Thermal imaging and quantitative trait loci (QTL) mapping. Environ. Exp. Bot. 2011, 71, 158–165. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press, Harcourt Brace& Company Publishers: London, UK; New York, NY, USA, 1995; p. 889. ISBN 978-0-12-473543-9. [Google Scholar] [CrossRef]
- Forde, B.G. Local and Long-Range Signaling Pathways Regulating Plant Responses to Nitrate. Annu. Rev. Plant Biol. 2002, 53, 203–224. [Google Scholar] [CrossRef]
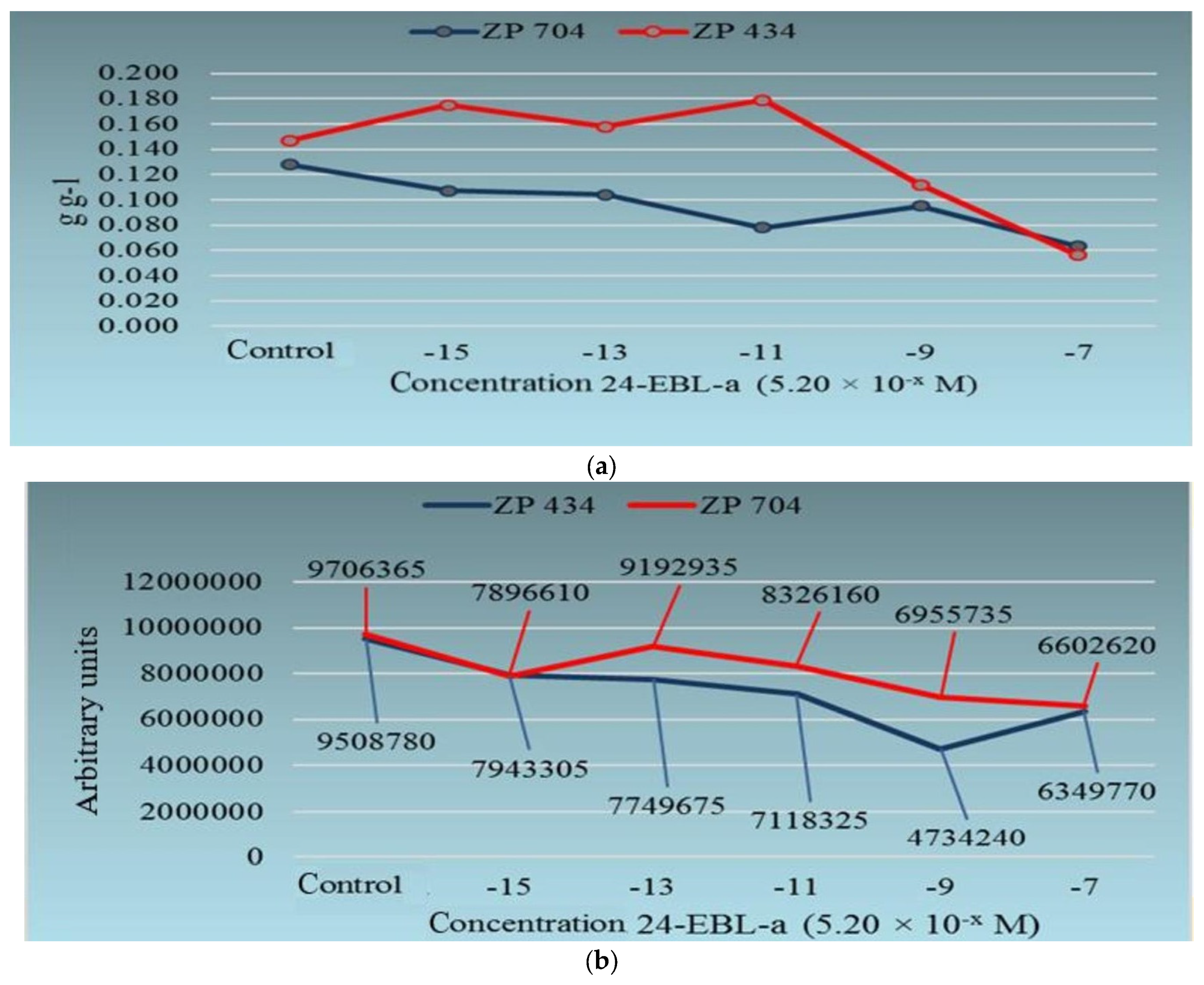
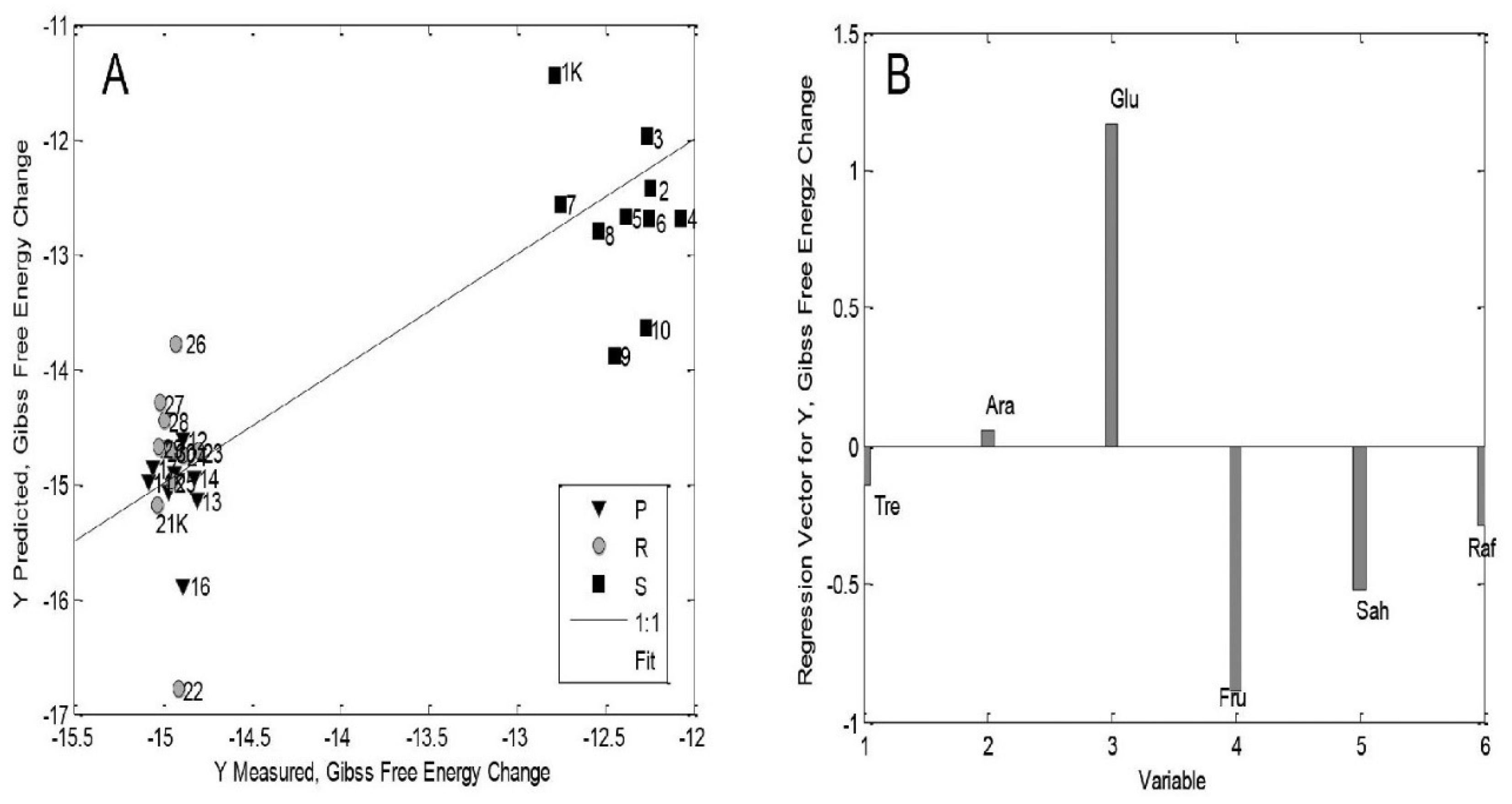
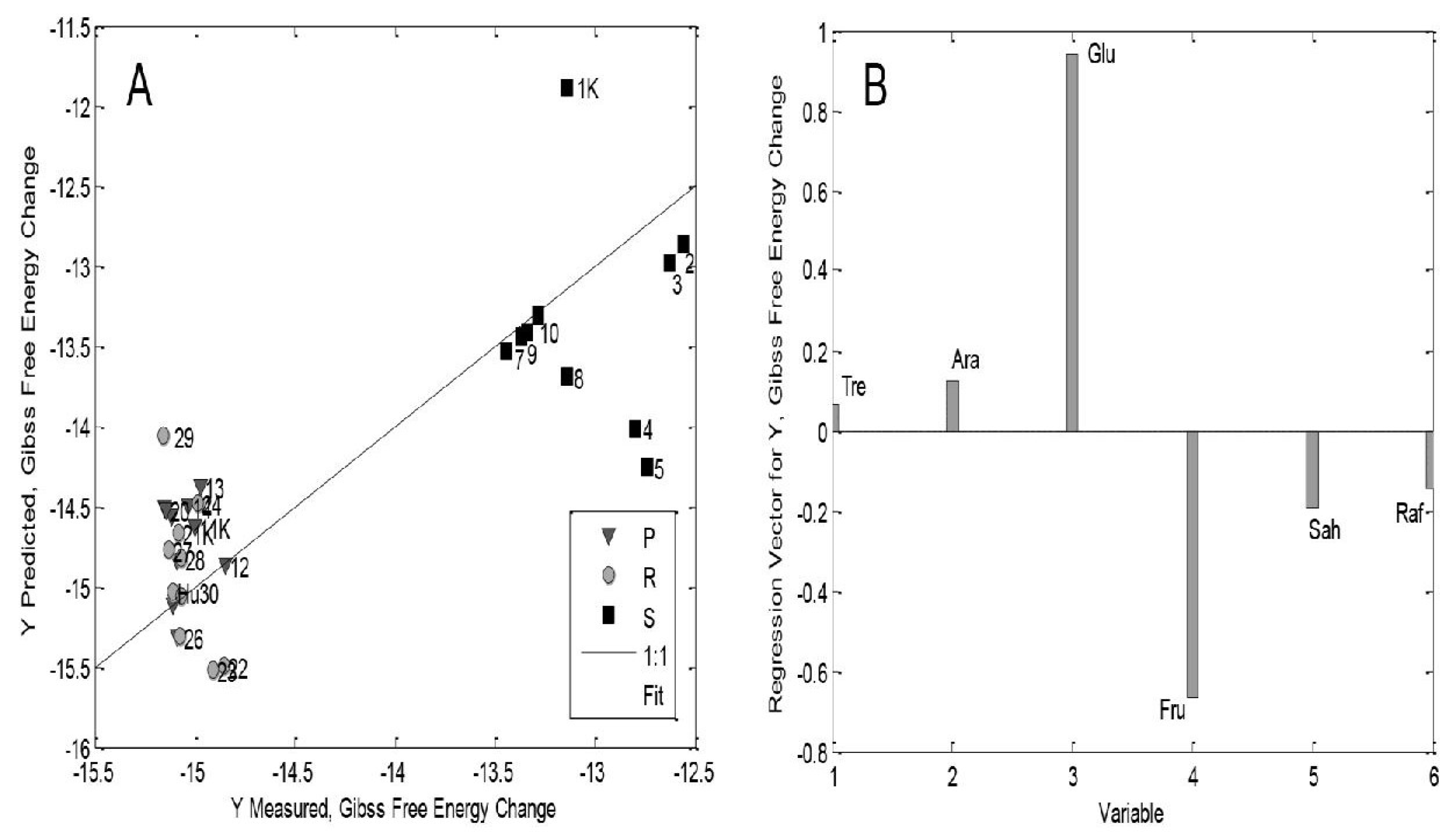
| Type of BR | Concentration of BRs | Other Treatments | Plant Species and/or Genotype/Ecotype; Age of Seedlings/Plants | Measured Parameters (with Unit of Measure)—9 Different Parts (S, R, St: Stem) | Higher (>, ≥)/Lower (˂, ≤) Values Than Control or Non-Significant Difference (n-s.d.) | Reference |
|---|---|---|---|---|---|---|
| 24-EBL | 10−6, 10−8 and 10−10 M | Ni (different concentrations) | Vigna radiata (nodulated) 45 d old | SOD, POD (units g−1 FW) | 24-EBL treat.: ≥ Ni treat.: ≥ 24-EBL + Ni treat.: > | [38] |
| 24-EBL | 10−6, 10−8 and 10−10 M | Ni (different concentrations) | Vigna radiata (nodulated) 45 d old | CAT (mM H2O2 degraded g−1 FW), Pro (mol g−1 FW) | 24-EBL treat.: n-s.d. Ni treat.: ≥ 24-EBL + Ni treat.: > | [38] |
| BRs | 10−5 M | Different concentrations of Cd, GSH, GB, and SA | Rice (japonica type) 12 d after 2nd leaf stage | SOD (units g−1 FW), | (Cd, GSH, BRs, GB, SA treat.): > (S), n-s.d. (R, St) | [37] |
| BRs | 10−5 M | Different concentrations of Cd, GSH, GB, and SA | Rice (japonica type) 12 d after 2nd leaf stage | POD (OD470 g−1 FW min−1) | (Cd, SA treat.): > (S); (GSH, BRs, GB treat.): n-s.d. (S); (GSH treat.): n-s.d. (R); (BRs treat.): n-s.d. (St); (BRs, GB, SA treat.): ˂ (R); (GSH, BRs, GB, SA treat.): ˂ (St); | [37] |
| Castasterone (CS) | 10−7, 10−9 and 10−11 M | Cu (different concentrations) | Brasicca juncea 7 d old | APX, DHAR, GR, GST, GPOX (μmol UA mg−1 protein) | Controle + Cu: ≥ CS: n-s.d. CS + Cu: ≥ | [34] |
| Castasterone (CS) | 10−7, 10−9 and 10−11 M | Cu (different concentrations) | Brasicca juncea 7 d old | Glu, AA (mg g−1 FW) | Controle + Cu: ≥ CS: ≥ CS + Cu: ≥ | [34] |
| 28-HBL | 10−7, 10−9 and 10−11 M | Zn (different concentrations) | Brasicca juncea 7 d old | POD, CAT, GR, APX, SOD (mmole UA mg protein−1) | Controle + Zn: >10−7 M (POD, GR, APX, SOD); Controle + Zn: >10−9 M (CAT) 28-HBL + Zn: >10−7 M (CAT, GR, APX, SOD) | [32] |
| 28-HBL/ 24-EBL | 0.5, 1.0, 2.0 10−6 M | PEG6000 (15% dilution)-induced drought | Raphanus sativus 3 + 7 d old | POD (μmol AA mg protein−1 min−1); CAT (μmol H2O2 mg protein−1 min−1) | Controle + PEG6000: > 24-EBL: > 2 × 10−6 M, >0.5 × 10−6 M PEG6000 + 24-EBL: >0.5 × 10−6 M 28-HBL: > 2 × 10−6 M, >0.5 × 10−6 M PEG6000 + 28-HBL: > 0.5 × 10−6 M | [27] |
| 28-HBL/ 24-EBL | 0.5, 1.0, 2.0 10−6 M | PEG6000 (15% dilution)-induced drought | Raphanus sativus 3 + 7 d old | APX (μmol AA mg protein−1 min−1); SOD (UA mg protein−1) | Controle + PEG6000: 24-EBL: >10−6 M, >0.5 × 10−6 M PEG6000 + 24-EBL: >0.5 × 10−6 M 28-HBL: >10−6 M, >0.5 × 10−6 M PEG6000 + 28-HBL: >0.5 × 10−6 M | [27] |
| 24-EBL | 10−8 M | H2O2 + cold (4 °C, 3 h during 3 d) | Brasicca juncea 10 d old | SOD (UA mg protein−1); CAT (UA mg protein−1); APX (μmol AA mg protein−1 min−1) | H2O2 + cold: n-s.d., >, > 24-EBL + H2O2 + cold: > | [26] |
| 28-HBL/ 24-EBL | 10−6, 10−8 and 10−10 M | Natural conditions | Brasicca juncea 10 d old | APX (μmol AA mg protein−1 min−1); CAT (UA mg protein−1) | >10−6 M 24-EBL and 28-HBL | [25] |
| 24-EBL | 10−7 M | Chlorpyrifos (0,06% dilution) | Rice (indica type) 12 d old | SOD (UA mg protein−1); GR (μmol min−1 mg protein−1); APX (μmol AA mg protein−1 min−1); CAT (UA mg protein−1); Pro (μmol g−1 FW) | SOD, GR, SOD: 24-EBL + CPF: >10−7 M; APX, Pro: 24-EBL +CPF: >10−9 M; | [30] |
| 24-EBL | 10−6, 10−8 and 10−10 M | Heat (40 °C, 3 h over 3 d) | Brassica juncea L. 10 d old | CAT (UA mg protein−1); APX (μmol AA mg protein−1 min−1); SOD (UA mg protein−1); | SOD, CAT: >10−7 M; APX: >10−10 M; | [28] |
| 24-EBL | 10−8 M | BZR, 50 mM and 100 mM of NaCl | A.haliana (ecotype Columbia 0) 21 d old | Tot, COX, and AOX respiration (resp.; nM CO2 min−1 X mg DW) | 24-EBL + NaCl/ BZR Tot resp.: ≥/˂ COX resp.: ≥/≤ AOX resp.: ≥/˂ | [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolić, B.; Jovanović, V.; Knežević, B.; Nikolić, Z.; Babović-Đorđević, M. Mode of Action of Brassinosteroids: Seed Germination and Seedling Growth and Development—One Hypothesis. Int. J. Mol. Sci. 2025, 26, 2559. https://doi.org/10.3390/ijms26062559
Nikolić B, Jovanović V, Knežević B, Nikolić Z, Babović-Đorđević M. Mode of Action of Brassinosteroids: Seed Germination and Seedling Growth and Development—One Hypothesis. International Journal of Molecular Sciences. 2025; 26(6):2559. https://doi.org/10.3390/ijms26062559
Chicago/Turabian StyleNikolić, Bogdan, Vladan Jovanović, Branislav Knežević, Zoran Nikolić, and Maja Babović-Đorđević. 2025. "Mode of Action of Brassinosteroids: Seed Germination and Seedling Growth and Development—One Hypothesis" International Journal of Molecular Sciences 26, no. 6: 2559. https://doi.org/10.3390/ijms26062559
APA StyleNikolić, B., Jovanović, V., Knežević, B., Nikolić, Z., & Babović-Đorđević, M. (2025). Mode of Action of Brassinosteroids: Seed Germination and Seedling Growth and Development—One Hypothesis. International Journal of Molecular Sciences, 26(6), 2559. https://doi.org/10.3390/ijms26062559





