4(3H)-Quinazolinone: A Natural Scaffold for Drug and Agrochemical Discovery
Abstract
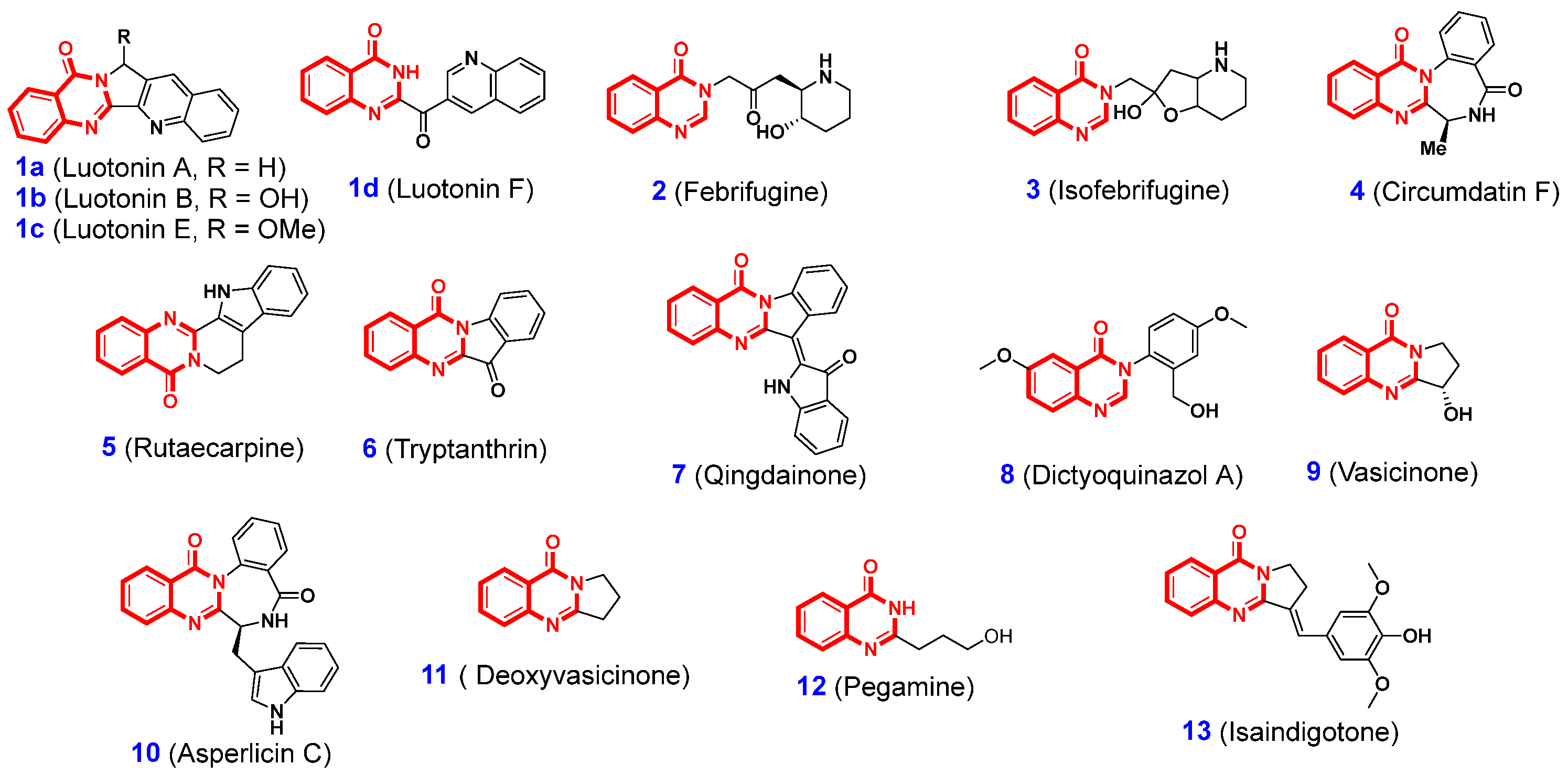
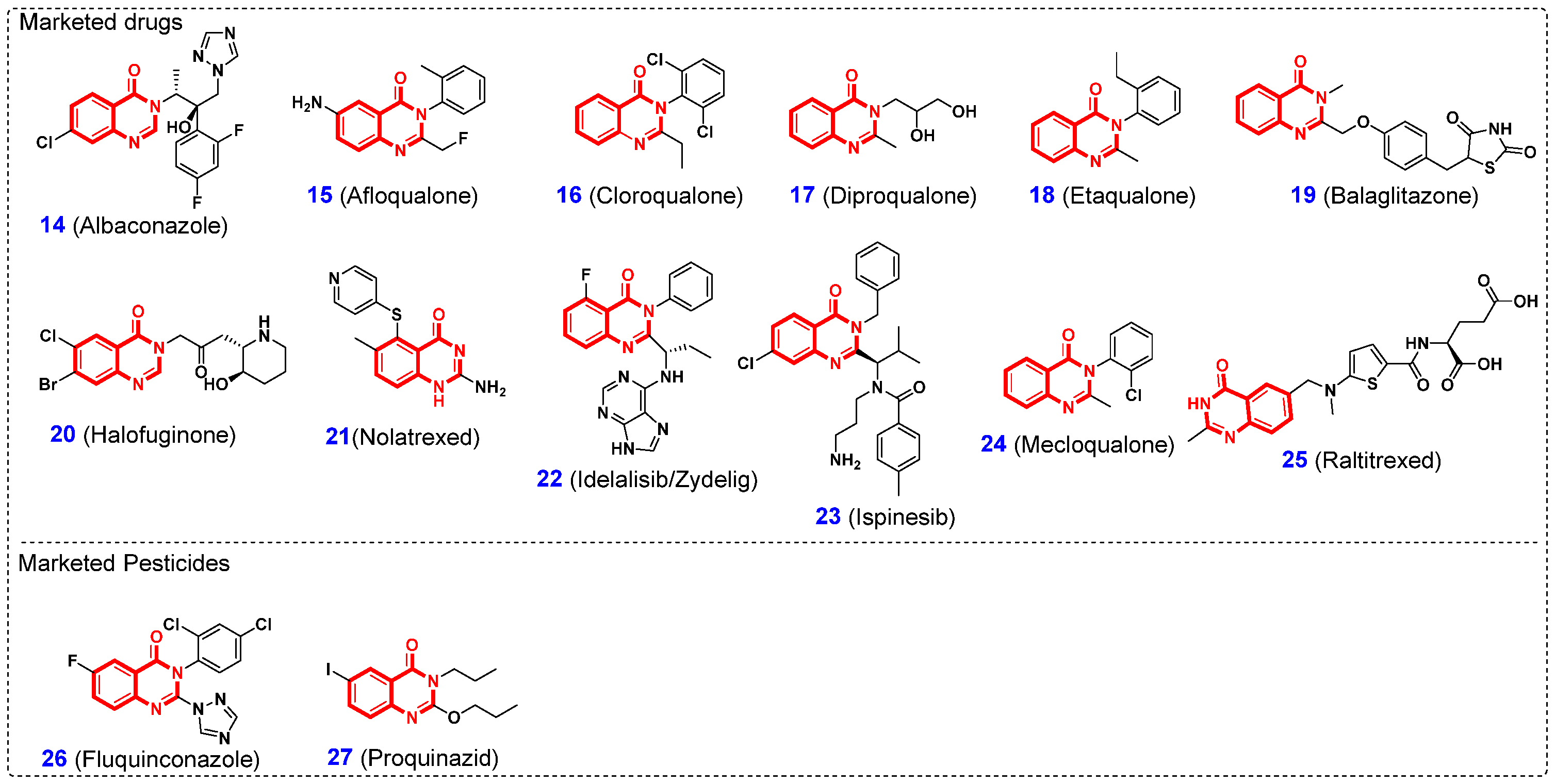
1. Introduction
2. Application of the 4(3H)-QLOs in Medicinal Chemistry
2.1. Anticancer Activity
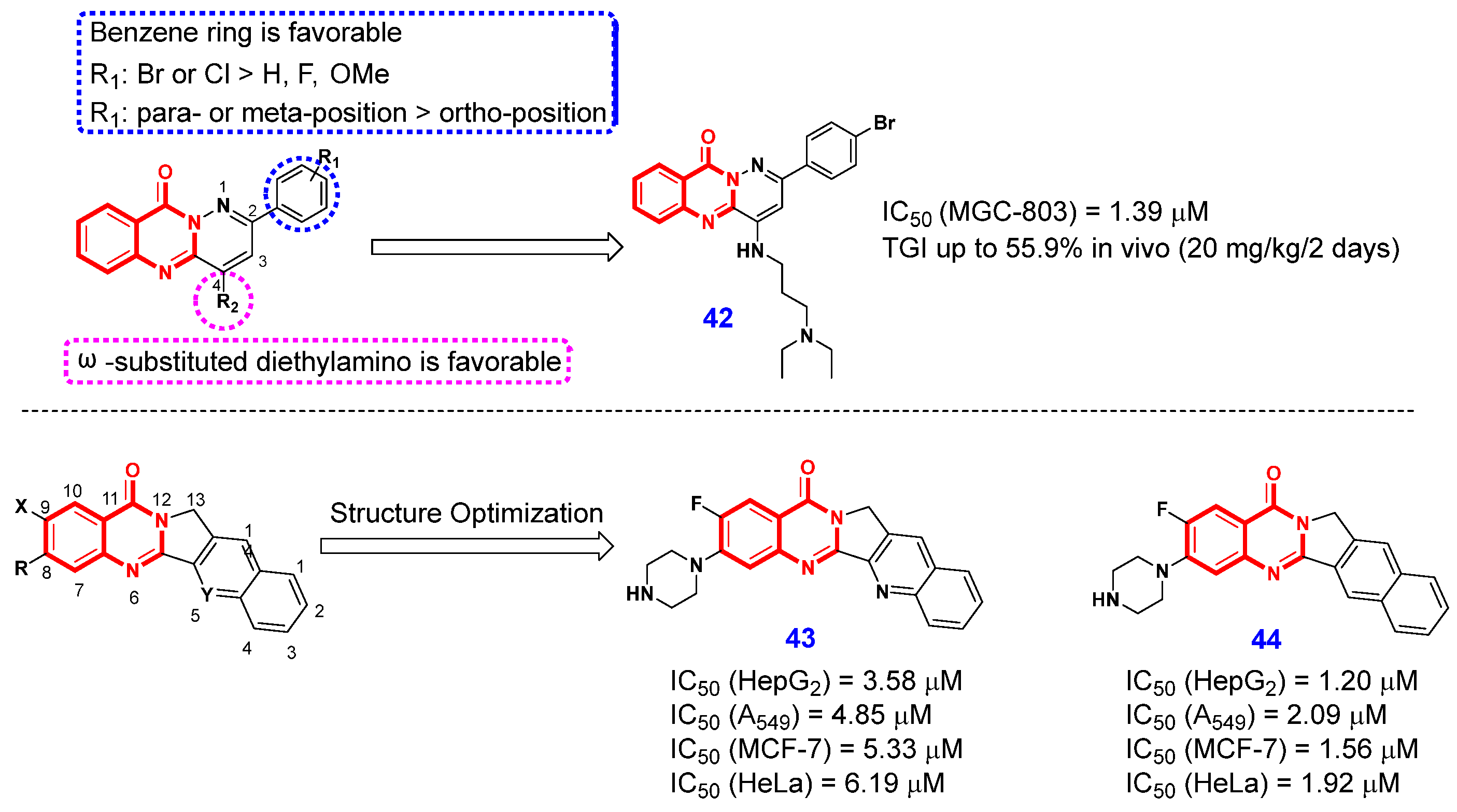
2.1.1. Inhibition of Protein Kinases
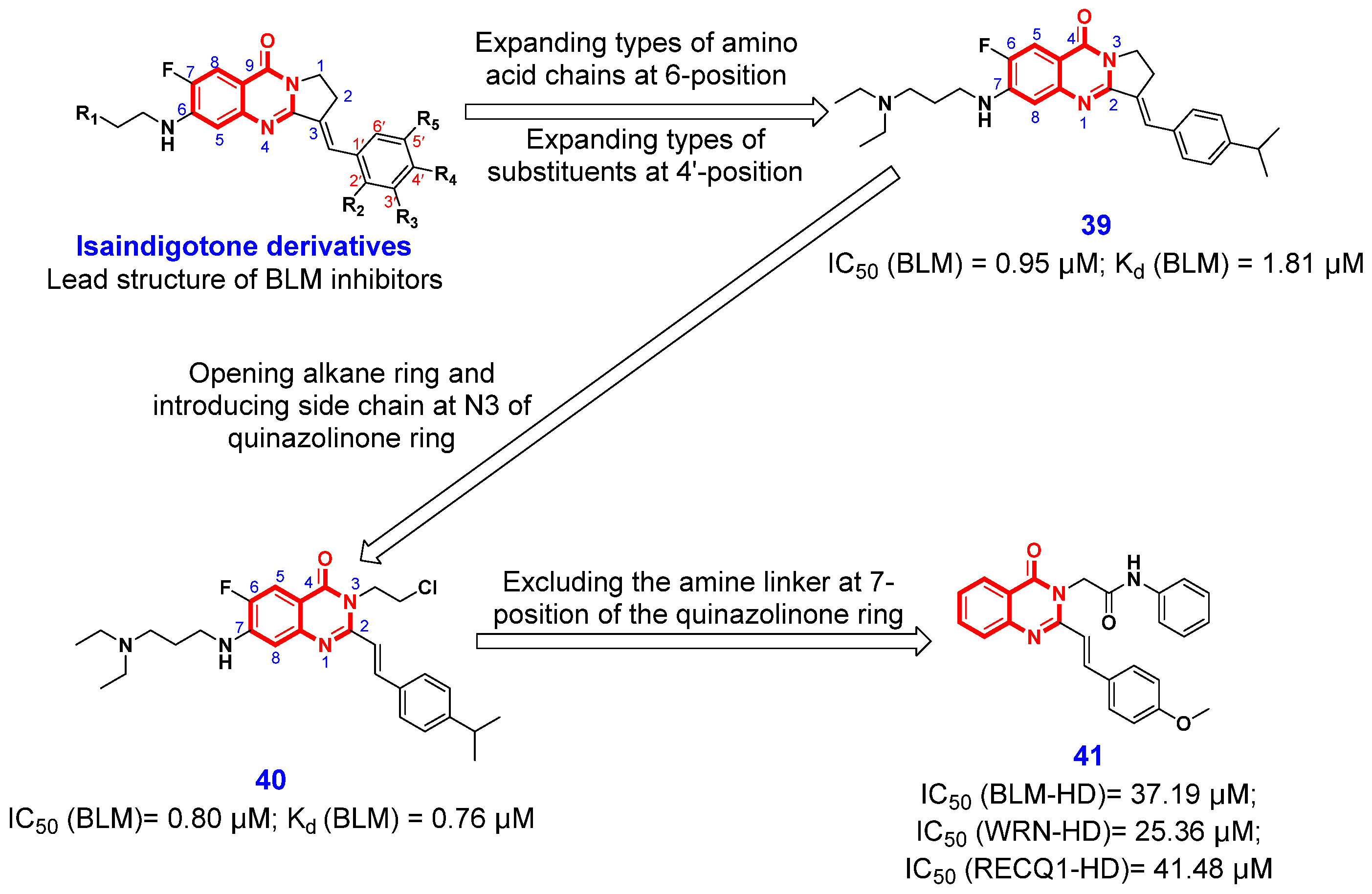
2.1.2. Inhibition of RecQ Helicases
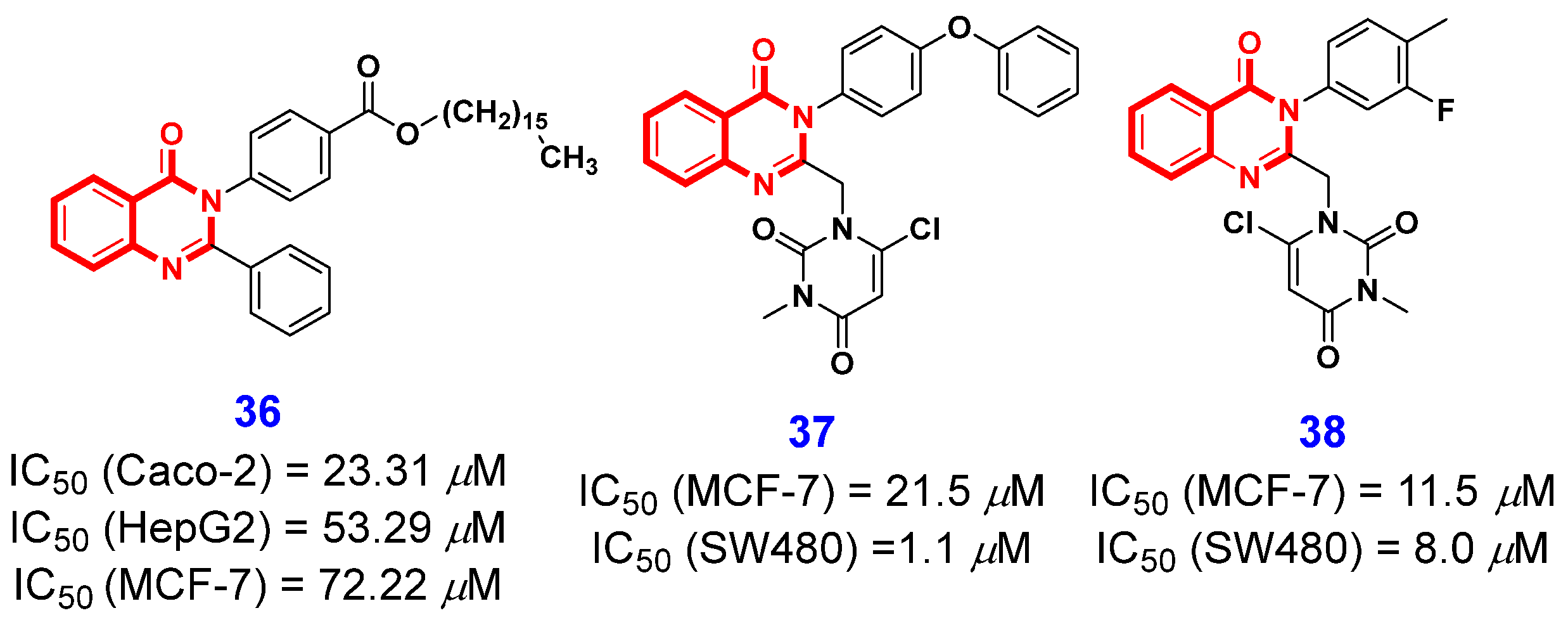
2.1.3. Inhibition of Topoisomerase 1
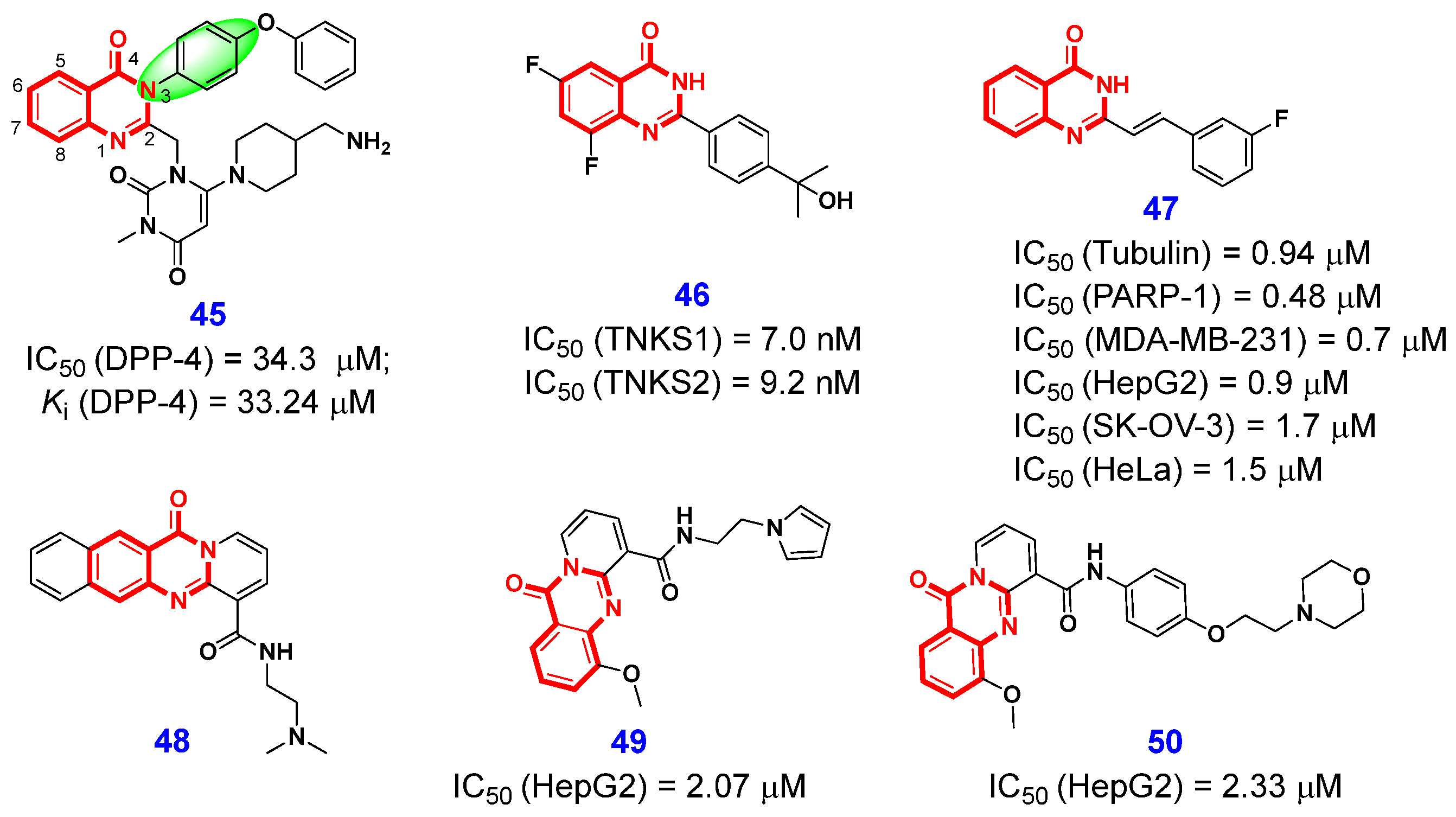
2.1.4. Inhibition of Dipeptidyl Peptidase-4
2.1.5. Inhibition of Tankyrase
2.1.6. Dual-Target Inhibitors
2.1.7. Inhibition of Other Anticancer Targets
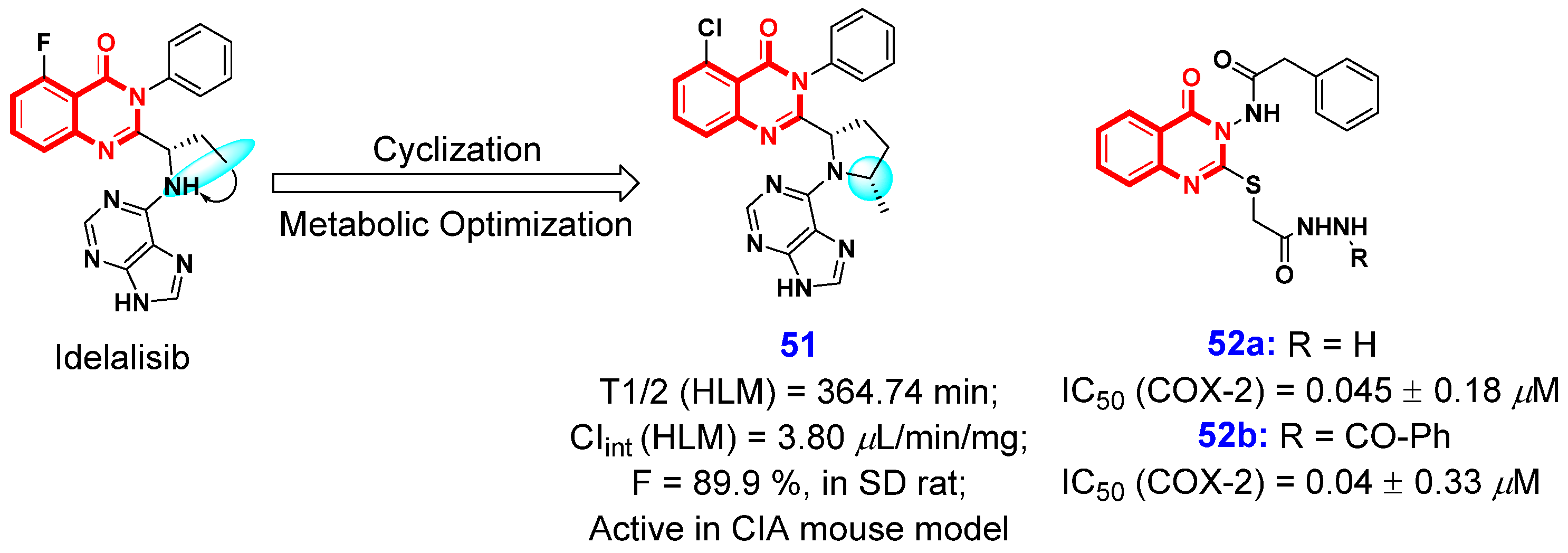
2.2. Anti-Inflammatory Activities
2.3. Antiviral Activities
2.4. Antimalarial Activity
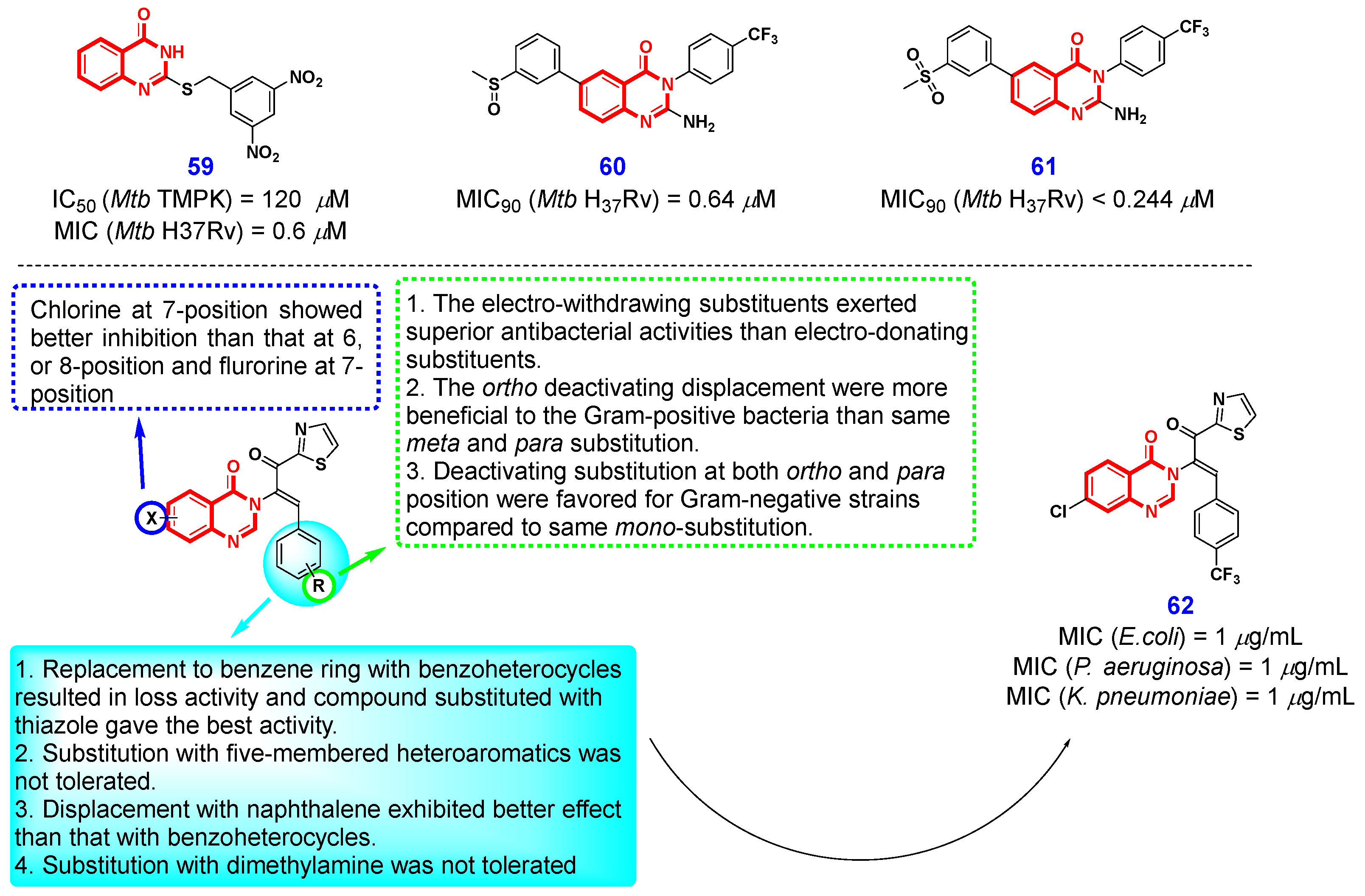
2.5. Antibacterial Activity
3. Application of the 4(3H)-QLOs in Agrochemical Chemistry
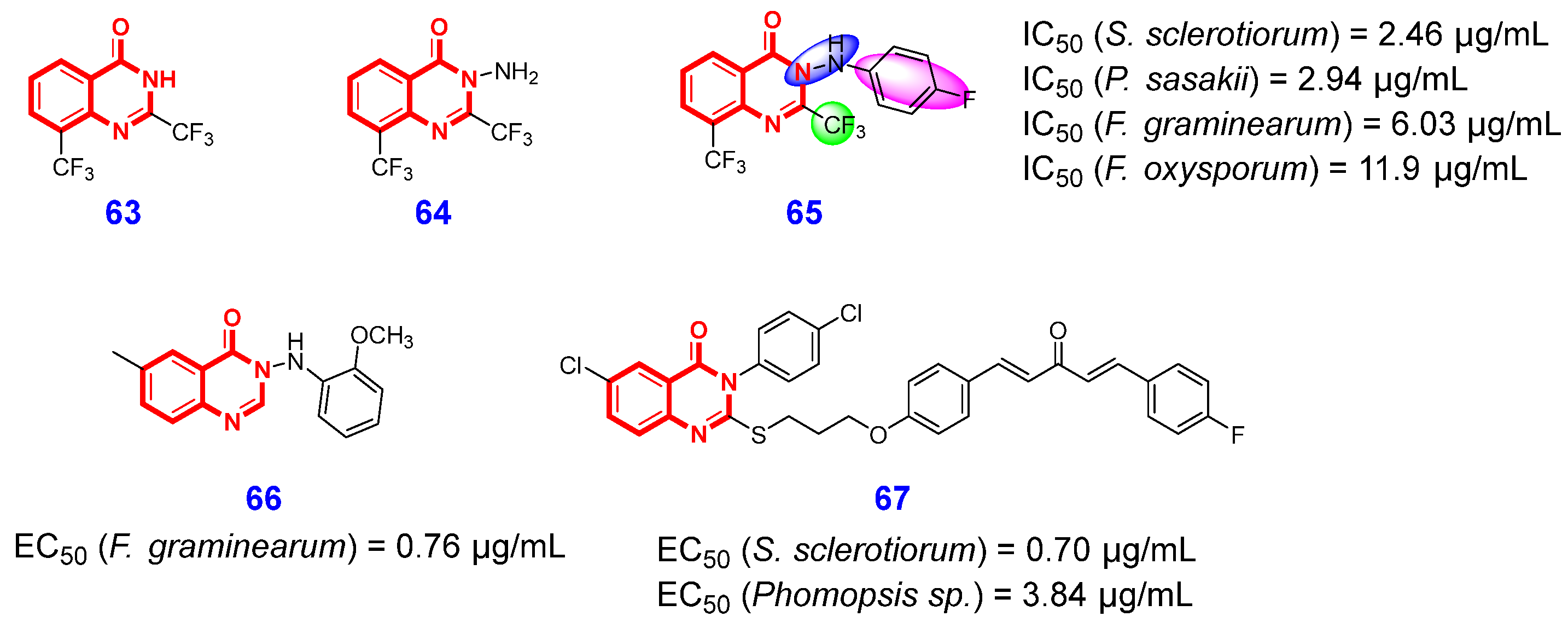
3.1. Antifungal Activities
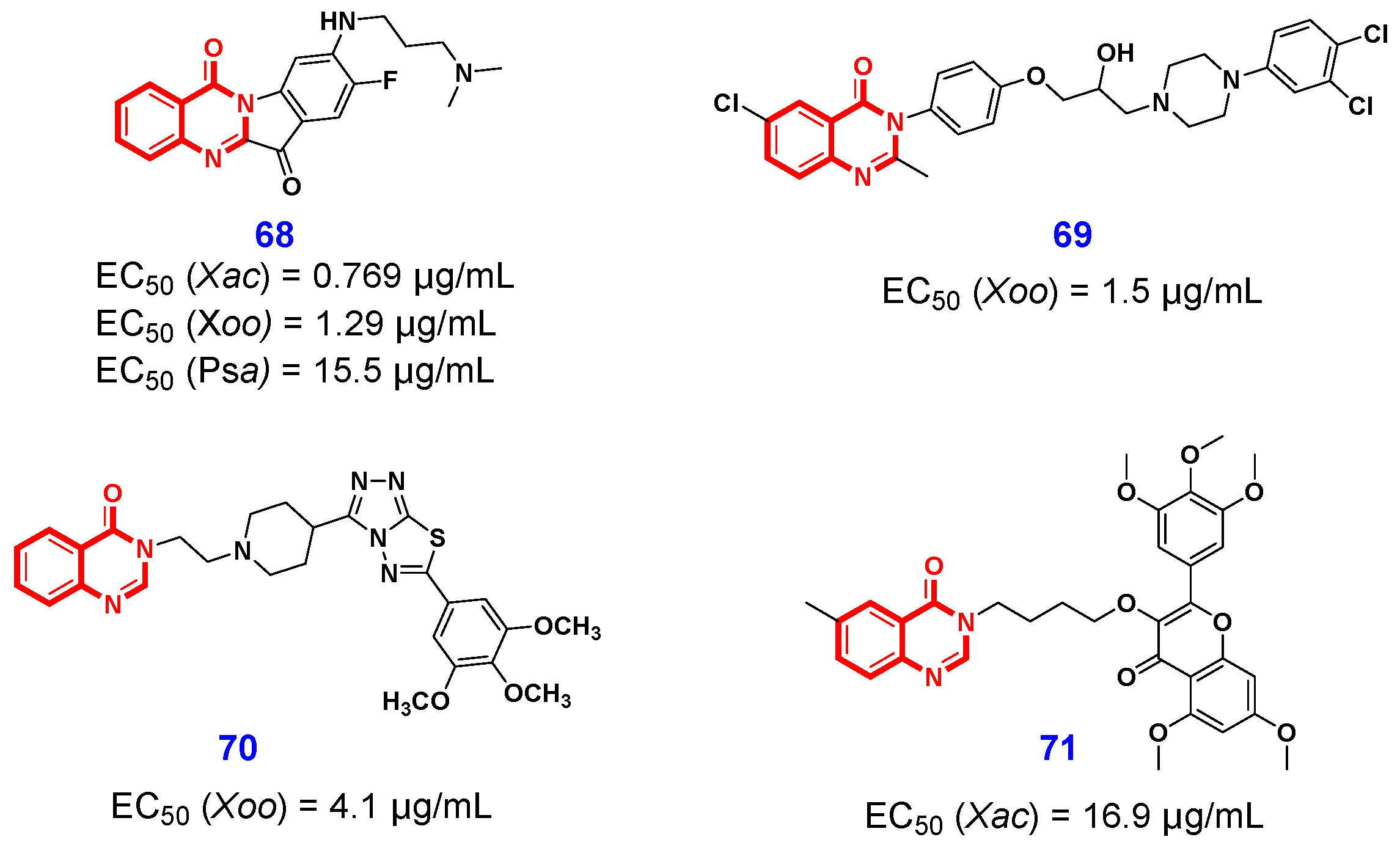
3.2. Antibacterial Activities
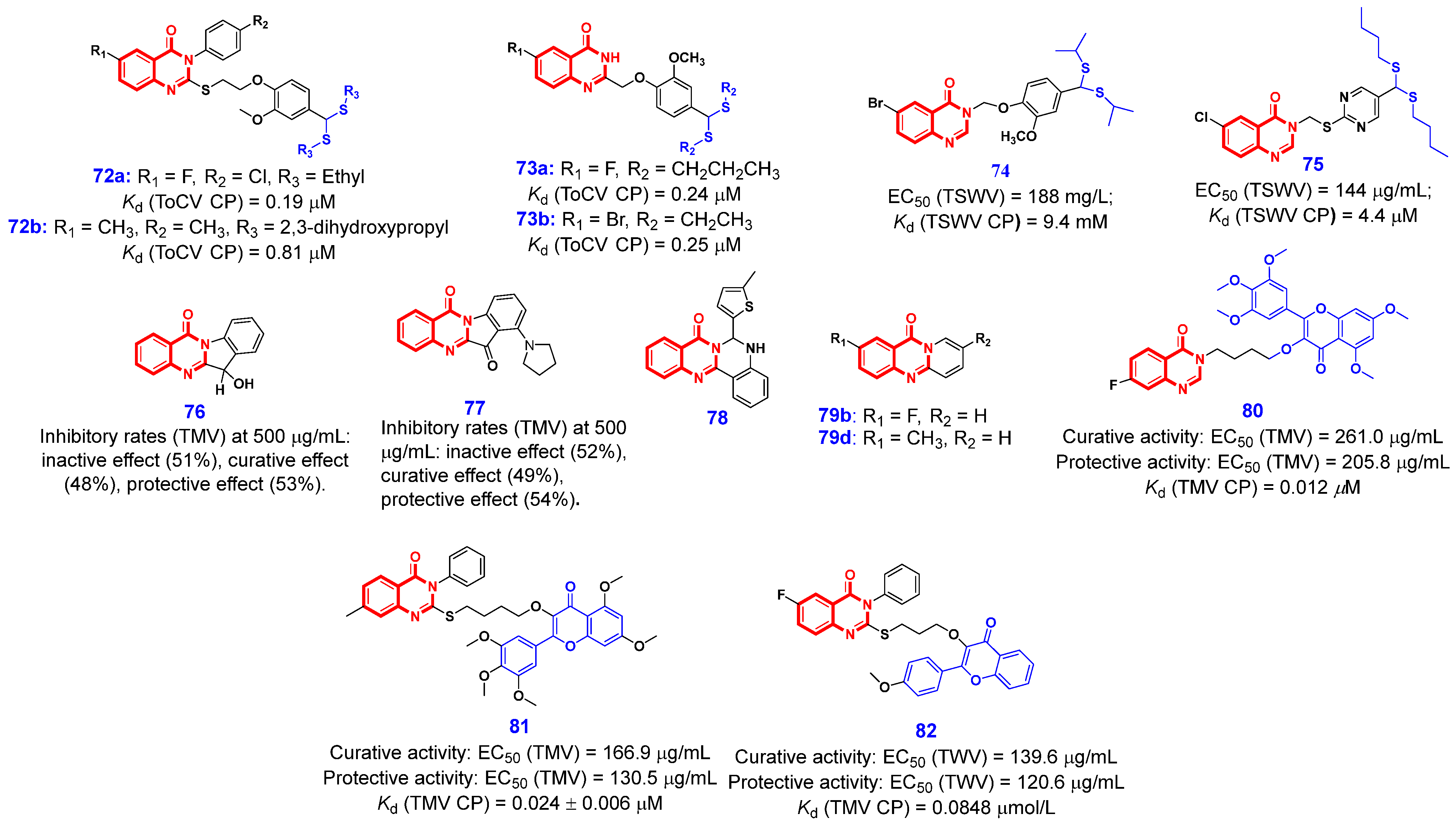
3.3. Antiviral Activities
3.4. Herbicidal Activities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| NPs | Natural products |
| QLOs | 4(3H)-quinazolinones |
| SAR | Structure–activity relationship |
| Plk1 | Polo-like kinase 1 |
| KD | Kinase domain |
| PBD | Polo-box domain |
| AKT | Serine–threonine kinase |
| EGFR | Epidermal growth factor receptor |
| BLM | Bloom syndrome protein |
| WRN | Werner syndrome protein |
| HRR | Homologous recombination repair |
| Kd | Dissociation constants |
| PARP | Poly (ADP-ribose) polymerase |
| Top I | Topoisomerase I |
| PK | Pharmacokinetic |
| TGI | Tumor growth inhibition |
| DPP-4 | Dipeptidyl peptidase-4 |
| TNKS | Tankyrases |
| 3D | Three-dimensional |
| Pol I | Polymerase I |
| PI3Ks | Phosphoinositol 3-kinases |
| SD | Sprague−Dawley |
| T1/2 | Half-life |
| CIA | Collagen-induced arthritis |
| COX | Cyclooxygenase |
| ZIKV | Zika virus |
| DENV | Dengue virus |
| TB | Tuberculosis |
| Mtb | Mycobacterium tuberculosis |
| TMPK | Thymidylate kinase |
| Xac | Xanthomonas axonopodis pv. Citri |
| Xoo | Xanthomonas oryzae pv. Oryzae |
| Psa | Pseudomonas syringae pv. Actinidiae |
| TC | thiodiazole copper |
| ToCV | Tomato chlorosis virus |
| TMV | Tobacco mosaic virus |
| NNM | ningnanmycin |
| CP | Coat protein |
| TSWV | Tomato spotted wilt virus |
| XCLSBM | Xiangcaoliusuobingmi |
| ACCase | Acetyl-CoA carboxylase |
References
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. BBA-Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Butler, M.S.; Robertson, A.A.B.; Cooper, M.A. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 2014, 31, 1612–1661. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug. Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Crane, E.A.; Gademann, K. Capturing biological activity in natural product fragments by chemical synthesis. Angew. Chem. Int. Ed. 2016, 55, 3882–3902. [Google Scholar] [CrossRef]
- Xiao, Z.; Morris-Natschke, S.L.; Lee, K.-H. Strategies for the optimization of natural leads to anticancer drugs or drug candidates. Med. Res. Rev. 2016, 36, 32–91. [Google Scholar] [CrossRef]
- Shen, B. A new golden age of natural products drug discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef]
- Sparks, T.C.; Hahn, D.R.; Garizi, N.V. Natural products, their derivatives, mimics and synthetic equivalents: Role in agrochemical discovery. Pest Manag. Sci. 2017, 73, 700–715. [Google Scholar] [CrossRef]
- Li, X.; Dong, W.; Nalin, A.P.; Wang, Y.; Pan, P.; Xu, B.; Zhang, Y.; Tun, S.; Zhang, J.; Wang, L.-S.; et al. The natural product chitosan enhances the anti-tumor activity of natural killer cells by activating dendritic cells. Oncoimmunology 2018, 7, e1431085. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting drug chemo-resistance in cancer using natural products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef]
- Newman, D.J. Natural products and drug discovery. Natl. Sci. Rev. 2022, 9, nwac206. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Dossi, R.; Frapolli, R.; Di Giandomenico, S.; Paracchini, L.; Bozzi, F.; Brich, S.; Castiglioni, V.; Borsotti, P.; Belotti, D.; Uboldi, S.; et al. Antiangiogenic activity of trabectedin in myxoid liposarcoma: Involvement of host TIMP-1 and TIMP-2 and tumor thrombospondin-1. Int. J. Cancer 2015, 136, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.; Gugel, K.H.; Hägele, K.; Hagenmaier, H.; Jessipow, S.; König, W.A.; Zähner, H. Stoffwechselprodukte von mikroorganismen. 98. mitteilung. phosphinothricin und phosphinothricyl-alanyl-alanin. Helv. Chim. Acta 1972, 55, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.S.V.; Pereira, B.B. Properties, toxicity and current applications of the biolarvicide spinosad. J. Toxicol. Environ. Health B Crit. Rev. 2020, 23, 13–26. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Yao, H.; Liu, J.; Xu, S.; Zhu, Z.; Xu, J. The structural modification of natural products for novel drug discovery. Expert Opin. Drug Discov. 2017, 12, 121–140. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Yao, H.; Xu, J. Insights into drug discovery from natural products through structural modification. Fitoterapia 2015, 103, 231–241. [Google Scholar] [CrossRef]
- Maier, M.E. Design and synthesis of analogues of natural products. Org. Biomol. Chem. 2015, 13, 5302–5343. [Google Scholar] [CrossRef]
- Guo, Z. The modification of natural products for medical use. Acta Pharm. Sin. B 2017, 7, 119–136. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, Y.; Peng, Y.; Zhang, X.; Li, S.; Peng, Y.; Peng, X.; Zhuo, L.; Jiang, W. Natural product evodiamine-inspired medicinal chemistry: Anticancer activity, structural optimization and structure-activity relationship. Eur. J. Med. Chem. 2023, 247, 115031. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Morris-Natschke, S.L.; Liu, Y.; Guo, X.; Xu, X.; Goto, M.; Li, J.; Yang, G.; Lee, K. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.-F.; Morris-Natschke, S.L.; Yang, G.Z.; Liu, Y.Q.; Guo, X.; Xu, X.-S.; Goto, M.; Li, J.C.; Zhang, J.Y.; Lee, K. Biologically active quinoline and quinazoline alkaloids part II. Med. Res. Rev. 2018, 38, 1614–1660. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.M.; Liang, X.A.; Kong, Y.; Jia, B. Structural diversity and biological activities of indole diketopiperazine alkaloids from fungi. J. Agric. Food Chem. 2016, 64, 6659–6671. [Google Scholar] [CrossRef]
- Meng, X.H.; Li, N.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. Plant resources, chemical constituents, and bioactivities of tea plants from the genus Camellia section Thea. J. Agric. Food Chem. 2019, 67, 5318–5349. [Google Scholar] [CrossRef]
- He, D.; Wang, M.; Zhao, S.; Shu, Y.; Zeng, H.; Xiao, C.; Lu, C.; Liu, Y. Pharmaceutical prospects of naturally occurring quinazolinone and its derivatives. Fitoterapia 2017, 119, 136–149. [Google Scholar] [CrossRef]
- Saeed, A. Isocoumarins, miraculous natural products blessed with diverse pharmacological activities. Eur. J. Med. Chem. 2016, 116, 290–317. [Google Scholar] [CrossRef]
- Song, C.; Yang, J.; Zhang, M.; Ding, G.; Jia, C.; Qin, J.; Guo, L. Marine natural products: The important resource of biological insecticide. Chem. Biodivers. 2021, 18, e2001020. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, L.; Ran, W.; Zhong, K.; Zhao, Y.; Gao, H. Antimicrobial activities of natural flavonoids against foodborne pathogens and their application in food industry. Food Chem. 2024, 460, 140476. [Google Scholar] [CrossRef]
- Huang, W.; Wan, Y.; Su, H.; Zhang, Z.; Liu, Y.; Sadeeq, M.; Xian, M.; Feng, X.; Xiong, P.; Hou, F. Recent advances in phenazine natural products: Biosynthesis and metabolic engineering. J. Agric. Food Chem. 2024, 72, 21364–21379. [Google Scholar] [CrossRef]
- Li, T.; Lv, M.; Wen, H.; Wang, J.; Wang, Z.; Xu, J.; Fang, S.; Xu, H. High value-added application of natural plant products in crop protection: Construction and pesticidal activities of piperine-type ester derivatives and their toxicology study. J. Agric. Food Chem. 2022, 70, 16126–16134. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, H.; Lange, G.; Müller, T.; Rosinger, C.; Willms, L.; Almsick, A. 4-Hydroxyphenylpyruvate dioxygenase inhibitors in combination with safeners: Solutions for modern and sustainable agriculture. Angew. Chem. Int. Ed. 2013, 52, 9388–9398. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Rezafungin: First approval. Drugs 2023, 83, 833–840. [Google Scholar] [CrossRef]
- Watkins, R.R.; File, T.M. Lefamulin: A novel semisynthetic pleuromutilin antibiotic for community-acquired bacterial pneumonia. Clin. Infect. Dis. 2020, 71, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- Duraes, F.; Sousa, E. Omadacycline: A newly approved antibacterial from the class of tetracyclines. Pharmaceuticals 2019, 12, 63. [Google Scholar] [CrossRef]
- Hughes, D.L. Patent review of manufacturing routes to fifth-generation cephalosporin drugs. Part 1, ceftolozane. Org. Process Res. Dev. 2017, 21, 430–443. [Google Scholar] [CrossRef]
- Alsibaee, A.M.; Al-Yousef, H.M.; Al-Salem, H.S. Quinazolinones, the winning horse in drug discovery. Molecules 2023, 28, 978. [Google Scholar] [CrossRef]
- Nomura, T.; Ma, Z.-Z.; Hano, Y.; Chen, Y.-J. Two new pyrroloquinazolinoquinoline alkaloids from Peganum nigellastrum. Heterocycles 1997, 46, 541–546. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, K.; Wang, Z.W.; Liu, Y.X.; Ma, D.J.; Wang, Q.M. Luotonin A and its derivatives as novel antiviral and antiphytopathogenic fungus agents. J. Agric. Food Chem. 2020, 68, 8764–8773. [Google Scholar] [CrossRef]
- Gettler, J.; Markovic, M.; Koóš, P.; Gracza, T. Recent advances in the research on luotonins A, B, and E. Molecules 2024, 29, 3522. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Cai, J.; Liu, D.; Yan, Y.; Zhang, H.; Li, L.; Wang, X.; Xiang, W.; Zhang, J. Discovery of febrifugine with specific anti-Phytophthora oomycete activity isolated from Dichroa febrifuga Lour. Ind. Crops Prod. 2022, 178, 114651. [Google Scholar] [CrossRef]
- Ishih, A.; Suzuki, T.; Watanabe, M.; Miyase, T.; Terada, M. Combination effects of chloroquine with the febrifugine and isofebrifugine mixture against a blood-induced infection with chloroquine-resistant Plasmodium berghei NK65 in ICR mice. Phytother. Res. 2003, 17, 1234–1236. [Google Scholar] [CrossRef]
- Rahbæk, L.; Breinholt, J. Circumdatins D, E, and F: Further fungal benzodiazepine analogues from Aspergillus ochraceus. J. Nat. Prod. 1999, 62, 904–905. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Li, J.; Xu, S. Rutaecarpine: A promising cardiovascular protective alkaloid from Evodia rutaecarpa (Wu Zhu Yu). Pharmacol. Res. 2019, 141, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Luo, C.; Cho, J.; Lai, D.; Shen, X.; Zhang, X.; Zhou, W. Tryptanthrin exerts anti-breast cancer effects both in vitro and in vivo through modulating the inflammatory tumor microenvironment. Acta Pharm. 2021, 71, 245–266. [Google Scholar] [CrossRef]
- Kaur, R.; Manjal, S.K.; Rawal, R.K.; Kumar, K. Recent synthetic and medicinal perspectives of tryptanthrin. Bioorg. Med. Chem. 2017, 25, 4533–4552. [Google Scholar] [CrossRef]
- Vivek-Ananth, R.P.; Rana, A.; Rajan, N.; Biswal, H.S.; Samal, A. In silico identification of potential natural product inhibitors of human proteases key to SARS-CoV-2 infection. Molecules 2020, 25, 3822. [Google Scholar] [CrossRef]
- Lee, I.K.; Yun, B.S.; Han, G.; Cho, D.H.; Kim, Y.H.; Yoo, I.D. Dictyoquinazols A, B, and C, new neuroprotective compounds from the mushroom Dictyophora indusiata. J. Nat. Prod. 2002, 65, 1769–1772. [Google Scholar] [CrossRef]
- Cambridge, G.W.; Jansen, A.B.A.; Jarman, D.A. Bronchodilating action of vasicinone and related compounds. Nature 1962, 196, 1217. [Google Scholar] [CrossRef]
- Zawalich, W.S.; Diaz, V.A. Asperlicin antagonizes stimulatory effects of cholecystokinin on isolated islets. Am. J. Physiol. Endocrinol. Metab. 1987, 252, E370–E374. [Google Scholar] [CrossRef]
- Wang, C.H.; Zeng, H.; Wang, Y.H.; Li, C.; Cheng, J.; Ye, Z.J.; He, X.J. Antitumor quinazoline alkaloids from the seeds of Peganum harmala. J. Asian Nat. Prod. Res. 2015, 17, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Khashimov, K.N.; Telezhenetskaya, M.V.; Rashkes, Y.V.; Yunusov, S.Y. Pegamine: A new alkaloid from Peganum harmala. Chem. Nat. Compd. 1970, 6, 462–464. [Google Scholar] [CrossRef]
- Molina, P.; Tárraga, A.; Gonzalez-Tejero, A.; Rioja, I.; Ubeda, A.; Terencio, M.C.; Alcaraz, M.J. Inhibition of leukocyte functions by the alkaloid isaindigotone from Isatis indigotica and some new synthetic derivatives. J. Nat. Prod. 2001, 64, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Capilla, J.; Yustes, C.; Mayayo, E.; Fernández, B.; Ortoneda, M.; Pastor, F.J.; Guarro, J. Efficacy of albaconazole (UR-9825) in treatment of disseminated Scedosporium prolificans infection in rabbits. Antimicrob. Agents Chemother. 2003, 47, 1948–1951. [Google Scholar] [CrossRef]
- Otsuka, M.; Furuuchi, S.; Harigaya, S. Formation route of sulfur-containing metabolites of afloqualone, a new centrally acting muscle relaxant, in rat. Chem. Pharm. Bull. 1983, 31, 2799–2809. [Google Scholar] [CrossRef] [PubMed]
- Tekale, S.U.; Munde, S.B.; Kauthale, S.S.; Pawar, R.P. An efficient, convenient, and solvent-free synthesis of 2,3-dihydroquinazolin-4(1H)-ones using montmorillonite-KSF clay as a heterogeneous catalyst. Org. Prep. Proced. Int. 2018, 50, 314–322. [Google Scholar] [CrossRef]
- Dubey, A.V.; Kumar, A.V. Cu(II)–glucose: Sustainable catalyst for the synthesis of quinazolinones in a biomass-derived solvent 2-methylTHF and application for the synthesis of diproqualone. ACS Sustain. Chem. Eng. 2018, 6, 14283–14291. [Google Scholar] [CrossRef]
- Yang, H.K.; Liu, J.L.; Dai, L.N.; Wang, Y.; Wei, J.M.; Amin, W. Detection and quantification of psychotropic drug etaqualone in human hair using GC–MS/MS. Leg. Med. 2021, 53, 101964. [Google Scholar]
- Agrawal, R.; Jain, P.; Dikshit, S.N. Balaglitazone: A second generation peroxisome proliferator-activated receptor (PPAR) gamma (γ) agonist. Mini Rev. Med. Chem. 1983, 12, 87–97. [Google Scholar] [CrossRef]
- Tsuchida, K.; Tsujita, T.; Hayashi, M.; Ojima, A.; Keleku-Lukwete, N.; Katsuoka, F.; Otsuki, A.; Kikuchi, H.; Oshima, Y.; Suzuki, M.; et al. Halofuginone enhances the chemo-sensitivity of cancer cells by suppressing NRF2 accumulation. Free Radic. Biol. Med. 2017, 103, 236–247. [Google Scholar] [CrossRef]
- Maitia, S.; MaitiDutta, S.; Chen, G.P. Regulations of expressions of rat/human sulfotransferases by anticancer drug, nolatrexed, and micronutrients. Anticancer Drug 2022, 33, e525–e533. [Google Scholar] [CrossRef]
- Cheah, C.Y.; Fowler, N.H. Idelalisib in the management of lymphoma. Blood 2016, 128, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.Q.; Wu, Y.; Cheng, J.F.; Chen, L.; Liu, R.; Ding, Y.; Wu, S.C.; Ma, J.H.; Sheng, C.Q. Ispinesib as an effective warhead for the design of autophagosome-tethering chimeras: Discovery of potent degraders of nicotinamide phosphoribosyltransferase (NAMPT). J. Med. Chem. 2022, 65, 7619–7628. [Google Scholar] [CrossRef]
- El-Azab, A.S.; ElTahir, K.E.H.; Attia, S.M. Synthesis and anticonvulsant evaluation of some novel 4(3H)-quinazolinones. Monatsh. Chem. 2011, 142, 837–848. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cunningham, D.; Maroun, J.; Cervantes, A.; Glimelius, B. Raltitrexed: Current clinical status and future directions. Ann. Oncol. 2002, 13, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Vera Palma, C.A.; Madariaga Burrows, R.P.; González, M.G.; Gamboa, B.R.; Moya-Elizondo, E.A. Integration between Pseudomonas protegens strains and fluquinconazole for the control of take-all in wheat. Crop Prot. 2019, 121, 163–172. [Google Scholar] [CrossRef]
- Gimeno-Alcañiz, J.V.; Esteve-Turrillas, F.A.; Abad-Fuentes, A.; Agulló, C.; Navarro-Fuertes, I.; Abad-Somovilla, A.; Mercader, J.V. Rapid immunochemical methods for the analysis of proquinazid in strawberry QuEChERS extracts. Anal. Bioanal. Chem. 2024, 416, 7395–7404. [Google Scholar] [CrossRef]
- Popat, K.; McQueen, K.; Feeley, T.W. The global burden of cancer. Best Pract. Res. Clin. Anaesthesiol. 2013, 27, 399–408. [Google Scholar] [CrossRef]
- de Cárcer, G. The mitotic cancer target polo-like kinase 1: Oncogene or tumor suppressor? Genes 2019, 10, 208. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Wang, J.; Ouyang, L.; Wang, Y. Polo-like kinase 1 inhibitors in human cancer therapy: Development and therapeutic potential. J. Med. Chem. 2022, 65, 10133–10160. [Google Scholar] [CrossRef]
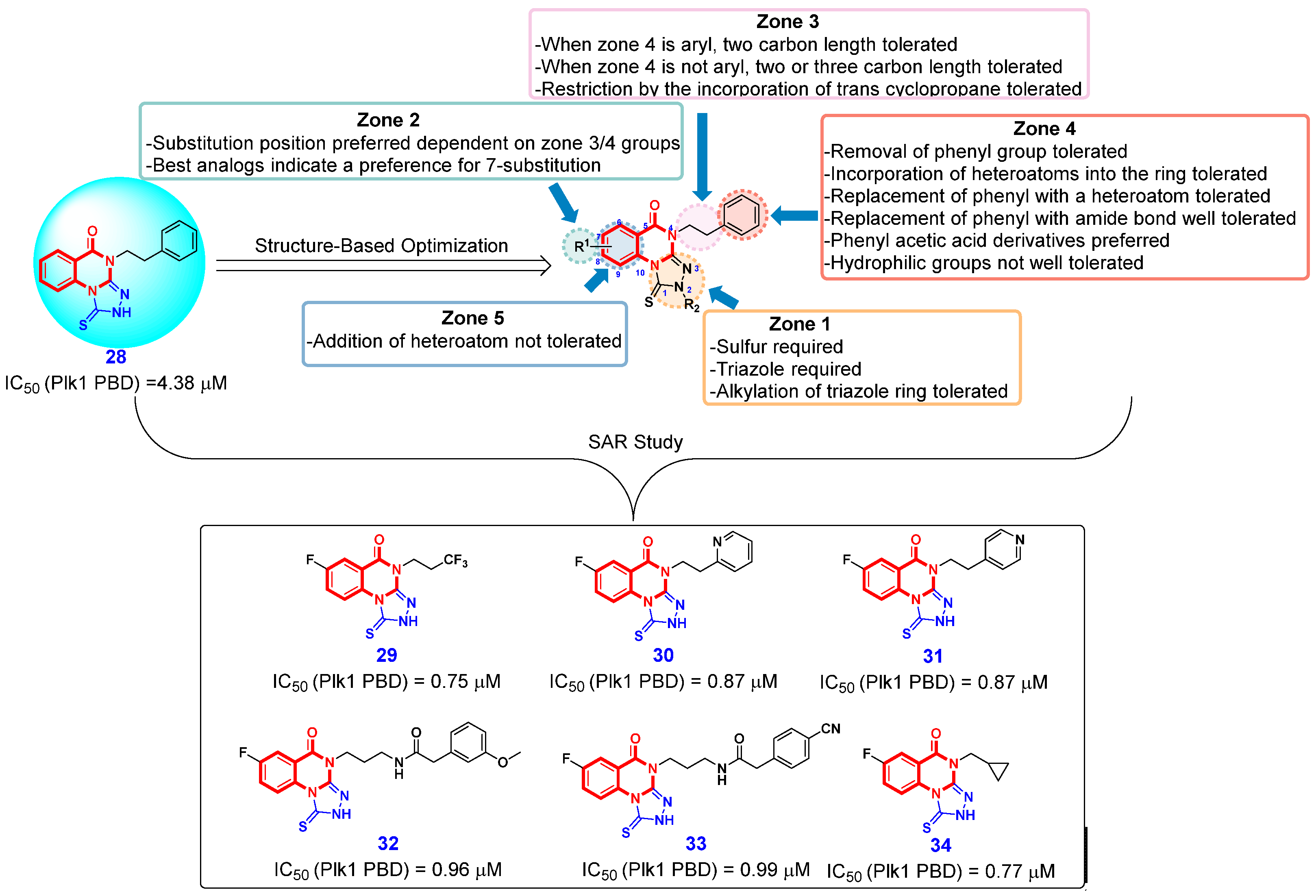
- Alverez, C.N.; Park, J.-E.; Toti, K.S.; Xia, Y.; Krausz, K.W.; Rai, G.; Bang, J.K.; Gonzalez, F.J.; Jacobson, K.A.; Lee, K.S. Identification of a new heterocyclic scaffold for inhibitors of the polo-box domain of polo-like kinase 1. J. Med. Chem. 2020, 63, 14087–14117. [Google Scholar] [CrossRef] [PubMed]
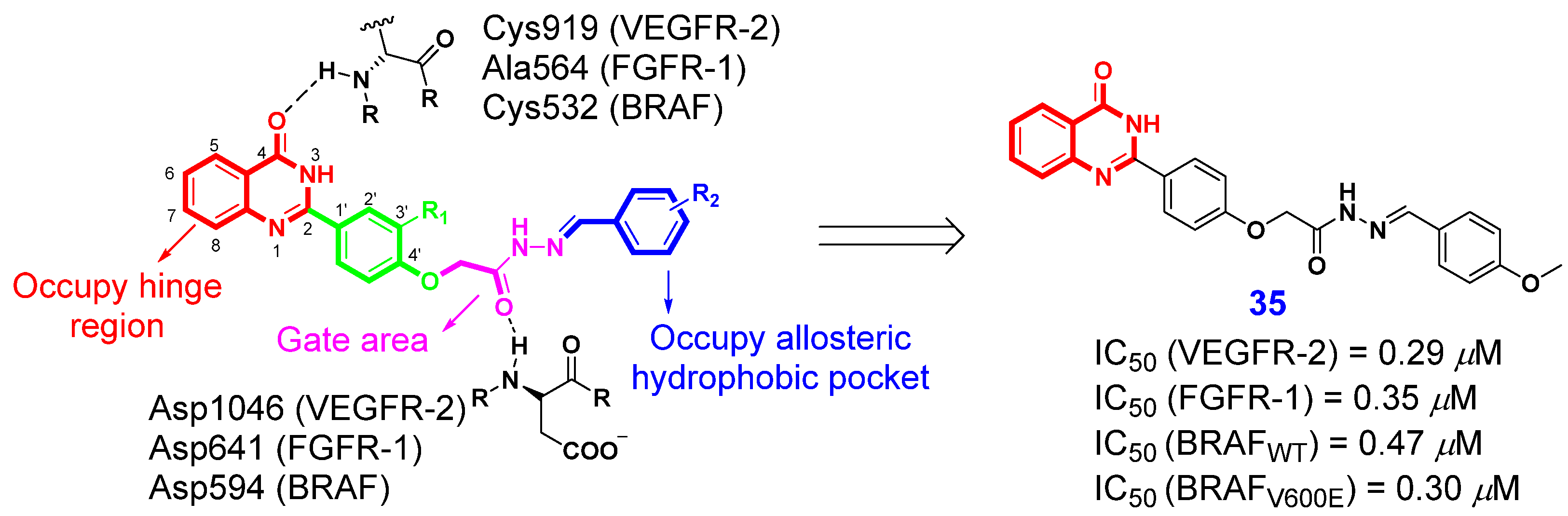
- Eldehna, W.M.; El Kerdawy, A.M.; Al-Ansary, G.H.; Al-Rashood, S.T.; Ali, M.M.; Mahmoud, A.E. Type IIA-Type IIB protein tyrosine kinase inhibitors hybridization as an efficient approach for potent multikinase inhibitor development: Design, synthesis, anti-proliferative activity, multikinase inhibitory activity and molecular modeling of novel indolinone-based ureides and amides. Eur. J. Med. Chem. 2019, 163, 37–53. [Google Scholar]
- Blanc1, J.; Geney, R.; Menet, C. Type II kinase inhibitors: An opportunity in cancer for rational design. Anticancer Agents Med. Chem. 2013, 13, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Karim, S.S.; Syam, Y.M.; El Kerdawy, A.M.; Abdel-Mohsen, H.T. Rational design and synthesis of novel quinazolinone N-acetohydrazides as type II multi-kinase inhibitors and potential anticancer agents. Bioorg. Chem. 2024, 142, 106920. [Google Scholar] [CrossRef]
- Brazil, D.P.; Park, J.; Hemmings, B.A. PKB binding proteins: Getting in on the Akt. Cell 2002, 111, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Morrow, J.K.; Du-Cuny, L.; Chen, L.; Meuillet, E.J.; Mash, E.A.; Powis, G.; Zhang, S. Recent development of anticancer therapeutics targeting Akt. Recent Pat. Anticancer Drug Discov. 2011, 6, 146–159. [Google Scholar] [CrossRef]
- McDowell, K.A.; Riggins, G.J.; Gallia, G.L. Targeting the AKT pathway in glioblastoma. Curr. Pharm. Des. 2011, 17, 2411–2420. [Google Scholar] [CrossRef]
- Cassinelli, G.; Zuco, V.; Gatti, L.; Lanzi, C.; Zaffaroni, N.; Colombo, D.; Perego, P. Targeting the Akt kinase to modulate survival, invasiveness and drug resistance of cancer cells. Curr. Med. Chem. 2013, 20, 1923–1945. [Google Scholar] [CrossRef]
- Brown, J.S.; Banerji, U. Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharmacol. Ther. 2017, 172, 101–115. [Google Scholar] [CrossRef]
- Noser, A.A.; El-Naggar, M.; Donia, T.; Abdelmonsef, A.H. Synthesis, in silico and in vitro assessment of new quinazolinones as anticancer agents via potential AKT inhibition. Molecules 2020, 25, 4780. [Google Scholar] [CrossRef]
- Gristina, V.; Malapelle, U.; Galvano, A.; Pisapia, P.; Pepe, F.; Rolfo, C.; Tortorici, S.; Bazan, V.; Troncone, G.; Russo, A. The significance of epidermal growth factor receptor uncommon mutations in nonsmall cell lung cancer: A systematic review and critical appraisal. Cancer Treat. Rev. 2020, 85, 101994. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Sharma, V.; Alam, O.; Manaithiya, A.; Alam, P.; Kahksha; Alam, M.T.; Imran, M. Novel quinazoline-based EGFR kinase inhibitors: A review focusing on SAR and molecular docking studies (2015–2019). Eur. J. Med. Chem. 2020, 204, 112640. [Google Scholar] [CrossRef] [PubMed]
- Ataollahi, E.; Behrouz, M.; Mardaneh, P.; Emami, M.; Zare, S.; Zafarian, H.; Khabnadideh, S.; Emami, L. Novel quinazolinone derivatives as anticancer agents: Design, synthesis, biological evaluation and computational studies. J. Mol. Struct. 2024, 1295, 136622. [Google Scholar] [CrossRef]
- O’Connor, M.J. Targeting the DNA damage response in cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef]
- Lu, H.; Davis, A.J. Human RecQ helicases in DNA double-strand break repair. Front. Cell Dev. Biol. 2021, 9, 640755. [Google Scholar] [CrossRef] [PubMed]
- Urban, V.; Dobrovolna, J.; Janscak, P. Distinct functions of human RecQ helicases during DNA replication. Biophys. Chem. 2017, 225, 20–26. [Google Scholar] [CrossRef]
- Croteau, D.L.; Popuri, V.; Opresko, P.L.; Bohr, V.A. Human RecQ helicases in DNA repair, recombination, and replication. Annu. Rev. Biochem. 2014, 83, 519–552. [Google Scholar] [CrossRef]
- Wu, L.; Hickson, I.D. The bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature 2003, 426, 870–874. [Google Scholar] [CrossRef]
- Davies, S.L.; North, P.S.; Hickson, I.D. Role for BLM in replication-fork restart and suppression of origin firing after replicative stress. Nat. Struct. Mol. Biol. 2007, 14, 677–679. [Google Scholar] [CrossRef]
- Nguyen, G.H.; Dexheimer, T.S.; Rosenthal, A.S.; Chu, W.K.; Singh, D.K.; Mosedale, G.; Bachrati, C.Z.; Schultz, L.; Sakurai, M.; Savitsky, P.; et al. A small molecule inhibitor of the BLM helicase modulates chromosome stability in human cells. Chem. Biol. 2013, 20, 55–62. [Google Scholar] [CrossRef]
- Yin, Q.-K.; Wang, C.-X.; Wang, Y.-Q.; Guo, Q.-L.; Zhang, Z.-L.; Ou, T.-M.; Huang, S.-L.; Li, D.; Wang, H.-G.; Tan, J.-H.; et al. Discovery of isaindigotone derivatives as novel bloom’s syndrome protein (BLM) helicase inhibitors that disrupt the BLM/DNA interactions and regulate the homologous recombination repair. J. Med. Chem. 2019, 62, 3147–3162. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-X.; Zhang, Z.-L.; Yin, Q.-K.; Tu, J.-L.; Wang, J.-E.; Xu, Y.-H.; Rao, Y.; Ou, T.-M.; Huang, S.-L.; Li, D.; et al. Design, synthesis, and evaluation of new quinazolinone derivatives that inhibit bloom’s syndrome protein (BLM) helicase, trigger DNA damage at the telomere region, and synergize with PARP inhibitors. J. Med. Chem. 2020, 63, 9752–9772. [Google Scholar] [CrossRef]
- Haggag, H.S.; Aboukhatwa, S.M.; Nafie, M.S.; Paul, A.; Sharafeldin, N.; Oliver, A.W.; El-Hamamsy, M.H. Design and synthesis of quinazolin-4-one derivatives as potential anticancer agents and investigation of their interaction with RecQ helicases. Bioorg. Chem. 2024, 144, 107086. [Google Scholar] [CrossRef] [PubMed]
- Martín-Encinas, E.; Selas, A.; Palacios, F.; Alonso, C. The design and discovery of topoisomerase I inhibitors as anticancer therapies. Expert Opin. Drug Discov. 2022, 17, 581–601. [Google Scholar] [CrossRef]
- Thomas, A.; Pommier, Y. Targeting topoisomerase I in the era of precision medicine. Clin. Cancer Res. 2019, 25, 6581–6589. [Google Scholar] [CrossRef]
- Khadka, D.B.; Cho, W.-J. 3-Arylisoquinolines as novel topoisomerase I inhibitors. Bioorg. Med. Chem. 2011, 19, 724–734. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Zhang, X.-R.; Lyu, L.; Wang, S.-Q.; Zhang, X.-T. Pyridazino[1,6-b]quinazolinones as new anticancer scaffold: Synthesis, DNA intercalation, topoisomerase I inhibition and antitumor evaluation in vitro and in vivo. Bioorg. Chem. 2020, 99, 103814. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.H.; Li, H.P.; Wang, J.; Peng, X.Z.; Hu, C.L.; Luo, L.C. Design, synthesis, and anticancer activities of 8,9-substituted Luotonin A analogs as novel topoisomerase I inhibitors. Med. Chem. Res. 2021, 30, 1512–1522. [Google Scholar] [CrossRef]
- Havre, P.A.; Dang, L.H.; Ohnuma, K.; Iwata, S.; Morimoto, C.; Dang, N.H. CD26 expression on T-anaplastic large cell lymphoma (ALCL) line karpas 299 is associated with increased expression of versican and MT1-MMP and enhanced adhesion. BMC Cancer 2013, 13, 517. [Google Scholar] [CrossRef]
- Hollande, C.; Boussier, J.; Ziai, J.; Nozawa, T.; Bondet, V.; Phung, W.; Lu, B.F.; Duffy, D.; Paradis, V.; Mallet, V.; et al. Inhibition of the dipeptidyl peptidase DPP4 (CD26) reveals IL-33-dependent eosinophil-mediated control of tumor growth. Nat. Immunol. 2019, 20, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Kraman, M.; Bambrough, P.J.; Arnold, J.N.; Roberts, E.W.; Magiera, L.; Jones, J.O.; Gopinathan, A.; Tuveson, D.A.; Fearon, D.T. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science 2010, 330, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, R.; Hong, Y.R.; Shah, C.; Ali, A.; Skelton IV, W.P.; Huo, J.; Dang, N.H.; Dang, L.H. Dipeptidyl peptidase 4 inhibitors as novel agents in improving survival in diabetic patients with colorectal cancer and lung cancer: A surveillance epidemiology and endpoint research medicare study. Cancer Med. 2019, 8, 3918–3927. [Google Scholar] [CrossRef]
- Emami, L.; Faghih, Z.; Sakhteman, A.; Rezaei, Z.; Faghih, Z.; Salehi, F.; Khabnadideh, S. Design, synthesis, molecular simulation, and biological activities of novel quinazolinone-pyrimidine hybrid derivatives as dipeptidyl peptidase-4 inhibitors and anticancer agents. New J. Chem. 2020, 44, 19515–19531. [Google Scholar] [CrossRef]
- Pascal, J.M. The comings and goings of PARP-1 in response to DNA damage. DNA Repair 2018, 71, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Kraus, W.L. The expanding universe of PARP1-mediated molecular and therapeutic mechanisms. Molecular Cell 2022, 82, 2315–2334. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Y.; Sykes, M.; Wang, S. Small-molecule inhibitors of tankyrases as prospective therapeutics for cancer. J. Med. Chem. 2022, 65, 5244–5273. [Google Scholar] [CrossRef]
- Buchstaller, H.-P.; Anlauf, U.; Dorsch, D.; Kuhn, D.; Lehmann, M.; Leuthner, B.; Musil, D.; Radtki, D.; Ritzert, C.; Rohdich, F.; et al. Discovery and optimization of 2-arylquinazolin-4-ones into a potent and selective tankyrase inhibitor modulating Wnt pathway activity. J. Med. Chem. 2019, 62, 7897–7909. [Google Scholar] [CrossRef]
- Huang, P.; Le, X.; Huang, F.; Yang, J.; Yang, H.; Ma, J.; Hu, G.; Li, Q.; Chen, Z. Discovery of a dual tubulin polymerization and cell division cycle 20 homologue inhibitor via structural modification on apcin. J. Med. Chem. 2020, 63, 4685–4700. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, Y.; Liu, Y.; Sun, D.; Zhen, Y.; Liu, J.; Fu, L.; Zhang, L.; Ouyang, L. Discovery of a novel dual-target inhibitor of ERK1 and ERK5 that induces regulated cell death to overcome compensatory mechanism in specific tumor types. J. Med. Chem. 2020, 63, 3976–3995. [Google Scholar] [CrossRef]
- He, J.; Tam, K.Y. Dual-target inhibitors of cholinesterase and GSK-3β to modulate alzheimer’s disease. Drug Discov. Today 2024, 29, 103914. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, Q.; Kong, X.; Huo, X.; Dong, Z.; Ma, Y.; Yang, K.; Niu, W.; Zhang, K. Design of balanced dual-target inhibitors of EGFR and microtubule. Bioorg. Chem. 2024, 143, 107087. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Ren, R.; Sun, X.; Zou, Y.; Shi, Y.; Di, B.; Niu, M.-M. Discovery of a dual tubulin and poly(ADP-ribose) polymerase-1 inhibitor by structure-based pharmacophore modeling, virtual screening, molecular docking, and biological evaluation. J. Med. Chem. 2021, 64, 15702–15715. [Google Scholar] [CrossRef]
- Peltonen, K.; Colis, L.; Liu, H.; Trivedi, R.; Moubarek, M.S.; Moore, H.M.; Bai, B.; Rudek, M.A.; Bieberich, C.J.; Laiho, M. A Targeting modality for destruction of RNA polymerase I that possesses anticancer activity. Cancer Cell 2014, 25, 77–90. [Google Scholar] [CrossRef]
- Shourkaei, F.A.; Ranjbar, P.R.; Foroumadi, A.; Shams, F. Design and synthesis of BMH-21-like quinazolinone derivatives as potential anti-cancer agents. J. Mol. Struct. 2024, 1308, 138083. [Google Scholar] [CrossRef]
- Foster, F.M.; Traer, C.J.; Abraham, S.M.; Fry, M.J. The phosphoinositide (PI) 3-kinase family. J. Cell Sci. 2003, 116, 3037–3040. [Google Scholar] [CrossRef] [PubMed]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- Perry, M.W.D.; Abdulai, R.; Mogemark, M.; Petersen, J.; Thomas, M.J.; Valastro, B.; Westin Eriksson, A. Evolution of PI3Kγ and δ inhibitors for inflammatory and autoimmune diseases. J. Med. Chem. 2019, 62, 4783–4814. [Google Scholar] [CrossRef]
- Xu, C.; Chen, S.; Deng, Y.; Song, J.; Li, J.; Chen, X.; Chang, P.; Yao, L.; Tang, H. Distinct roles of PI3Kδ and PI3Kγ in a toluene diisocyanate-induced murine asthma model. Toxicology 2021, 454, 152747. [Google Scholar] [CrossRef]
- Tang, Y.; Zheng, F.; Bao, X.; Zheng, Y.; Hu, X.; Lou, S.; Zhao, H.; Cui, S. Discovery of highly selective and orally bioavailable PI3Kδ inhibitors with anti-inflammatory activity for treatment of acute lung injury. J. Med. Chem. 2023, 66, 11905–11926. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Dai, G.; Su, W.; Xiao, K.; Weng, J.; Zhang, Z.; Wang, Q.; Yuan, T.; Shi, F.; Zhang, Z.; et al. Discovery, optimization, and evaluation of potent and highly selective PI3Kγ–PI3Kδ dual inhibitors. J. Med. Chem. 2019, 62, 4936–4948. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.J.; Li, D.; Zheng, W.; Shi, M.S.; Chen, Y.; Tang, M.H.; Yang, T.; Zhao, M.; Deng, D.X.; Zhang, C.F.; et al. Discovery, optimization, and evaluation of quinazolinone derivatives with novel linkers as orally efficacious phosphoinositide-3-kinase delta inhibitors for treatment of inflammatory diseases. J. Med. Chem. 2021, 64, 8951–8970. [Google Scholar] [CrossRef] [PubMed]
- Rouzer, C.A.; Marnett, L.J. Structural and chemical biology of the interaction of cyclooxygenase with substrates and non-steroidal anti-inflammatory drugs. Chem. Rev. 2020, 120, 7592–7641. [Google Scholar] [CrossRef] [PubMed]
- Sakr, A.; Rezq, S.; Ibrahim, S.M.; Soliman, E.; Baraka, M.M.; Romero, D.G.; Kothayer, H. Design and synthesis of novel quinazolinones conjugated ibuprofen, indole acetamide, or thioacetohydrazide as selective COX-2 inhibitors: Anti-inflammatory, analgesic and anticancer activities. J. Enzyme Inhib. Med. Chem. 2021, 36, 1810–1828. [Google Scholar] [CrossRef]
- Moreira, J.; Peixoto, T.M.; Siqueira, A.M.; Lamas, C.C. Sexually acquired Zika virus: A systematic review. Clin. Microbiol. Infect. 2017, 23, 296–305. [Google Scholar] [CrossRef]
- Nitsche, C.; Holloway, S.; Schirmeister, T.; Klein, C.D. Biochemistry and medicinal chemistry of the dengue virus protease. Chem. Rev. 2014, 114, 11348–11381. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Ashraf-Uz-Zaman, M.; Li, X.; Yao, Y.; Mishra, C.B.; Moku, B.K.; Song, Y. Quinazolinone compounds have potent antiviral activity against Zika and Dengue virus. J. Med. Chem. 2023, 66, 10746–10760. [Google Scholar] [CrossRef]
- Poespoprodjo, J.R.; Douglas, N.M.; Ansong, D.; Kho, S.; Anstey, N.M. Malaria. Lancet 2023, 402, 2328–2345. [Google Scholar] [CrossRef]
- Laleu, B.; Akao, Y.; Ochida, A.; Duffy, S.; Lucantoni, L.; Shackleford, D.M.; Chen, G.; Katneni, K.; Chiu, F.C.K.; White, K.L.; et al. Discovery and structure–activity relationships of quinazolinone-2-carboxamide derivatives as novel orally efficacious antimalarials. J. Med. Chem. 2021, 64, 12582–12602. [Google Scholar] [CrossRef] [PubMed]
- Nara, H.; Sato, K.; Naito, T.; Mototani, H.; Oki, H.; Yamamoto, Y.; Kuno, H.; Santou, T.; Kanzaki, N.; Terauchi, J.; et al. Discovery of novel, highly potent, and selective quinazoline-2-carboxamide-based matrix metalloproteinase (MMP)-13 inhibitors without a zinc binding group using a structure-based design approach. J. Med. Chem. 2014, 57, 8886–8902. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Tuberculosis Report 2019; WHO: Geneva, Switzerland, 2019.
- Naik, M.; Raichurkar, A.; Bandodkar, B.S.; Varun, B.V.; Bhat, S.; Kalkhambkar, R.; Murugan, K.; Menon, R.; Bhat, J.; Paul, B.; et al. Structure guided lead generation for M. Tuberculosis thymidylate kinase (Mtb TMK): Discovery of 3-cyanopyridone and 1,6-naphthyridin-2-one as potent inhibitors. J. Med. Chem. 2015, 58, 753–766. [Google Scholar] [CrossRef]
- Kumar, B.V.S.; Khetmalis, Y.M.; Chaitanya, K.S.; Chandu, A.; Shetye, G.; Ma, R.; Murugesan, S.; Franzblau, S.G.; Sekhar, K.V.G.C. Design, synthesis and biological evaluation of novel pyrido-[2,3-d]-pyrimidin-2-amine analogues as antimycobacterial agents. J. Mol. Struct. 2024, 1303, 137600. [Google Scholar] [CrossRef]
- Jian, Y.; Forbes, H.E.; Hulpia, F.; Risseeuw, M.D.P.; Caljon, G.; Munier-Lehmann, H.; Boshoff, H.I.M.; Van Calenbergh, S. 2-((3,5-Dinitrobenzyl)thio)quinazolinones: Potent antimycobacterial agents activated by deazaflavin (F420)-dependent nitroreductase (Ddn). J. Med. Chem. 2021, 64, 440–457. [Google Scholar] [CrossRef]
- Harbut, M.B.; Yang, B.; Liu, R.; Yano, T.; Vilchèze, C.; Cheng, B.; Lockner, J.; Guo, H.; Yu, C.; Franzblau, S.G.; et al. Small molecules targeting mycobacterium tuberculosis Type II NADH dehydrogenase exhibit antimycobacterial activity. Angew. Chem. Int. Ed. 2018, 57, 3478–3482. [Google Scholar] [CrossRef]
- Akester, J.N.; Njaria, P.; Nchinda, A.; Le Manach, C.; Myrick, A.; Singh, V.; Lawrence, N.; Njoroge, M.; Taylor, D.; Moosa, A.; et al. Synthesis, structure-activity relationship, and mechanistic studies of aminoquinazolinones displaying antimycobacterial activity. ACS Infect. Dis. 2020, 6, 1951–1964. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, M.F.; Zhou, C.-H. Identification of unique quinazolone thiazoles as novel structural scaffolds for potential Gram-negative bacterial conquerors. J. Med. Chem. 2021, 64, 7630–7645. [Google Scholar] [CrossRef]
- Chen, T.; Xiong, H.; Yang, J.F.; Zhu, X.L.; Qu, R.Y.; Yang, G.F. Diaryl ether: A privileged scaffold for drug and agrochemical discovery. J. Agric. Food Chem. 2020, 68, 9839–9877. [Google Scholar] [CrossRef]
- Di Liberto, M.G.; Stegmayer, M.I.; Svetaz, L.A.; Derita, M.G. Evaluation of Argentinean medicinal plants and isolation of their bioactive compounds as an alternative for the control of postharvest fruits phytopathogenic fungi. Rev. Bras. Farmacogn. 2019, 29, 686–688. [Google Scholar] [CrossRef]
- Xu, H.; Fan, L.L. Antifungal agents. Part 4: Synthesis and antifungal activities of novel indole [1,2-c]-1,2,4-benzotriazine derivatives against phytopathogenic fungi in vitro. Eur. J. Med. Chem. 2011, 46, 364–369. [Google Scholar] [CrossRef]
- Peng, J.-W.; Yin, X.-D.; Li, H.; Ma, K.-Y.; Zhang, Z.-J.; Zhou, R.; Wang, Y.-L.; Hu, G.-F.; Liu, Y.-Q. Design, synthesis, and structure-activity relationship of quinazolinone derivatives as potential fungicides. J. Agric. Food Chem. 2021, 69, 4604–4614. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chai, J.; Gu, Y.; Zhang, D.; Meng, F.; Si, X.; Yang, C.; Xue, W. Expedient discovery for novel antifungal leads inhibiting Fusarium graminearum: 3-(phenylamino)quinazolin-4(3H)-ones deriving from systematic optimizations on a tryptanthrin structure. J. Agric. Food Chem. 2022, 70, 13165–13175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhan, W.; Yuan, C.; Zhang, T.; Mao, P.; Sun, Z.; An, Y.; Xue, W. Design, synthesis and antifungal activity of novel 1,4-pentadiene-3-one containing quinazolinone. Int. J. Mol. Sci. 2023, 24, 2599. [Google Scholar] [CrossRef]
- Wang, Y.; Pruitt, R.N.; Nurnberger, T.; Wang, Y.C. Evasion of plant immunity by microbial pathogens. Nat. Rev. Microbiol. 2022, 20, 449–464. [Google Scholar] [CrossRef]
- Tarkowski, P.; Vereecke, D. Threats and opportunities of plant pathogenic bacteria. Biotechnol. Adv. 2014, 32, 215–229. [Google Scholar] [CrossRef]
- Zhang, G.; Li, C.; Li, Y.; Chen, D.; Li, Z.; Wang, Z.; Ouyang, G. Design, synthesis, and mechanism of novel 9-aliphatic amine tryptanthrin derivatives against phytopathogenic bacteria. J. Agric. Food Chem. 2023, 71, 14232–14242. [Google Scholar] [CrossRef]
- Shao, L.; Zhao, S.; Yang, S.; Zhou, X.; Li, Y.; Li, C.; Chen, D.; Li, Z.; Ouyang, G.; Wang, Z. Design, synthesis, antibacterial evaluation, three-dimensional quantitative structure–activity relationship, and mechanism of novel quinazolinone derivatives. J. Agric. Food Chem. 2023, 71, 3939–3949. [Google Scholar] [CrossRef]
- Wu, N.; Yang, Y.; Tian, G.; An, L.; Liu, S.; Yan, T.; Yi, M.; Bao, X. Synthesis, X-Ray crystal structure, and antimicrobial studies of new quinazolin-4(3H)-one derivatives containing the 1,2,4-triazolo[3,4-b][1,3,4]thiadiazole moiety and 4-piperidinyl linker. J. Agric. Food Chem. 2023, 71, 19277–19287. [Google Scholar] [CrossRef]
- Liu, T.; Peng, F.; Cao, X.; Liu, F.; Wang, Q.; Liu, L.; Xue, W. Design, synthesis, antibacterial activity, antiviral activity, and mechanism of myricetin derivatives containing a quinazolinone moiety. ACS Omega 2021, 6, 30826–30833. [Google Scholar] [CrossRef] [PubMed]
- Karasev, A.V.; Gray, S.M. Continuous and emerging challenges of potato virus Y in potato. Annu. Rev. Phytopathol. 2013, 51, 571–586. [Google Scholar] [CrossRef]
- Boquel, S.; Zhang, J.; Goyer, C.; Giguere, M.A.; Clark, C.; Pelletier, Y. Effect of insecticide-treated potato plants on aphid behavior and potato virus Y acquisition. Pest Manag. Sci. 2015, 71, 1106–1112. [Google Scholar] [CrossRef]
- Su, B.; Cai, C.; Deng, M.; Wang, Q.M. Spatial configuration and 3D conformation directed design, synthesis, antiviral activity, and structure-activity relationships (SARs) of phenanthroindolizidine analogues. J. Agric. Food Chem. 2016, 64, 2039–2045. [Google Scholar] [CrossRef]
- Satyanarayana, T.; Gowda, S.; Mawassi, M.; Albiach-Marti, M.R.; Ayllon, M.A.; Robertson, C.; Garnsey, S.M.; Dawson, W.O. Closterovirus encoded HSP70 homolog and p61 in addition to both coat proteins function in efficient virion assembly. Virology 2000, 278, 253–265. [Google Scholar] [CrossRef]
- Alzhanova, D.V.; Hagiwara, Y.; Peremyslov, V.V.; Dolja, V.V. Genetic analysis of the cell-to-cell movement of beet yellows closterovirus. Virology 2000, 268, 192–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Zhao, L.; Zhu, C.; Wu, Z.; Zhang, G.; Gan, X.; Liu, D.; Pan, J.; Hu, D.; Song, B. Facile synthesis of novel vanillin derivatives incorporating a bis(2-hydroxyethyl)dithhioacetal moiety as antiviral agents. J. Agric. Food Chem. 2017, 65, 4582–4588. [Google Scholar] [CrossRef]
- Ran, L.; Yang, H.; Luo, L.; Huang, M.; Hu, D. Discovery of Potent and Novel Quinazolinone Sulfide Inhibitors with Anti-ToCV Activity. J. Agric. Food Chem. 2020, 68, 5302–5308. [Google Scholar] [CrossRef] [PubMed]
- Zu, G.; Gan, X.; Xie, D.; Yang, H.; Zhang, A.; Li, S.; Hu, D.; Song, B. Design, synthesis, and anti-ToCV activity of novel 4(3H)-quinazolinone derivatives bearing dithioacetal moiety. J. Agric. Food Chem. 2020, 68, 5539–5544. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Xie, D.; Song, B.; Hu, D. First report on anti-TSWV activities of quinazolinone derivatives containing a dithioacetal moiety. J. Agric. Food Chem. 2021, 69, 12135–12142. [Google Scholar] [CrossRef]
- Zu, G.; Chen, J.; Song, B.; Hu, D. Synthesis, anti-tomato spotted wilt virus activities, and interaction mechanisms of novel dithioacetal derivatives containing a 4(3H)-quinazolinone pyrimidine ring. J. Agric. Food Chem. 2021, 69, 14459–14466. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Guo, J.; Wang, Z.; Liu, Y.; Li, Y.; Ma, D.; Wang, Q. Discovery of tryptanthrins as novel antiviral and anti-phytopathogenic-fungus agents. J. Agric. Food Chem. 2020, 68, 5586–5595. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, F.; Zhu, Y.; Cao, X.; Wang, Q.; Liu, F.; Liu, L.; Xue, W. Design, synthesis, biological activity evaluation and mechanism of action of myricetin derivatives containing thioether quinazolinone. Arab. J. Chem. 2022, 15, 104019. [Google Scholar] [CrossRef]
- Gong, C.; Meng, K.; Sun, Z.; Zeng, W.; An, Y.; Zou, H.; Qiu, Y.; Liu, D.; Xue, W. Flavonol derivatives containing a quinazolinone moiety: Design, synthesis, and antiviral activity. Chem. Biodivers. 2024, 21, e202301737. [Google Scholar] [CrossRef]
- Wang, C.; Chen, K.; Li, N.; Fu, S.; Li, P.; Ji, L.; Liu, G.; Wang, X.; Lei, K. Design, synthesis, mode of action and herbicidal evaluation of quinazolin-4(3H)-one derivatives based on aryloxyphenoxypropionate motif. Agronomy 2022, 12, 1840. [Google Scholar] [CrossRef]
- Li, N.; Chen, K.; Han, S.; Wang, S.; He, Y.; Wang, X.; Li, P.; Ji, L.; Liu, R.; Lei, K. Synthesis, herbicidal activity, and molecular mode of action evaluation of novel aryloxyphenoxypropionate/amide derivatives containing a quinazolinone moiety. J. Agric. Food Chem. 2024, 72, 9445–9456. [Google Scholar] [CrossRef]
- Wang, S.M.; Li, N.; Han, S.B.; Fu, S.Y.; Chen, K.; Cheng, W.J.; Lei, K. Synthesis, herbicidal activity, and molecular mode of action evaluation of novel quinazolinone-phenoxypropionate hybrids containing a diester moiety. Agronomy 2024, 14, 2124. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Wang, S.; Fu, S.; Kim, J.; Park, P.; Liu, R.; Lei, K. 4(3H)-Quinazolinone: A Natural Scaffold for Drug and Agrochemical Discovery. Int. J. Mol. Sci. 2025, 26, 2473. https://doi.org/10.3390/ijms26062473
Chen K, Wang S, Fu S, Kim J, Park P, Liu R, Lei K. 4(3H)-Quinazolinone: A Natural Scaffold for Drug and Agrochemical Discovery. International Journal of Molecular Sciences. 2025; 26(6):2473. https://doi.org/10.3390/ijms26062473
Chicago/Turabian StyleChen, Ke, Shumin Wang, Shuyue Fu, Junehyun Kim, Phumbum Park, Rui Liu, and Kang Lei. 2025. "4(3H)-Quinazolinone: A Natural Scaffold for Drug and Agrochemical Discovery" International Journal of Molecular Sciences 26, no. 6: 2473. https://doi.org/10.3390/ijms26062473
APA StyleChen, K., Wang, S., Fu, S., Kim, J., Park, P., Liu, R., & Lei, K. (2025). 4(3H)-Quinazolinone: A Natural Scaffold for Drug and Agrochemical Discovery. International Journal of Molecular Sciences, 26(6), 2473. https://doi.org/10.3390/ijms26062473






