Short-Chain Fatty Acids and the Gut–Retina Connection: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
- Keyword Search: We used specific keywords and phrases, utilizing the MeSH feature of PubMed, such as “short chain fatty acids”, “butyrate”, “nervous system cells”, “neurons”, “ganglion cells”, “amacrine cells”, “bipolar cells”, “horizontal cells”, “photoreceptors”, “astrocytes”, “microglia”, “Müller cells”, “retina”, “macula lutea”, “retinal diseases”, “molecular mechanisms”, “epigenetic regulation”, “histone acetylation”, “histone deacetylase inhibitors”, “gene expression”, “signal transduction”, “immune modulation”, “oxidative stress”, “anti-inflammatory”, “neuroprotection”, “cell survival”, “photoreceptor survival”, “gut-brain axis”, “epigenetic mechanisms”, “inflammation”, “histone deacetylase inhibition”, “acetyl-CoA metabolism”, “retinal ganglion cells”, “retinal degeneration”, “macular degeneration”, “dysbiosis”, “intestinal microbiology”, “gut microbiota”, “microbiome”.
- Boolean Operators: Operators like AND, OR, and NOT were used to refine search results (e.g., (“short chain fatty acids” OR “butyrate”) AND (“retina” OR “macula lutea”)).
- Filters Used: Articles in the English language were used, and the rest were discarded. Abstracts were reviewed to select studies that met the inclusion criteria. Only articles that had the full text available were utilized for review and citation. Any duplicates were then removed.
- Snowballing Approach: To ensure a comprehensive review of the literature, we employed the snowballing technique. This involved systematically examining the reference lists of key articles identified during the initial search to uncover additional studies pertinent to our research topic. By tracing these references, we aimed to identify relevant publications that may not have been captured in our primary search strategy, thereby enhancing the breadth and depth of our review.
2.2. Study Selection and Data Analysis
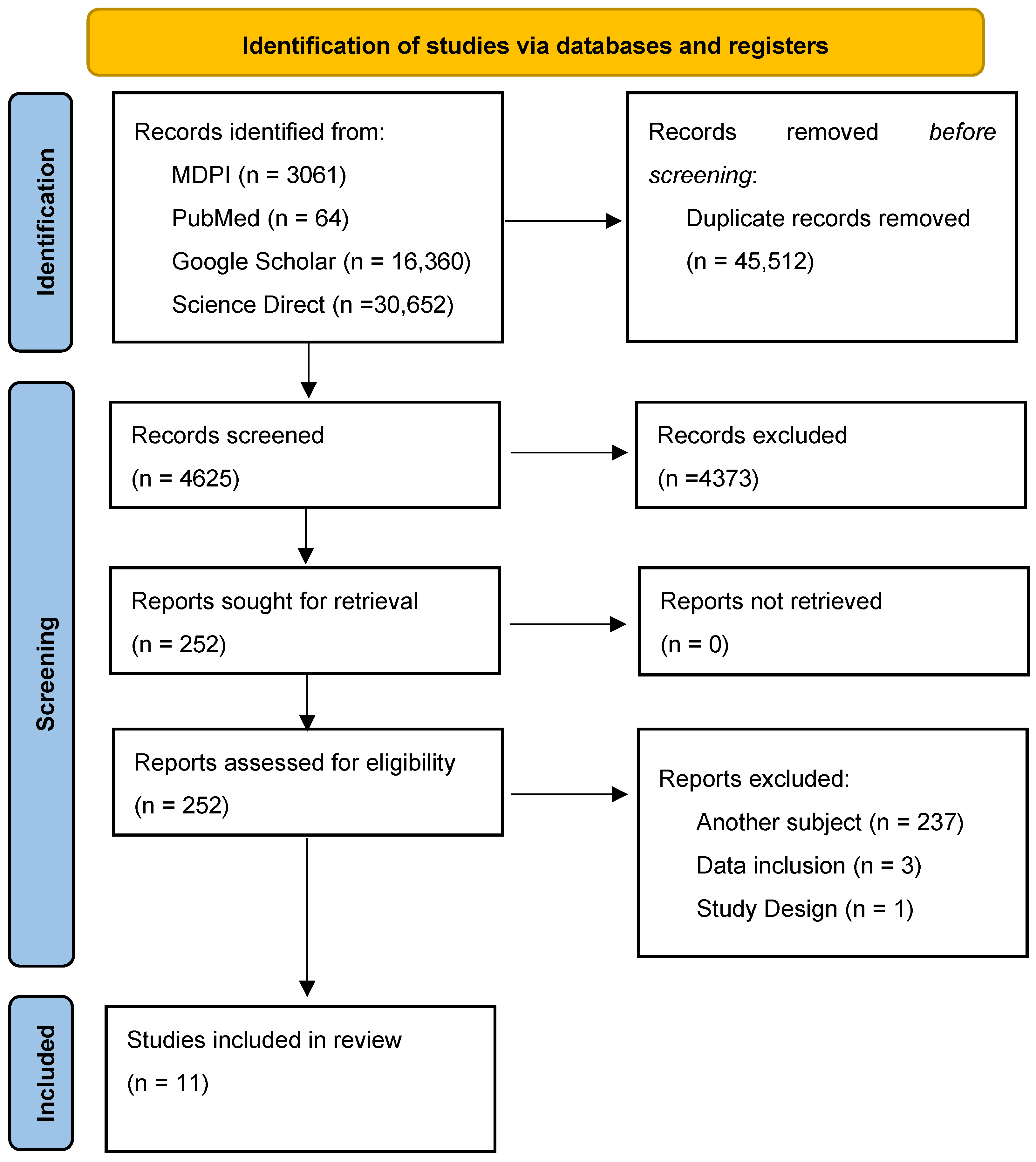
3. Results
3.1. Overview of Included Studies
3.2. Alterations in SCFA Levels in Retinal Diseases
| Study | Study Design | Species Investigated | Sample Size (n) | Model | Sample Type | Analysis Methodology | Dominant Bacterial Genera | SCFA | Statistical Significance (p-Value) |
|---|---|---|---|---|---|---|---|---|---|
| Rowan et al., 2017 [29] | Experimental (in vivo) | C57BL/6J mice | 60 | HG/LG diet-AMD | Fecal, retinal tissue | 16S rRNA sequencing, metabolomics, histology | Bacteroides, Prevotella | Butyrate and acetate increased with a high-fiber diet | <0.05 |
| Xiao et al., 2020 [30] | Experimental (in vitro and in vivo) | C57BL/6J mice, HUVECs | 100 mice (in vivo), several replicates (in vitro) | Laser–CNV | Choroid tissue, | Western blot, Choroid Sprouting Assay, RT-PCR, Immunofluorescence Histochemistry | NA | Dose-dependent reduction in neovascularization and SCFA-modulated pathways | <0.05 |
| Chen et al., 2021 [31] | Experimental (in vitro and in vivo) | C57BL/6J mice | ~50 mice (in vivo), multiple replicates (in vitro) | LPS-uveitis | Retinal tissues, RAC culture | GC-MS, ELISA, Western blot, flow cytometry | NA | SCFA (butyrate, propionate) inhibited inflammatory cytokine production | <0.05 |
| Chen et al., 2022 [32] | Experimental (human and mouse models) | C57BL/6J mice, human POAG patients | ~50 mice, 22 patients | POAG | Fecal, serum, retinal tissues | Metagenomics, GC-MS, 16S rRNA sequencing | Dysgonamonadaceae (enriched in POAG), Barnesiellaceae (enriched in controls) | SCFA increase observed in POAG; reduction post-antibiotic treatment | <0.05 |
| Dos Reis et al., 2022 [33] | Experimental (in vitro and in vivo) | Wistar rats, ARPE-19 cells, CAM | 18 rats, multiple replicates | Laser–CNV | Retinal tissue, CAM, cultured cells | SEM, OCT, CAM assay, HPLC, histopathology | NA | Controlled release of NaBu for 35 days with antiangiogenic effect | <0.05 |
| Shen et al., 2022 [34] | Experimental (in vivo, human data included) | Sprague Dawley rats, human subjects | 20 rats; 20 human subjects | STZ-T1DM | Retinal, plasma, vitreous fluid | GC-MS, ELISA, 16S rDNA sequencing, histopathology | Ruminococcaceae, Prevotellaceae, Alloprevotella, Bifidobacterium pseudolongum | Yes (STZ-induced T1DM reduced SCFAs; restored with LA and ALA) | <0.05 |
| Zhang et al., 2023 [35] | Experimental (in vivo) | C57BL/6J mice | 47 mice | Laser–CNV | Retinal, fecal samples | RNA sequencing, 16S rRNA sequencing, LC-MS | Akkermansia, Bifidobacterium | Increased SCFA levels (butyrate, propionate) in metformin group | <0.05 |
| Huang et al., 2023 [36] | Experimental (in vivo) | C57BL/6J mice | ~20 mice | STZ-T1DM | Plasma, fecal, retinal tissue | OCT, HE staining, electroretinography, LC-MS/MS, 16S rRNA sequencing | Dubosiella, Ileibacterium, Lachnospiraceae | Butyric acid, caproic acid, and 4-methylvaleric acid increased with NaB | <0.05 |
| Baldi et al., 2024 [37] | Randomized clinical trial | Human | 45 | N/A | Stool, plasma | 16S rRNA sequencing, GC-MS, OCT | Faecalibacterium, Lachnospiraceae | Total SCFA levels reduced in nAMD group compared to HC; partially restored with intervention | <0.05 |
| Vergroesen et al., 2024 [38] | Observational | Human | 1472 | N/A | Stool samples | 16S rRNA sequencing, meta-analysis | Butyrivibri, Caproiciproducens, Clostridium sensu stricto 1 | Decreased butyrate-producing taxa in glaucoma group | <0.05 |
| Qin et al., 2024 [39] | Cross-sectional and longitudinal cohort | Human | 161 | N/A | Stool, plasma, PBMCs | 16S rRNA sequencing, GC-MS, transcriptomics | Butyricicoccus, Ruminococcus torques | Acetate and butyrate reduced in DR patients | <0.05 |
3.3. Effects of SCFA Supplementation on Retinal Health
3.4. Mechanisms of the Gut–Retina Axis: Evidence from the Reviewed Studies
4. Discussion
4.1. The Impact of Gut Microbiota on SCFA Production and Ocular Health
4.2. SCFA Levels in Retinal Diseases
4.3. SCFA Supplementation as a Therapeutic Target
4.4. Limitataions of Included Studies
4.5. Limitataions of Our Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCFA | Short-chain fatty acid |
| AMD | Age-related macular degeneration |
| DR | Diabetic retinopathy |
| GPCRs | G-protein coupled receptors |
| HDAC | Histone Deacetylase Inhibitors |
| CNV | Choroidal neovascularization |
| HUVEC | Humans umbilical vein endothelial cells |
| ALA | Alpha-linolenic acid |
| LA | Linoleic acid |
| GCL | Ganglion cell layer |
| ERG | Electroretinography |
| NaBu | Sodium butyrate |
| CAM | Chorioallantoic membrane |
References
- Yang, X.; Chen, H.; Zhang, T.; Yin, X.; Man, J.; He, Q.; Lu, M. Global, regional, and national burden of blindness and vision loss due to common eye diseases along with its attributable risk factors from 1990 to 2019: A systematic analysis from the global burden of disease study 2019. Aging 2021, 13, 19614–19642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160, Erratum in Lancet Glob. Health 2021, 9, e408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zou, M.; Chen, A.; Liu, Z.; Jin, L.; Zheng, D.; Congdon, N.; Jin, G. The burden, causes, and determinants of blindness and vision impairment in Asia: An analysis of the Global Burden of Disease Study. J. Glob. Health 2024, 14, 04100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schultz, N.M.; Bhardwaj, S.; Barclay, C.; Gaspar, L.; Schwartz, J. Global Burden of Dry Age-Related Macular Degeneration: A Targeted Literature Review. Clin. Ther. 2021, 43, 1792–1818. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Saifi, A.I.; Nagrale, P.; Ansari, K.K.; Saifi, I.; Chaurasia, S. Advancement in Understanding Glaucoma: A Comprehensive Review. Cureus 2023, 15, e46254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Luu, K.T.; Seal, J.; Green, M.; Winskill, C.; Attar, M. Effect of Anti-VEGF Therapy on the Disease Progression of Neovascular Age-Related Macular Degeneration: A Systematic Review and Model-Based Meta-Analysis. J. Clin. Pharmacol. 2022, 62, 594–608. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nozaki, M.; Ando, R.; Kimura, T.; Kato, F.; Yasukawa, T. The Role of Laser Photocoagulation in Treating Diabetic Macular Edema in the Era of Intravitreal Drug Administration: A Descriptive Review. Medicina 2023, 59, 1319. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tîrziu, A.T.; Susan, M.; Susan, R.; Sonia, T.; Harich, O.O.; Tudora, A.; Varga, N.I.; Tiberiu-Liviu, D.; Avram, C.R.; Boru, C.; et al. From Gut to Eye: Exploring the Role of Microbiome Imbalance in Ocular Diseases. J. Clin. Med. 2024, 13, 5611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Napolitano, P.; Filippelli, M.; Davinelli, S.; Bartollino, S.; dell’Omo, R.; Costagliola, C. Influence of gut microbiota on eye diseases: An overview. Ann. Med. 2021, 53, 750–761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Qiu, P.; Shen, T. Gut microbiota and eye diseases: A review. Medicine 2024, 103, e39866. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cavuoto, K.M.; Banerjee, S.; Galor, A. Relationship between the microbiome and ocular health. Ocul. Surf. 2019, 17, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Sheppard, J.D. Gut Microbiome and Its Influence On Ocular Surface and Ocular Surface Diseases. Eye Contact Lens 2022, 48, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wan, Z.; Zhang, Y.; Wang, T.; Xue, Y.; Peng, Q. Composition and diversity of gut microbiota in diabetic retinopathy. Front. Microbiol. 2022, 13, 926926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.; Wang, Z.; Ma, H.; Ji, S.; Chen, Z.; Cui, Z.; Chen, J.; Tang, S. Dysbiosis and Implication of the Gut Microbiota in Diabetic Retinopathy. Front. Cell. Infect. Microbiol. 2021, 11, 646348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, J.; Dong, H.; Wang, T.; Yu, H.; Yu, J.; Ma, S.; Song, X.; Sun, Q.; Xu, Y.; Liu, M. What is the impact of microbiota on dry eye: A literature review of the gut-eye axis. BMC Ophthalmol. 2024, 24, 262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Moniri, N.H.; Farah, Q. Short-chain free-fatty acid G protein-coupled receptors in colon cancer. Biochem. Pharmacol. 2021, 186, 114483. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H.; Mo, Y. The gut-retina axis: A new perspective in the prevention and treatment of diabetic retinopathy. Front. Endocrinol. 2023, 14, 1205846. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, J.; Zhang, J.Y.; Luo, W.; He, P.C.; Skondra, D. The Emerging Role of Gut Microbiota in Age-Related Macular Degeneration. Am. J. Pathol. 2023, 193, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rowan, S.; Jiang, S.; Korem, T.; Szymanski, J.; Chang, M.L.; Szelog, J.; Cassalman, C.; Dasuri, K.; McGuire, C.; Nagai, R.; et al. Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2017, 114, E4472–E4481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, X.; Chen, M.; Xu, Y.; Huang, S.; Liang, J.; Cao, Y.; Chen, H. Sodium Butyrate Inhibits Neovascularization Partially via TNXIP/VEGFR2 Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 6415671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, N.; Wu, J.; Wang, J.; Piri, N.; Chen, F.; Xiao, T.; Zhao, Y.; Sun, D.; Kaplan, H.J.; Shao, H. Short chain fatty acids inhibit endotoxin-induced uveitis and inflammatory responses of retinal astrocytes. Exp. Eye Res. 2021, 206, 108520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.; Wang, Y.; Liu, Y.; Li, F.; Chen, Y.; Fang, X.; Wen, T.; Xu, S.; Kermany, D.; Deng, S.; et al. Dysbiosis of gut microbiome contributes to glaucoma pathogenesis. MedComm–Future Med. 2022, 1, e28. [Google Scholar] [CrossRef]
- Reis, J.S.D.; Dos Reis Teixeira, A.; De Vasconcelos Quaresma, A.; Almeida, T.C.; Arribada, R.G.; Neto, J.T.; Da Silva, F.H.R.; Silva-Cunha, A.; Lima De Moura, S.A.; Da Silva, G.N.; et al. Sodium butyrate-loaded nanoparticles coated with chitosan for the treatment of neovascularization in age-related macular degeneration: Ocular biocompatibility and antiangiogenic activity. Eur. J. Pharm. Biopharm. 2022, 179, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, L.; Wang, Y.; Chen, Z.; Ma, J.; Fang, X.; Das, U.N.; Yao, K. Beneficial Actions of Essential Fatty Acids in Streptozotocin-Induced Type 1 Diabetes Mellitus. Front. Nutr. 2022, 9, 890277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.Y.; Xiao, J.; Xie, B.; Barba, H.; Boachie-Mensah, M.; Shah, R.N.; Nadeem, U.; Spedale, M.; Dylla, N.; Lin, H.; et al. Oral Metformin Inhibits Choroidal Neovascularization by Modulating the Gut-Retina Axis. Investig. Ophthalmol. Vis. Sci. 2023, 64, 21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.; Wang, Z.; Ye, B.; Ma, J.H.; Ji, S.; Sheng, W.; Ye, S.; Ou, Y.; Peng, Y.; Yang, X.; et al. Sodium butyrate ameliorates diabetic retinopathy in mice via the regulation of gut microbiota and related short-chain fatty acids. J. Transl. Med. 2023, 21, 451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baldi, S.; Pagliai, G.; Di Gloria, L.; Pallecchi, M.; Barca, F.; Pieri, B.; Bartolucci, G.; Ramazzotti, M.; Amedei, A.; Palendri, G.; et al. Beneficial Effects of Micronutrient Supplementation in Restoring the Altered Microbiota and Gut-Retina Axis in Patients with Neovascular Age-Related Macular Degeneration-A Randomized Clinical Trial. Nutrients 2024, 16, 3971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vergroesen, J.E.; Jarrar, Z.A.; Weiss, S.; Frost, F.; Ansari, A.S.; Nguyen, P.; Kraaij, R.; Medina-Gomez, C.; Völzke, H.; Tost, F.; et al. Glaucoma Patients Have a Lower Abundance of Butyrate-Producing Taxa in the Gut. Investig. Ophthalmol. Vis. Sci. 2024, 65, 7, Erratum in Investig. Ophthalmol. Vis. Sci. 2025, 66, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, X.; Sun, J.; Chen, S.; Xu, Y.; Lu, L.; Lu, M.; Li, J.; Ma, Y.; Lou, F.; Zou, H. Gut microbiota predict retinopathy in patients with diabetes: A longitudinal cohort study. Appl. Microbiol. Biotechnol. 2024, 108, 497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donohoe, D.R.; Collins, L.B.; Wali, A.; Bigler, R.; Sun, W.; Bultman, S.J. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell 2012, 48, 612–626. [Google Scholar] [CrossRef]
- Serban, D.; Dascalu, A.M.; Arsene, A.L.; Tribus, L.C.; Vancea, G.; Pantea Stoian, A.; Costea, D.O.; Tudosie, M.S.; Stana, D.; Cristea, B.M.; et al. Gut Microbiota Dysbiosis in Diabetic Retinopathy-Current Knowledge and Future Therapeutic Targets. Life 2023, 13, 968. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, X.; Xu, M.; Zhao, Z.; Wang, Y.; Liu, Y.; Zhang, T.; Wan, X.; Jiang, M.; Luo, X.; Shen, Y.; et al. Bifidobacterium promotes retinal ganglion cell survival by regulating the balance of retinal glial cells. CNS Neurosci. Ther. 2023, 29 (Suppl. S1), 146–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Labetoulle, M.; Baudouin, C.; Benitez Del Castillo, J.M.; Rolando, M.; Rescigno, M.; Messmer, E.M.; Aragona, P. How gut microbiota may impact ocular surface homeostasis and related disorders. Prog. Retin. Eye Res. 2024, 100, 101250. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Li, J.J.; Zou, Y.; Zou, B.; Wei, L. Microbiota and Ocular Diseases. Front. Cell. Infect. Microbiol. 2021, 11, 759333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, S.; Liu, J.; Xiang, J.; Yan, R.; Li, S.; Fan, Q.; Lu, L.; Wu, J.; Xue, Y.; Fu, T.; et al. Restorative Effects of Short-Chain Fatty Acids on Corneal Homeostasis Disrupted by Antibiotic-Induced Gut Dysbiosis. Am. J. Pathol. 2024; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.C.; Popova, E.Y.; James, J.; Briones, M.R.; Zhang, S.S.; Barnstable, C.J. Histone Deacetylase 1 Is Essential for Rod Photoreceptor Differentiation by Regulating Acetylation at Histone H3 Lysine 9 and Histone H4 Lysine 12 in the Mouse Retina. J. Biol. Chem. 2017, 292, 2422–2440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, W.; Wang, Q.; Wan, D.; Sun, Y.; Wang, L.; Chen, H.; Liu, C.; Petersen, R.B.; Li, J.; Xue, W.; et al. Histone HIST1H1C/H1.2 regulates autophagy in the development of diabetic retinopathy. Autophagy 2017, 13, 941–954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, J.; Chen, N.; Grau, E.; Johnson, L.; Liu, Y.; Li, C.; Scott, P.A.; Kim, C.; Sun, D.; Kaplan, H.J.; et al. Short chain fatty acids inhibit corneal inflammatory responses to TLR ligands via the ocular G-protein coupled receptor 43. Ocul. Surf. 2024, 32, 48–57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scuderi, G.; Troiani, E.; Minnella, A.M. Gut Microbiome in Retina Health: The Crucial Role of the Gut-Retina Axis. Front. Microbiol. 2022, 12, 726792. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, X.; Chen, P.S.; Dallas, S.; Wilson, B.; Block, M.L.; Wang, C.C.; Kinyamu, H.; Lu, N.; Gao, X.; Leng, Y.; et al. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int. J. Neuropsychopharmacol. 2008, 11, 1123–1134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, F.; Lv, Y.W.; Long, J.; Chen, J.M.; He, J.M.; Ruan, X.Z.; Zhu, H.B. Butyrate Improves the Metabolic Disorder and Gut Microbiome Dysbiosis in Mice Induced by a High-Fat Diet. Front. Pharmacol. 2019, 10, 1040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Study | SCFA(s) Investigated | Model (Species) | Route of Administration | Dosage/Duration | Key Retinal Findings | Other Findings |
|---|---|---|---|---|---|---|
| Xiao et al., 2020 [30] | Sodium butyrate | Laser-induced CNV (mice), HUVECs (in vitro) | Intravitreal injection, in vitro | In vivo: Not specified in abstract; In vitro: various | Reduced CNV lesion size, inhibited HUVEC proliferation and tube formation, upregulated TXNIP, downregulated VEGFR2 | |
| Chen et al., 2021 [31] | SCFAs (acetate, propionate, butyrate) | LPS-induced uveitis (mice) | Intraperitoneal injection | In vivo: 500 mg/kg, in vitro: 1, 5, 10 mM | Reduced production of IL-6, TNF-alfa, CXCL1, and CXCL12 by LPS-stimulated RACs in vitro; reduced severity of uveitis in vivo | Enhanced antigen presenting ability of RACs in vitro; reduced immune cell migration in vitro |
| Dos Reis et al., 2022 [33] | Sodium butyrate | Wistar rats, ARPE-19 cells, CAM | Intravitreal injection, in vitro | 34.4 µg/mL (nanoparticles) | Nanoparticles: no retinal toxicity, antiangiogenic in CAM assay; Free NaBu: retinal damage | Nanoparticles: controlled release of NaBu for 35 days |
| Shen et al., 2022 [34] | LA and ALA | STZ-induced T1DM (rats) | Intraperitoneal injection | 100 μg/day every other day for 3 weeks | Prevented retinal thinning, reduced cell number reduction | Improved lipid profiles; modulated gut microbiota; reduced inflammation |
| Zhang et al., 2023 [35] | Oral metformin (indirectly increased butyrate and propionate) | Laser-induced CNV (mice) | Oral gavage | 300 mg/kg | Reduced CNV lesion size, decreased Iba1+ macrophages/microglia around the lesion. | Altered gut microbiome composition; increased fecal SCFAs; modulated RPE/choroid gene expression |
| Huang et al., 2023 [36] | Sodium butyrate | STZ-induced T1DM (mice) | Oral gavage | 500 mg/kg daily for 12 weeks | Ameliorated retinal thinning (inner/middle layers), inhibited microglial activation, improved ERG parameters | Reduced blood glucose, food, and water consumption; enhanced tight junction protein expression in the small intestine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciurariu, E.; Tirziu, A.-T.; Varga, N.-I.; Hirtie, B.; Alexandru, A.; Ivan, C.-S.; Nicolescu, L. Short-Chain Fatty Acids and the Gut–Retina Connection: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 2470. https://doi.org/10.3390/ijms26062470
Ciurariu E, Tirziu A-T, Varga N-I, Hirtie B, Alexandru A, Ivan C-S, Nicolescu L. Short-Chain Fatty Acids and the Gut–Retina Connection: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(6):2470. https://doi.org/10.3390/ijms26062470
Chicago/Turabian StyleCiurariu, Elena, Andreea-Talida Tirziu, Norberth-Istvan Varga, Bogdan Hirtie, Alexandru Alexandru, Cristiana-Smaranda Ivan, and Laura Nicolescu. 2025. "Short-Chain Fatty Acids and the Gut–Retina Connection: A Systematic Review" International Journal of Molecular Sciences 26, no. 6: 2470. https://doi.org/10.3390/ijms26062470
APA StyleCiurariu, E., Tirziu, A.-T., Varga, N.-I., Hirtie, B., Alexandru, A., Ivan, C.-S., & Nicolescu, L. (2025). Short-Chain Fatty Acids and the Gut–Retina Connection: A Systematic Review. International Journal of Molecular Sciences, 26(6), 2470. https://doi.org/10.3390/ijms26062470







