Depletion of Fkbp5 Protects Against the Rapid Decline in Ovarian Reserve Induced by Prenatal Stress in Female Offspring of Wild-Type Mice
Abstract
1. Introduction
2. Results
2.1. PNS Administration Affects the Follicular Reserve in Adult Fkbp5+/+ Mice
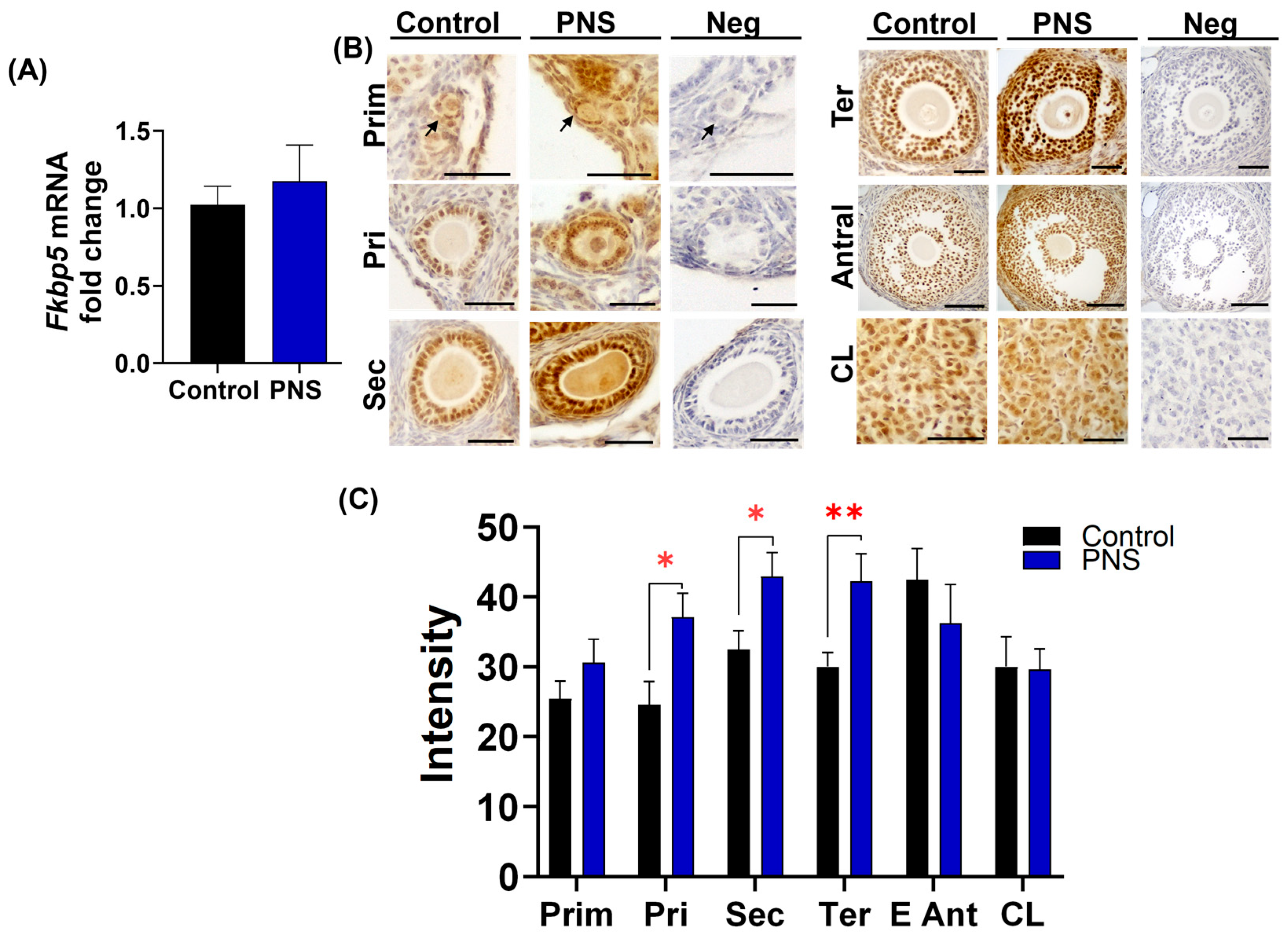
2.2. PNS Increases FKBP51 Expression in Granulosa Cells in Adult Offspring Ovary
2.3. PNS Reduces Hsd11b2 Levels in Adult Fkbp5+/+ Mice
2.4. PNS Effects on Progesterone Signaling in Adult Offspring
2.5. The Impact of PNS on Androgen Signaling in Adult Offspring
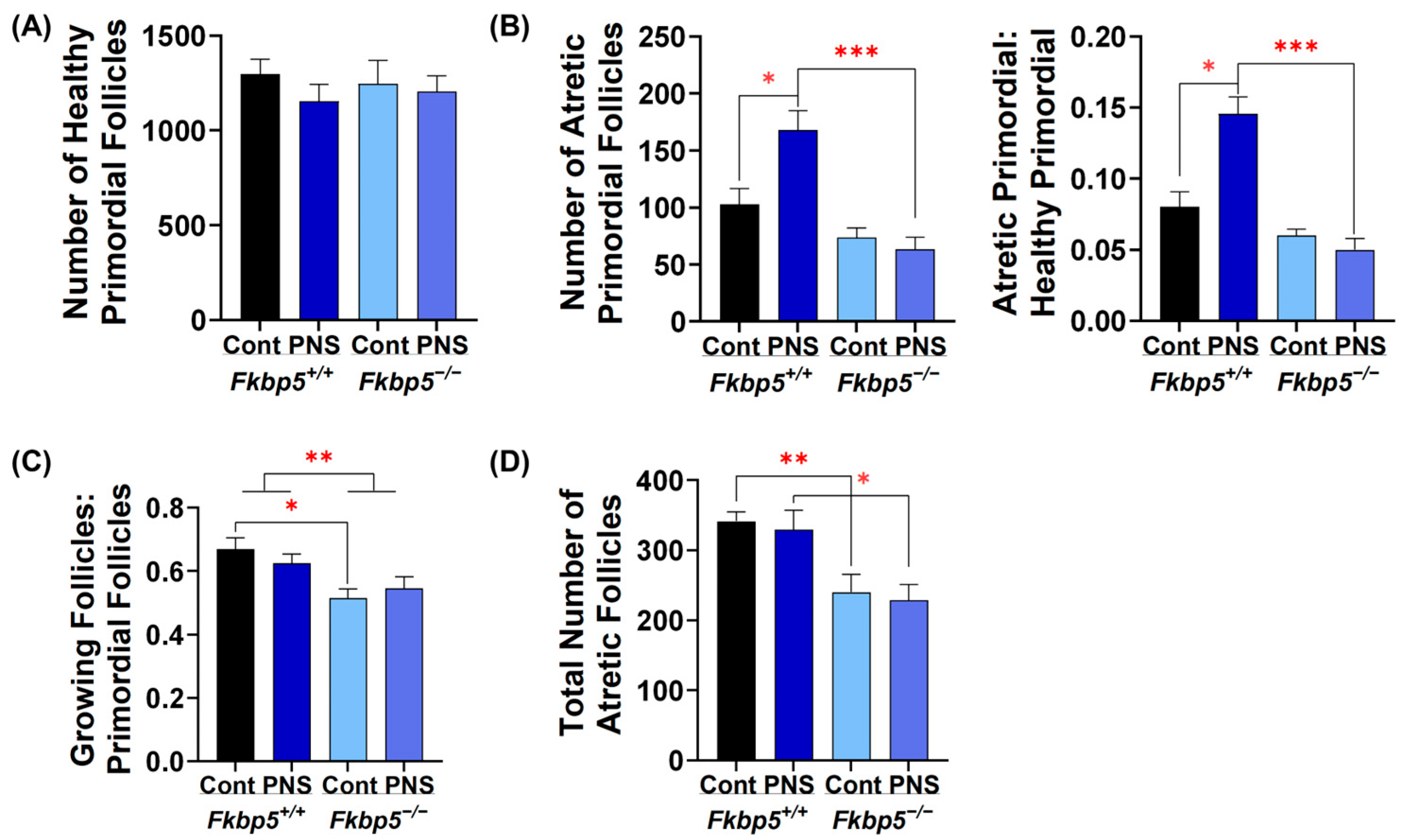
2.6. PNS Administration Increases Primordial Follicle Atresia in Peripubertal Fkbp5+/+ Offspring
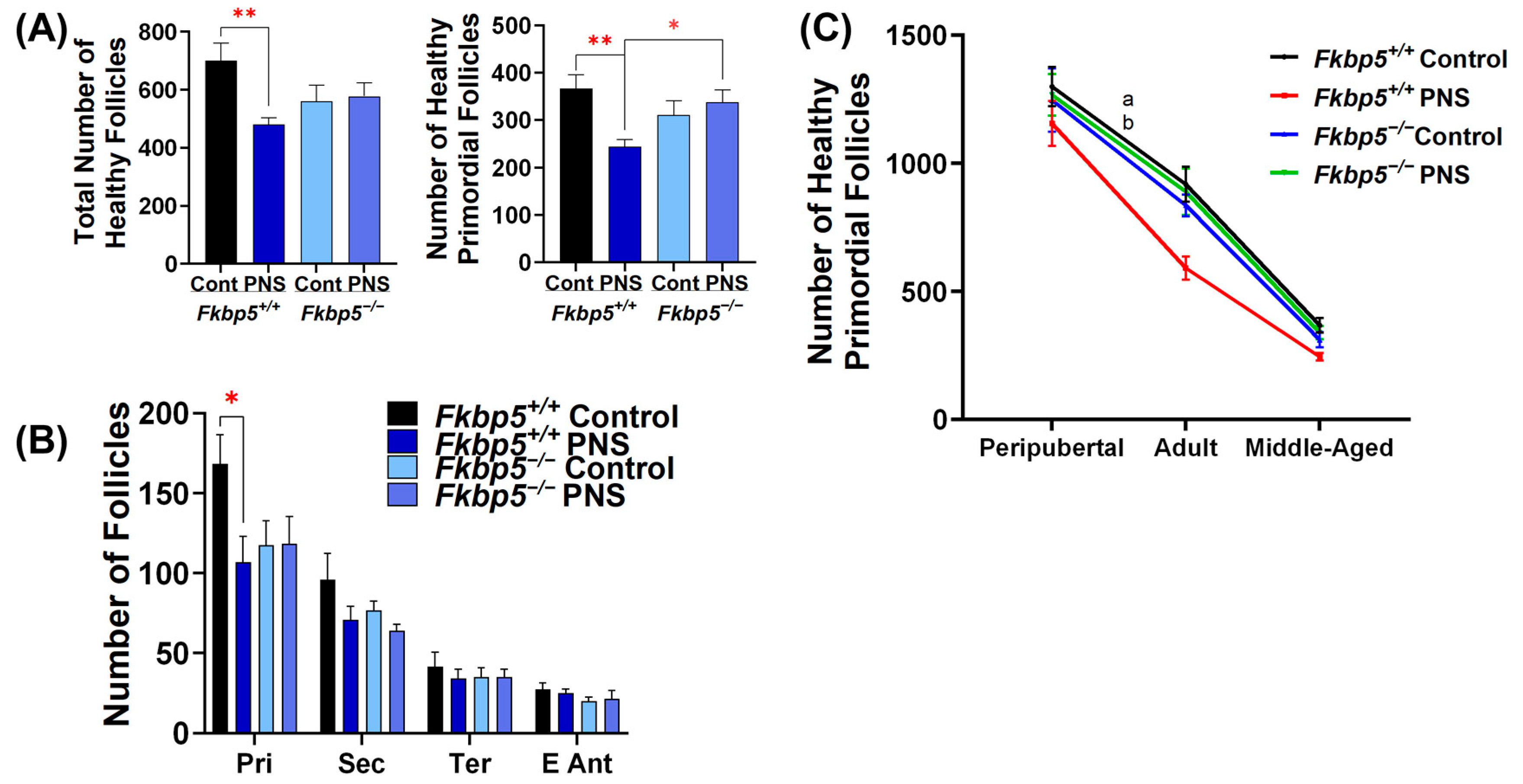
2.7. Fkbp5 Depletion Reduces Follicle Recruitment in Peripubertal Control Offspring
2.8. PNS Delays Pubertal Onset in Fkbp5+/+ Mice
2.9. PNS Causes a Rapid Decline in Ovarian Reserve in Middle-Aged Fkbp5+/+ Mice
2.10. The Effect of PNS on FKBP51 Expression in the Middle-Aged Offspring Ovary
2.11. PNS Disruptions in Cholesterol Transport in Middle-Aged Fkbp5+/+ Offspring Ovaries
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Determination of Estrous Cycle Phase
4.4. Sampling Procedure
4.5. Histomorphometric Analysis of Folliculogenesis in Ovaries
4.6. Reverse Transcription and Quantitative Real Time (q)-PCR Analysis
4.7. Immunohistochemistry and Quantification of Staining Intensity Analysis
4.8. Hormone Assessment
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Graham, B.M. Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry 2017, 4, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Musanejad, E.; Haghpanah, T.; Mirzaie, V.; Ezzatabadipour, M. Effects of ethanol and nicotine co-administration on follicular atresia and placental histo-morphology in the first-generation mice pups during intrauterine development and lactation periods. Toxicol. Rep. 2021, 8, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Pooley, A.E.; Benjamin, R.C.; Sreedhar, S.; Eagle, A.L.; Robison, A.J.; Mazei-Robison, M.S.; Breedlove, S.M.; Jordan, C.L. Sex differences in the traumatic stress response: The role of adult gonadal hormones. Biol. Sex. Differ. 2018, 9, 32. [Google Scholar] [CrossRef]
- Zhai, Q.Y.; Wang, J.J.; Tian, Y.; Liu, X.; Song, Z. Review of psychological stress on oocyte and early embryonic development in female mice. Reprod. Biol. Endocrinol. 2020, 18, 101. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31. [Google Scholar] [CrossRef]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010, 35, 109–125. [Google Scholar]
- Richards, J.S.; Pangas, S.A. The ovary: Basic biology and clinical implications. J. Clin. Investig. 2010, 120, 963–972. [Google Scholar] [CrossRef]
- Demyttenaere, K.; Nijs, P.; Evers-Kiebooms, G.; Koninckx, P.R. Coping and the ineffectiveness of coping influence the outcome of in vitro fertilization through stress responses. Psychoneuroendocrinology 1992, 17, 655–665. [Google Scholar] [CrossRef]
- Ebbesen, S.M.; Zachariae, R.; Mehlsen, M.Y.; Thomsen, D.; Højgaard, A.; Ottosen, L.; Petersen, T.; Ingerslev, H.J. Stressful life events are associated with a poor in-vitro fertilization (IVF) outcome: A prospective study. Hum. Reprod. 2009, 24, 2173–2182. [Google Scholar] [CrossRef]
- Prasad, S.; Tiwari, M.; Pandey, A.N.; Shrivastav, T.G.; Chaube, S.K. Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 2016, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.L.; Parks, J.C.; Russ, J.E.; Khan, S.A.; Schoolcraft, W.B.; Yuan, Y.; Katz-Jaffe, M.G. Increased Systemic Antioxidant Power Ameliorates the Aging-Related Reduction in Oocyte Competence in Mice. Int. J. Mol. Sci. 2021, 22, 13019. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Di Emidio, G.; Vitti, M.; Di Carlo, M.; Santini, S., Jr.; D’Alessandro, A.M.; Falone, S.; Amicarelli, F. Sirtuin Functions in Female Fertility: Possible Role in Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2015, 2015, 659687. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Prasad, S.; Tripathi, A.; Pandey, A.N.; Ali, I.; Singh, A.K.; Shrivastav, T.G.; Chaube, S.K. Apoptosis in mammalian oocytes: A review. Apoptosis 2015, 20, 1019–1025. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 2020, 11, 617843. [Google Scholar] [CrossRef]
- Nakamura, K.; Sheps, S.; Arck, P.C. Stress and reproductive failure: Past notions, present insights and future directions. J. Assist. Reprod. Genet. 2008, 25, 47–62. [Google Scholar] [CrossRef]
- Guzeloglu-Kayisli, O.; Semerci, N.; Guo, X.; Larsen, K.; Ozmen, A.; Arlier, S.; Mutluay, D.; Nwabuobi, C.; Sipe, B.; Buhimschi, I.; et al. Decidual cell FKBP51-progesterone receptor binding mediates maternal stress-induced preterm birth. Proc. Natl. Acad. Sci. USA 2021, 118, e2010282118. [Google Scholar] [CrossRef]
- Wood, C.E.; Rudolph, A.M. Can maternal stress alter fetal adrenocorticotropin secretion? Endocrinology 1984, 115, 298–301. [Google Scholar] [CrossRef]
- Pepe, G.J.; Albrecht, E.D. Actions of Placental and Fetal Adrenal Steroid Hormones in Primate Pregnancy*. Endocr. Rev. 1995, 16, 608–648. [Google Scholar] [CrossRef]
- Schoof, E.; Girstl, M.; Frobenius, W.; Kirschbaum, M.; Repp, R.; Knerr, I.; Rascher, W.; Dötsch, J. Course of placental 11beta-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase mRNA expression during human gestation. Eur. J. Endocrinol. 2001, 145, 187–192. [Google Scholar] [CrossRef]
- Liu, M.Y.; Wei, L.L.; Zhu, X.H.; Ding, H.C.; Liu, X.H.; Li, H.; Li, Y.Y.; Han, Z.; Li, L.D.; Du, Z.W.; et al. Prenatal stress modulates HPA axis homeostasis of offspring through dentate TERT independently of glucocorticoids receptor. Mol. Psychiatry 2023, 28, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.D.S.; Rodrigues, P.D.S.; Santos, D.N.L.; Silva-Sampaio, A.C.; Kirsten, T.B.; Suffredini, I.B.; Coque, A.C.; da Silva, R.A.; Bernardi, M.M. Prenatal restraint stress downregulates the hypothalamic kisspeptidergic system transcripts genes, reduces the estrogen plasma levels, delayed the onset of puberty, and reduced the sexual behavior intensity in female rats. Physiol. Behav. 2023, 260, 114055. [Google Scholar] [CrossRef] [PubMed]
- Barra, R.; Cruz, G.; Mayerhofer, A.; Paredes, A.; Lara, H.E. Maternal sympathetic stress impairs follicular development and puberty of the offspring. Reproduction 2014, 148, 137–145. [Google Scholar] [CrossRef] [PubMed]
- García-Vargas, D.; Juárez-Rojas, L.; Rojas Maya, S.; Retana-Márquez, S. Prenatal stress decreases sperm quality, mature follicles and fertility in rats. Syst. Biol. Reprod. Med. 2019, 65, 223–235. [Google Scholar] [CrossRef]
- Zhou, Q.; Suzuki, A.; Iinuma, M.; Wang, K.Y.; Kubo, K.Y.; Azuma, K. Effects of Maternal Chewing on Prenatal Stress-Induced Cognitive Impairments in the Offspring via Multiple Molecular Pathways. Int. J. Mol. Sci. 2020, 21, 5627. [Google Scholar] [CrossRef]
- Ristić, N.; Nestorović, N.; Manojlović-Stojanoski, M.; Trifunović, S.; Ajdžanović, V.; Filipović, B.; Pendovski, L.; Milošević, V. Adverse effect of dexamethasone on development of the fetal rat ovary. Fundam. Clin. Pharmacol. 2019, 33, 199–207. [Google Scholar] [CrossRef]
- Ristić, N.; Nestorović, N.; Manojlović-Stojanoski, M.; Trifunović, S.; Ajdžanović, V.; Filipović, B.; Pendovski, L.; Milošević, V. Prenatal dexamethasone exposure and developmental programming of the ovary of the offspring: A structural study in the rat. Reprod. Fertil. Dev. 2021, 33, 245–255. [Google Scholar] [CrossRef]
- Poulain, M.; Frydman, N.; Duquenne, C.; N’Tumba-Byn, T.; Benachi, A.; Habert, R.; Rouiller-Fabre, V.; Livera, G. Dexamethasone induces germ cell apoptosis in the human fetal ovary. J. Clin. Endocrinol. Metab. 2012, 97, E1890–E1897. [Google Scholar] [CrossRef]
- Hähle, A.; Merz, S.; Meyners, C.; Hausch, F. The Many Faces of FKBP51. Biomolecules 2019, 9, 35. [Google Scholar] [CrossRef]
- Vermeer, H.; Hendriks-Stegeman, B.I.; van der Burg, B.; van Buul-Offers, S.C.; Jansen, M. Glucocorticoid-induced increase in lymphocytic FKBP51 messenger ribonucleic acid expression: A potential marker for glucocorticoid sensitivity, potency, and bioavailability. J. Clin. Endocrinol. Metab. 2003, 88, 277–284. [Google Scholar] [CrossRef]
- Hubler, T.R.; Denny, W.B.; Valentine, D.L.; Cheung-Flynn, J.; Smith, D.F.; Scammell, J.G. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology 2003, 144, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Schatz, F.; Guzeloglu-Kayisli, O.; Basar, M.; Buchwalder, L.F.; Ocak, N.; Guzel, E.; Guller, S.; Semerci, N.; Kayisli, U.A.; Lockwood, C.J. Enhanced Human Decidual Cell–Expressed FKBP51 May Promote Labor-Related Functional Progesterone Withdrawal. Am. J. Pathol. 2015, 185, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Guzeloglu Kayisli, O.; Kayisli, U.A.; Basar, M.; Semerci, N.; Schatz, F.; Lockwood, C.J. Progestins Upregulate FKBP51 Expression in Human Endometrial Stromal Cells to Induce Functional Progesterone and Glucocorticoid Withdrawal: Implications for Contraceptive- Associated Abnormal Uterine Bleeding. PLoS ONE 2015, 10, e0137855. [Google Scholar] [CrossRef] [PubMed]
- Malekpour, M.; Shekouh, D.; Safavinia, M.E.; Shiralipour, S.; Jalouli, M.; Mortezanejad, S.; Azarpira, N.; Ebrahimi, N.D. Role of FKBP5 and its genetic mutations in stress-induced psychiatric disorders: An opportunity for drug discovery. Front. Psychiatry 2023, 14, 1182345. [Google Scholar] [CrossRef]
- Sabbagh, J.J.; O’Leary, J.C., III; Blair, L.J.; Klengel, T.; Nordhues, B.A.; Fontaine, S.N.; Binder, E.B.; Dickey, C.A. Age-Associated Epigenetic Upregulation of the FKBP5 Gene Selectively Impairs Stress Resiliency. PLoS ONE 2014, 9, e107241. [Google Scholar] [CrossRef]
- Zannas, A.S.; Jia, M.; Hafner, K.; Baumert, J.; Wiechmann, T.; Pape, J.C.; Arloth, J.; Ködel, M.; Martinelli, S.; Roitman, M. Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-κB–driven inflammation and cardiovascular risk. Proc. Natl. Acad. Sci. USA 2019, 116, 11370–11379. [Google Scholar] [CrossRef]
- Criado-Marrero, M.; Gebru, N.T.; Gould, L.A.; Smith, T.M.; Kim, S.; Blackburn, R.J.; Dickey, C.A.; Blair, L.J. Early Life Stress and High FKBP5 Interact to Increase Anxiety-Like Symptoms through Altered AKT Signaling in the Dorsal Hippocampus. Int. J. Mol. Sci. 2019, 20, 2738. [Google Scholar] [CrossRef]
- Wang, L.; Wojcieszak, J.; Kumar, R.; Zhao, Z.; Sun, X.; Xie, S.; Winblad, B.; Pavlov, P.F. FKBP51-Hsp90 Interaction-Deficient Mice Exhibit Altered Endocrine Stress Response and Sex Differences Under High-Fat Diet. Mol. Neurobiol. 2023, 61, 1479–1494. [Google Scholar] [CrossRef]
- Camaioni, A.; Ucci, M.A.; Campagnolo, L.; De Felici, M.; Klinger, F.G.; On behalf of the Italian Society of Embryology, Reproduction and Research. The process of ovarian aging: It is not just about oocytes and granulosa cells. J. Assist. Reprod. Genet. 2022, 39, 783–792. [Google Scholar] [CrossRef]
- Moghadam, A.R.E.; Moghadam, M.T.; Hemadi, M.; Saki, G. Oocyte quality and aging. JBRA Assist. Reprod. 2022, 26, 105–122. [Google Scholar] [CrossRef]
- Arabin, B.; Hellmeyer, L.; Maul, J.; Metz, G.A.S. Awareness of maternal stress, consequences for the offspring and the need for early interventions to increase stress resilience. J. Perinat. Med. 2021, 49, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Faught, E.; Santos, H.B.; Vijayan, M.M. Loss of the glucocorticoid receptor causes accelerated ovarian ageing in zebrafish. Proc. Biol. Sci. 2020, 287, 20202190. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Huang, Y.; Wen, J.; Su, Q.; Huang, Y.; Cai, L.; Lin, W.; Zong, L.; Huang, H.; Qian, X.; et al. Early life exposure to famine and reproductive aging among Chinese women. Menopause 2019, 26, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.A.; Bernal, A.B.; Vickers, M.H.; Gohir, W.; Petrik, J.J.; Sloboda, D.M. Early life exposure to undernutrition induces ER stress, apoptosis, and reduced vascularization in ovaries of adult rat offspring. Biol. Reprod. 2015, 92, 110. [Google Scholar] [CrossRef]
- Sominsky, L.; Meehan, C.L.; Walker, A.K.; Bobrovskaya, L.; McLaughlin, E.A.; Hodgson, D.M. Neonatal immune challenge alters reproductive development in the female rat. Horm. Behav. 2012, 62, 345–355. [Google Scholar] [CrossRef]
- Aiken, C.E.; Tarry-Adkins, J.L.; Spiroski, A.M.; Nuzzo, A.M.; Ashmore, T.J.; Rolfo, A.; Sutherland, M.J.; Camm, E.J.; Giussani, D.A.; Ozanne, S.E. Chronic gestational hypoxia accelerates ovarian aging and lowers ovarian reserve in next-generation adult rats. FASEB J. 2019, 33, 7758–7766. [Google Scholar] [CrossRef]
- Cimadomo, D.; Fabozzi, G.; Vaiarelli, A.; Ubaldi, N.; Ubaldi, F.M.; Rienzi, L. Impact of Maternal Age on Oocyte and Embryo Competence. Front. Endocrinol. 2018, 9, 327. [Google Scholar] [CrossRef]
- Kordowitzki, P. Oxidative Stress Induces Telomere Dysfunction and Shortening in Human Oocytes of Advanced Age Donors. Cells 2021, 10, 1866. [Google Scholar] [CrossRef]
- Nagarajan, S.; Seddighzadeh, B.; Baccarelli, A.; Wise, L.A.; Williams, M.; Shields, A.E. Adverse maternal exposures, methylation of glucocorticoid-related genes and perinatal outcomes: A systematic review. Epigenomics 2016, 8, 925–944. [Google Scholar] [CrossRef]
- Katz-Jaffe, M.G.; Lane, S.L.; Parks, J.C.; McCallie, B.R.; Makloski, R.; Schoolcraft, W.B. Antioxidant Intervention Attenuates Aging-Related Changes in the Murine Ovary and Oocyte. Life 2020, 10, 250. [Google Scholar] [CrossRef]
- Blair, L.J.; Nordhues, B.A.; Hill, S.E.; Scaglione, K.M.; O’Leary, J.C., III; Fontaine, S.N.; Breydo, L.; Zhang, B.; Li, P.; Wang, L.; et al. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J. Clin. Investig. 2013, 123, 4158–4169. [Google Scholar] [CrossRef] [PubMed]
- Sopinka, N.M.; Patterson, L.D.; Redfern, J.C.; Pleizier, N.K.; Belanger, C.B.; Midwood, J.D.; Crossin, G.T.; Cooke, S.J. Manipulating glucocorticoids in wild animals: Basic and applied perspectives. Conserv. Physiol. 2015, 3, cov031. [Google Scholar] [CrossRef] [PubMed]
- Laryea, G.; Schütz, G.; Muglia, L.J. Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Mol. Endocrinol. 2013, 27, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.H.; Yang, B.Q.; Hu, Y.; Fan, Y.Y.; Zhang, L.X.; Zhou, J.C.; Wang, Y.Q.; Lu, C.L.; Ma, X. Dexamethasone altered steroidogenesis and changed redox status of granulosa cells. Endocrine 2014, 47, 639–647. [Google Scholar] [CrossRef]
- Michael, A.E.; Pester, L.A.; Curtis, P.; Shaw, R.W.; Edwards, C.R.; Cooke, B.A. Direct inhibition of ovarian steroidogenesis by cortisol and the modulatory role of 11 beta-hydroxysteroid dehydrogenase. Clin. Endocrinol. 1993, 38, 641–644. [Google Scholar] [CrossRef]
- Huang, T.-J.; Shirley Li, P. Dexamethasone Inhibits Luteinizing Hormone-Induced Synthesis of Steroidogenic Acute Regulatory Protein in Cultured Rat Preovulatory Follicles1. Biol. Reprod. 2001, 64, 163–170. [Google Scholar] [CrossRef]
- Bhaumik, S.; Lockett, J.; Cuffe, J.; Clifton, V.L. Glucocorticoids and Their Receptor Isoforms: Roles in Female Reproduction, Pregnancy, and Foetal Development. Biology 2023, 12, 1104. [Google Scholar] [CrossRef]
- Akison, L.; Robker, R. The Critical Roles of Progesterone Receptor (PGR) in Ovulation, Oocyte Developmental Competence and Oviductal Transport in Mammalian Reproduction. Reprod. Domest. Anim. 2012, 47, 288–296. [Google Scholar] [CrossRef]
- Runnebaum, B.; Zander, J. Progesterone and 20 alpha-dihydroprogesterone in human myometrium during pregnancy. Acta Endocrinol. Suppl. 1971, 150, 3–45. [Google Scholar]
- Jamnongjit, M.; Hammes, S.R. Ovarian steroids: The good, the bad, and the signals that raise them. Cell Cycle 2006, 5, 1178–1183. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The Molecular Biology, Biochemistry, and Physiology of Human Steroidogenesis and Its Disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Allan, C.M.; Jimenez, M.; Lim, P.R.; Davey, R.A.; Zajac, J.D.; Illingworth, P.; Handelsman, D.J. Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology 2007, 148, 3674–3684. [Google Scholar] [CrossRef] [PubMed]
- Stechschulte, L.A.; Sanchez, E.R. FKBP51—A selective modulator of glucocorticoid and androgen sensitivity. Curr. Opin. Pharmacol. 2011, 11, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Burris-Hiday, S.D.; Scott, E.E. Steroidogenic cytochrome P450 17A1 structure and function. Mol. Cell. Endocrinol. 2021, 528, 111261. [Google Scholar] [CrossRef]
- Fitzpatrick, S.L.; Richards, J.S. Regulation of cytochrome P450 aromatase messenger ribonucleic acid and activity by steroids and gonadotropins in rat granulosa cells. Endocrinology 1991, 129, 1452–1462. [Google Scholar] [CrossRef]
- Lintern-Moore, S.; Moore, G.P. The initiation of follicle and oocyte growth in the mouse ovary. Biol. Reprod. 1979, 20, 773–778. [Google Scholar] [CrossRef]
- Ojeda, S.; Urbanski, H.; Knobil, E.; Neill, J. Puberty in the rat. Physiol. Reprod. 1994, 2, 363–409. [Google Scholar]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian aging: Mechanisms and clinical consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Findlay, J.K.; Hutt, K.J.; Hickey, M.; Anderson, R.A. How Is the Number of Primordial Follicles in the Ovarian Reserve Established?1. Biol. Reprod. 2015, 93, 111. [Google Scholar] [CrossRef]
- Traylor, C.S.; Johnson, J.D.; Kimmel, M.C.; Manuck, T.A. Effects of psychological stress on adverse pregnancy outcomes and nonpharmacologic approaches for reduction: An expert review. Am. J. Obstet. Gynecol. MFM 2020, 2, 100229. [Google Scholar] [CrossRef]
- Wu, Y.; De Asis-Cruz, J.; Limperopoulos, C. Brain structural and functional outcomes in the offspring of women experiencing psychological distress during pregnancy. Mol. Psychiatry 2024, 29, 2223–2240. [Google Scholar] [CrossRef] [PubMed]
- Klimek, M.; Entringer, S.; Matras, A.; Blukacz, M.; Nenko, I.; Galbarczyk, A.; Jasienska, G. Early-life adversities and later-life reproductive patterns in women with fully traced reproductive history. Sci. Rep. 2023, 13, 9328. [Google Scholar] [CrossRef] [PubMed]
- Bräuner, E.V.; Koch, T.; Doherty, D.A.; Dickinson, J.E.; Juul, A.; Hart, R.; Hickey, M. The association between in utero exposure to maternal psychological stress and female reproductive function in adolescence: A prospective cohort study. Compr. Psychoneuroendocrinol. 2021, 5, 100026. [Google Scholar] [CrossRef]
- Yano, K.; Matsuzaki, T.; Iwasa, T.; Mayila, Y.; Yanagihara, R.; Tungalagsuvd, A.; Munkhzaya, M.; Tokui, T.; Kamada, S.; Hayashi, A.; et al. The influence of psychological stress in early life on sexual maturation and sexual behavior in male and female rats. Reprod. Med. Biol. 2020, 19, 135–141. [Google Scholar] [CrossRef]
- Atrooz, F.; Alkadhi, K.A.; Salim, S. Understanding stress: Insights from rodent models. Curr. Res. Neurobiol. 2021, 2, 100013. [Google Scholar] [CrossRef] [PubMed]
- Creutzberg, K.C.; Sanson, A.; Viola, T.W.; Marchisella, F.; Begni, V.; Grassi-Oliveira, R.; Riva, M.A. Long-lasting effects of prenatal stress on HPA axis and inflammation: A systematic review and multilevel meta-analysis in rodent studies. Neurosci. Biobehav. Rev. 2021, 127, 270–283. [Google Scholar] [CrossRef]
- Seewoo, B.J.; Hennessy, L.A.; Feindel, K.W.; Etherington, S.J.; Croarkin, P.E.; Rodger, J. Validation of Chronic Restraint Stress Model in Young Adult Rats for the Study of Depression Using Longitudinal Multimodal MR Imaging. eNeuro 2020, 7, 1–22. [Google Scholar] [CrossRef]
- Hoeijmakers, L.; Harbich, D.; Schmid, B.; Lucassen, P.J.; Wagner, K.V.; Schmidt, M.V.; Hartmann, J. Depletion of FKBP51 in female mice shapes HPA axis activity. PLoS ONE 2014, 9, e95796. [Google Scholar] [CrossRef]
- O’Leary III, J.C.; Dharia, S.; Blair, L.J.; Brady, S.; Johnson, A.G.; Peters, M.; Cheung-Flynn, J.; Cox, M.B.; de Erausquin, G.; Weeber, E.J. A new anti-depressive strategy for the elderly: Ablation of FKBP5/FKBP51. PLoS ONE 2011, 6, e24840. [Google Scholar] [CrossRef]
- Maiarù, M.; Tochiki, K.K.; Cox, M.B.; Annan, L.V.; Bell, C.G.; Feng, X.; Hausch, F.; Géranton, S.M. The stress regulator FKBP51 drives chronic pain by modulating spinal glucocorticoid signaling. Sci. Transl. Med. 2016, 8, ra319–ra325. [Google Scholar] [CrossRef]
- Touma, C.; Gassen, N.C.; Herrmann, L.; Cheung-Flynn, J.; Büll, D.R.; Ionescu, I.A.; Heinzmann, J.-M.; Knapman, A.; Siebertz, A.; Depping, A.-M.; et al. FK506 Binding Protein 5 Shapes Stress Responsiveness: Modulation of Neuroendocrine Reactivity and Coping Behavior. Biol. Psychiatry 2011, 70, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Isola, J.V.V.; Ocañas, S.R.; Hubbart, C.R.; Ko, S.; Mondal, S.A.; Hense, J.D.; Carter, H.N.C.; Schneider, A.; Kovats, S.; Alberola-Ila, J.; et al. A single-cell atlas of the aging mouse ovary. Nat. Aging 2024, 4, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Ansere, V.A.; Ali-Mondal, S.; Sathiaseelan, R.; Garcia, D.N.; Isola, J.V.V.; Henseb, J.D.; Saccon, T.D.; Ocañas, S.R.; Tooley, K.B.; Stout, M.B.; et al. Cellular hallmarks of aging emerge in the ovary prior to primordial follicle depletion. Mech. Ageing Dev. 2021, 194, 111425. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, C.; Hanson, H.E.; Martin, L.B. FKBP5 expression is related to HPA flexibility and the capacity to cope with stressors in female and male house sparrows. Horm. Behav. 2021, 135, 105038. [Google Scholar] [CrossRef]
- Criado-Marrero, M.; Rein, T.; Binder, E.B.; Porter, J.T.; Koren, J.; Blair, L.J. Hsp90 and FKBP51: Complex regulators of psychiatric diseases. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160532. [Google Scholar] [CrossRef]
- Galigniana, N.M.; Ballmer, L.T.; Toneatto, J.; Erlejman, A.G.; Lagadari, M.; Galigniana, M.D. Regulation of the glucocorticoid response to stress-related disorders by the Hsp90-binding immunophilin FKBP51. J. Neurochem. 2012, 122, 4–18. [Google Scholar] [CrossRef]
- Gershon, E.; Dekel, N. Newly Identified Regulators of Ovarian Folliculogenesis and Ovulation. Int. J. Mol. Sci. 2020, 21, 4565. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Wu, M.; Wei, J.; Sun, X.; Wang, A.; Hu, G.; Jia, J. Secoisolariciresinol Diglucoside Improves Ovarian Reserve in Aging Mouse by Inhibiting Oxidative Stress. Front. Mol. Biosci. 2021, 8, 806412. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Ahmed, S.M.; El-Gammal, Z.; Shouman, S.; Ahmed, A.; Mansour, R.; El-Badri, N. Oocyte Aging: The Role of Cellular and Environmental Factors and Impact on Female Fertility. Adv. Exp. Med. Biol. 2020, 1247, 109–123. [Google Scholar] [CrossRef]
- Cincotta, S.A.; Richardson, N.; Foecke, M.H.; Laird, D.J. Differential susceptibility of male and female germ cells to glucocorticoid-mediated signaling. eLife 2024, 12, RP90164. [Google Scholar] [CrossRef]
- Findlay, J.K.; Dunning, K.R.; Gilchrist, R.B.; Hutt, K.J.; Russell, D.L.; Walters, K.A. Selection of primordial follicles. In The Ovary, 3rd ed.; Pckley, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 5–8. [Google Scholar]
- Liew, S.H.; Nguyen, Q.N.; Strasser, A.; Findlay, J.K.; Hutt, K.J. The ovarian reserve is depleted during puberty in a hormonally driven process dependent on the pro-apoptotic protein BMF. Cell Death Dis. 2017, 8, e2971. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Canning, J.; Kaneko, T.; Pru, J.K.; Tilly, J.L. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 2004, 428, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Wallace, W.H.; Kelsey, T.W. Human ovarian reserve from conception to the menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef] [PubMed]
- Bristol-Gould, S.K.; Kreeger, P.K.; Selkirk, C.G.; Kilen, S.M.; Mayo, K.E.; Shea, L.D.; Woodruff, T.K. Fate of the initial follicle pool: Empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev. Biol. 2006, 298, 149–154. [Google Scholar] [CrossRef]
- Kerr, J.B.; Myers, M.; Anderson, R.A. The dynamics of the primordial follicle reserve. Reproduction 2013, 146, R205–R215. [Google Scholar] [CrossRef]
- Xu, M.; Sun, J.; Wang, Q.; Zhang, Q.; Wei, C.; Lai, D. Chronic restraint stress induces excessive activation of primordial follicles in mice ovaries. PLoS ONE 2018, 13, e0194894. [Google Scholar] [CrossRef]
- Liew, S.H.; Vaithiyanathan, K.; Cook, M.; Bouillet, P.; Scott, C.L.; Kerr, J.B.; Strasser, A.; Findlay, J.K.; Hutt, K.J. Loss of the proapoptotic BH3-only protein BCL-2 modifying factor prolongs the fertile life span in female mice. Biol. Reprod. 2014, 90, 77. [Google Scholar] [CrossRef]
- Ireland, J.L.; Scheetz, D.; Jimenez-Krassel, F.; Themmen, A.P.; Ward, F.; Lonergan, P.; Smith, G.W.; Perez, G.I.; Evans, A.C.; Ireland, J.J. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol. Reprod. 2008, 79, 1219–1225. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, L.; Cai, H.; Liu, L.; Wang, H.; Zhang, J.; Xia, G.; Wang, J.; Wang, F.; Wang, C. SP1 impacts the primordial to primary follicle transition by regulating cholesterol metabolism in granulosa cells. FASEB J. 2023, 37, e22767. [Google Scholar] [CrossRef]
- Kahsar-Miller, M.D.; Conway-Myers, B.A.; Boots, L.R.; Azziz, R. Steroidogenic acute regulatory protein (StAR) in the ovaries of healthy women and those with polycystic ovary syndrome. Am. J. Obstet. Gynecol. 2001, 185, 1381–1387. [Google Scholar] [CrossRef]
- Song, T.; Chen, J.; Yang, S.; Liu, B.; Zhang, L.; Zhang, Q.; Cheng, J.C.; Fang, L. Resveratrol stimulates StAR expression and progesterone production by GPER-mediated downregulation of Snail expression in human granulosa cells. J. Food Drug Anal. 2023, 31, 315–325. [Google Scholar] [CrossRef]
- Ding, M.; Lu, Y.; Wen, Q.; Xing, C.; Huang, X.; Zhang, Y.; Wang, W.; Zhang, C.; Zhang, M.; Meng, F.; et al. Ovarian PERK/NRF2/CX43/StAR/progesterone pathway activation mediates female reproductive dysfunction induced by cold exposure. Sci. Rep. 2024, 14, 10248. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Waddell, B.J. Increased Fetal Glucocorticoid Exposure Delays Puberty Onset in Postnatal Life. Endocrinology 2000, 141, 2422–2428. [Google Scholar] [CrossRef] [PubMed]
- Paus, T.; Keshavan, M.; Giedd, J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008, 9, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Koebele, S.V.; Bimonte-Nelson, H.A. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas 2016, 87, 5–17. [Google Scholar] [CrossRef]
- Nold, V.; Richter, N.; Hengerer, B.; Kolassa, I.T.; Allers, K.A. FKBP5 polymorphisms induce differential glucocorticoid responsiveness in primary CNS cells—First insights from novel humanized mice. Eur. J. Neurosci. 2021, 53, 402–415. [Google Scholar] [CrossRef]
- Blair, L.J.; Criado-Marrero, M.; Zheng, D.; Wang, X.; Kamath, S.; Nordhues, B.A.; Weeber, E.J.; Dickey, C.A. The Disease-Associated Chaperone FKBP51 Impairs Cognitive Function by Accelerating AMPA Receptor Recycling. eNeuro 2019, 6, 1–17. [Google Scholar] [CrossRef]
- Caligioni, C.S. Assessing Reproductive Status/Stages in Mice. Curr. Protoc. Neurosci. 2009, 48, A.4I.1–A.4I.8. [Google Scholar] [CrossRef]
- Lee, E.B.; Chakravarthi, V.P.; Wolfe, M.W.; Rumi, M.A.K. ERβ Regulation of Gonadotropin Responses during Folliculogenesis. Int. J. Mol. Sci. 2021, 22, 10348. [Google Scholar] [CrossRef]
- Uslu, B.; Dioguardi, C.C.; Haynes, M.; Miao, D.Q.; Kurus, M.; Hoffman, G.; Johnson, J. Quantifying growing versus non-growing ovarian follicles in the mouse. J. Ovarian Res. 2017, 10, 3. [Google Scholar] [CrossRef]
- Guzeloglu-Kayisli, O.; Lalioti, M.D.; Aydiner, F.; Sasson, I.; Ilbay, O.; Sakkas, D.; Lowther, K.M.; Mehlmann, L.M.; Seli, E. Embryonic poly(A)-binding protein (EPAB) is required for oocyte maturation and female fertility in mice. Biochem. J. 2012, 446, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Kuyatt, B.L.; Reidy, C.A.; Hui, K.Y.; Jordan, W.H. Quantitation of smooth muscle proliferation in cultured aorta. A color image analysis method for the Macintosh. Anal. Quant. Cytol. Histol. 1993, 15, 83–87. [Google Scholar] [PubMed]
- Lehr, H.-A.; van der Loss, C.M.; Teeling, P.; Gown, A.M. Complete Chromogen Separation and Analysis in Double Immunohistochemical Stains Using Photoshop-based Image Analysis. J. Histochem. Cytochem. 1999, 47, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, N.; Gantner, C.W.; Hunt, C.P.J.; Ermine, C.M.; Frausin, S.; Viventi, S.; Ovchinnikov, D.A.; Kirik, D.; Parish, C.L.; Thompson, L.H. A combined cell and gene therapy approach for homotopic reconstruction of midbrain dopamine pathways using human pluripotent stem cells. Cell Stem Cell 2022, 29, 434–448.e435. [Google Scholar] [CrossRef]



| Experimental Group | n | Day of VO Mean ± SEM |
|---|---|---|
| Fkbp5+/+ Control | 15 | 29.20 ± 0.56 b |
| Fkbp5+/+ PNS | 8 | 32.63 ± 0.96 a |
| Fkbp5−/− Control | 12 | 31.08 ± 0.80 |
| Fkbp5−/− PNS | 12 | 33.33 ± 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, M.; Cetinkaya-Un, B.; Sarkar, P.; Kayisli, U.A.; Semerci-Gunay, N.; Teng, M.; Lockwood, C.J.; Guzeloglu-Kayisli, O. Depletion of Fkbp5 Protects Against the Rapid Decline in Ovarian Reserve Induced by Prenatal Stress in Female Offspring of Wild-Type Mice. Int. J. Mol. Sci. 2025, 26, 2471. https://doi.org/10.3390/ijms26062471
Moore M, Cetinkaya-Un B, Sarkar P, Kayisli UA, Semerci-Gunay N, Teng M, Lockwood CJ, Guzeloglu-Kayisli O. Depletion of Fkbp5 Protects Against the Rapid Decline in Ovarian Reserve Induced by Prenatal Stress in Female Offspring of Wild-Type Mice. International Journal of Molecular Sciences. 2025; 26(6):2471. https://doi.org/10.3390/ijms26062471
Chicago/Turabian StyleMoore, Monica, Busra Cetinkaya-Un, Papri Sarkar, Umit A. Kayisli, Nihan Semerci-Gunay, Michael Teng, Charles J. Lockwood, and Ozlem Guzeloglu-Kayisli. 2025. "Depletion of Fkbp5 Protects Against the Rapid Decline in Ovarian Reserve Induced by Prenatal Stress in Female Offspring of Wild-Type Mice" International Journal of Molecular Sciences 26, no. 6: 2471. https://doi.org/10.3390/ijms26062471
APA StyleMoore, M., Cetinkaya-Un, B., Sarkar, P., Kayisli, U. A., Semerci-Gunay, N., Teng, M., Lockwood, C. J., & Guzeloglu-Kayisli, O. (2025). Depletion of Fkbp5 Protects Against the Rapid Decline in Ovarian Reserve Induced by Prenatal Stress in Female Offspring of Wild-Type Mice. International Journal of Molecular Sciences, 26(6), 2471. https://doi.org/10.3390/ijms26062471








