Catestatin in Cardiovascular Diseases
Abstract
1. Introduction
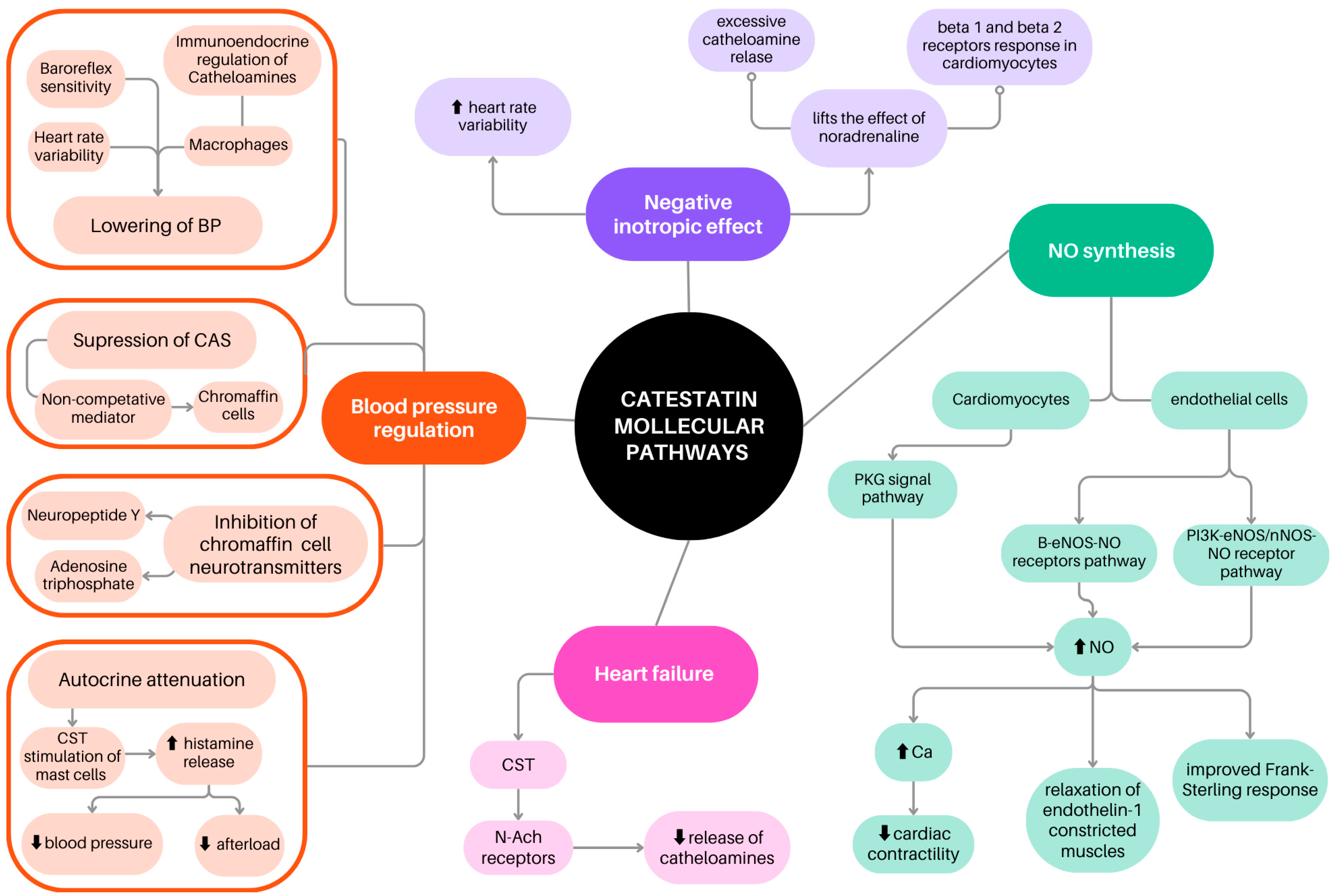
2. Catestatin in the Regulation of Blood Pressure
3. Inotropic Effect of Catestatin and Association Between Catestatin and Heart Rate Variability
4. Catestatin İnfluence on NO Synthesis and Metabolism
5. Catestatin in Coronary Artery Disease and Atherosclerosis Development
5.1. Catestatin and Angiogenesis After Myocardial İnfarction
5.2. Catestatin Antiarrhythmic Potential
5.3. Catestatin as a Potential Coronary Artery Disease Course Marker
6. Catestatin in Heart Failure
7. Catestatin in Other Diseases
7.1. Catestatin in Pre-Eclampsia
7.2. Catestatin in Acute Pulmonary Embolism
7.3. Catestatin in Chronic Kidney Disease
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 95% CI | 95% confidence interval |
| ACS | Acute coronary syndrome |
| ANS | Autonomic nervous system |
| CAD | Coronary artery disease |
| CgA | Chromogranin A |
| CKD | Chronic kidney disease |
| CTS | Catestatin |
| CTO | Chronic total occlusion of coronary artery |
| CVD | Cardiovascular disease |
| DM | Diabetes mellitus |
| HA | Arterial hypertension |
| HF | Heart failure |
| HR | Hazard ratio |
| HRV | Heart rate variability |
| LDL | Low-density lipoproteins |
| LVP | Left ventricular pressure |
| MACE | Major adverse cardiovascular events |
| MI | Myocardial infarction |
| SCD | Sudden cardiac death |
| STEMI | Myocardial infarction with ST-segment elevation |
| UAP | Unstable angina pectoris |
| VEGF | vascular endothelial growth factor |
| VF | Ventricular fibrillation |
| VT | Ventricular tachycardia |
References
- Townsend, N.; Kazakiewicz, D.; Lucy Wright, F.; Timmis, A.; Huculeci, R.; Torbica, A.; Gale, C.P.; Achenbach, S.; Weidinger, F.; Vardas, P. Epidemiology of cardiovascular disease in Europe. Nat. Rev. Cardiol. 2022, 19, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. The Emerging Roles of Chromogranins and Derived Polypeptides in Atherosclerosis, Diabetes, and Coronary Heart Disease. Int. J. Mol. Sci. 2021, 22, 6118. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, S.; Chirumbolo, S.; Franzini, M.; Tirelli, U.; Valdenassi, L. Oxygen-ozone therapy for myocardial ischemic stroke and cardiovascular disorders. Med. Gas Res. 2025, 15, 36–43. [Google Scholar] [CrossRef]
- Gorący, J.; Gorący, A.; Wójcik-Grzeszczuk, A.; Gorący, I.; Rosik, J. Analysis of Genetic Variants in the Glucocorticoid Receptor Gene. Biomedicines 2022, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.C.W.; Zheng, B.B.; Tang, M.L.; Chu, H.; Zhao, Y.T.; Weng, C. Global Burden of Cardiovascular Diseases and its Risk Factors, 1990-2021: A Systematic Analysis for the Global Burden of Disease Study 2021. QJM 2025, hcaf022. [Google Scholar] [CrossRef]
- Garg, R.; Agarwal, A.; Katekar, R.; Dadge, S.; Yadav, S.; Gayen, J.R. Chromogranin A-derived peptides pancreastatin and catestatin: Emerging therapeutic target for diabetes. Amino Acids 2023, 55, 549–561. [Google Scholar] [CrossRef]
- Zalewska, E.; Kmieć, P.; Sworczak, K. Role of Catestatin in the Cardiovascular System and Metabolic Disorders. Front. Cardiovasc. Med. 2022, 9, 909480. [Google Scholar] [CrossRef]
- Bozic, J.; Kumric, M.; Ticinovic Kurir, T.; Urlic, H.; Martinovic, D.; Vilovic, M.; Tomasovic Mrcela, N.; Borovac, J.A. Catestatin as a Biomarker of Cardiovascular Diseases: A Clinical Perspective. Biomedicines 2021, 9, 1757. [Google Scholar] [CrossRef]
- Meng, Q.H.; Halfdanarson, T.R.; Bornhorst, J.A.; Jann, H.; Shaheen, S.; Shi, R.Z.; Schwabe, A.; Stade, K.; Halperin, D.M. Circulating Chromogranin A as a Surveillance Biomarker in Patients with Carcinoids-The CASPAR Study. Clin. Cancer Res. 2024, 30, 5559–5567. [Google Scholar] [CrossRef]
- Vanli Tonyali, N.; Karabay, G.; Arslan, B.; Aktemur, G.; Tokgoz Cakir, B.; Seyhanli, Z.; Demir Çendek, B.; Yilmaz Ergani, S.; Eroglu, H.; Mermi, S.; et al. Maternal Serum Catestatin Levels in Gestational Diabetes Mellitus: A Potential Biomarker for Risk Assessment and Diagnosis. J. Clin. Med. 2025, 14, 435. [Google Scholar] [CrossRef]
- Wołowiec, Ł.; Banach, J.; Budzyński, J.; Wołowiec, A.; Kozakiewicz, M.; Bieliński, M.; Jaśniak, A.; Olejarczyk, A.; Grześk, G. Prognostic Value of Plasma Catestatin Concentration in Patients with Heart Failure with Reduced Ejection Fraction in Two-Year Follow-Up. J. Clin. Med. 2023, 12, 4208. [Google Scholar] [CrossRef]
- Loh, Y.P.; Cheng, Y.; Mahata, S.K.; Corti, A.; Tota, B. Chromogranin A and derived peptides in health and disease. J. Mol. Neurosci. 2012, 48, 347–356. [Google Scholar] [CrossRef]
- Muntjewerff, E.M.; Christoffersson, G.; Mahata, S.K.; van den Bogaart, G. Putative regulation of macrophage-mediated inflammation by catestatin. Trends Immunol. 2022, 43, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, Y.; Mularczyk, M.; Marycz, K.; Bourebaba, L. Catestatin peptide of chromogranin A as a potential new target for several risk factors management in the course of metabolic syndrome. Biomed. Pharmacother. 2021, 134, 111113. [Google Scholar] [CrossRef]
- Al Ghorani, H.; Kulenthiran, S.; Lauder, L.; Böhm, M.; Mahfoud, F. Hypertension trials update. J. Hum. Hypertens. 2021, 35, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, N.R. Catestatin is a novel endogenous peptide that regulates cardiac function and blood pressure. Cardiovasc. Res. 2008, 80, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Angelone, T.; Quintieri, A.M.; Brar, B.K.; Limchaiyawat, P.T.; Tota, B.; Mahata, S.K.; Cerra, M.C. The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology 2008, 149, 4780–4793. [Google Scholar] [CrossRef]
- Ying, W.; Tang, K.; Avolio, E.; Schilling, J.M.; Pasqua, T.; Liu, M.A.; Cheng, H.; Gao, H.; Zhang, J.; Mahata, S.; et al. Immunosuppression of Macrophages Underlies the Cardioprotective Effects of CST (Catestatin). Hypertension 2021, 77, 1670–1682. [Google Scholar] [CrossRef]
- Kübler, W.; Haass, M. Cardioprotection: Definition, classification, and fundamental principles. Heart 1996, 75, 330–333. [Google Scholar] [CrossRef]
- Jianqiang, G.; Li, S.; Guo, J. GW27-e0952 Effect Of Catecholamine Release-Inhibitory Peptide Catestatin on Sympathetic Activity Of Hypertension. J. Am. Coll. Cardiol. 2016, 68, C33–C34. [Google Scholar] [CrossRef]
- Krüger, P.-G.; Mahata, S.K.; Helle, K.B. Catestatin (CgA344–364) stimulates rat mast cell release of histamine in a manner comparable to mastoparan and other cationic charged neuropeptides. Regul. Pept. 2003, 114, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.J.; Gupta, R.; Mahapatra, N.R.; Goswami, S.K. Catestatin reverses the hypertrophic effects of norepinephrine in H9c2 cardiac myoblasts by modulating the adrenergic signaling. Mol. Cell Biochem. 2020, 464, 205–219. [Google Scholar] [CrossRef]
- Dev, N.B.; Gayen, J.R.; O’Connor, D.T.; Mahata, S.K. Chromogranin a and the autonomic system: Decomposition of heart rate variability and rescue by its catestatin fragment. Endocrinology 2010, 151, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Fan, Y.; Wang, Z.; Huang, F.; Li, Z.; Sun, Z.; Hua, S.; Jin, W.; Chen, Y. Catestatin Protects Against Diastolic Dysfunction by Attenuating Mitochondrial Reactive Oxygen Species Generation. J. Am. Heart Assoc. 2023, 12, e029470. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Ma, Q.; Jia, H.; Ma, H.; Du, Z.; Liu, Y.; Zhang, X.; Guan, Y. Electrophysiological Mechanism of Catestatin Antiarrhythmia: Enhancement of. J. Am. Heart Assoc. 2024, 13, e035415. [Google Scholar] [CrossRef]
- Lener, D.; Noflatscher, M.; Kirchmair, E.; Bauer, A.; Holfeld, J.; Gollmann-Tepeköylü, C.; Kirchmair, R.; Theurl, M. The angiogenic neuropeptide catestatin exerts beneficial effects on human coronary vascular cells and cardiomyocytes. Peptides 2023, 168, 171077. [Google Scholar] [CrossRef] [PubMed]
- Bralewska, M.; Pietrucha, T.; Sakowicz, A. Reduction in CgA-Derived CST Protein Level in HTR-8/SVneo and BeWo Trophoblastic Cell Lines Caused by the Preeclamptic Environment. Int. J. Mol. Sci. 2023, 24, 7124. [Google Scholar] [CrossRef]
- Muntjewerff, E.M.; Parv, K.; Mahata, S.K.; van Riessen, N.K.; Phillipson, M.; Christoffersson, G.; van den Bogaart, G. The anti-inflammatory peptide Catestatin blocks chemotaxis. J. Leukoc. Biol. 2022, 112, 273–278. [Google Scholar] [CrossRef]
- Chu, S.Y.; Peng, F.; Wang, J.; Liu, L.; Meng, L.; Zhao, J.; Han, X.N.; Ding, W.H. Catestatin in defense of oxidative-stress-induced apoptosis: A novel mechanism by activating the beta2 adrenergic receptor and PKB/Akt pathway in ischemic-reperfused myocardium. Peptides 2020, 123, 170200. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Yang, C.; Su, X.; Yang, W.; Dai, Y.; Han, H.; Jiang, J.; Lu, L.; Wang, H.; et al. Decreased circulating catestatin levels are associated with coronary artery disease: The emerging anti-inflammatory role. Atherosclerosis 2019, 281, 78–88. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Ge, L.; Wang, T.; Ma, Z.; Han, Y.; Duan, Y.; Xu, X.; Liu, W.; Yuan, J.; et al. Catestatin prevents endothelial inflammation and promotes thrombus resolution in acute pulmonary embolism in mice. Biosci. Rep. 2019, 39, BSR20192236. [Google Scholar] [CrossRef] [PubMed]
- Gaede, A.H.; Pilowsky, P.M. Catestatin in rat RVLM is sympathoexcitatory, increases barosensitivity, and attenuates chemosensitivity and the somatosympathetic reflex. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1538–R1545. [Google Scholar] [CrossRef]
- Gaede, A.H.; Pilowsky, P.M. Catestatin, a chromogranin A-derived peptide, is sympathoinhibitory and attenuates sympathetic barosensitivity and the chemoreflex in rat CVLM. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R365–R372. [Google Scholar] [CrossRef]
- Avolio, E.; Mahata, S.K.; Mantuano, E.; Mele, M.; Alò, R.; Facciolo, R.M.; Talani, G.; Canonaco, M. Antihypertensive and neuroprotective effects of catestatin in spontaneously hypertensive rats: Interaction with GABAergic transmission in amygdala and brainstem. Neuroscience 2014, 270, 48–57. [Google Scholar] [CrossRef]
- O’Connor, D.T.; Kailasam, M.T.; Kennedy, B.P.; Ziegler, M.G.; Yanaihara, N.; Parmer, R.J. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J. Hypertens. 2002, 20, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Durakoğlugil, M.E.; Ayaz, T.; Kocaman, S.A.; Kırbaş, A.; Durakoğlugil, T.; Erdoğan, T.; Çetin, M.; Şahin, O.Z.; Çiçek, Y. The relationship of plasma catestatin concentrations with metabolic and vascular parameters in untreated hypertensive patients: Influence on high-density lipoprotein cholesterol. Anatol. J. Cardiol. 2015, 15, 577–585. [Google Scholar] [CrossRef]
- Meng, L.; Ye, X.J.; Ding, W.H.; Yang, Y.; Di, B.B.; Liu, L.; Huo, Y. Plasma catecholamine release-inhibitory peptide catestatin in patients with essential hypertension. J. Cardiovasc. Med. 2011, 12, 643–647. [Google Scholar] [CrossRef]
- O’Connor, D.T.; Zhu, G.; Rao, F.; Taupenot, L.; Fung, M.M.; Das, M.; Mahata, S.K.; Mahata, M.; Wang, L.; Zhang, K.; et al. Heritability and genome-wide linkage in US and australian twins identify novel genomic regions controlling chromogranin a: Implications for secretion and blood pressure. Circulation 2008, 118, 247–257. [Google Scholar] [CrossRef]
- Choi, Y.; Miura, M.; Nakata, Y.; Sugasawa, T.; Nissato, S.; Otsuki, T.; Sugawara, J.; Iemitsu, M.; Kawakami, Y.; Shimano, H.; et al. A common genetic variant of the chromogranin A-derived peptide catestatin is associated with atherogenesis and hypertension in a Japanese population. Endocr. J. 2015, 62, 797–804. [Google Scholar] [CrossRef]
- Rao, F.; Wen, G.; Gayen, J.R.; Das, M.; Vaingankar, S.M.; Rana, B.K.; Mahata, M.; Kennedy, B.P.; Salem, R.M.; Stridsberg, M.; et al. Catecholamine release-inhibitory peptide catestatin (chromogranin A(352-372)): Naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation 2007, 115, 2271–2281. [Google Scholar] [CrossRef]
- Xu, W.X.; Fan, Y.Y.; Song, Y.; Liu, X.; Liu, H.; Guo, L.J. Prognostic differences of catestatin among young and elderly patients with acute myocardial infarction. World J. Emerg. Med. 2022, 13, 169–174. [Google Scholar] [CrossRef]
- Xu, W.; Yu, H.; Wu, H.; Li, S.; Chen, B.; Gao, W. Plasma Catestatin in Patients with Acute Coronary Syndrome. Cardiology 2017, 136, 164–169. [Google Scholar] [CrossRef]
- Liu, L.; Ding, W.; Zhao, F.; Shi, L.; Pang, Y.; Tang, C. Plasma levels and potential roles of catestatin in patients with coronary heart disease. Scand. Cardiovasc. J. 2013, 47, 217–224. [Google Scholar] [CrossRef]
- Chu, S.Y.; Peng, F.; Wang, J.; Liu, L.; Zhao, J.; Han, X.N.; Ding, W.H. Catestatin as a predictor for cardiac death in heart failure with mildly reduced and preserved ejection fraction. ESC Heart Fail. 2025, 12, 517–524. [Google Scholar] [CrossRef]
- Borovac, J.A.; Glavas, D.; Susilovic Grabovac, Z.; Supe Domic, D.; Stanisic, L.; D’Amario, D.; Kwok, C.S.; Bozic, J. Circulating sST2 and catestatin levels in patients with acute worsening of heart failure: A report from the CATSTAT-HF study. ESC Heart Fail. 2020, 7, 2818–2828. [Google Scholar] [CrossRef]
- Zhu, D.; Xie, H.; Wang, X.; Liang, Y.; Yu, H.; Gao, W. Catestatin-A Novel Predictor of Left Ventricular Remodeling After Acute Myocardial Infarction. Sci. Rep. 2017, 7, 44168. [Google Scholar] [CrossRef]
- Palmrich, P.; Schirwani-Hartl, N.; Haberl, C.; Haslinger, P.; Heinzl, F.; Zeisler, H.; Binder, J. Catestatin-A Potential New Therapeutic Target for Women with Preeclampsia? An Analysis of Maternal Serum Catestatin Levels in Preeclamptic Pregnancies. J. Clin. Med. 2023, 12, 5931. [Google Scholar] [CrossRef]
- Tüten, N.; Güralp, O.; Gök, K.; Hamzaoglu, K.; Oner, Y.O.; Makul, M.; Bulut, H.; Irmak, K.; Tüten, A.; Malik, E. Serum catestatin level is increased in women with preeclampsia. J. Obstet. Gynaecol. 2022, 42, 55–60. [Google Scholar] [CrossRef]
- Bralewska, M.; Biesiada, L.; Grzesiak, M.; Rybak-Krzyszkowska, M.; Huras, H.; Gach, A.; Pietrucha, T.; Sakowicz, A. Chromogranin A demonstrates higher expression in preeclamptic placentas than in normal pregnancy. BMC Pregnancy Childbirth 2021, 21, 680. [Google Scholar] [CrossRef]
- Özalp, M.; Yaman, H.; Demir, Ö.; Aytekin Garip, S.; Aran, T.; Osmanağaoğlu, M.A. The role of maternal serum catestatin in the evaluation of preeclampsia and fetal cardiac functions. Turk. J. Obstet. Gynecol. 2021, 18, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Luketin, M.; Mizdrak, M.; Boric-Skaro, D.; Martinovic, D.; Tokic, D.; Vilovic, M.; Supe-Domic, D.; Ticinovic Kurir, T.; Bozic, J. Plasma Catestatin Levels and Advanced Glycation End Products in Patients on Hemodialysis. Biomolecules 2021, 11, 456. [Google Scholar] [CrossRef]
- Izci, S.; Acar, E.; Inanir, M. Plasma catestatin level predicts sPESI score and mortality in acute pulmonary embolism. Arch. Med. Sci. Atheroscler. Dis. 2020, 5, e49–e56. [Google Scholar] [CrossRef] [PubMed]
- Pàmies, A.; Llop, D.; Ibarretxe, D.; Rosales, R.; Girona, J.; Masana, L.; Vallvé, J.C.; Paredes, S. Enhanced Association of Novel Cardiovascular Biomarkers Fetuin-A and Catestatin with Serological and Inflammatory Markers in Rheumatoid Arthritis Patients. Int. J. Mol. Sci. 2024, 25, 9910. [Google Scholar] [CrossRef]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart Rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2021, 17, e160721189770. [Google Scholar] [CrossRef]
- Borovac, J.A.; Glavas, D.; Susilovic Grabovac, Z.; Supe Domic, D.; D’Amario, D.; Bozic, J. Catestatin in Acutely Decompensated Heart Failure Patients: Insights from the CATSTAT-HF Study. J. Clin. Med. 2019, 8, 1132. [Google Scholar] [CrossRef]
- Dev, N.B.; Mir, S.A.; Gayen, J.R.; Siddiqui, J.A.; Mustapic, M.; Vaingankar, S.M. Cardiac Electrical Activity in a Genomically “Humanized” Chromogranin A Monogenic Mouse Model with Hyperadrenergic Hypertension. J. Cardiovasc. Transl. Res. 2014, 7, 483–493. [Google Scholar] [CrossRef]
- Kiranmayi, M.; Chirasani, V.R.; Allu, P.K.; Subramanian, L.; Martelli, E.E.; Sahu, B.S.; Vishnuprabu, D.; Kumaragurubaran, R.; Sharma, S.; Bodhini, D.; et al. Catestatin Gly364Ser Variant Alters Systemic Blood Pressure and the Risk for Hypertension in Human Populations via Endothelial Nitric Oxide Pathway. Hypertension 2016, 68, 334–347. [Google Scholar] [CrossRef]
- Sahu, B.S.; Obbineni, J.M.; Sahu, G.; Allu, P.K.; Subramanian, L.; Sonawane, P.J.; Singh, P.K.; Sasi, B.K.; Senapati, S.; Maji, S.K.; et al. Functional genetic variants of the catecholamine-release-inhibitory peptide catestatin in an Indian population: Allele-specific effects on metabolic traits. J. Biol. Chem. 2012, 287, 43840–43852. [Google Scholar] [CrossRef]
- Bassino, E.; Fornero, S.; Gallo, M.P.; Ramella, R.; Mahata, S.K.; Tota, B.; Levi, R.; Alloatti, G. A novel catestatin-induced antiadrenergic mechanism triggered by the endothelial PI3K-eNOS pathway in the myocardium. Cardiovasc. Res. 2011, 91, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Ralapanawa, U.; Sivakanesan, R. Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J. Epidemiol. Glob. Health 2021, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Alique, M.; Luna, C.; Carracedo, J.; Ramírez, R. LDL biochemical modifications: A link between atherosclerosis and aging. Food Nutr. Res. 2015, 59, 29240. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, C.; Cosín-Sales, J. LDL cholesterol and atherosclerosis: The evidence. Clin. Investig. Arterioscler. 2021, 33 (Suppl. S1), 25–32. [Google Scholar] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Muntjewerff, E.M.; Dunkel, G.; Nicolasen, M.J.T.; Mahata, S.K.; van den Bogaart, G. Catestatin as a Target for Treatment of Inflammatory Diseases. Front. Immunol. 2018, 9, 2199. [Google Scholar] [CrossRef]
- Xu, W.; Yu, H.; Li, W.; Gao, W.; Guo, L.; Wang, G. Plasma Catestatin: A Useful Biomarker for Coronary Collateral Development with Chronic Myocardial Ischemia. PLoS ONE 2016, 11, e0149062. [Google Scholar] [CrossRef][Green Version]
- Bhar-Amato, J.; Davies, W.; Agarwal, S. Ventricular Arrhythmia after Acute Myocardial Infarction: ’The Perfect Storm’. Arrhythm. Electrophysiol. Rev. 2017, 6, 134–139. [Google Scholar] [CrossRef]
- Pei, Z.; Ma, D.; Ji, L.; Zhang, J.; Su, J.; Xue, W.; Chen, X.; Wang, W. Usefulness of catestatin to predict malignant arrhythmia in patients with acute myocardial infarction. Peptides 2014, 55, 131–135. [Google Scholar]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Barone Gibbs, B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data from the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar]
- Mahata, S.K.; Kiranmayi, M.; Mahapatra, N.R. Catestatin: A Master Regulator of Cardiovascular Functions. Curr. Med. Chem. 2018, 25, 1352–1374. [Google Scholar] [CrossRef]
- Polyakova, E.A.; Mikhaylov, E.N.; Sonin, D.L.; Cheburkin, Y.V.; Galagudza, M.M. Neurohumoral, cardiac and inflammatory markers in the evaluation of heart failure severity and progression. J. Geriatr. Cardiol. 2021, 18, 47–66. [Google Scholar] [PubMed]
- Penna, C.; Alloatti, G.; Gallo, M.P.; Cerra, M.C.; Levi, R.; Tullio, F.; Bassino, E.; Dolgetta, S.; Mahata, S.K.; Tota, B.; et al. Catestatin improves post-ischemic left ventricular function and decreases ischemia/reperfusion injury in heart. Cell Mol. Neurobiol. 2010, 30, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Liu, T.; Zhong, P.; Xiong, F.; Cui, B.; Wu, J.; Wu, G. Chronic catestatin treatment reduces atrial fibrillation susceptibility via improving calcium handling in post-infarction heart failure rats. Peptides 2023, 159, 170904. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Bralewska, M.; Pietrucha, T.; Sakowicz, A. The Role of Catestatin in Preeclampsia. Int. J. Mol. Sci. 2024, 25, 2461. [Google Scholar] [CrossRef]
- Hassine, M.; Kallala, M.Y.; Mahjoub, M.; Boussaada, M.; Bouchahda, N.; Gamra, H. Pulmonary embolism: The Pulmonary Embolism Severity Index (PESI) score and mortality predictors. Pan Afr. Med. J. 2023, 45, 48. [Google Scholar]
- Shah, I.K.; Merfeld, J.M.; Chun, J.; Tak, T. Pathophysiology and Management of Pulmonary Embolism. Int. J. Angiol. 2022, 31, 143–149. [Google Scholar] [CrossRef]
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 2018, 33 (Suppl. S3), iii28–iii34. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Adamczak, M.; de Oliveira, R.B.; Massy, Z.A.; Sarafidis, P.; Agarwal, R.; Mark, P.B.; Kotanko, P.; Ferro, C.J.; et al. Cardiovascular complications in chronic kidney disease: A review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc. Res. 2023, 119, 2017–2032. [Google Scholar] [CrossRef]
| Study and Its Reference | Methodology | Results Summary |
|---|---|---|
| Zhang et al. 2024 [25] | Fourty-two male adult Sprague–Dawley rats randomly divided into CTS and NON-CTS equinumerous groups. Ventricular arrhythmias were induced by ligation of the LAD and electrical stimulation. | CTS notably reduced induced ventricular arrhythmia caused by ischaemia and electric stimulation in rats. In the CTS group: ↑Ito, ↑IK, ↑IK1, and ↓ICa-L activity. |
| Lener et al. 2023 [26] | Matrigel assays; human coronary artery endothelial cells (HCAECs) and human coronary artery smooth muscle cells (HCASMCs) | CTS induces chemotaxis of HCAECs (relative CI CTS 1 nM 1.79 ± 0.1, n = 3, p < 0.01 vs. control) similar to VEGF (relative CI VEGF 50 ng/mL 2.13 ± 0.09, n = 3, p < 0.01 vs. control). CTS stimulates HCAEC proliferation (relative proliferation CTS 1 nM 1.62 ± 0.05, n = 5, p < 0.01). CTS had similar effect on capillary tube formation as VEGF (relative tube formation CTS 1 nM 2.42 ± 0.1, n = 3, p < 0.001 vs. control; relative tube formation VEGF 50 ng/mL 2.06 ± 0.1, n = 3, p < 0.001 vs. control). CTS activated ERK 1/2 signaling pathway in HCAEC. CTS stimulates HCASMC proliferation (relative proliferation CTS 10 nM 1.6 ± 0.09, n = 3, p < 0.001 vs. control, relative proliferation CTS 1 nM 1.27± 0.04, n = 3, p = n.s. control) CTS stimulated ERK 1/2 signaling and activation of the PI3-kinase-Akt pathway in HCASMC. CTS reduced H2O2 induced apoptosis (relative apoptosis CTS 1 nM 0.76 ± 0.05, p < 0.05 vs. control) |
| Qiu et al. 2023 [24] | SG: C57BL/six male mice with TAC/DOCA induced HFpEF G1: 28 days of CTS treatment, n = 8 G2: 28 days of placebo, n = 8 C57BL/six male mice after thoracotomy without TAC/DOCA G3: 28 days of CTS treatment, n = 8 G4: 28 days of placebo, n = 8 ECHO evaluation, conductance catheter pressure-volume analysis, microscopy and genetic analysis of cardiomyocytes 7 weeks after surgery. | CTS Protects Diastolic Dysfunction in mice with HFpEF. In the TAC/DOCA (G2) group, ↑E/A velocity ratio; ↓E wave deceleration time; ↑E/e′ suggesting diastolic dysfunction. ↑LV end-systolic pressure, ↑LV chamber stiffness, ↑LV concentric hypertrophy, ↑Volume of cardiomyocytes. Those effects were reduced in TAC/DOCA + CTS (G1) group. Mitochondrial ROS generation reduction in TAC/DOCA + CTS (G1) compared to G2). Restoration of mitochondrial respiratory chain by CTS treatment. |
| Bralewska et al. 2023 [27] | HTR-8/SVneo (CRL-3271) and BeWO (CCL-98) trophoblast cell lines incubated in pre-eclamptic environment (hypoxia, pro-inflammatory, oxidative stress) | Throphoblast cells produce CgA and CTS. Pre-eclamptic environment promotes ↓CHGA gene expression (p < 0.001); ↓CTS level in trophoblast; ↑apoptosis. There is negative correlation between CTS level and apoptotic index for both HTR-8/SVneo (R = 0.4) and BeWo cells (R = 0.5). CTS acts as antiapoptotic factor in vitro. |
| Muntjewerff et al. 2022 [28] | Transwell migration assays on human blood monocytes and neutrophiles; aortic ring model from Cx3cr1+/gfp transgenic mice. | CTS itself has a weak chemotactic effect on monocytes and neutrophiles, however it counteracts the chemoattraction of leukocytes by inflammatory chemokines CCL2, CXCL2, and IL-8 CTS promotes angiogenesis. |
| Ying et al. 2021 [18] | TG: CTS-KO C57BL/6 male mice, n = 8 CG: CTS-WT C57BL/6 male mice, n = 8 | In CTS-KO mice: ↑SBP, ↑DSB, ↑MAP. Pro-inflammatory: serum cytokines ↑TNF-α, ↑IFN-γ, ↑CCL2, ↑CCL3, ↑CXCL, genes upregulation: Tnfa, Ifng, Emr1, Itgam, Itgax, Nos2a, IL12b CcL2, and CxcL1. Anti-inflammatory: serum ↓IL-10, genes downregulation: IL10, IL4, Mrc1, Arg1, Clec7a and Clec10a. Adrenal and plasma ↑catecholamines. ↑Sympathetic nerve activity. Those effects were reversed by exogenous CTS administration. IPC-induced cardioprotection impairment. ↑phosphorylation (Ser177/181) of IKK-β (inflammatory NF-κB signaling pathway). Macrophages production: ↓TNF-α, ↓CCL-2, ↓CCL-3, ↓CXCL-1, ↓IL-1β, ↑IL-10 after CTS administration. Macrophages themselves produce CgA and CTS. |
| Alam et al. 2020 [22] | H9c2 myoblasts stimulated for sarcomere reorganization by Troponin T antibodies and norepinephrine. | CTS attenuates myoblasts hypertrophy and suppresses the generation of ROS induced by norepinephrine; however, CTS does not protect cells from apoptotic signalling induced by norepinephrine. |
| Chu et al. 2020 [29] | 8 weeks old male Sprague–Dawley rats TG: Langendorff global ischemia/reperfusion model; CG: healthy rats; primary culture of cardiomyocytes from neonatal rats. | Average LV infarct size was33.66 ± 3.61% in I/R group. Posttreatment with CTS reduced infarct size to 20.25 ± 3.23% (p = 0.011 vs. I/R group). ↓LDH in myocardium in I/R group than in CG (6843.5 ± 1136.0) U/g vs. (102,470.0 ± 1066.1)U/g, p < 0.001. CTS intervention reduced the ↓LDH (8994.4 ± 963.8)U/g vs. (6843.5 ± 1136.0)U/g, p < 0.001). CTS post-treatment decreased oxidative-stress and reduced apoptosis of cardiomyocytes after I/R. CTS reduced apoptosis in cardiomyocytes culture induced by H2O2 through activating the β2 adrenergic receptor and PI3K/Akt pathway. |
| Chen et al. 2019 [30] | Atherosclerosis model: 8 weeks old male ApoE-KO mice divided into groups: G1 (CG) PBS i.p. G2: CTS i.p. G3 CTS + DX600 (ACE2 inhibitor) i.p. Human aortic endothelial cells (HAECs) and human umbilical vein endothelial cells (HUVECs) | CTS reduced TNF-α-induced expression of IL-6, MMP-2 and adhesion molecules (ICAM-1, VCAM-1, and E-selectin) in HAECs. CTS promoted expression and activity of ACE2 in HUVECs. CTS reduced adhesion events and increased the rolling velocity of leukocytes, those effect were blocked by ACE2 inhibitor though. Plaque area of the aorta was reduced in the CTS-treated mice vs. controls. |
| Chen et al. 2019 [31] | Eight-week-old C57/BL6 mice randomly divided into control (n = 20), control CTS (n = 20), APE (n = 20), and APE CTS (n = 20) Human pulmonary artery endothelial cells (HPAECs). APE in mice was induced by injection of collagen and epinephrine through the inferior vena cava | Plasma CTS lower in APE than in CG (p < 0.01) Negative correlation between CTS and Platelets level (Pearson correlation test r = 0.6732). Survival rate 30 min after APE onset was higher in APE CTS than in APE group (80% vs. 30%, p < 0.01). CTS had anti-thrombotic activity in APE mice. CTS inhibited APE-induced release of inflammatory neutrophils and macrophages. CTS blocked TLR-4 p38 phosphorylation in HPAECs. |
| Study and Its Reference | Methodology | Results Summary |
|---|---|---|
| Coronary artery disease | ||
| Xu et al. 2022 [41] | Cohort study among 165 patients with AMI; 4 years follow-up for MACEs MACEs group n = 24. Young = age <60 years old Elderly = age ≥60 years old | Lower CTS level in MACEs group (0.74 ± 0.49 ng/mL vs. 1.10 ± 0.79 ng/mL, p = 0.033); MACEs rate was higher in the elderly group than in the young group (23.8% [15/63] vs. 8.8% [9/102], p = 0.008). CTS level was lower in the MACEs group than in the non-MACEs group (0.76 ± 0.50 ng/mL vs. 1.31 ± 0.77 ng/mL, p = 0.012) and CTS was associated with MACEs (Kaplan Meier, p = 0.007) among the elderly group, but not in the young group (Kaplan Meier, p= 0.893). In the Cox proportional hazards regression CTS was independent factor for MACEs in elderly patients (hazard ratio 0.19, 95% confidence interval 0.06–0.62, p = 0.006). |
| Chen et al. 2019 [30] | Cross-sectional study Stage 1 TG: 224 patients with CAD and CG: 204 healthy controls Stage 2 association between CTS and atherosclerosis severity in 921 CAD patients | CTS lower in CAD patients than in CG 1.14 (1.05–1.24) ng/mL vs. 2.15 (1.92–2.39) ng/mL, p < 0.001. Negative correlation between CTS and atherosclerosis severity (r = −0.208, p < 0.001) |
| Xu et al. 2017 [42] | Cohort study among 170 patients with suspected ACS who underwent coronarography TG: STEMI n = 46; UAP n = 89 CG: No CAD n = 35 2 years follow-up for MACEs | Plasma CTS in STEMI group (0.80 ± 0.62 ng/mL) and UAP group (0.99 ± 0.63 ng/mL) were lower than in CG (1.38 ± 0.98 ng/mL; p = 0.001). In multivariable linear regression, body mass index, presence of hypertension, and type of CAD were independently related to the plasma CTS level. However, there were no significant differences in MACEs between patients with high and low levels of CTS |
| Liu et al. 2013 [43] | Cohort study on 120 CAD and TG: SAP n = 15; UAP n = 47; NSTEMI n = 22; STEMI n = 36 CG: 30 healthy individuals CTS measurement at admission Median follow-up time: 1045 days | CTS higher in TG than in CG (1.02 ± 0.70 vs. 0.41 ± 0.14, p < 0.05) CTS higher in SAP than in CG (0.72 ± 0.50 vs. 0.41 ± 0.14, p < 0.05) CTS higher in UAP than in CG (0.88 ± 0.58 vs. 0.41 ± 0.14, p < 0.05) CTS higher in NSTEMI than in CG (1.05 ± 0.48 vs. 0.41 ± 0.14, p < 0.05) CTS higher in STEMI than in CG (1.31 ± 0.91 vs. 0.41 ± 0.14, p < 0.05) CTS correlated positively with NE (Spearman correlation coefficient r = 0.51, p = 0.00) and NTproBNP (r = 0.24, p = 0.01) Plasma CTS on admission was not associated with adverse cardiovascular events. |
| Heart failure | ||
| Chu et al. 2024 [44] | A cohort study on 199 HF patients according to modified Framingham criteria. LVEF ≤ 40% n = 100; LVEF > 40% n = 102; Determination of CTS predictive value in HFrEF and HFmrEF/HFpEF respestively. | Plasma CTS level had a moderate predictive ability for CV death with a C statistic of 0.59 (95% CI 0.45–0.74), sensitivity 51.8%, specificity 71.8% in the HFrEF population and better prognostic value with C statistic of 0.72 (95% CI 0.59–0.85), sensitivity 70,8%, specificity 71,8% in the HFmrEF/HFpEF population. |
| Qiu et al. 2023 [24] | A cross-sectional study on 81 patients with HFpEF and 76 non–heart failure controls. | Serum CTS level was higher in HFpEF group than in CG (11.21 [interquartile range, 6.81–19.12] ng/mL vs. 23.62 [interquartile range, 11.53–34.81] ng/mL; p < 0.001). Serum CTS level was positively correlated with NT-proBNP level (r = 0.41; p < 0.001) and E/e′ ratio (r = 0.25; p = 0.002). |
| Borovac et al. 2020 [45] | Cohort study on 96 acute decompensated HF followed up until discharge Survivors n = 90 Non-survivors n = 6 | Serum CTS higher in non-survivors than in survivors 19.8 (IQR 9.9–28.0) vs. 5.6 (IQR 3.4–9.8) ng/mL, p < 0.001. CTS was an independent predictor of in-hospital death (FC 6.58, 95% CI 1.66–21.78, p = 0.003). In ROC analysis CTS AUC (0.905 95% CI 0.792–1.000, p < 0.001). |
| Zhu et al. 2017 [46] | A cohort study on 72 patients with STEMI followed-up for 65 months and 30 healthy controls. Serum CTS measurement. ECHO. | CTS levels correlated with the changes of LVEDD (p < 0.0001), EF (p = 0.0002), E (p = 0.0003), A (p < 0.0001), E’ (p < 0.0001), E/A (p < 0.0001), as well as E/E’ (p < 0.0001). |
| Pre-eclampsia | ||
| Palmrich et al. 2023 [47] | Cross-sectional study among 100 pregnant women. TG: 50 pre-eclamptic singleton pregnancy patients CG: 50 healthy pregnant women Serum CTS level comparison. | CTS serum level in pre-eclamptic group lower than in CG (median CTS: 3.03 ng/mL, IQR [1.24–7.21 ng/mL] vs. 4.82 ng/mL, IQR [1.82–10.02 ng/mL]; p = 0.010). |
| Tüten et al. 2022 [48] | Cross-sectional study among 2oo pregnant women. TG: 50 women with mild preeclampsia, 50 women with severe preeclampsia, CG: 100 healthy pregnant women | Mean serum CTS increased in the preeclampsia group than in CG (290.7 ± 95.5 pg/mL vs. 182.8 ± 72.0 pg/mL). No significant differences in CTS level between mild and severe preeclampsia groups (282.7 ± 97.9 pg/mL vs. 298.7 ± 93.4 pg/mL, p = 0.431). Serum CTS had positive correlations with systolic and diastolic blood pressure, urea, uric acid, and creatinine. |
| Bralewska et al. 2021 [49] | A cohort study of 205 pregnant women. TG: 102 pre-eclamtic patients CG: 103 healthy pregnant women Placental expression of the CgA gene and placental CTS level comparison. | Placental expression of chromogranin A higher in pre-eclamptic patients than in CG (−0.25 ± 1.7 vs. −0.82 ± 1.5, p = 0.011). Mean CTS level lower in pre-eclamptic group than in CG (6.4 ± 1.0 vs. 6.7 ± 1.4, p = 0.04). |
| Özalp et al. 2021 [50] | Cross-sectional study TG: 27 women with early-onset pre-eclampsia, 28 women with late-onset pre-eclampsia CG: 28 healthy pregnant women. Maternal serum CTS measurement and fetal ECHO. | The fetal E/A ratio positively correlated with the maternal serum CTS levels in both the pre-eclampsia group and CG (p < 0.001, p < 0.001). Fetal isovolumetric relaxation time and MPI values negatively correlated with maternal CTS in pre-eclampsia and CG (p < 0.001, p = 0.001, p < 0.001, and p = 0.002, respectively). No significant CTS level difference between TG and CG. |
| Chronic kidney disease | ||
| Luketin et al. 2021 [51] | Cross-sectional study TG: 91 adult patients with end-term chronic kidney disease haemodialyzed > 1 year CG: 70 healthy adult individuals | Plasma CTS higher in HD than CG (32.85 ± 20.18 vs. 5.39 ± 1.24 ng/mL, p < 0.001). Positive correlations between CTS and AGEs (r = 0.492, p < 0.001) and between CTS and both the Dialysis Malnutrition Score (r = 0.295, p = 0.004) and Malnutrition-Inflammation Score (r = 0.290, p = 0.005). |
| Pulmonary embolism | ||
| Izci et al. 2020 [52] | Prospectively study TG: 160 patients with contrasted CT-confirmed pulmonary embolism female n = 76; male n = 84 CTS measurement within 24 h after admission. CG: 97 healthy individuals female n = 55; male n = 42 Serum CTS measurement 0, 3 and 7 days after admission; ECHO | Plasma CTS higher in APE group than in CG (17.5 ± 6.1 ng/mL vs. 27.3 ± 5.7 ng/mL, p < 0.001). Plasma CTS higher in the sPESI ≥ 1 (n = 72) than in the patients with sPESI < 1 (37.3 ± 6.1 vs. 24.2 ± 5.3 ng/mL, p < 0.001). Positive correlation between CTS level and sPESI score (±0.581, p < 0.001). ROC curve analysis with cut-off level of 31.2 ng/mL, and the CTS level predicted mortality with a sensitivity of 100% and specificity of 52.6% (AUC = 0.883, 95% CI: 0.689–0.921). CTS level correlated with right ventricular dysfunction. Negative endpoints were associated with higher CTS levels after admission. |
| Rheumatoid arthritis | ||
| Pàmies et al. 2024 [53] | A cohort study of 199 rheumatoid arthritis patients. female n = 132; male n = 67 | RF-positive patients had higher CTS levels than RF-negative patients (p < 0.001). Positive correlations between: CTS and LDL-C (ρ = 0.32, p = 0.009); CTS and IL-32 (ρ = 0.20, p = 0.003); CTS and fet-a (ρ = 0.20, p = 0.004). ↑CTS in women with DM2 (p = 0.04). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulpa, J.; Paduch, J.; Szczepanik, M.; Gorący-Rosik, A.; Rosik, J.; Tchórz, M.; Pawlik, A.; Gorący, J. Catestatin in Cardiovascular Diseases. Int. J. Mol. Sci. 2025, 26, 2417. https://doi.org/10.3390/ijms26062417
Kulpa J, Paduch J, Szczepanik M, Gorący-Rosik A, Rosik J, Tchórz M, Pawlik A, Gorący J. Catestatin in Cardiovascular Diseases. International Journal of Molecular Sciences. 2025; 26(6):2417. https://doi.org/10.3390/ijms26062417
Chicago/Turabian StyleKulpa, Joanna, Jarosław Paduch, Marcin Szczepanik, Anna Gorący-Rosik, Jakub Rosik, Magdalena Tchórz, Andrzej Pawlik, and Jarosław Gorący. 2025. "Catestatin in Cardiovascular Diseases" International Journal of Molecular Sciences 26, no. 6: 2417. https://doi.org/10.3390/ijms26062417
APA StyleKulpa, J., Paduch, J., Szczepanik, M., Gorący-Rosik, A., Rosik, J., Tchórz, M., Pawlik, A., & Gorący, J. (2025). Catestatin in Cardiovascular Diseases. International Journal of Molecular Sciences, 26(6), 2417. https://doi.org/10.3390/ijms26062417







