Gene Expression and Activity of Selected Antioxidant and DNA Repair Enzymes in the Prefrontal Cortex of Sheep as Affected by Kynurenic Acid
Abstract
1. Introduction
2. Results
2.1. Antioxidant Enzymes: mRNA Expression and Activity
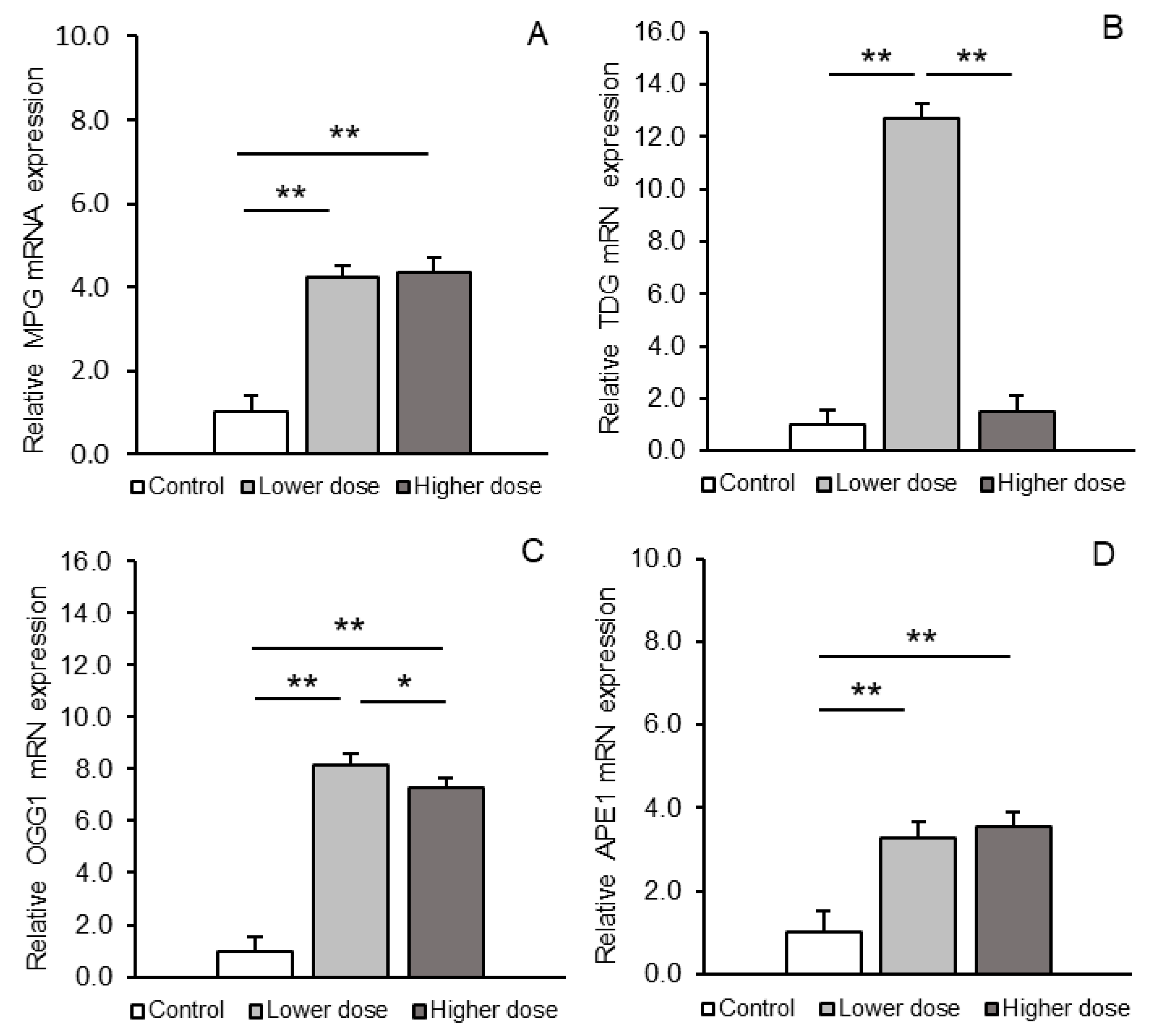
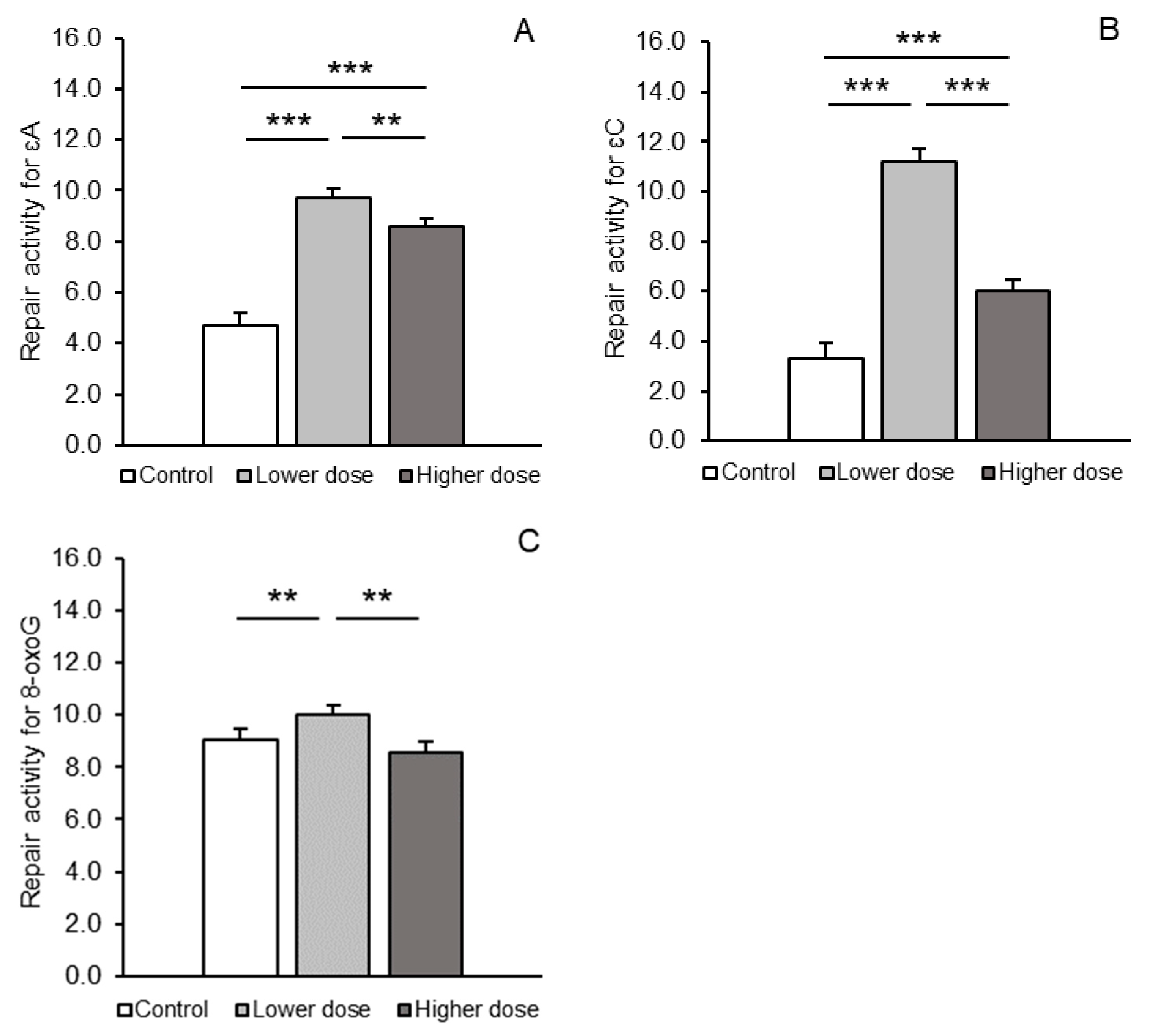
2.2. BER Pathway Enzymes: mRNA Expression and Activity
3. Discussion
4. Materials and Methods
4.1. Animal Management
4.2. Third Ventricle Cannulation
4.3. Experimental Design and Tissue Collection
4.4. Analysis of Relative mRNA Abundance
4.5. Determination of Antioxidant Enzyme Activity
4.6. Determination of BER Pathway Enzymes Activities
4.7. Statistical Analysis
5. Conclusions and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, M.A.; Gonçalves, H.M.R.; Davis, F. Reactive oxygen species in biological media are they friend or foe? Major in vivo and in vitro sensing challenges. Talanta 2023, 260, 124648. [Google Scholar] [CrossRef] [PubMed]
- Jaganjac, M.; Milkovic, L.; Zarkovic, N.; Zarkovic, K. Oxidative stress and regeneration. Free Radic. Biol. Med. 2022, 181, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Rummel, N.G.; Butterfield, D.A. Altered Metabolism in Alzheimer Disease Brain: Role of Oxidative Stress. Antioxid. Redox Signal. 2021, 36, 1289–1305. [Google Scholar] [CrossRef]
- Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L.; Trist, B.G. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. Engl. 2020, 60, 9215–9246. [Google Scholar] [CrossRef]
- Nguyen, N.H. Anti-oxidative effects of superoxide dismutase 3 on inflammatory diseases. J. Mol. Med. 2020, 98, 59–69. [Google Scholar] [CrossRef]
- Ishihara, Y.; Itoh, K. Microglial inflammatory reactions regulated by oxidative stress. J. Clin. Biochem. Nutr. 2023, 72, 23–27. [Google Scholar] [CrossRef]
- Al-Hajaya, Y.; Karpinska, B.; Foyer, C.H.; Baker, A. Nuclear and peroxisomal targeting of catalase. Plant Cell Environ. 2022, 45, 1096–1108. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Bratovcic, A. Antioxidant enzymes and their role in preventing cell damage. Acta Sci. Nutr. Health 2020, 4, 132–138. [Google Scholar] [CrossRef]
- Faria-Pereira, A.; Morais, V.A. Synapses: The Brain’s Energy-Demanding Sites. Int. J. Mol. Sci. 2022, 23, 3627. [Google Scholar] [CrossRef]
- Basu, K.A. DNA Damage, Mutagenesis and Cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, C.; Feng, C.; Yan, C.; Yu, Y.; Chen, Z.; Guo, C.; Wang, X. Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep. 2022, 27, 45–52. [Google Scholar] [CrossRef]
- Trasviña-Arenas, C.H.; Demir, M.; Lin, W.J.; David, S.S. Structure, function and evolution of the Helix-hairpin-Helix DNA glycosylase superfamily: Piecing together the evolutionary puzzle of DNA base damage repair mechanisms. DNA Repair 2021, 108, 103231. [Google Scholar] [CrossRef]
- Gohil, D.; Sarker, A.H.; Roy, R. Base Excision Repair: Mechanisms and Impact in Biology; Disease; and Medicine. Int. J. Mol. Sci. 2023, 24, 14186. [Google Scholar] [CrossRef]
- Roszkowicz-Ostrowska, K.; Młotkowska, P.; Kowalczyk, P.; Marciniak, E.; Barszcz, M.; Misztal, T. Central Stimulatory Effect of Kynurenic Acid on BDNF-TrkB Signaling and BER Enzymatic Activity in the Hippocampal CA1 Field in Sheep. Int. J. Mol. Sci. 2023, 24, 136. [Google Scholar] [CrossRef] [PubMed]
- Młotkowska, P.; Misztal, T.; Paweł Kowalczyk, P.; Marciniak, E. Effect of kynurenic acid on enzymatic activity of the DNA base excision repair pathway in specific areas of the sheep brain. Sci. Rep. 2024, 14, 15506. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.; Rashidi, N.; Nurgali, K.; Apostolopoulos, V. The Role of Tryptophan Metabolites in Neuropsychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 9968. [Google Scholar] [CrossRef]
- Osuch, B.; Kucharska, T.; Chmielewska, N.; Maciejak, P.; Szyndler, J.; Płaźniket, A. The role of mitophagy in selected neurodegenerative diseases. Adv. Psych. Neurol. 2019, 28, 154–161. [Google Scholar] [CrossRef]
- Sas, K.; Szabó, E.; Vécsei, L. Mitochondria, Oxidative Stress and the Kynurenine System, with a Focus on Ageing and Neuroprotection. Molecules 2018, 17, 191. [Google Scholar] [CrossRef]
- Misztal, T.; Roszkowicz-Ostrowska, K.; Kowalczyk, P.; Młotkowska, P.; Marciniak, E. Kynurenic acid modulates the expression of genes and the activity of cellular antioxidant enzymes in the hypothalamus and hippocampus in sheep. Int. J. Mol. Sci. 2024, 25, 9428. [Google Scholar] [CrossRef]
- Allen, L.M.; Lesyshyn, R.A.; O’Dell, S.J.; Allen, T.A.; Fortin, N.J. The hippocampus, prefrontal cortex, and perirhinal cortex are critical to incidental order memory. Behav. Brain Res. 2020, 379, 112215. [Google Scholar] [CrossRef]
- Tang, W.; Shin, J.D.; Jadhav, S.P. Multiple time-scales of decision-making in the hippocampus and prefrontal cortex. eLife 2020, 10, 66227. [Google Scholar] [CrossRef]
- Ramírez Ortega, D.; Ugalde Muñiz, P.E.; Blanco Ayala, T.; Vázquez Cervantes, G.I.; Lugo Huitrón, R.; Pineda, B.; González Esquivel, D.F.; Pérez de la Cruz, G.; Pedraza Chaverrí, J.; Sánchez Chapul, L.; et al. On the Antioxidant Properties of L-Kynurenine: An Efficient ROS Scavenger and Enhancer of Rat Brain Antioxidant Defense. Antioxidants 2022, 11, 31. [Google Scholar] [CrossRef]
- Kim, H.H.; Jeong, S.H.; Ha, S.E.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Won, C.K.; Heo, J.D.; Kim, H.W.; Kim, G.S. Cellular Regulation of Kynurenic Acid-Induced Cell Apoptosis Pathways in AGS Cells. Int. J. Mol. Sci. 2022, 23, 8894. [Google Scholar] [CrossRef]
- Hardeland, R.; Zsizsik, B.K. Kynurenic acid as a free radical scavenger: Measurements of educt and product fluorescence and of light emission from an excited intermediate state. In Biological Rhythms and Antioxidative Protection; Hardeland, R., Ed.; Cuvillier: Göttingen, Germany, 1997; pp. 153–160. [Google Scholar]
- Zsizsik, B.K.; Hardeland, R. A putative mechanism of kynurenic acid oxidation by free radicals: Scavenging of two hydroxyl radicals and superoxide anion, release of •NO and CO2. In Actions and Redox Properties of Melatonin and Other Aromatic Amino Acid Metabolites; Hardeland, R., Ed.; Cuvillier: Gottingen, Germany, 2001; pp. 164–167. [Google Scholar]
- Lugo-Huitrón, R.; Blanco-Ayala, T.; Ugalde-Muñiz, P.; Carrillo-Mora, P.; Pedraza-Chaverrí, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzón, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Obara-Michlewska, M. The tryptophan metabolism, kynurenine pathway and oxidative stress—Implications for glioma pathobiology. Neurochem. Int. 2022, 158, 105363. [Google Scholar] [CrossRef] [PubMed]
- Silver Ferreira, F.; Schmitz, F.; Marques, E.P.; Siebert, C.; Wyse, A.T.S. Intrastriatal quinolinic acid administration impairs redox homeostasis and induces inflammatory changes: Prevention by kynurenic acid. Neurotox. Res. 2020, 38, 50–58. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general Overview of mechanisms and implications in human disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Rios, C.; Santamaria, A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem. Res. 1991, 16, 1139–1143. [Google Scholar] [CrossRef]
- Guillemin, G.J. Quinolinic acid; the inescapable neurotoxin. FEBS J. 2012, 279, 1356–1365. [Google Scholar] [CrossRef]
- La Cruz, V.P.D.; Carrillo-Mora, P.; Santamaría, A. Quinolinic acid; an endogenous molecule combining excitotoxicity; oxidative stress and other toxic mechanisms. Int. J. Tryptophan Res. 2012, 5, 1–8. [Google Scholar] [CrossRef]
- Stone, T.W.; Darlington, L.G.; Badawy, A.A.; Williams, R.O. The Complex World of Kynurenic Acid: Reflections on Biological Issues and Therapeutic Strategy. Int. J. Mol. Sci. 2024, 20, 9040. [Google Scholar] [CrossRef]
- Jobert, L.; Nilsen, H. Regulatory mechanisms of RNA function: Emerging roles of DNA repair enzymes. Cell. Mol. Life Sci. 2014, 71, 2451–2465. [Google Scholar] [CrossRef]
- Urenjak, J.; Obrenovitch, T.P. Neuroprotective potency of kynurenic acid against excitotoxicity. Neuroreport 2000, 11, 1341–1344. [Google Scholar] [CrossRef]
- Scharcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2019, 112, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Ostapiuk, A.; Urbanska, E.M. Kynurenic acid in neurodegenerative disorders—Unique neuroprotection or double-edged sword? CNS Neurosci. Ther. 2021, 28, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Liu, L.; Jian, Z.; Cui, T.; Yang, Y.; Guo, S.; Yi, X.; Wang, G.; Li, C.; et al. Role of the aryl hydrocarbon receptor signaling pathway in promoting mitochondrial biogenesis against oxidative damage in human melanocytes. J. Dermatol. Sci. 2019, 96, 33–41. [Google Scholar] [CrossRef]
- Cortés Malagón, E.M.; López Ornelas, A.; Olvera Gómez, I.; Bonilla Delgado, J. The Kynurenine Pathway, Aryl Hydrocarbon Receptor, and Alzheimer’s Disease. Brain Sci. 2024, 14, 950. [Google Scholar] [CrossRef]
- Dittmann, K.H.; Rothmund, M.C.; Paasch, A.; Mayer, C.; Fehrenbacher, B.; Schaller, M.; Frauenstein, K.; Fritsche, E.; Haamann-Stemmann, T.; Braeuning, A.; et al. The nuclear aryl hydocarbon receptor is involved in regulation of DNA repair and cell survival following treatment with ionizing radiation. Toxicol. Lett. 2016, 240, 122. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic acid: The janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front. Immunol. 2018, 10, 8957. [Google Scholar] [CrossRef]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells. 2021, 10, 1603. [Google Scholar] [CrossRef]
- Levine, R.L.; Yang, I.Y.; Hossain, M.; Pandya, G.A.; Grollman, A.P.; Moriya, M. Mutagenesis induced by a single 1;N6-ethenodeoxyadenosine adduct in human cells. Cancer Res. 2000, 60, 4098–4104. [Google Scholar]
- Moriya, M.; Pandya, G.A.; Johnson, F.; Grollman, A.P. Cellular response to exocyclic DNA adducts. IARC Sci. Publ. 1999, 150, 263–270. [Google Scholar]
- Memisoglu, A.; Samson, L. Base excision repair in yeast and mammals. Mutat. Res. 2000, 451, 39–51. [Google Scholar] [CrossRef]
- Hahm, J.Y.; Park, J.; Jang, E.S.; Chi, S.W. 8-Oxoguanine: From oxidative damage to epigenetic and epitranscriptional modification. Exp. Mol. Med. 2022, 54, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
- Chiorcea-Paquim, A.M. 8-oxoguanine and 8- oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC–ECD Determination. Molecules 2022, 27, 1620. [Google Scholar] [CrossRef] [PubMed]
- Kasai, H.; Iwamoto-Tanaka, N.; Miyamoto, T.; Kawanami, K.; Kawanami, S.; Kido, R.; Ikeda, M. Life style and urinary 8-hydroxydeoxygua-nosine; a marker of oxidative DNA damage: Effects of exercise; working conditions; meat intake; body mass index; and smoking. Jpn. J. Cancer Res. 2001, 92, 9–15. [Google Scholar] [CrossRef]
- Halliwell, B. Can oxidative DNA damage be used as a biomarker of cancer risk in humans? Problems; resolutions; and preliminary results from nutritional supplementation studies. Free Radic. Res. 1998, 29, 469–486. [Google Scholar] [CrossRef]
- Marnett, L.J. Oxyradicals and DNA damage. Carcinogenesis 2000, 21, 361–370. [Google Scholar] [CrossRef]
- Pao, P.C.; Patnaik, D.; Watson, L.A.; Gao, F.; Pan, L.; Wang, J.; Chinnakkaruppan, A.; Penney, J.; Hugh, P.; Huang, W.; et al. HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat. Commun. 2020, 18, 2484. [Google Scholar] [CrossRef]
- Lodato, M.A.; Ziegenfuss, J.S. The two faces of DNA oxidation in genomic and functional mosaicism during aging in human neurons. Front. Aging 2022, 3, 991460. [Google Scholar] [CrossRef]
- Liu, T.C.; Guo, K.W.; Chu, J.W.; Hsiao, Y.Y. Understanding APE1 cellular functions by the structural preference of exonuclease activities. Comput. Struct. Biotechnol. J. 2021, 19, 3682–3691. [Google Scholar] [CrossRef]
- Yang, J.L.; Lin, Y.T.; Chuang, P.C.; Bohr, V.A.; Mattson, M.P. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolec. Med. 2014, 16, 161–174. [Google Scholar] [CrossRef]
- Strzetelski, J. IZ PIB–INRA Feeding Recommendations for Ruminants and Feed Tables; National Research Institute of Animal Production: Kraków, Poland, 2014. (In Polish) [Google Scholar]
- Welento, J.; Szteyn, S.; Milart, Z. Observations on the stereotaxic configuration of the hypothalamus nuclei in the sheep. Anat. Anz. 1969, 124, 1–27. [Google Scholar]
- Traczyk, W.; Przekop, F. Methods of investigation of the function of the hypothalamus and hypophysis in chronic experiments in sheep. Acta Physiol. Pol. 1963, 14, 227–236. [Google Scholar] [PubMed]
- Moroni, F.; Cozzi, A.; Sili, M.; Mannaioni, G. Kynurenic acid: A metabolite with multiple actions and multiple targets in brain and periphery. J. Neural Trans. 2012, 119, 133–139. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pairwise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Fridovich, I. Oxygen radicals; hydrogen peroxide and oxygen toxicity. In Free Radicals in Biology; Pryor, W.A., Ed.; Academic Press: New York, NY, USA, 1976; pp. 239–277. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Hopkins, J.; Tudhope, G.R. Glutathione peroxidase in human red cells in health and disease. Br. J. Haematol. 1973, 25, 563–575. [Google Scholar] [CrossRef]


| Gene | Primers (5’–3’) | Genbank Acc. No. | Amplicon Size |
|---|---|---|---|
| MPG | F: GCTGAGGGCCAGCCAACACCTGC R: CGCCCCTTTACCCACGGAGCCCA | XM_027962019.2 | 121 |
| TDG | F: TAATGGGCAGTGGATGACCC R: TAATGGGCAGTGGATGACCC | XM_027967675.3 | 128 |
| OGG1 | F: CTCAGAAATTCCAAGGTGTTC R: CCGCTCCACCATGCCAGTG | XM_012099510.5 | 113 |
| APE1 | F: GAATGCTGGCTTCACTCCACA R: AAAGGTGTAGGCATACGCCGT | XM_004010390.5 | 115 |
| SOD2 | F: GCAAGGAACAACAGGTCTTATCC R: ACTTGGTGTAAGGCTGACGG | NM_001280703.1 | 181 |
| CAT | F: GAGCCCACCTGCAAAGTTCT R: CTCCTACTGGATTACCGGCG | XM_004016396.6 | 148 |
| GPX1 | F: TGTCGTACTCGGCTTCCC R: AGCGGATGCGCCTTCTCG | XM_004018462.1 | 163 |
| GAPDH | F: GGGTCATCATCTCTGCACCT R: GGTCATAAGTCCCTCCACGA | NM_001190390.1 | 131 |
| PPIC | F: ACGGCCAAGGTCTTCTTTG R: TATCCTTTCTCTCCCGTTGC | NM_001076910 | 131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marciniak, E.; Osuch, B.; Młotkowska, P.; Kowalczyk, P.; Roszkowicz-Ostrowska, K.; Misztal, T. Gene Expression and Activity of Selected Antioxidant and DNA Repair Enzymes in the Prefrontal Cortex of Sheep as Affected by Kynurenic Acid. Int. J. Mol. Sci. 2025, 26, 2381. https://doi.org/10.3390/ijms26062381
Marciniak E, Osuch B, Młotkowska P, Kowalczyk P, Roszkowicz-Ostrowska K, Misztal T. Gene Expression and Activity of Selected Antioxidant and DNA Repair Enzymes in the Prefrontal Cortex of Sheep as Affected by Kynurenic Acid. International Journal of Molecular Sciences. 2025; 26(6):2381. https://doi.org/10.3390/ijms26062381
Chicago/Turabian StyleMarciniak, Elżbieta, Bartosz Osuch, Patrycja Młotkowska, Paweł Kowalczyk, Katarzyna Roszkowicz-Ostrowska, and Tomasz Misztal. 2025. "Gene Expression and Activity of Selected Antioxidant and DNA Repair Enzymes in the Prefrontal Cortex of Sheep as Affected by Kynurenic Acid" International Journal of Molecular Sciences 26, no. 6: 2381. https://doi.org/10.3390/ijms26062381
APA StyleMarciniak, E., Osuch, B., Młotkowska, P., Kowalczyk, P., Roszkowicz-Ostrowska, K., & Misztal, T. (2025). Gene Expression and Activity of Selected Antioxidant and DNA Repair Enzymes in the Prefrontal Cortex of Sheep as Affected by Kynurenic Acid. International Journal of Molecular Sciences, 26(6), 2381. https://doi.org/10.3390/ijms26062381







