Chitin Synthase Is Required for Cuticle Formation and Molting in the Chinese Mitten Crab Eriocheir sinensis
Abstract
1. Introduction
2. Results
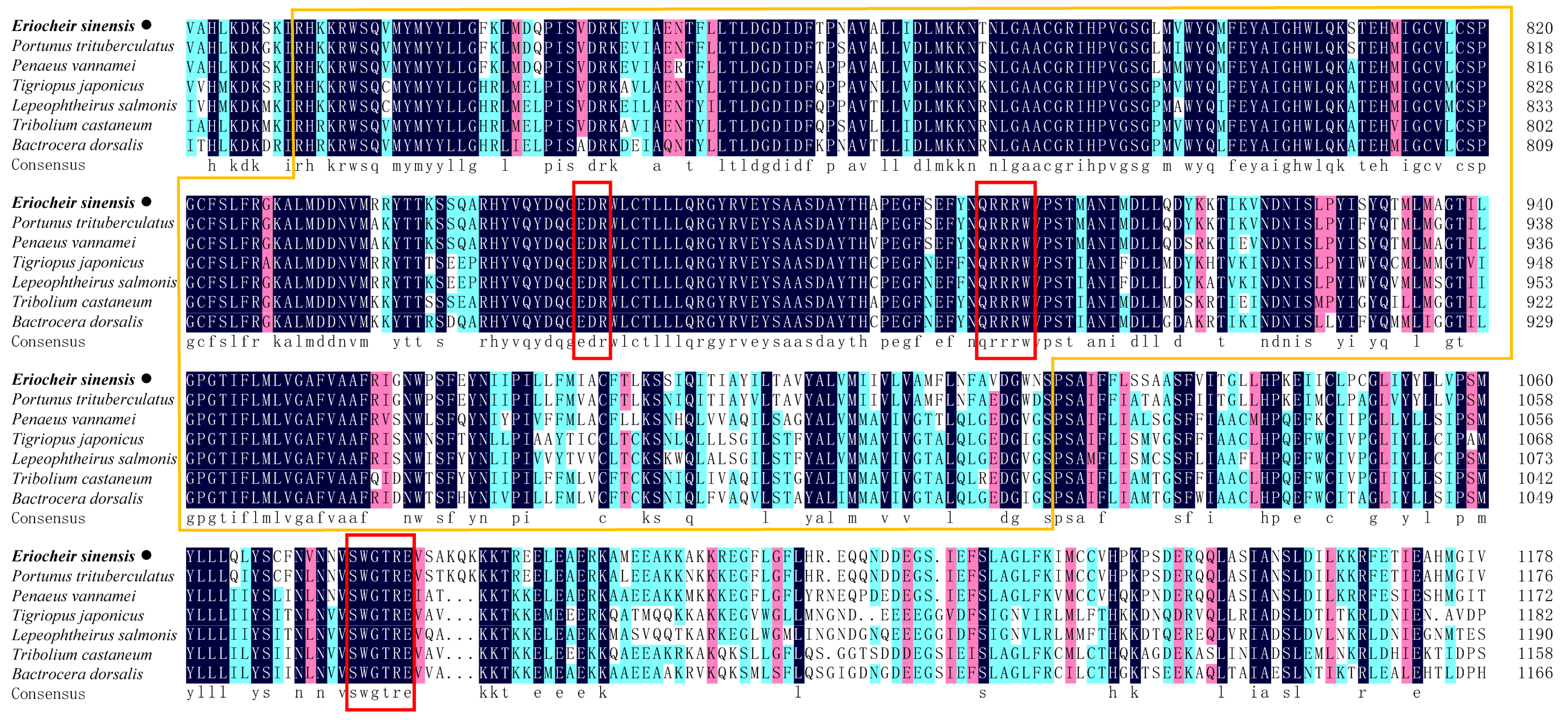
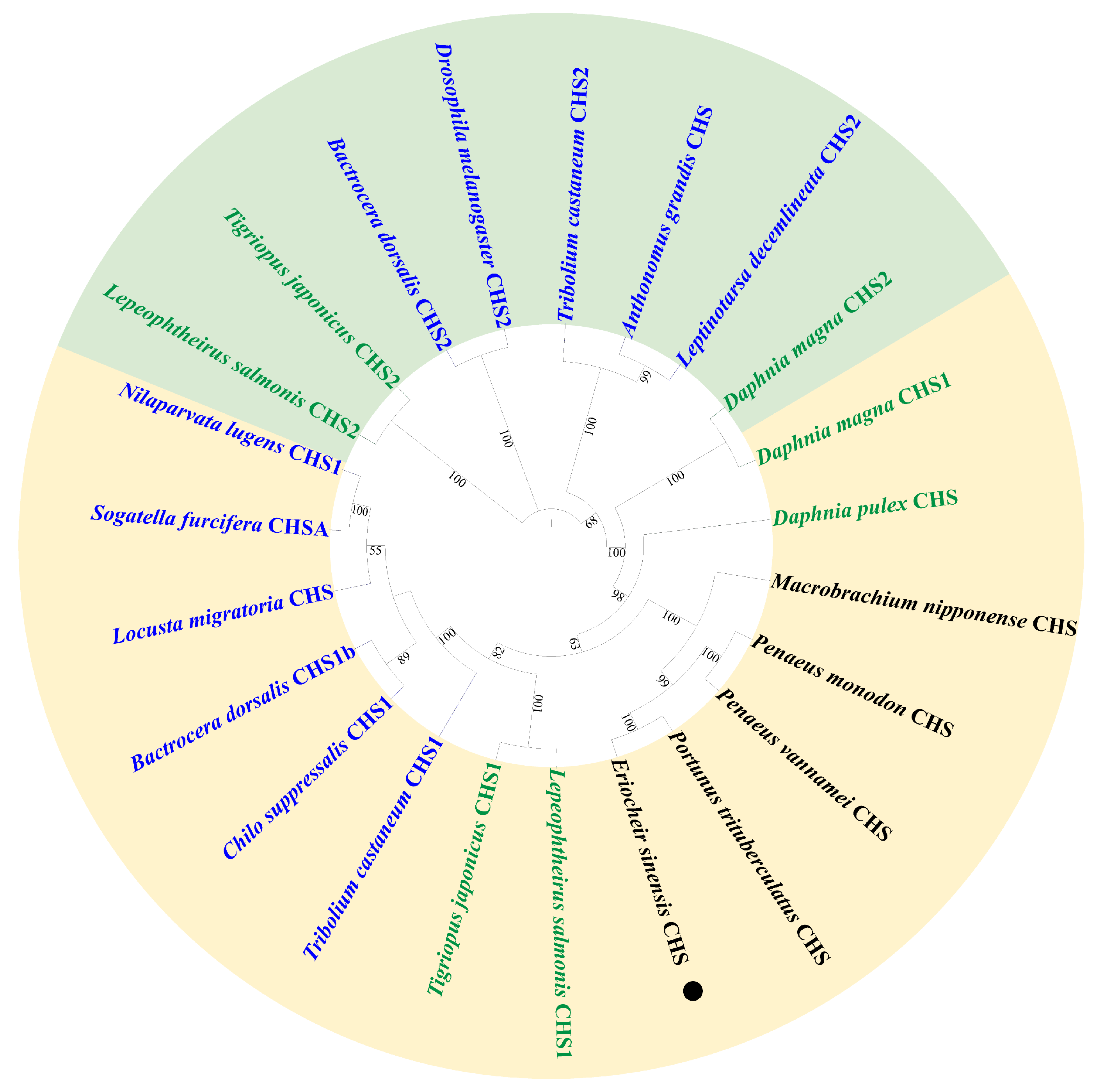
2.1. Identification and Characterization of EsCHS
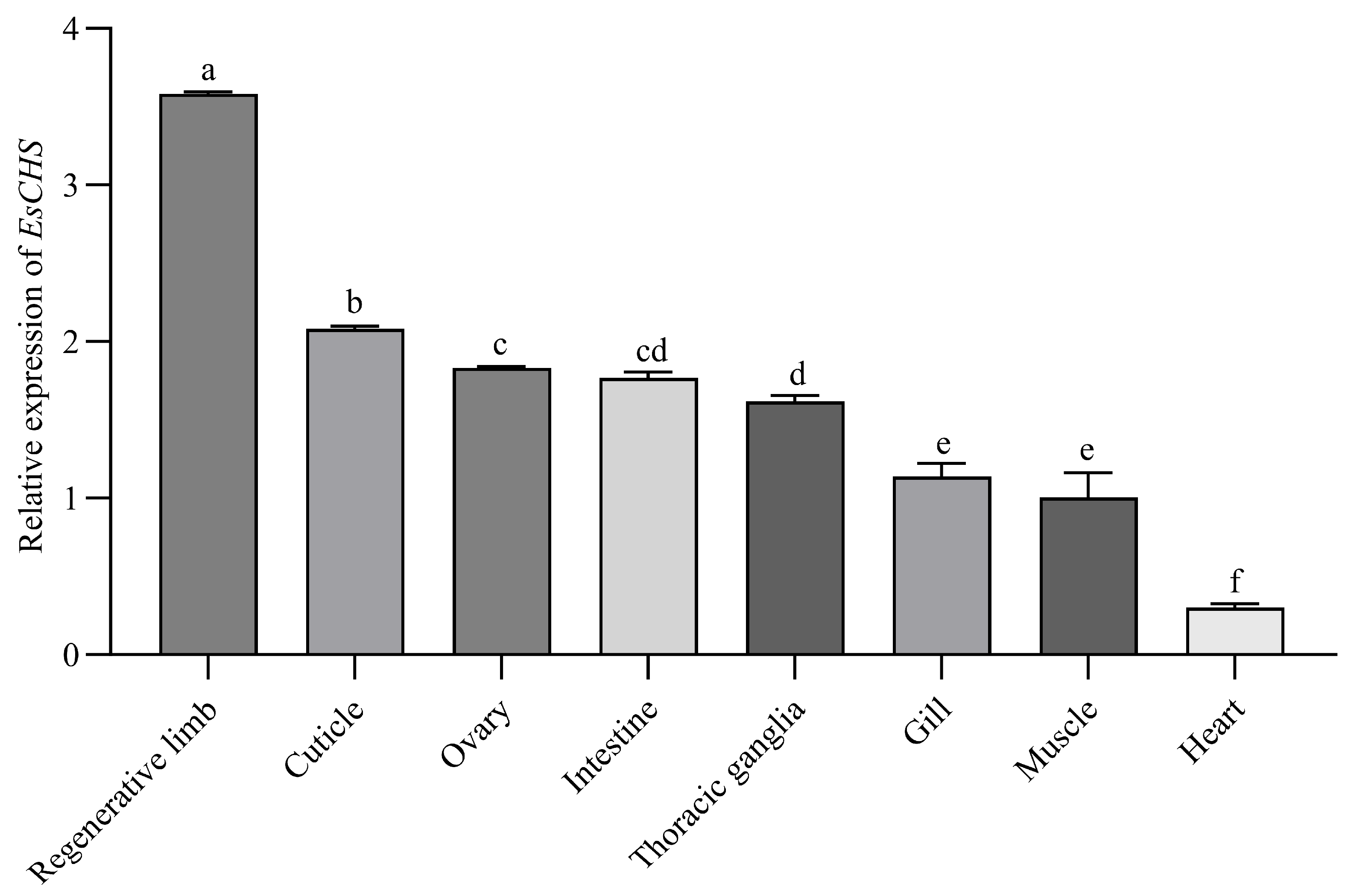
2.2. Expression of EsCHS in Different Tissues
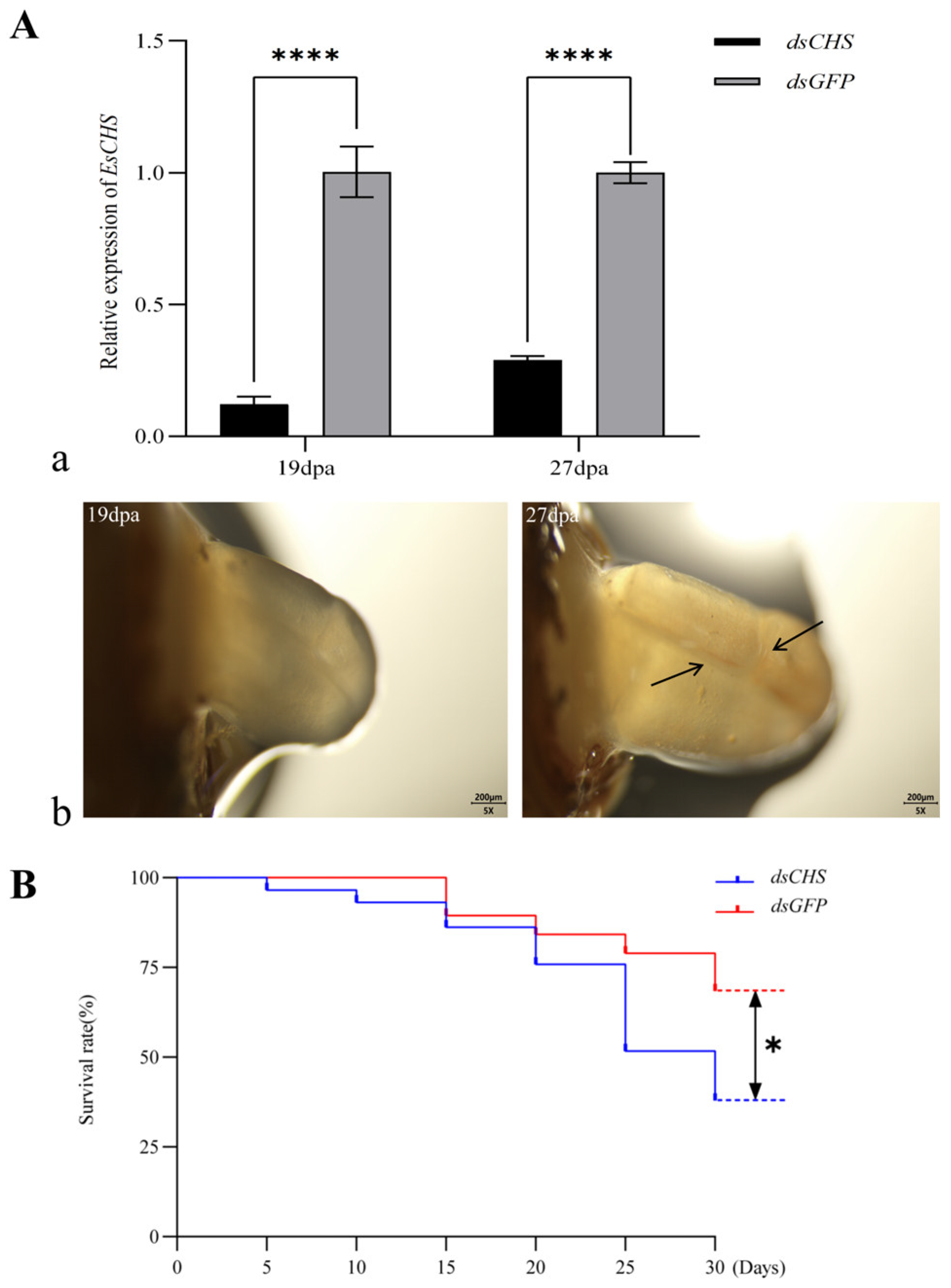
2.3. Expression of EsCHS in Molting Cycle
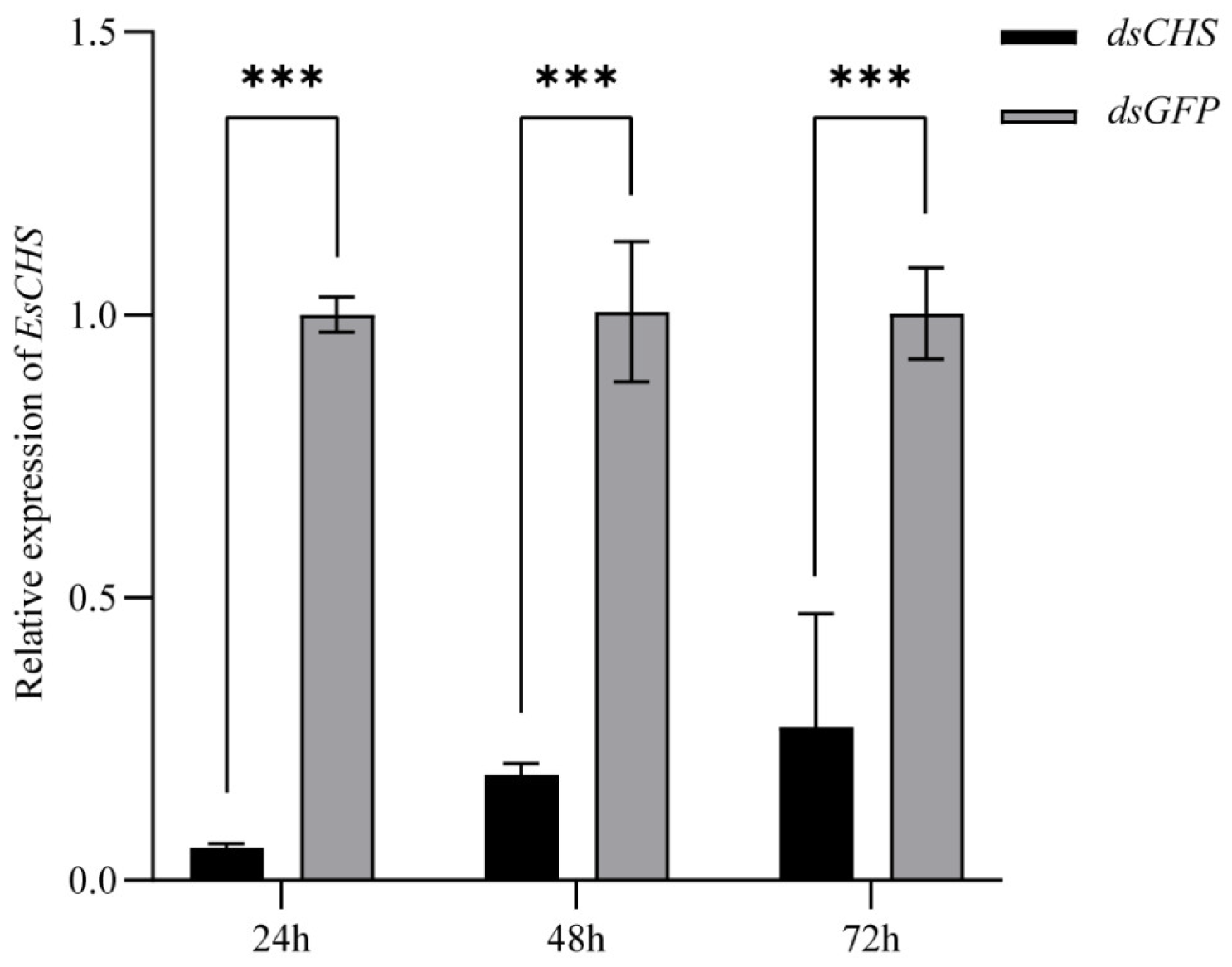
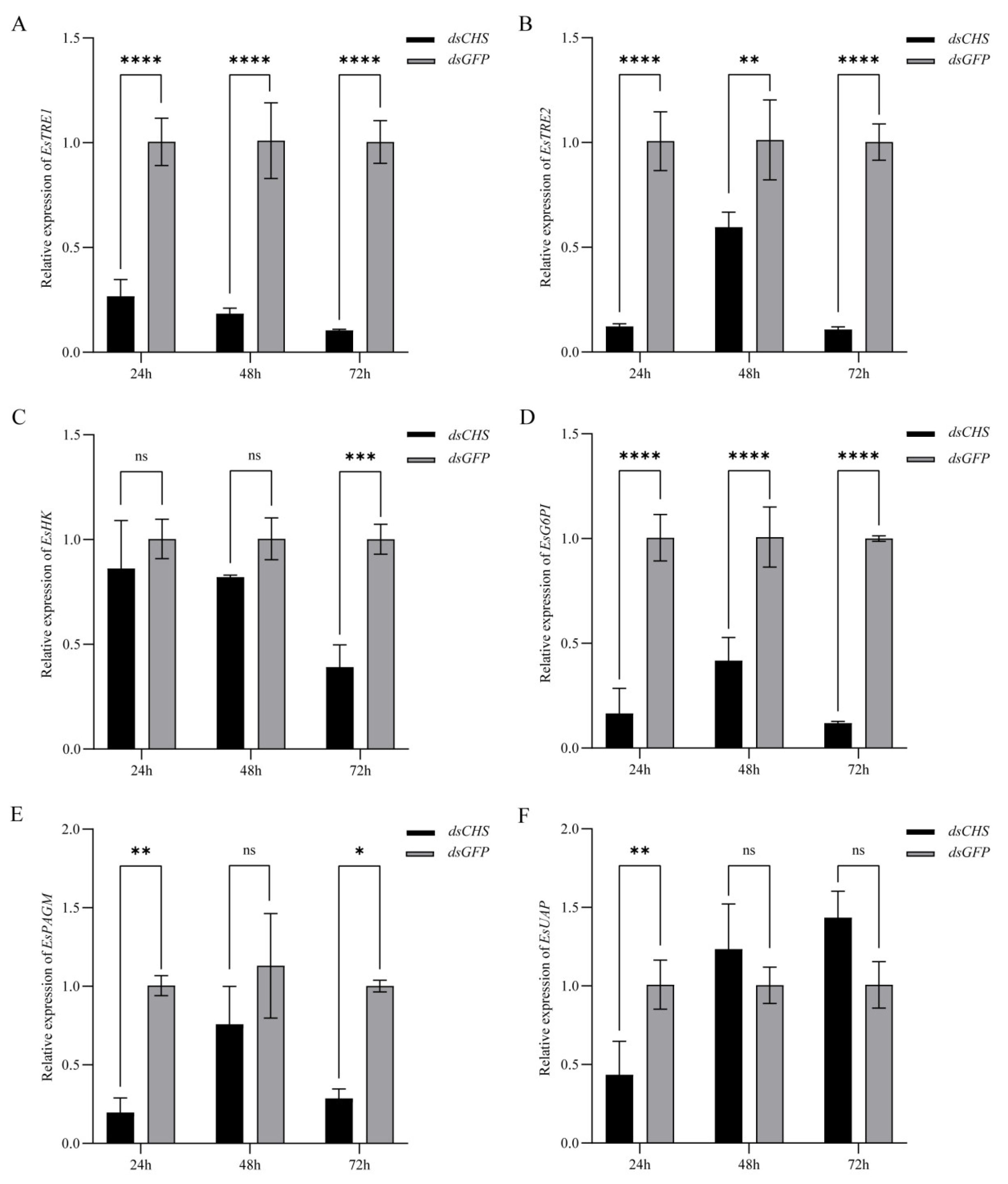
2.4. Expression of EsCHS and Chitin Biosynthesis-Related Genes After RNAi
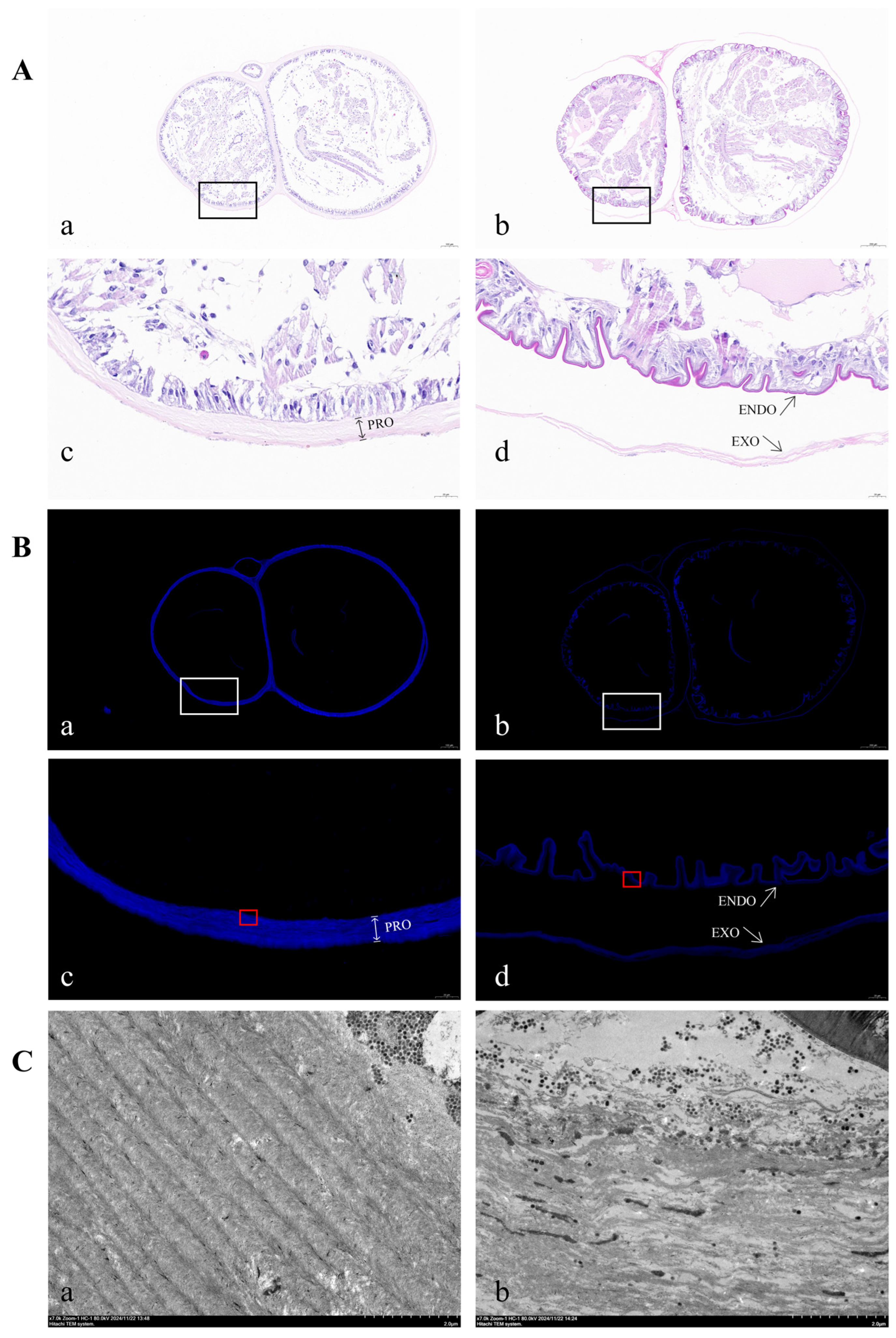
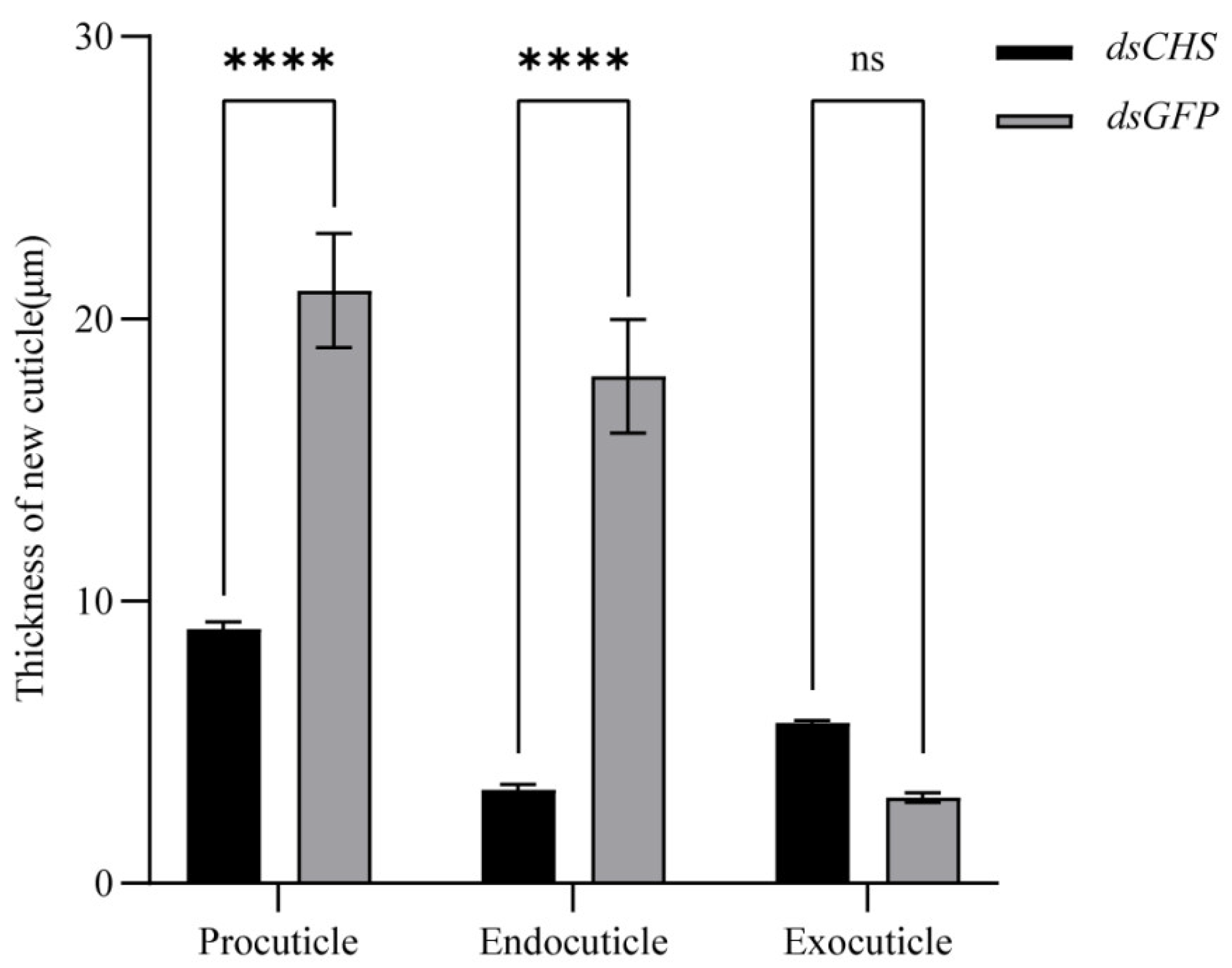
2.5. Functional Impact of EsCHS Knockdown
3. Discussion
4. Materials and Methods
4.1. Specimen and Culture Conditions
4.2. RNA Extraction and Sequence Verification
4.3. Bioinformatical Analysis
4.4. Expressional Analysis of EsCHS by Quantitative Real-Time PCR
4.5. Function Analysis of EsCHS by RNA Interference
4.5.1. Short-Term RNA Interference
4.5.2. Long-Term RNA Interference
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, K.Y.; Merzendorfer, H.; Zhang, W.; Zhang, J.; Muthukrishnan, S. Biosynthesis, Turnover, and Functions of Chitin in Insects. Annu. Rev. Entomol. 2016, 61, 177–196. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, J.; Li, F.; Xiang, J. Chitin Synthesis and Degradation in Crustaceans: A Genomic View and Application. Mar. Drugs 2021, 19, 153. [Google Scholar] [CrossRef]
- Yu, A.; Beck, M.; Merzendorfer, H.; Yang, Q. Advances in understanding insect chitin biosynthesis. Insect Biochem. Mol. Biol. 2024, 164, 104058. [Google Scholar] [CrossRef]
- Arakane, Y.; Hogenkamp, D.G.; Zhu, Y.C.; Kramer, K.J.; Specht, C.A.; Beeman, R.W.; Kanost, M.R.; Muthukrishnan, S. Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem. Mol. Biol. 2004, 34, 291–304. [Google Scholar] [CrossRef]
- Arakane, Y.; Specht, C.A.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Chitin synthases are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 2008, 38, 959–962. [Google Scholar] [CrossRef]
- De Giorgio, E.; Giannios, P.; Espinàs, M.L.; Llimargas, M. A dynamic interplay between chitin synthase and the proteins Expansion/Rebuf reveals that chitin polymerisation and translocation are uncoupled in Drosophila. PLoS Biol. 2023, 21, e3001978. [Google Scholar] [CrossRef]
- Rocha, J.; Garcia-Carreño, F.L.; Muhlia-Almazán, A.; Peregrino-Uriarte, A.B.; Yépiz-Plascencia, G.; Córdova-Murueta, J.H. Cuticular chitin synthase and chitinase mRNA of whiteleg shrimp Litopenaeus vannamei during the molting cycle. Aquaculture 2012, 330–333, 111–115. [Google Scholar] [CrossRef]
- Wang, P.; Guo, A.; Zhang, Y.; Lv, Y.; Ning, Q. Gene Cloning and Expression Analysis of Cuticle Chitin Synthase in Macrobrachium nipponense. J. Fish. China 2015, 39, 9. [Google Scholar]
- Uddowla, M.H.; Kim, A.R.; Park, W.G.; Kim, H.W. cDNAs Encoding Chitin Synthase from Shrimp (Pandalopsis japonica): Molecular Characterization and Expression Analysis. OMICS Int. 2015, 6, 298. [Google Scholar]
- Imaizumi, K.; Sano, M.; Kondo, H.; Hirono, I. Insights Into a Chitin Synthase of Kuruma Shrimp Penaeus japonicus and Its Role in Peritrophic Membrane and Cuticle Formation. Mar. Biotechnol. 2023, 25, 837–845. [Google Scholar] [CrossRef]
- Park, K.; Kwak, T.S.; Kwak, I.S. Integrated analysis of exoskeletal surface profile and chitin-related gene expression on Macrophthalmus japonicus mud crabs exposed to hexabromocyclododecane. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2022, 257, 109331. [Google Scholar] [CrossRef]
- Kato, K.; Okamura, K.; Hiki, K.; Kintsu, H.; Nohara, K.; Yamagishi, T.; Nakajima, N.; Watanabe, H.; Yamamoto, H. Potential differences in chitin synthesis ability cause different sensitivities to diflubenzuron among three strains of Daphnia magna. Aquat. Toxicol. 2022, 243, 106071. [Google Scholar] [CrossRef]
- Harðardóttir, H.M.; Male, R.; Nilsen, F.; Eichner, C.; Dondrup, M.; Dalvin, S. Chitin synthesis and degradation in Lepeophtheirus salmonis: Molecular characterization and gene expression profile during synthesis of a new exoskeleton. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 227, 123–133. [Google Scholar] [CrossRef]
- Harðardóttir, H.M.; Male, R.; Nilsen, F.; Dalvin, S. Chitin Synthases Are Critical for Reproduction, Molting, and Digestion in the Salmon Louse (Lepeophtheirus salmonis). Life 2021, 11, 47. [Google Scholar] [CrossRef]
- Campli, G.; Volovych, O.; Kim, K.; Veldsman, W.P.; Drage, H.B.; Sheizaf, I.; Lynch, S.; Chipman, A.D.; Daley, A.C.; Robinson-Rechavi, M.; et al. The moulting arthropod: A complete genetic toolkit review. Biol. Rev. Camb. Philos. Soc. 2024, 99, 2338–2375. [Google Scholar] [CrossRef]
- Li, X.; Xu, Z.; Zhou, G.; Lin, H.; Zhou, J.; Zeng, Q.; Mao, Z.; Gu, X. Molecular characterization and expression analysis of five chitinases associated with molting in the Chinese mitten crab, Eriocheir sinensis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2015, 187, 110–120. [Google Scholar] [CrossRef]
- Li, X.; Chu, J.; Diao, P.; Xu, Z.; Xu, Y.; Zhou, G.; Zhou, J.; Bi, K. Identification and expression profiles of chitin deacetylase family genes in the Chinese mitten crab, Eriocheir sinensis. Aquac. Res. 2021, 52, 3198–3211. [Google Scholar] [CrossRef]
- Chen, W.; Cao, P.; Liu, Y.; Yu, A.; Wang, D.; Chen, L.; Sundarraj, R.; Yuchi, Z.; Gong, Y.; Merzendorfer, H.; et al. Structural basis for directional chitin biosynthesis. Nature 2022, 610, 402–408. [Google Scholar] [CrossRef]
- Braden, L.; Michaud, D.; Igboeli, O.O.; Dondrup, M.; Hamre, L.; Dalvin, S.; Purcell, S.L.; Kongshaug, H.; Eichner, C.; Nilsen, F.; et al. Identification of critical enzymes in the salmon louse chitin synthesis pathway as revealed by RNA interference-mediated abrogation of infectivity. Int. J. Parasitol. 2020, 50, 873–889. [Google Scholar] [CrossRef]
- Zimoch, L.; Merzendorfer, H. Immunolocalization of chitin synthase in the tobacco hornworm. Cell Tissue Res. 2002, 308, 287–297. [Google Scholar] [CrossRef]
- Adler, P.N. The localization of chitin synthase mediates the patterned deposition of chitin in developing Drosophila bristles. Cold Spring Harb. Lab. 2019, 718841. [Google Scholar] [CrossRef]
- Li, X.; Diao, P.; Chu, J.; Zhou, G.; Zhou, J.; Lin, H.; Chen, J.; Zeng, Q. Molecular characterization and function of chitin deacetylase-like from the Chinese mitten crab, Eriocheir sinensis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2021, 256, 110612. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Y.; Zhou, M.; Tang, Y.; Chen, R.; Chen, Y.; Wen, Y.; Wang, S. RNAi-mediated CHS-2 silencing affects the synthesis of chitin and the formation of the peritrophic membrane in the midgut of Aedes albopictus larvae. Parasit. Vectors 2023, 16, 259. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Hou, X.; Wang, J.; Yue, W.; Huang, S.; Xu, G.; Yan, J.; Lu, G.; Hofreiter, M.; et al. “Omics” data unveil early molecular response underlying limb regeneration in the Chinese mitten crab, Eriocheir sinensis. Sci. Adv. 2022, 8, eabl4642. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Zhou, Q.; Tian, Y.; Zuo, J.; Yuan, Z.; Liu, Y.; Li, J.; Sun, J. Hippo Signaling Regulates Blastema Formation During Limb Regeneration in Chinese Mitten Crab (Eriocheir sinensis). Mar. Biotechnol. 2023, 25, 204–213. [Google Scholar] [CrossRef]
- Henriques, B.S.; Garcia, E.S.; Azambuja, P.; Genta, F.A. Determination of Chitin Content in Insects: An Alternate Method Based on Calcofluor Staining. Front. Physiol. 2020, 11, 117. [Google Scholar] [CrossRef]
- Flaven-Pouchon, J.; Moussian, B. Fluorescent Microscopy-Based Detection of Chitin in Intact Drosophila melanogaster. Front. Physiol. 2022, 13, 856369. [Google Scholar] [CrossRef]
- Zeng, B.; Chen, F.R.; Sun, H.; Liu, Y.; Wu, S.F.; Bass, C.; Gao, C.F. Molecular and functional analysis of chitin synthase genes in Chilo suppressalis (Lepidoptera: Crambidae). Insect Sci. 2023, 30, 661–676. [Google Scholar] [CrossRef]
- Hwang, D.S.; Lee, M.C.; Kyung, D.H.; Kim, H.S.; Han, J.; Kim, I.C.; Puthumana, J.; Lee, J.S. WAFs lead molting retardation of naupliar stages with down-regulated expression profiles of chitin metabolic pathway and related genes in the copepod Tigriopus japonicus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 193, 9–17. [Google Scholar] [CrossRef]
- Wang, D.; Yang, G.; Ru, S.; Zhang, Z.; Li, Y.; Wang, J. Herbicide prometryn adversely affects the development and reproduction of Tigriopus japonicus by disturbing the ecdysone signal pathway and chitin metabolic pathway. Aquat. Toxicol. 2023, 254, 106378. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Hu, Y.; Lu, S.; Deng, Y.; Zhang, H.; Zhao, Y.; Yu, Y.; Huang, H.; Zhou, J.; Li, X. Chitin Synthase Is Required for Cuticle Formation and Molting in the Chinese Mitten Crab Eriocheir sinensis. Int. J. Mol. Sci. 2025, 26, 2358. https://doi.org/10.3390/ijms26052358
Zhang T, Hu Y, Lu S, Deng Y, Zhang H, Zhao Y, Yu Y, Huang H, Zhou J, Li X. Chitin Synthase Is Required for Cuticle Formation and Molting in the Chinese Mitten Crab Eriocheir sinensis. International Journal of Molecular Sciences. 2025; 26(5):2358. https://doi.org/10.3390/ijms26052358
Chicago/Turabian StyleZhang, Ting, Yuning Hu, Siyu Lu, Yanfei Deng, Huimin Zhang, Yanhua Zhao, Yawen Yu, Hongbin Huang, Jun Zhou, and Xuguang Li. 2025. "Chitin Synthase Is Required for Cuticle Formation and Molting in the Chinese Mitten Crab Eriocheir sinensis" International Journal of Molecular Sciences 26, no. 5: 2358. https://doi.org/10.3390/ijms26052358
APA StyleZhang, T., Hu, Y., Lu, S., Deng, Y., Zhang, H., Zhao, Y., Yu, Y., Huang, H., Zhou, J., & Li, X. (2025). Chitin Synthase Is Required for Cuticle Formation and Molting in the Chinese Mitten Crab Eriocheir sinensis. International Journal of Molecular Sciences, 26(5), 2358. https://doi.org/10.3390/ijms26052358








