Differentially Expressed Genes in Rat Brain Regions with Different Degrees of Ischemic Damage
Abstract
1. Introduction
2. Results
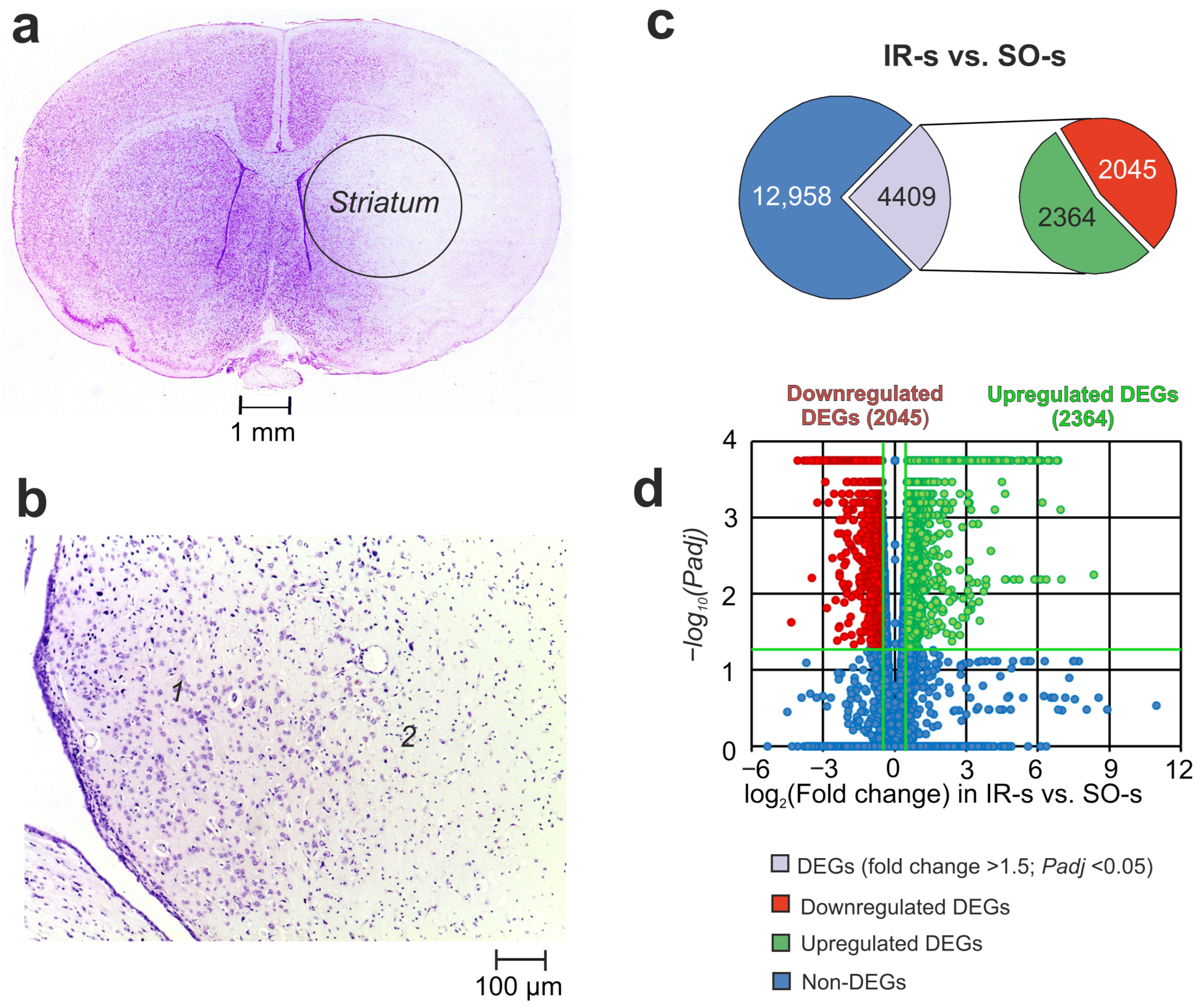
2.1. Histological Examination of Brain Samples
2.2. RNA-Seq Analysis of the Effect of IR on the Striatum of Rats 24 h After tMCAO
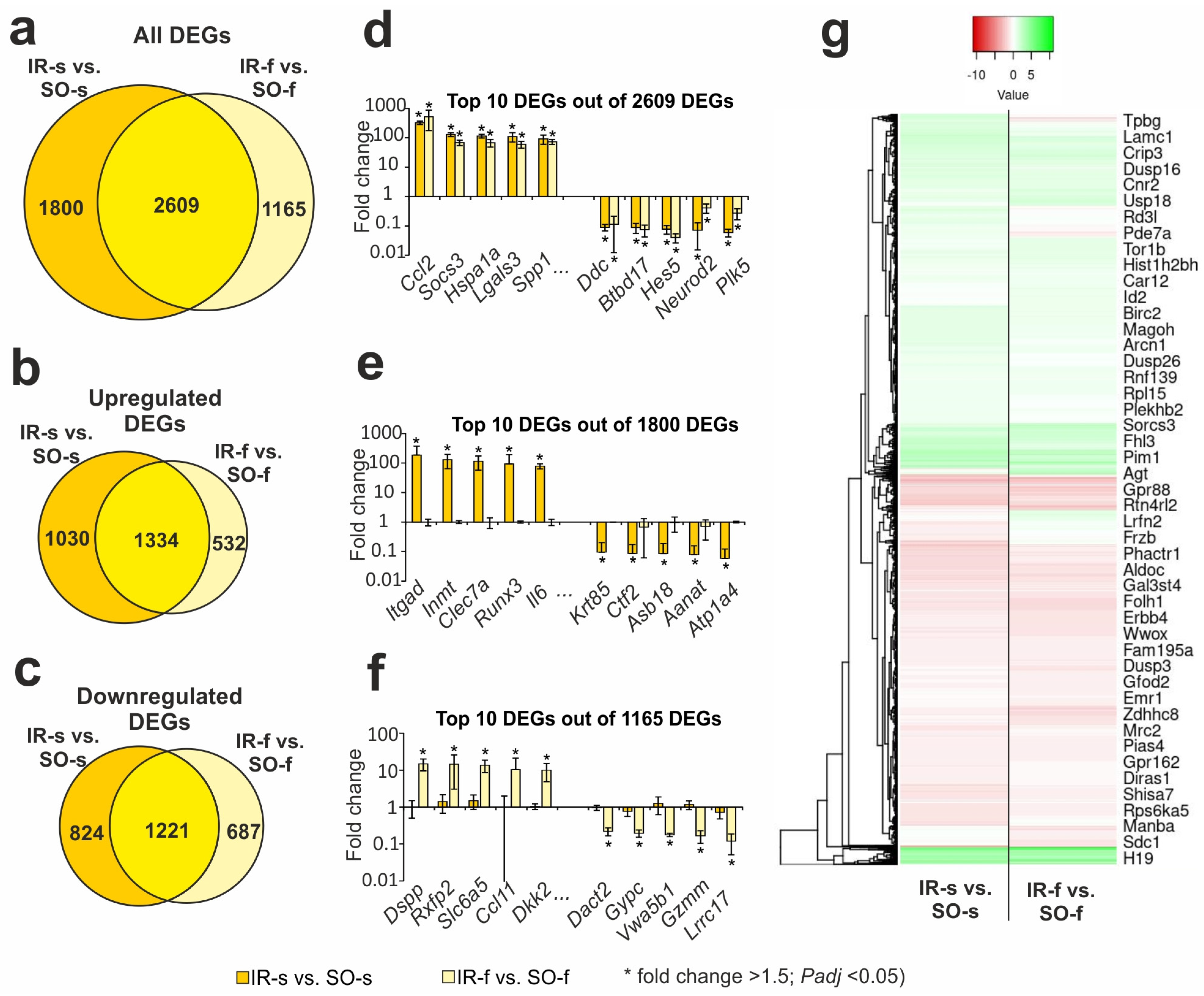
2.3. Comparison of RNA-Seq Results in Different Groups in Striatum and FC 24 h After tMCAO
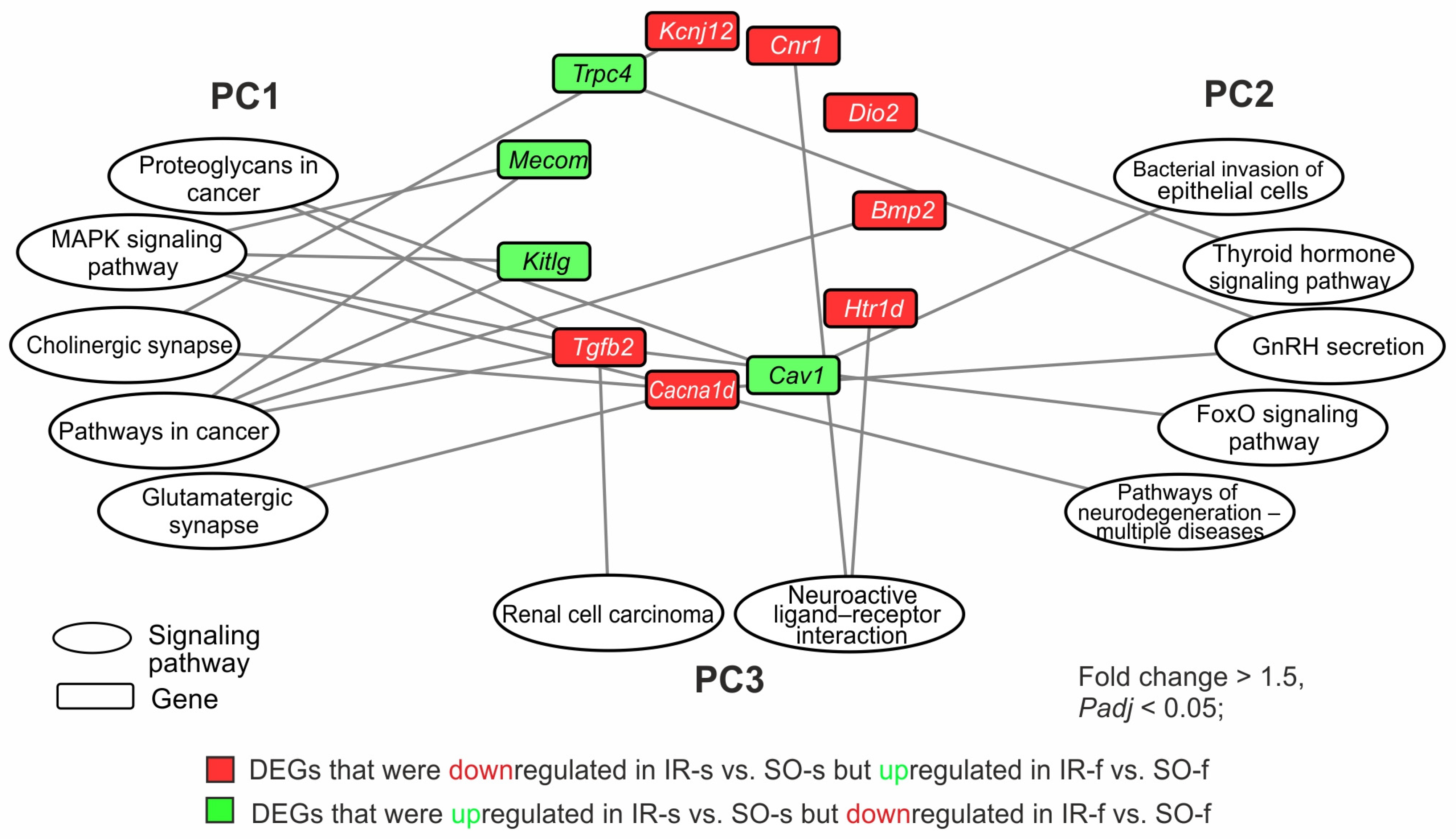
2.4. DEGs Exhibited Opposite Changes at the mRNA Level in the Two Brain Tissues 24 h After tMCAO
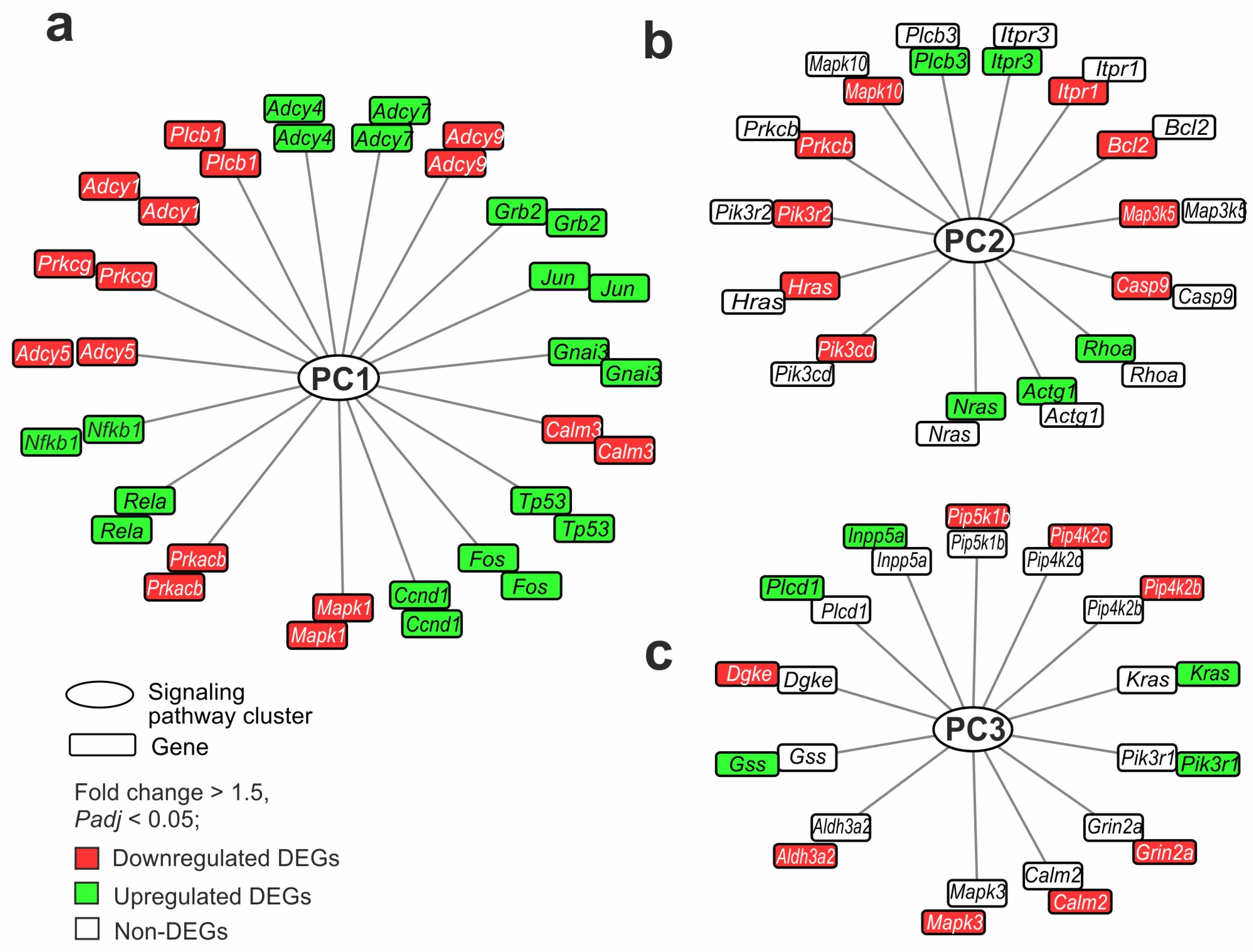
2.5. Functional Annotations of DEGs Altered in Different Comparison Groups
2.6. Gene Regulatory Networks Characterizing Common and Specific Effects of Ischemia on Striatum and FC Cells 24 h After tMCAO
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. tMCAO Model
4.3. Sample Collection and RNA Isolation
4.4. RNA-Seq
4.5. cDNA Synthesis and Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.6. RNA-Seq Data Analysis
4.7. Real-Time RT-PCR Data Analysis
4.8. Functional Analysis
4.9. Availability of Data and Material
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Capirossi, C.; Laiso, A.; Renieri, L.; Capasso, F.; Limbucci, N. Epidemiology, organization, diagnosis and treatment of acute ischemic stroke. Eur. J. Radiol. Open 2023, 11, 100527. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Santomauro, D.F.; Aali, A.; Abate, Y.H.; Abbafati, C.; Abbastabar, H.; Abd ElHafeez, S.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdollahi, A.; et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wang, W.; Zhang, Y.; Xiong, Y.; Tao, C.; Ma, L.; Ma, J.; You, C.; Wang, C. Global, Regional, and National Burden of Stroke, 1990–2021: A Systematic Analysis for Global Burden of Disease 2021. Stroke 2024, 55, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Filippenkov, I.B.; Khrunin, A.V.; Mozgovoy, I.V.; Dergunova, L.V.; Limborska, S.A. Are Ischemic Stroke and Alzheimer’s Disease Genetically Consecutive Pathologies? Biomedicines 2023, 11, 2727. [Google Scholar] [CrossRef]
- Elman-Shina, K.; Efrati, S. Ischemia as a common trigger for Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1012779. [Google Scholar] [CrossRef]
- Stephenson, D.T.; Rash, K.; Clemens, J.A. Amyloid precursor protein accumulates in regions of neurodegeneration following focal cerebral ischemia in the rat. Brain Res. 1992, 593, 128–135. [Google Scholar] [CrossRef]
- Qi, J.P.; Wu, H.; Yang, Y.; Wang, D.D.; Chen, Y.X.; Gu, Y.H.; Liu, T. Cerebral ischemia and Alzheimer’s disease: The expression of amyloid-β and apolipoprotein E in human hippocampus. J. Alzheimer’s Dis. 2007, 12, 335–341. [Google Scholar] [CrossRef]
- Ziaunys, M.; Sakalauskas, A.; Mikalauskaite, K.; Smirnovas, V. Polymorphism of alpha-synuclein amyloid fibrils depends on ionic strength and protein concentration. Int. J. Mol. Sci. 2021, 22, 12382. [Google Scholar] [CrossRef]
- Carapeto, A.P.; Marcuello, C.; Faísca, P.F.N.; Rodrigues, M.S. Morphological and Biophysical Study of S100A9 Protein Fibrils by Atomic Force Microscopy Imaging and Nanomechanical Analysis. Biomolecules 2024, 14, 1091. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Pan, Y.; Bai, B.; Chen, J. Global gene expression profile of cerebral ischemia-reperfusion injury in rat MCAO model. Oncotarget 2017, 8, 74607–74622. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini-Giampietro, D.E.; Bennett, M.V.L.; Zukin, R.S. Are Ca2+-permeable kainate/AMPA receptors more abundant in immature brain? Neurosci. Lett. 1992, 144, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Szydlowska, K.; Tymianski, M. Calcium, ischemia and excitotoxicity. Cell Calcium 2010, 47, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar] [CrossRef]
- Jurcau, A.; Ardelean, A.I. Oxidative Stress in Ischemia/Reperfusion Injuries following Acute Ischemic Stroke. Biomedicines 2022, 10, 574. [Google Scholar] [CrossRef]
- Lenglet, S.; Montecucco, F.; Mach, F.; Schaller, K.; Gasche, Y.; Copin, J.C. Analysis of the expression of nine secreted matrix metalloproteinases and their endogenous inhibitors in the brain of mice subjected to ischaemic stroke. Thromb. Haemost. 2014, 112, 363–378. [Google Scholar] [CrossRef]
- Kestner, R.I.; Mayser, F.; Vutukuri, R.; Hansen, L.; Günther, S.; Brunkhorst, R.; Devraj, K.; Pfeilschifter, W. Gene Expression Dynamics at the Neurovascular Unit During Early Regeneration After Cerebral Ischemia/Reperfusion Injury in Mice. Front. Neurosci. 2020, 14, 280. [Google Scholar] [CrossRef]
- Krueger, M.; Bechmann, I.; Immig, K.; Reichenbach, A.; Härtig, W.; Michalski, D. Blood-brain barrier breakdown involves four distinct stages of vascular damage in various models of experimental focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2015, 35, 292–303. [Google Scholar] [CrossRef]
- Bartelt-Kirbach, B.; Slowik, A.; Beyer, C.; Golenhofen, N. Upregulation and phosphorylation of HspB1/Hsp25 and HspB5/αB-crystallin after transient middle cerebral artery occlusion in rats. Cell Stress Chaperones 2017, 22, 653–663. [Google Scholar] [CrossRef]
- Li, W.; Liu, D.; Xu, J.; Zha, J.; Wang, C.; An, J.; Xie, Z.; Qiao, S. Astrocyte-Derived TNF-α-Activated Platelets Promote Cerebral Ischemia/Reperfusion Injury by Regulating the RIP1/RIP3/AKT Signaling Pathway. Mol. Neurobiol. 2022, 59, 5734–5749. [Google Scholar] [CrossRef]
- Dergunova, L.V.; Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Mozerov, S.A.; Gubsky, L.V.; Limborska, S.A. Genome-wide transcriptome analysis using RNA-Seq reveals a large number of differentially expressed genes in a transient MCAO rat model. BMC Genom. 2018, 19, 655. [Google Scholar] [CrossRef] [PubMed]
- Filippenkov, I.B.; Remizova, J.A.; Denisova, A.E.; Stavchansky, V.V.; Golovina, K.D.; Gubsky, L.V.; Limborska, S.A.; Dergunova, L.V. Differential gene expression in the contralateral hemisphere of the rat brain after focal ischemia. Sci. Rep. 2023, 13, 573. [Google Scholar] [CrossRef]
- Ford, G.; Xu, Z.; Gates, A.; Jiang, J.; Ford, B.D. Expression Analysis Systematic Explorer (EASE) analysis reveals differential gene expression in permanent and transient focal stroke rat models. Brain Res. 2006, 1071, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Jackman, K.; Kunz, A.; Iadecola, C. Modeling focal cerebral ischemia in vivo. In Neurodegeneration; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 793, pp. 195–209. [Google Scholar] [CrossRef]
- Ma, R.; Xie, Q.; Li, Y.; Chen, Z.; Ren, M.; Chen, H.; Li, H.; Li, J.; Wang, J. Animal models of cerebral ischemia: A review. Biomed. Pharmacother. 2020, 131, 110686. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xia, K.; Zhong, C.; Chen, X.; Yang, F.; Chen, L.; You, J. Neuroprotective effects of GPR68 against cerebral ischemia-reperfusion injury via the NF-κB/Hif-1α pathway. Brain Res. Bull. 2024, 216, 111050. [Google Scholar] [CrossRef]
- Poddar, R.; Rajagopal, S.; Winter, L.; Allan, A.M.; Paul, S. A peptide mimetic of tyrosine phosphatase STEP as a potential therapeutic agent for treatment of cerebral ischemic stroke. J. Cereb. Blood Flow Metab. 2019, 39, 1069–1084. [Google Scholar] [CrossRef]
- Guo, H.; Adah, D.; James, P.B.; Liu, Q.; Li, G.; Ahmadu, P.; Chai, L.; Wang, S.; Liu, Y.; Hu, L. Xueshuantong Injection (Lyophilized) Attenuates Cerebral Ischemia/Reperfusion Injury by the Activation of Nrf2-VEGF Pathway. Neurochem. Res. 2018, 43, 1096–1103. [Google Scholar] [CrossRef]
- He, Y.; Zhang, H.; Deng, J.; Cai, Z.; Gu, M.; Zhao, C.; Guo, Y. The functions of fluoxetine and identification of fluoxetine-mediated circular RNAs and messenger RNAs in cerebral ischemic stroke. Bioengineered 2021, 12, 2364–2376. [Google Scholar] [CrossRef]
- Filippenkov, I.B.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Sudarkina, O.Y.; Dmitrieva, V.G.; Gubsky, L.V.; Myasoedov, N.F.; Limborska, S.A.; et al. Novel Insights into the Protective Properties of ACTH(4-7)PGP (Semax) Peptide at the Transcriptome Level Following Cerebral Ischaemia–Reperfusion in Rats. Genes 2020, 11, 681. [Google Scholar] [CrossRef]
- Filippenkov, I.B.; Shpetko, Y.Y.; Stavchansky, V.V.; Denisova, A.E.; Gubsky, L.V.; Andreeva, L.A.; Myasoedov, N.F.; Limborska, S.A.; Dergunova, L.V. ACTH-like Peptides Compensate Rat Brain Gene Expression Profile Disrupted by Ischemia a Day After Experimental Stroke. Biomedicines 2024, 12, 2830. [Google Scholar] [CrossRef]
- Zucha, D.; Abaffy, P.; Kirdajova, D.; Jirak, D.; Kubista, M.; Anderova, M.; Valihrach, L. Spatiotemporal transcriptomic map of glial cell response in a mouse model of acute brain ischemia. Proc. Natl. Acad. Sci. USA 2024, 121, e2404203121. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Gong, Y.; Chang, W.; Yan, J.; Hu, Y.; Pan, Z.; Huang, P. Unraveling Spatiotemporal Metabolic Perturbation of Amino Acids Associated with Ischemia–Reperfusion Injury by MALDI MS Imaging. Mol. Neurobiol. 2024, 39621233. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Aswendt, M.; Lee, A.G.; Ishizaka, S.; Cao, Z.; Wang, E.H.; Levy, S.L.; Smerin, D.L.; McNab, J.A.; Zeineh, M.; et al. RNA-Sequencing Analysis Revealed a Distinct Motor Cortex Transcriptome in Spontaneously Recovered Mice After Stroke. Stroke 2018, 49, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Volume 7, pp. 113–170. [Google Scholar] [CrossRef]
- Adams, K.L.; Gallo, V. The diversity and disparity of the glial scar. Nat. Neurosci. 2018, 21, 9–15. [Google Scholar] [CrossRef]
- Kaya, T.; Mattugini, N.; Liu, L.; Ji, H.; Cantuti-Castelvetri, L.; Wu, J.; Schifferer, M.; Groh, J.; Martini, R.; Besson-Girard, S.; et al. CD8+ T cells induce interferon-responsive oligodendrocytes and microglia in white matter aging. Nat. Neurosci. 2022, 25, 1446–1457. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Corley, S.M.; MacKenzie, K.L.; Beverdam, A.; Roddam, L.F.; Wilkins, M.R. Differentially expressed genes from RNA-Seq and functional enrichment results are affected by the choice of single-end versus paired-end reads and stranded versus non-stranded protocols. BMC Genom. 2017, 18, 399. [Google Scholar] [CrossRef]
- Rosati, D.; Palmieri, M.; Brunelli, G.; Morrione, A.; Iannelli, F.; Frullanti, E.; Giordano, A. Differential gene expression analysis pipelines and bioinformatic tools for the identification of specific biomarkers: A review. Comput. Struct. Biotechnol. J. 2024, 23, 1154–1168. [Google Scholar] [CrossRef]
- Ryang, Y.-M.; Dang, J.; Kipp, M.; Petersen, K.-U.; Fahlenkamp, A.V.; Gempt, J.; Wesp, D.; Rossaint, R.; Beyer, C.; Coburn, M. Solulin reduces infarct volume and regulates gene-expression in transient middle cerebral artery occlusion in rats. BMC Neurosci. 2011, 12, 113. [Google Scholar] [CrossRef]
- Gass, P.; Spranger, M.; Herdegen, T.; Bravo, R.; Köck, P.; Hacke, W.; Kiessling, M. Induction of FOS and JUN proteins after focal ischemia in the rat: Differential effect of the N-methyl-d-aspartate receptor antagonist MK-801. Acta Neuropathol. 1992, 84, 545–553. [Google Scholar] [CrossRef]
- Almeida, A.; Sánchez-Morán, I.; Rodríguez, C. Mitochondrial–nuclear p53 trafficking controls neuronal susceptibility in stroke. IUBMB Life 2021, 73, 582–591. [Google Scholar] [CrossRef]
- Maes, M.; Nikiforov, N.G.; Plaimas, K.; Suratanee, A.; Alfieri, D.F.; Reiche, E.M.V. New drug targets to prevent death due to stroke: A review based on results of protein-protein interaction network, enrichment, and annotation analyses. Int. J. Mol. Sci. 2021, 22, 12108. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C.J.; Nwaolu, U.; Martin, L.; Huang, B.J.; Mang, J.; Salinas, D.; Schlaff, C.D.; Ghenbot, S.; Lansford, J.L.; Potter, B.K.; et al. Systemic inflammation following traumatic injury and its impact on neuroinflammatory gene expression in the rodent brain. J. Neuroinflamm. 2024, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Noda, M. Thyroid Hormone in the CNS: Contribution of Neuron–Glia Interaction. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2018; Volume 106, pp. 313–331. [Google Scholar] [CrossRef]
- Shafia, S.; Khoramirad, A.; Akhoundzadeh, K. Thyroid hormones and stroke, the gap between clinical and experimental studies. Brain Res. Bull. 2024, 213, 110983. [Google Scholar] [CrossRef] [PubMed]
- Gkantzios, A.; Karapepera, V.; Tsiptsios, D.; Liaptsi, E.; Christidi, F.; Gkartzonika, E.; Karatzetzou, S.; Kokkotis, C.; Kyrtsopoulos, M.; Tsiakiri, A.; et al. Investigating the Predictive Value of Thyroid Hormone Levels for Stroke Prognosis. Neurol. Int. 2023, 15, 926–953. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, Y.H.; Wang, X.M.; Chen, H.S. PI3Kδ inhibition alleviates the brain injury during cerebral ischemia reperfusion via suppressing pericyte contraction in a TNF-α dependent manner. Exp. Neurol. 2024, 375, 114728. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Yin, J.; Su, D.; Pan, Z.; Li, P.; Wang, X. MicroRNA-15b deteriorates hypoxia/reoxygenation-induced cardiomyocyte apoptosis by downregulating Bcl-2 and MAPK3. J. Investig. Med. 2018, 66, 39–45. [Google Scholar] [CrossRef]
- Minutoli, L.; Antonuccio, P.; Polito, F.; Bitto, A.; Squadrito, F.; Di Stefano, V.; Nicotina, P.A.; Fazzari, C.; Maisano, D.; Romeo, C.; et al. Mitogen-activated protein kinase 3/mitogen-activated protein kinase 1 activates apoptosis during testicular ischemia-reperfusion injury in a nuclear factor-κB-independent manner. Eur. J. Pharmacol. 2009, 604, 27–35. [Google Scholar] [CrossRef]
- Wang, H.; Pang, W.; Xu, X.; You, B.; Zhang, C.; Li, D. Cryptotanshinone Attenuates Ischemia/Reperfusion-induced Apoptosis in Myocardium by Upregulating MAPK3. J. Cardiovasc. Pharmacol. 2021, 77, 370–377. [Google Scholar] [CrossRef]
- Zhao, L.; Ding, L.D.; Xia, Z.H.; Sheng, P.; Shen, M.M.; Cai, Z.M.; Yan, B.C. A Network-Based Approach to Investigate the Neuroprotective Effects and Mechanisms of Action of Huangqi-Chuanxiong and Sanleng-Ezhu Herb Pairs in the Treatment of Cerebral Ischemic Stroke. Front. Pharmacol. 2022, 13, 844186. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, Y.; Pang, X.; Wang, X.; Zeng, H. Mechanisms of polydatin against spinal cord ischemia–reperfusion injury based on network pharmacology, molecular docking and molecular dynamics simulation. Bioorganic Chem. 2023, 140, 106840. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhu, R.; Peng, J.; Liu, H.; Pan, W.; Jin, Y.; Pei, J.; Zhang, L. Molecular mechanisms and potential targets of lycopene for alleviating renal ischemia-reperfusion injury revealed by network pharmacology and animal experiments. Int. Immunopharmacol. 2024, 143, 113421. [Google Scholar] [CrossRef] [PubMed]
- Hossmann, K.A. The two pathophysiologies of focal brain ischemia: Implications for translational stroke research. J. Cereb. Blood Flow Metab. 2012, 32, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Kossatz, E.; Silva-Peña, D.; Suárez, J.; de Fonseca, F.R.; Maldonado, R.; Robledo, P. Octadecylpropyl sulfamide reduces neurodegeneration and restores the memory deficits induced by hypoxia-ischemia in mice. Front. Pharmacol. 2018, 9, 376. [Google Scholar] [CrossRef]
- Zhang, M.; Martin, B.R.; Adler, M.W.; Razdan, R.K.; Ganea, D.; Tuma, R.F. Modulation of the balance between cannabinoid CB1 and CB2 receptor activation during cerebral ischemic/reperfusion injury. Neuroscience 2008, 152, 753–760. [Google Scholar] [CrossRef]
- Gereben, B.; Salvatore, D. Pretranslational regulation of type 2 deiodinase. Thyroid 2005, 15, 855–864. [Google Scholar] [CrossRef]
- Taroza, S.; Rastenytė, D.; Burkauskas, J.; Podlipskytė, A.; Kažukauskienė, N.; Patamsytė, V.; Mickuvienė, N. Deiodinases, organic anion transporter polypeptide polymorphisms and symptoms of anxiety and depression after ischemic stroke. J. Stroke Cerebrovasc. Dis. 2020, 29, 105040. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Z.; Luo, W.; Li, J.; Liao, J.; Chen, F.; Wang, D.; Lin, Y. Identifying immune cell infiltration and effective diagnostic biomarkers for ischemic stroke using bioinformatics analysis. PLoS ONE 2024, 19, e0310108. [Google Scholar] [CrossRef]
- Sun, K.; Li, H.; Dong, Y.; Cao, L.; Li, D.; Li, J.; Zhang, M.; Yan, D.; Yang, B. The Use of Identified Hypoxia-related Genes to Generate Models for Predicting the Prognosis of Cerebral Ischemia–reperfusion Injury and Developing Treatment Strategies. Mol. Neurobiol. 2024, 62, 3098–3124. [Google Scholar] [CrossRef]
- Cong, L.; He, Y.; Wu, Y.; Li, Z.; Ding, S.; Liang, W.; Xiao, X.; Zhang, H.; Wang, L. Discovery and validation of molecular patterns and immune characteristics in the peripheral blood of ischemic stroke patients. PeerJ 2024, 12, e17208. [Google Scholar] [CrossRef]
- Lv, M.; He, W.; Liang, T.; Yang, J.; Huang, X.; Liu, S.; Liang, X.; Long, J.; Su, L. Exploring biomarkers for ischemic stroke through integrated microarray data analysis. Brain Res. 2022, 1790, 147982. [Google Scholar] [CrossRef] [PubMed]
- Khrunin, A.V.; Khvorykh, G.V.; Arapova, A.S.; Kulinskaya, A.E.; Koltsova, E.A.; Petrova, E.A.; Kimelfeld, E.I.; Limborska, S.A. The Study of the Association of Polymorphisms in LSP1, GPNMB, PDPN, TAGLN, TSPO, and TUBB6 Genes with the Risk and Outcome of Ischemic Stroke in the Russian Population. Int. J. Mol. Sci. 2023, 24, 6831. [Google Scholar] [CrossRef] [PubMed]
- Khrunin, A.V.; Khvorykh, G.V.; Rozhkova, A.V.; Koltsova, E.A.; Petrova, E.A.; Kimelfeld, E.I.; Limborska, S.A. Examination of Genetic Variants Revealed from a Rat Model of Brain Ischemia in Patients with Ischemic Stroke: A Pilot Study. Genes 2021, 12, 1938. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Niu, L.; Feng, P.; Liu, S.; Han, H.; Zhang, B.; Wang, Y.; Wang, M. Identification of Novel Biomarkers for Ischemic Stroke Through Integrated Bioinformatics Analysis and Machine Learning. J. Mol. Neurosci. 2025, 75, 13. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, T.; Wang, C.; Lin, Y.; Hu, W.; Yue, M.; Li, H. Revealing the potential role of hub metabolism-related genes and their correlation with immune cells in acute ischemic stroke. IET Syst. Biol. 2024, 18, 129–142. [Google Scholar] [CrossRef]
- Filippenkov, I.B.; Remizova, J.A.; Denisova, A.E.; Stavchansky, V.V.; Golovina, K.D.; Gubsky, L.V.; Limborska, S.A.; Dergunova, L.V. Comparative Use of Contralateral and Sham-Operated Controls Reveals Traces of a Bilateral Genetic Response in the Rat Brain after Focal Stroke. Int. J. Mol. Sci. 2022, 23, 7308. [Google Scholar] [CrossRef]
- Koizumi, J.-I.; Yoshida, Y.; Nakazawa, T.; Ooneda, G. Experimental studies of ischemic brain edema. Jpn. J. Stroke 1986, 8, 1–8. [Google Scholar] [CrossRef]
- Stavchansky, V.V.; Yuzhakov, V.V.; Sevan’kaeva, L.E.; Fomina, N.K.; Koretskaya, A.E.; Denisova, A.E.; Mozgovoy, I.V.; Gubsky, L.V.; Filippenkov, I.B.; Myasoedov, N.F.; et al. Melanocortin Derivatives Induced Vascularization and Neuroglial Proliferation in the Rat Brain under Conditions of Cerebral Ischemia. Curr. Issues Mol. Biol. 2024, 46, 2071–2092. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software Environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: https://dataview.ncbi.nlm.nih.gov/object/PRJNA1119923?reviewer=plp0lftpig94lmq8vmup84at3d (accessed on 25 June 2024).
- National Center for Biotechnology Information. Available online: https://dataview.ncbi.nlm.nih.gov/object/PRJNA1128447?reviewer=vkci32ofqame6mkpbivjt1pqn8 (accessed on 25 June 2024).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippenkov, I.B.; Shpetko, Y.Y.; Stavchansky, V.V.; Denisova, A.E.; Yuzhakov, V.V.; Fomina, N.K.; Gubsky, L.V.; Limborska, S.A.; Dergunova, L.V. Differentially Expressed Genes in Rat Brain Regions with Different Degrees of Ischemic Damage. Int. J. Mol. Sci. 2025, 26, 2347. https://doi.org/10.3390/ijms26052347
Filippenkov IB, Shpetko YY, Stavchansky VV, Denisova AE, Yuzhakov VV, Fomina NK, Gubsky LV, Limborska SA, Dergunova LV. Differentially Expressed Genes in Rat Brain Regions with Different Degrees of Ischemic Damage. International Journal of Molecular Sciences. 2025; 26(5):2347. https://doi.org/10.3390/ijms26052347
Chicago/Turabian StyleFilippenkov, Ivan B., Yana Yu. Shpetko, Vasily V. Stavchansky, Alina E. Denisova, Vadim V. Yuzhakov, Natalia K. Fomina, Leonid V. Gubsky, Svetlana A. Limborska, and Lyudmila V. Dergunova. 2025. "Differentially Expressed Genes in Rat Brain Regions with Different Degrees of Ischemic Damage" International Journal of Molecular Sciences 26, no. 5: 2347. https://doi.org/10.3390/ijms26052347
APA StyleFilippenkov, I. B., Shpetko, Y. Y., Stavchansky, V. V., Denisova, A. E., Yuzhakov, V. V., Fomina, N. K., Gubsky, L. V., Limborska, S. A., & Dergunova, L. V. (2025). Differentially Expressed Genes in Rat Brain Regions with Different Degrees of Ischemic Damage. International Journal of Molecular Sciences, 26(5), 2347. https://doi.org/10.3390/ijms26052347






