Unraveling the Roles of UBE3A in Neurodevelopment and Neurodegeneration
Abstract
:1. Introduction
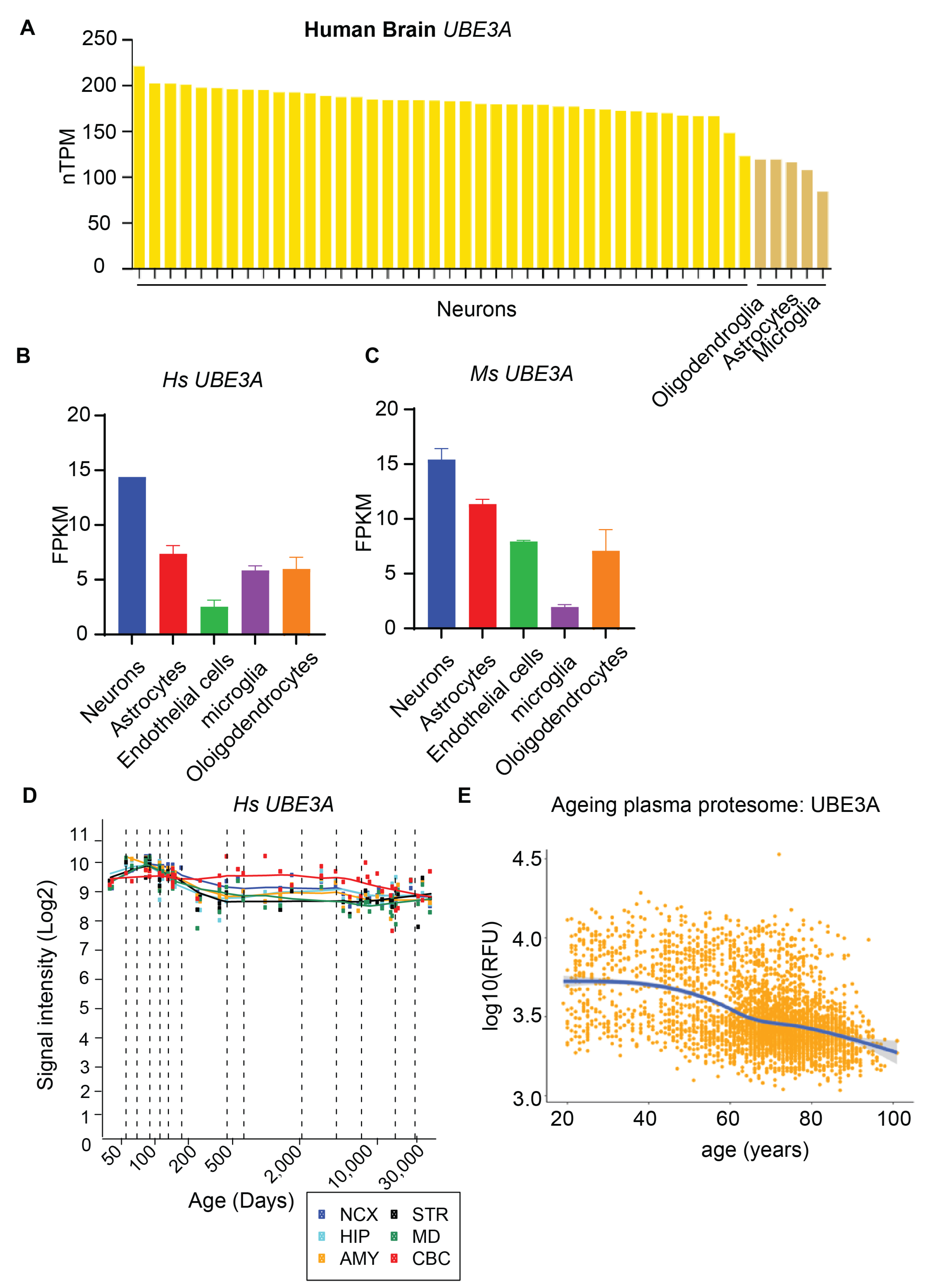
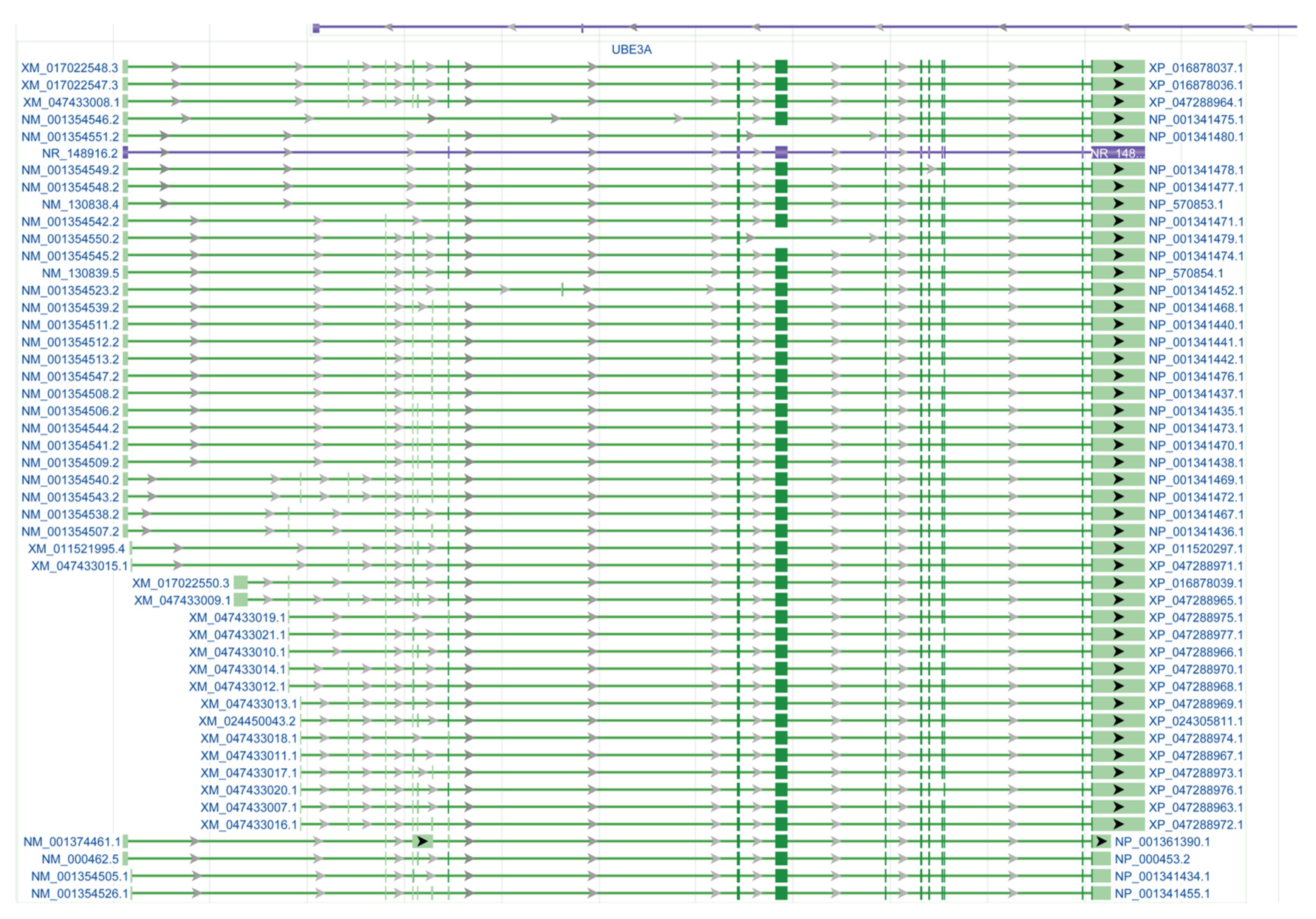
2. UBE3A Expression
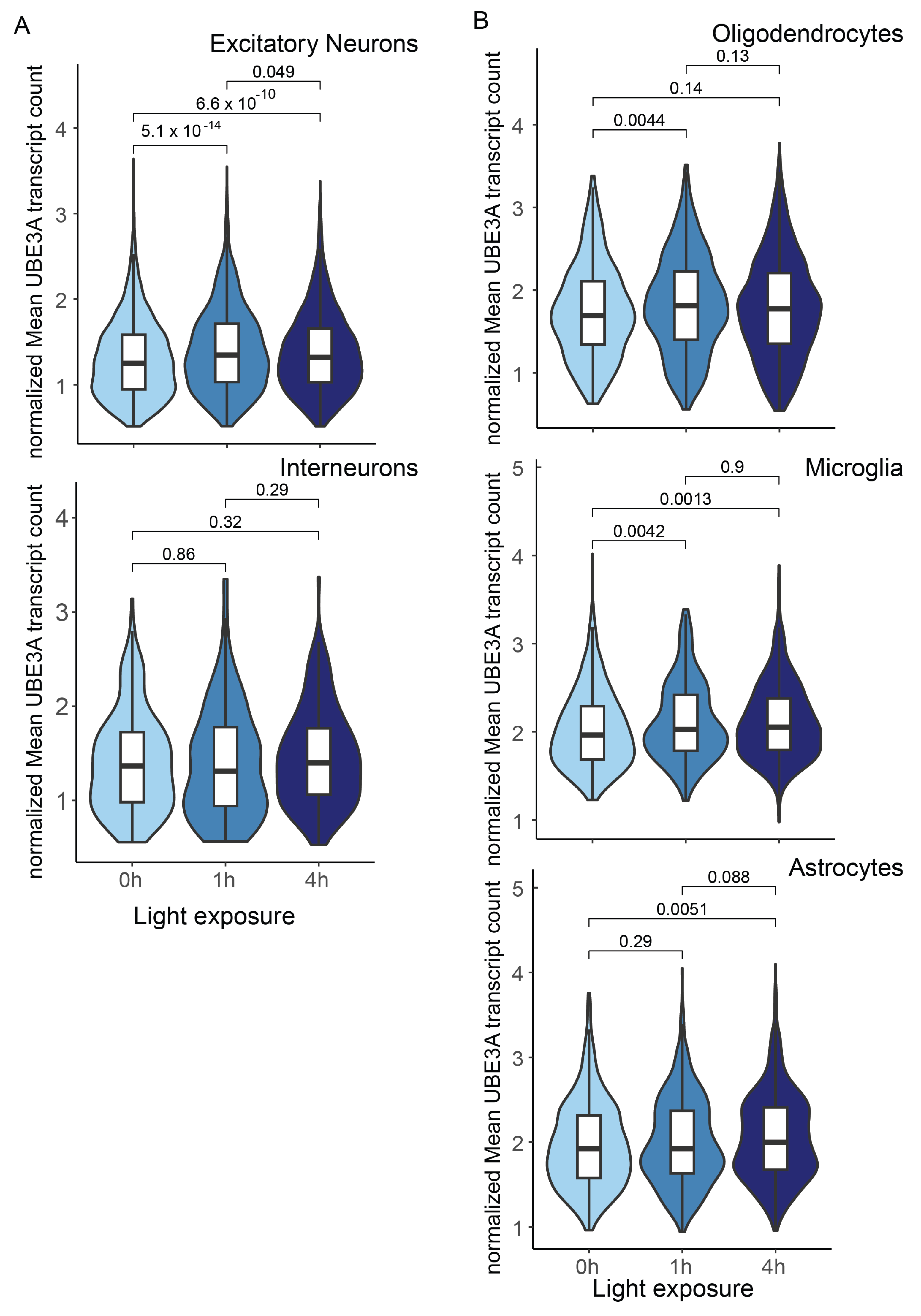
3. The Activity-Dependent UBE3A Expression in Neurons
4. The Unconventional Secretory Form of UBE3A
5. The Roles of UBE3A During Neurodevelopment
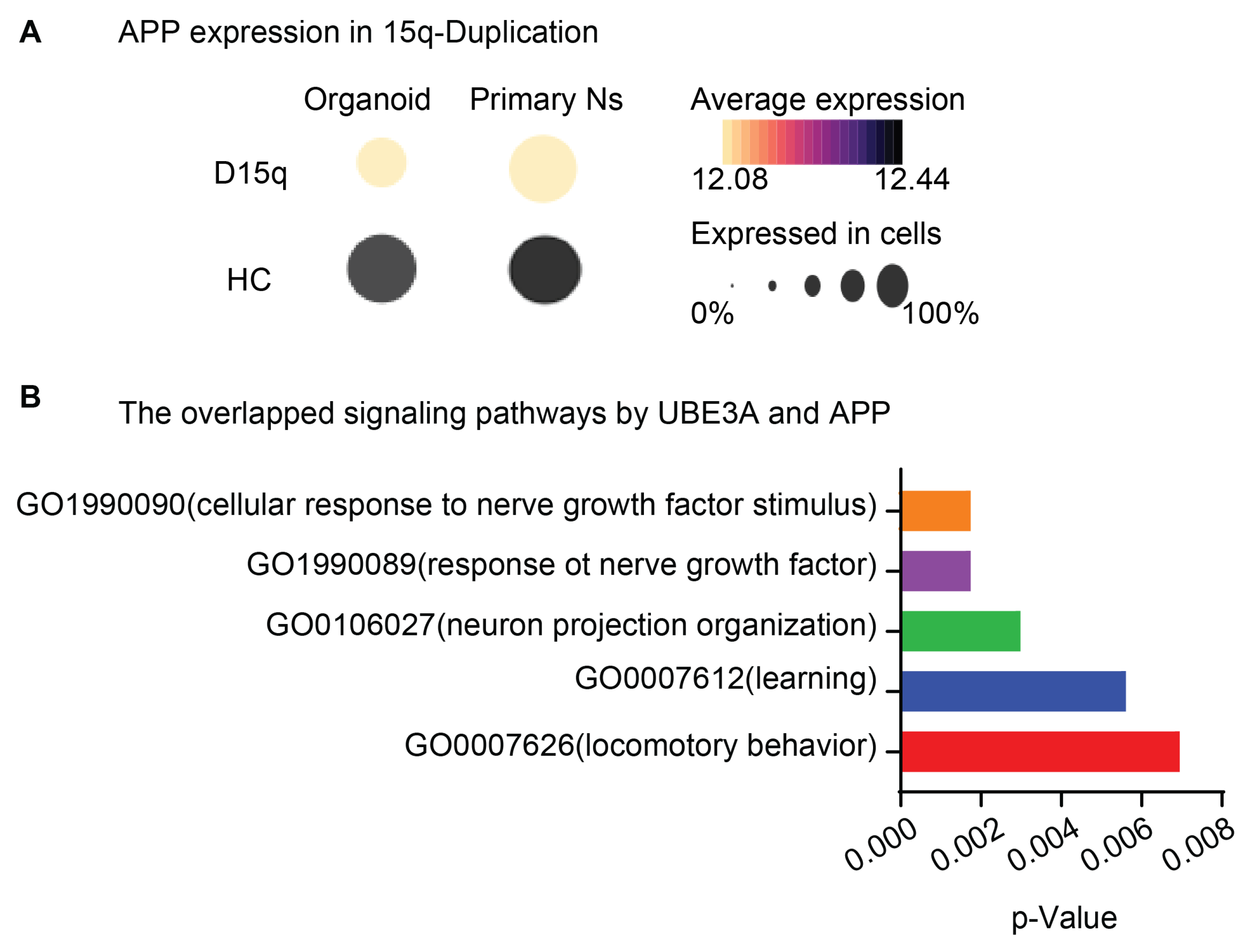
6. The Roles of UBE3A in Neurodegeneration
7. The Glial Function of UBE3A: Neuronal Spine Morphology, Seizures, and More
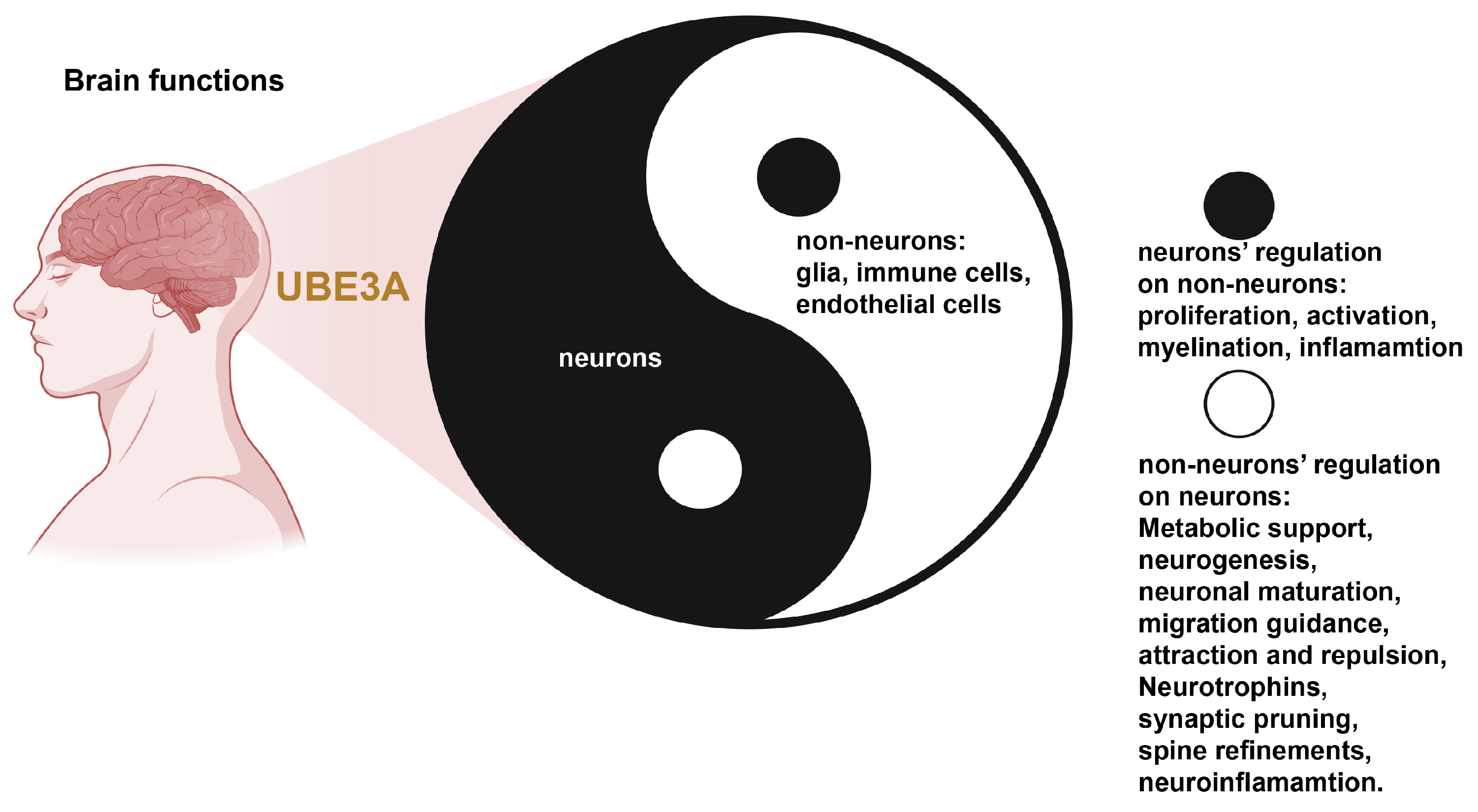
8. Conclusions: A Yin–Yang Model of UBE3A Functions in Neurons and Glia
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khago, D.; Fucci, I.J.; Byrd, R.A. The Role of Conformational Dynamics in the Recognition and Regulation of Ubiquitination. Molecules 2020, 25, 5933. [Google Scholar] [CrossRef]
- Barroso-Gomila, O.; Merino-Cacho, L.; Muratore, V.; Perez, C.; Taibi, V.; Maspero, E.; Azkargorta, M.; Iloro, I.; Trulsson, F.; Vertegaal, A.C.O.; et al. BioE3 identifies specific substrates of ubiquitin E3 ligases. Nat. Commun. 2023, 14, 7656. [Google Scholar]
- Chaudhary, P.; Proulx, J.; Park, I.W. Ubiquitin-protein ligase E3A (UBE3A) mediation of viral infection and human diseases. Virus Res. 2023, 335, 199191. [Google Scholar] [PubMed]
- Owais, A.; Mishra, R.K.; Kiyokawa, H. The HECT E3 Ligase E6AP/UBE3A as a Therapeutic Target in Cancer and Neurological Disorders. Cancers 2020, 12, 2108. [Google Scholar] [CrossRef]
- Samanta, D. Epilepsy in Angelman syndrome: A scoping review. Brain Dev. 2021, 43, 32–44. [Google Scholar] [PubMed]
- Chamberlain, S.J.; Brannan, C.I. The Prader-Willi syndrome imprinting center activates the paternally expressed murine Ube3a antisense transcript but represses paternal Ube3a. Genomics 2001, 73, 316–322. [Google Scholar] [PubMed]
- Rougeulle, C.; Glatt, H.; Lalande, M. The Angelman syndrome candidate gene, UBE3A/E6-AP, is imprinted in brain. Nat. Genet. 1997, 17, 14–15. [Google Scholar]
- Judson, M.C.; Sosa-Pagan, J.O.; Del Cid, W.A.; Han, J.E.; Philpot, B.D. Allelic specificity of Ube3a expression in the mouse brain during postnatal development. J. Comp. Neurol. 2014, 522, 1874–1896. [Google Scholar]
- Dindot, S.V.; Christian, S.; Murphy, W.J.; Berent, A.; Panagoulias, J.; Schlafer, A.; Ballard, J.; Radeva, K.; Robinson, R.; Myers, L.; et al. An ASO therapy for Angelman syndrome that targets an evolutionarily conserved region at the start of the UBE3A-AS transcript. Sci. Transl. Med. 2023, 15, eabf4077. [Google Scholar]
- Meng, L.; Ward, A.J.; Chun, S.; Bennett, C.F.; Beaudet, A.L.; Rigo, F. Towards a therapy for Angelman syndrome by targeting a long non-coding RNA. Nature 2015, 518, 409–412. [Google Scholar]
- Huang, H.S.; Allen, J.A.; Mabb, A.M.; King, I.F.; Miriyala, J.; Taylor-Blake, B.; Sciaky, N.; Dutton, J.W., Jr.; Lee, H.M.; Chen, X.; et al. Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature 2011, 481, 185–189. [Google Scholar] [PubMed]
- Meng, L.; Person, R.E.; Huang, W.; Zhu, P.J.; Costa-Mattioli, M.; Beaudet, A.L. Truncation of Ube3a-ATS unsilences paternal Ube3a and ameliorates behavioral defects in the Angelman syndrome mouse model. PLoS Genet. 2013, 9, e1004039. [Google Scholar]
- Vihma, H.; Li, K.; Welton-Arndt, A.; Smith, A.L.; Bettadapur, K.R.; Gilmore, R.B.; Gao, E.; Cotney, J.L.; Huang, H.C.; Collins, J.L.; et al. Ube3a unsilencer for the potential treatment of Angelman syndrome. Nat. Commun. 2024, 15, 5558. [Google Scholar] [PubMed]
- Offensperger, F.; Muller, F.; Jansen, J.; Hammler, D.; Gotz, K.H.; Marx, A.; Sirois, C.L.; Chamberlain, S.J.; Stengel, F.; Scheffner, M. Identification of Small-Molecule Activators of the Ubiquitin Ligase E6AP/UBE3A and Angelman Syndrome-Derived E6AP/UBE3A Variants. Cell Chem. Biol. 2020, 27, 1510–1520.e1516. [Google Scholar]
- Huang, B.; Zhou, L.; Liu, R.; Wang, L.; Xue, S.; Shi, Y.; Jeong, G.H.; Jeong, I.H.; Li, S.; Yin, J.; et al. Activation of E6AP/UBE3A-Mediated Protein Ubiquitination and Degradation Pathways by a Cyclic gamma-AA Peptide. J. Med. Chem. 2022, 65, 2497–2506. [Google Scholar]
- Weston, K.P.; Gao, X.; Zhao, J.; Kim, K.S.; Maloney, S.E.; Gotoff, J.; Parikh, S.; Leu, Y.C.; Wu, K.P.; Shinawi, M.; et al. Identification of disease-linked hyperactivating mutations in UBE3A through large-scale functional variant analysis. Nat. Commun. 2021, 12, 6809. [Google Scholar]
- Salminen, I.; Read, S.; Hurd, P.; Crespi, B. Genetic variation of UBE3A is associated with schizotypy in a population of typical individuals. Psychiatry Res. 2019, 275, 94–99. [Google Scholar]
- Maheshwari, M.; Shekhar, S.; Singh, B.K.; Jamal, I.; Vatsa, N.; Kumar, V.; Sharma, A.; Jana, N.R. Deficiency of Ube3a in Huntington’s disease mice brain increases aggregate load and accelerates disease pathology. Hum. Mol. Genet. 2014, 23, 6235–6245. [Google Scholar]
- Bhat, K.P.; Yan, S.; Wang, C.E.; Li, S.; Li, X.J. Differential ubiquitination and degradation of huntingtin fragments modulated by ubiquitin-protein ligase E3A. Proc. Natl. Acad. Sci. USA 2014, 111, 5706–5711. [Google Scholar]
- Gu, X.; Hou, Y.; Chen, Y.; Ou, R.; Cao, B.; Wei, Q.; Zhang, L.; Song, W.; Zhao, B.; Wu, Y.; et al. Enrichment of rare variants in E3 ubiquitin ligase genes in Early onset Parkinson’s disease. Neurobiol. Aging 2022, 109, 273–278. [Google Scholar]
- Olabarria, M.; Pasini, S.; Corona, C.; Robador, P.; Song, C.; Patel, H.; Lefort, R. Dysfunction of the ubiquitin ligase E3A Ube3A/E6-AP contributes to synaptic pathology in Alzheimer’s disease. Commun. Biol. 2019, 2, 111. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Vatsa, N.; Kumar, V.; Shekhar, S.; Sharma, A.; Jana, N.R. Ube3a deficiency inhibits amyloid plaque formation in APPswe/PS1deltaE9 mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2017, 26, 4042–4054. [Google Scholar] [CrossRef] [PubMed]
- Erickson, C.A.; Wink, L.K.; Baindu, B.; Ray, B.; Schaefer, T.L.; Pedapati, E.V.; Lahiri, D.K. Analysis of peripheral amyloid precursor protein in Angelman Syndrome. Am. J. Med. Genet. A 2016, 170, 2334–2337. [Google Scholar] [CrossRef] [PubMed]
- Sonzogni, M.; Zhai, P.; Mientjes, E.J.; van Woerden, G.M.; Elgersma, Y. Assessing the requirements of prenatal UBE3A expression for rescue of behavioral phenotypes in a mouse model for Angelman syndrome. Mol. Autism 2020, 11, 70. [Google Scholar] [CrossRef]
- Silva-Santos, S.; van Woerden, G.M.; Bruinsma, C.F.; Mientjes, E.; Jolfaei, M.A.; Distel, B.; Kushner, S.A.; Elgersma, Y. Ube3a reinstatement identifies distinct developmental windows in a murine Angelman syndrome model. J. Clin. Investig. 2015, 125, 2069–2076. [Google Scholar] [CrossRef]
- Gu, B.; Carstens, K.E.; Judson, M.C.; Dalton, K.A.; Rougie, M.; Clark, E.P.; Dudek, S.M.; Philpot, B.D. Ube3a reinstatement mitigates epileptogenesis in Angelman syndrome model mice. J. Clin. Investig. 2019, 129, 163–168. [Google Scholar] [CrossRef]
- Wolter, J.M.; Mao, H.; Fragola, G.; Simon, J.M.; Krantz, J.L.; Bazick, H.O.; Oztemiz, B.; Stein, J.L.; Zylka, M.J. Cas9 gene therapy for Angelman syndrome traps Ube3a-ATS long non-coding RNA. Nature 2020, 587, 281–284. [Google Scholar] [CrossRef]
- Grier, M.D.; Carson, R.P.; Lagrange, A.H. Toward a Broader View of Ube3a in a Mouse Model of Angelman Syndrome: Expression in Brain, Spinal Cord, Sciatic Nerve and Glial Cells. PLoS ONE 2015, 10, e0124649. [Google Scholar] [CrossRef]
- Burette, A.C.; Judson, M.C.; Li, A.N.; Chang, E.F.; Seeley, W.W.; Philpot, B.D.; Weinberg, R.J. Subcellular organization of UBE3A in human cerebral cortex. Mol. Autism 2018, 9, 54. [Google Scholar]
- Williams, K.; Irwin, D.A.; Jones, D.G.; Murphy, K.M. Dramatic Loss of Ube3A Expression during Aging of the Mammalian Cortex. Front. Aging Neurosci. 2010, 2, 18. [Google Scholar] [CrossRef]
- Avagliano Trezza, R.; Sonzogni, M.; Bossuyt, S.N.V.; Zampeta, F.I.; Punt, A.M.; van den Berg, M.; Rotaru, D.C.; Koene, L.M.C.; Munshi, S.T.; Stedehouder, J.; et al. Loss of nuclear UBE3A causes electrophysiological and behavioral deficits in mice and is associated with Angelman syndrome. Nat. Neurosci. 2019, 22, 1235–1247. [Google Scholar] [PubMed]
- Valluy, J.; Bicker, S.; Aksoy-Aksel, A.; Lackinger, M.; Sumer, S.; Fiore, R.; Wust, T.; Seffer, D.; Metge, F.; Dieterich, C.; et al. A coding-independent function of an alternative Ube3a transcript during neuronal development. Nat. Neurosci. 2015, 18, 666–673. [Google Scholar] [PubMed]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [PubMed]
- Greer, P.L.; Hanayama, R.; Bloodgood, B.L.; Mardinly, A.R.; Lipton, D.M.; Flavell, S.W.; Kim, T.K.; Griffith, E.C.; Waldon, Z.; Maehr, R.; et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell 2010, 140, 704–716. [Google Scholar]
- Filonova, I.; Trotter, J.H.; Banko, J.L.; Weeber, E.J. Activity-dependent changes in MAPK activation in the Angelman Syndrome mouse model. Learn. Mem. 2014, 21, 98–104. [Google Scholar]
- Nawaz, Z.; Lonard, D.M.; Smith, C.L.; Lev-Lehman, E.; Tsai, S.Y.; Tsai, M.J.; O’Malley, B.W. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol. Cell. Biol. 1999, 19, 1182–1189. [Google Scholar]
- Olivares, A.M.; Moreno-Ramos, O.A.; Haider, N.B. Role of Nuclear Receptors in Central Nervous System Development and Associated Diseases. J. Exp. Neurosci. 2015, 9 (Suppl. S2), 93–121. [Google Scholar]
- Chen, J.F.; Liu, K.; Hu, B.; Li, R.R.; Xin, W.; Chen, H.; Wang, F.; Chen, L.; Li, R.X.; Ren, S.Y.; et al. Enhancing myelin renewal reverses cognitive dysfunction in a murine model of Alzheimer’s disease. Neuron 2021, 109, 2292–2307.e2295. [Google Scholar]
- Ren, S.Y.; Xia, Y.; Yu, B.; Lei, Q.J.; Hou, P.F.; Guo, S.; Wu, S.L.; Liu, W.; Yang, S.F.; Jiang, Y.B.; et al. Growth hormone promotes myelin repair after chronic hypoxia via triggering pericyte-dependent angiogenesis. Neuron 2024, 112, 2177–2196 e2176. [Google Scholar]
- Montani, C.; Balasco, L.; Pagani, M.; Alvino, F.G.; Barsotti, N.; de Guzman, A.E.; Galbusera, A.; de Felice, A.; Nickl-Jockschat, T.K.; Migliarini, S.; et al. Sex-biasing influence of autism-associated Ube3a gene overdosage at connectomic, behavioral, and transcriptomic levels. Sci. Adv. 2024, 10, eadg1421. [Google Scholar] [CrossRef] [PubMed]
- Yang, X. Characterizing spine issues: If offers novel therapeutics to Angelman syndrome. Dev. Neurobiol. 2020, 80, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Yashiro, K.; Riday, T.T.; Condon, K.H.; Roberts, A.C.; Bernardo, D.R.; Prakash, R.; Weinberg, R.J.; Ehlers, M.D.; Philpot, B.D. Ube3a is required for experience-dependent maturation of the neocortex. Nat. Neurosci. 2009, 12, 777–783. [Google Scholar] [PubMed]
- Dindot, S.V.; Antalffy, B.A.; Bhattacharjee, M.B.; Beaudet, A.L. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum. Mol. Genet. 2008, 17, 111–118. [Google Scholar]
- Hrvatin, S.; Hochbaum, D.R.; Nagy, M.A.; Cicconet, M.; Robertson, K.; Cheadle, L.; Zilionis, R.; Ratner, A.; Borges-Monroy, R.; Klein, A.M.; et al. Single-cell analysis of experience-dependent transcriptomic states in the mouse visual cortex. Nat. Neurosci. 2018, 21, 120–129. [Google Scholar]
- Lin, C.W.; Cheng, Y.C.; Yang, C.H.; Huang, H.S. Light activates Ube3a, an Angelman syndrome-associated gene, by mediating the chromatin structures during postnatal development of mouse retina. J. Neurochem. 2023, 167, 766–777. [Google Scholar] [CrossRef]
- Hawtin, R.; Couthouis, J.; Khan, S.; Wang, G.; Panagoulias, J.; Gold, L.; Daugherty, S.; Kunecki, D.; Berry-Kravis, E.; Berent, A. Proteomic Profiling of Angelman Syndrome for Disease-associated Biomarker Discovery (P8-8.001). Neurology 2024, 102 (Suppl. S1), 3529. [Google Scholar]
- Dodge, A.; Willman, J.; Willman, M.; Nenninger, A.W.; Morrill, N.K.; Lamens, K.; Greene, H.; Weeber, E.J.; Nash, K.R. Identification of UBE3A Protein in CSF and Extracellular Space of the Hippocampus Suggest a Potential Novel Function in Synaptic Plasticity. Autism Res. 2021, 14, 645–655. [Google Scholar]
- Dodge, A.; Morrill, N.K.; Weeber, E.J.; Nash, K.R. Recovery of Angelman syndrome rat deficits with UBE3A protein supplementation. Mol. Cell. Neurosci. 2022, 120, 103724. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, G.; Liu, Y.; Standley, S.; Ji, A.; Tunuguntla, R.; Wang, Y.; Claus, C.; Luo, Y.; Baudry, M.; et al. UBE3A Regulates Synaptic Plasticity and Learning and Memory by Controlling SK2 Channel Endocytosis. Cell Rep. 2015, 12, 449–461. [Google Scholar]
- Khatri, N.; Gilbert, J.P.; Huo, Y.; Sharaflari, R.; Nee, M.; Qiao, H.; Man, H.Y. The Autism Protein Ube3A/E6AP Remodels Neuronal Dendritic Arborization via Caspase-Dependent Microtubule Destabilization. J. Neurosci. 2018, 38, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Margolis, S.S.; Salogiannis, J.; Lipton, D.M.; Mandel-Brehm, C.; Wills, Z.P.; Mardinly, A.R.; Hu, L.; Greer, P.L.; Bikoff, J.B.; Ho, H.Y.; et al. EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell 2010, 143, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Gossan, N.C.; Zhang, F.; Guo, B.; Jin, D.; Yoshitane, H.; Yao, A.; Glossop, N.; Zhang, Y.Q.; Fukada, Y.; Meng, Q.J. The E3 ubiquitin ligase UBE3A is an integral component of the molecular circadian clock through regulating the BMAL1 transcription factor. Nucleic Acids Res. 2014, 42, 5765–5775. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lou, S.S.; Wang, T.; Wu, R.J.; Li, G.; Zhao, M.; Lu, B.; Li, Y.Y.; Zhang, J.; Cheng, X.; et al. UBE3A-mediated PTPA ubiquitination and degradation regulate PP2A activity and dendritic spine morphology. Proc. Natl. Acad. Sci. USA 2019, 116, 12500–12505. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.X.; Yuan, Q.; Fukuda, M.; Yu, W.; Yan, H.; Lim, G.G.Y.; Nai, M.H.; D’Agostino, G.A.; Tran, H.D.; Itahana, Y.; et al. Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science 2019, 366, 1486–1492. [Google Scholar] [CrossRef]
- Tomaic, V.; Banks, L. Angelman syndrome-associated ubiquitin ligase UBE3A/E6AP mutants interfere with the proteolytic activity of the proteasome. Cell Death Dis. 2015, 6, e1625. [Google Scholar] [CrossRef]
- Xu, X.; Li, C.; Gao, X.; Xia, K.; Guo, H.; Li, Y.; Hao, Z.; Zhang, L.; Gao, D.; Xu, C.; et al. Excessive UBE3A dosage impairs retinoic acid signaling and synaptic plasticity in autism spectrum disorders. Cell Res. 2018, 28, 48–68. [Google Scholar] [CrossRef]
- Elu, N.; Osinalde, N.; Beaskoetxea, J.; Ramirez, J.; Lectez, B.; Aloria, K.; Rodriguez, J.A.; Arizmendi, J.M.; Mayor, U. Detailed Dissection of UBE3A-Mediated DDI1 Ubiquitination. Front. Physiol. 2019, 10, 534. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Muller, U.C.; Deller, T.; Korte, M. Not just amyloid: Physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 2017, 18, 281–298. [Google Scholar] [CrossRef]
- Patel, H.; Hodges, A.K.; Curtis, C.; Lee, S.H.; Troakes, C.; Dobson, R.J.B.; Newhouse, S.J. Transcriptomic analysis of probable asymptomatic and symptomatic alzheimer brains. Brain Behav. Immun. 2019, 80, 644–656. [Google Scholar] [CrossRef]
- Ghose, U.; Sproviero, W.; Winchester, L.; Amin, N.; Zhu, T.; Newby, D.; Ulm, B.S.; Papathanasiou, A.; Shi, L.; Liu, Q.; et al. Genome wide association neural networks (GWANN) identify genes linked to family history of Alzheimer’s disease. Brief. Bioinform. 2025, 26, bbae704. [Google Scholar] [CrossRef]
- Li, X.W.; Duan, T.T.; Chu, J.Y.; Pan, S.Y.; Zeng, Y.; Hu, F.F. SCAD-Brain: A public database of single cell RNA-seq data in human and mouse brains with Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1157792. [Google Scholar] [CrossRef]
- Reddy, J.S.; Heath, L.; Linden, A.V.; Allen, M.; Lopes, K.P.; Seifar, F.; Wang, E.; Ma, Y.; Poehlman, W.L.; Quicksall, Z.S.; et al. Bridging the gap: Multi-omics profiling of brain tissue in Alzheimer’s disease and older controls in multi-ethnic populations. Alzheimers Dement. 2024, 20, 7174–7192. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, J.; Frackowiak, J.; Mazur-Kolecka, B.; Schanen, N.C.; Cook, E.H., Jr.; Sigman, M.; Brown, W.T.; Kuchna, I.; Wegiel, J.; Nowicki, K.; et al. Abnormal intracellular accumulation and extracellular Abeta deposition in idiopathic and Dup15q11.2-q13 autism spectrum disorders. PLoS ONE 2012, 7, e35414. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Hyeon, S.J.; Das, T.; Suh, Y.S.; Kim, Y.K.; Lee, J.S.; Song, E.J.; Ryu, H.; Yu, K. UBE4B, a microRNA-9 target gene, promotes autophagy-mediated Tau degradation. Nat. Commun. 2021, 12, 3291. [Google Scholar] [CrossRef]
- Yang, X.; Duckhorn, J.; Marshall, J.; Huang, Y.A. Interlinked destinies: How ubiquitin-proteasome and autophagy systems underpin neurocognitive outcomes. Exp. Neurol. 2024, 379, 114869. [Google Scholar] [CrossRef]
- Vivanti, G.; Tao, S.; Lyall, K.; Robins, D.L.; Shea, L.L. The prevalence and incidence of early-onset dementia among adults with autism spectrum disorder. Autism Res. 2021, 14, 2189–2199. [Google Scholar] [CrossRef]
- Baron, C.A.; Tepper, C.G.; Liu, S.Y.; Davis, R.R.; Wang, N.J.; Schanen, N.C.; Gregg, J.P. Genomic and functional profiling of duplicated chromosome 15 cell lines reveal regulatory alterations in UBE3A-associated ubiquitin-proteasome pathway processes. Hum. Mol. Genet. 2006, 15, 853–869. [Google Scholar] [CrossRef]
- Perez, Y.; Velmeshev, D.; Wang, L.; White, M.; Siebert, C.; Baltazar, J.; Dutton, N.G.; Wang, S.; Haeussler, M.; Chamberlain, S.; et al. Single cell analysis of dup15q syndrome reveals developmental and postnatal molecular changes in autism. bioRxiv, 2023; bioRxiv:2023.09.22.559056. [Google Scholar]
- Del Prete, D.; Rice, R.C.; Rajadhyaksha, A.M.; D’Adamio, L. Amyloid Precursor Protein (APP) May Act as a Substrate and a Recognition Unit for CRL4CRBN and Stub1 E3 Ligases Facilitating Ubiquitination of Proteins Involved in Presynaptic Functions and Neurodegeneration. J. Biol. Chem. 2016, 291, 17209–17227. [Google Scholar] [CrossRef]
- Schmidt, M.F.; Gan, Z.Y.; Komander, D.; Dewson, G. Ubiquitin signalling in neurodegeneration: Mechanisms and therapeutic opportunities. Cell Death Differ. 2021, 28, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Maniv, I.; Sarji, M.; Bdarneh, A.; Feldman, A.; Ankawa, R.; Koren, E.; Magid-Gold, I.; Reis, N.; Soteriou, D.; Salomon-Zimri, S.; et al. Altered ubiquitin signaling induces Alzheimer’s disease-like hallmarks in a three-dimensional human neural cell culture model. Nat. Commun. 2023, 14, 5922. [Google Scholar] [CrossRef]
- Myeku, N.; Clelland, C.L.; Emrani, S.; Kukushkin, N.V.; Yu, W.H.; Goldberg, A.L.; Duff, K.E. Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat. Med. 2016, 22, 46–53. [Google Scholar] [CrossRef]
- Riday, T.T.; Dankoski, E.C.; Krouse, M.C.; Fish, E.W.; Walsh, P.L.; Han, J.E.; Hodge, C.W.; Wightman, R.M.; Philpot, B.D.; Malanga, C.J. Pathway-specific dopaminergic deficits in a mouse model of Angelman syndrome. J. Clin. Investig. 2012, 122, 4544–4554. [Google Scholar] [CrossRef]
- Mulherkar, S.A.; Jana, N.R. Loss of dopaminergic neurons and resulting behavioural deficits in mouse model of Angelman syndrome. Neurobiol. Dis. 2010, 40, 586–592. [Google Scholar] [CrossRef]
- Hayrapetyan, V.; Castro, S.; Sukharnikova, T.; Yu, C.; Cao, X.; Jiang, Y.H.; Yin, H.H. Region-specific impairments in striatal synaptic transmission and impaired instrumental learning in a mouse model of Angelman syndrome. Eur. J. Neurosci. 2014, 39, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Berrios, J.; Stamatakis, A.M.; Kantak, P.A.; McElligott, Z.A.; Judson, M.C.; Aita, M.; Rougie, M.; Stuber, G.D.; Philpot, B.D. Loss of UBE3A from TH-expressing neurons suppresses GABA co-release and enhances VTA-NAc optical self-stimulation. Nat. Commun. 2016, 7, 10702. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, J.; Wang, J.; Mao, L.; Tang, B.; Vanderklish, P.W.; Liao, X.; Xiong, Z.Q.; Liao, L. HAP1 is an in vivo UBE3A target that augments autophagy in a mouse model of Angelman syndrome. Neurobiol. Dis. 2019, 132, 104585. [Google Scholar] [CrossRef]
- Singh, B.K.; Vatsa, N.; Nelson, V.K.; Kumar, V.; Kumar, S.S.; Mandal, S.C.; Pal, M.; Jana, N.R. Azadiradione Restores Protein Quality Control and Ameliorates the Disease Pathogenesis in a Mouse Model of Huntington’s Disease. Mol. Neurobiol. 2018, 55, 6337–6346. [Google Scholar] [CrossRef]
- Shekhar, S.; Vatsa, N.; Kumar, V.; Singh, B.K.; Jamal, I.; Sharma, A.; Jana, N.R. Topoisomerase 1 inhibitor topotecan delays the disease progression in a mouse model of Huntington’s disease. Hum. Mol. Genet. 2017, 26, 420–429. [Google Scholar] [CrossRef]
- Jana, S.; Giri, B.; Das, S.; Manna, A.; Mandal, S.C.; Ranjan Jana, N. Azadiradione up-regulates the expression of parvalbumin and BDNF via Ube3a. Gene 2024, 897, 148081. [Google Scholar] [PubMed]
- Cummings, C.J.; Reinstein, E.; Sun, Y.; Antalffy, B.; Jiang, Y.; Ciechanover, A.; Orr, H.T.; Beaudet, A.L.; Zoghbi, H.Y. Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice. Neuron 1999, 24, 879–892. [Google Scholar] [PubMed]
- Mishra, A.; Dikshit, P.; Purkayastha, S.; Sharma, J.; Nukina, N.; Jana, N.R. E6-AP promotes misfolded polyglutamine proteins for proteasomal degradation and suppresses polyglutamine protein aggregation and toxicity. J. Biol. Chem. 2008, 283, 7648–7656. [Google Scholar]
- Mishra, A.; Maheshwari, M.; Chhangani, D.; Fujimori-Tonou, N.; Endo, F.; Joshi, A.P.; Jana, N.R.; Yamanaka, K. E6-AP association promotes SOD1 aggresomes degradation and suppresses toxicity. Neurobiol. Aging 2013, 34, 1310.e11–1310.e23. [Google Scholar]
- Yasuda, S.; Tsuchiya, H.; Kaiho, A.; Guo, Q.; Ikeuchi, K.; Endo, A.; Arai, N.; Ohtake, F.; Murata, S.; Inada, T.; et al. Stress- and ubiquitylation-dependent phase separation of the proteasome. Nature 2020, 578, 296–300. [Google Scholar]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar]
- Bekes, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar]
- Kurihara, T.; Asahi, T.; Sawamura, N. Cereblon-mediated degradation of the amyloid precursor protein via the ubiquitin-proteasome pathway. Biochem. Biophys. Res. Commun. 2020, 524, 236–241. [Google Scholar] [PubMed]
- Krumm, N.; O’Roak, B.J.; Shendure, J.; Eichler, E.E. A de novo convergence of autism genetics and molecular neuroscience. Trends Neurosci. 2014, 37, 95–105. [Google Scholar]
- Hogart, A.; Wu, D.; LaSalle, J.M.; Schanen, N.C. The comorbidity of autism with the genomic disorders of chromosome 15q11.2-q13. Neurobiol. Dis. 2010, 38, 181–191. [Google Scholar]
- Glessner, J.T.; Wang, K.; Cai, G.; Korvatska, O.; Kim, C.E.; Wood, S.; Zhang, H.; Estes, A.; Brune, C.W.; Bradfield, J.P.; et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature 2009, 459, 569–573. [Google Scholar] [PubMed]
- Yi, J.J.; Berrios, J.; Newbern, J.M.; Snider, W.D.; Philpot, B.D.; Hahn, K.M.; Zylka, M.J. An Autism-Linked Mutation Disables Phosphorylation Control of UBE3A. Cell 2015, 162, 795–807. [Google Scholar] [CrossRef] [PubMed]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Cicek, A.E.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef]
- Smith, S.E.; Zhou, Y.D.; Zhang, G.; Jin, Z.; Stoppel, D.C.; Anderson, M.P. Increased gene dosage of Ube3a results in autism traits and decreased glutamate synaptic transmission in mice. Sci. Transl. Med. 2011, 3, 103ra97. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yu, F.; Yun, E.; Lin, J.W.; Man, H.Y. mRNA nuclear retention reduces AMPAR expression and promotes autistic behavior in UBE3A-overexpressing mice. EMBO Rep. 2024, 25, 1282–1309. [Google Scholar] [CrossRef]
- Punt, A.M.; Judson, M.C.; Sidorov, M.S.; Williams, B.N.; Johnson, N.S.; Belder, S.; den Hertog, D.; Davis, C.R.; Feygin, M.S.; Lang, P.F.; et al. Molecular and behavioral consequences of Ube3a gene overdosage in mice. JCI Insight 2022, 7, e158953. [Google Scholar] [CrossRef]
- Krishnan, V.; Stoppel, D.C.; Nong, Y.; Johnson, M.A.; Nadler, M.J.; Ozkaynak, E.; Teng, B.L.; Nagakura, I.; Mohammad, F.; Silva, M.A.; et al. Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1. Nature 2017, 543, 507–512. [Google Scholar] [CrossRef]
- Copping, N.A.; Christian, S.G.B.; Ritter, D.J.; Islam, M.S.; Buscher, N.; Zolkowska, D.; Pride, M.C.; Berg, E.L.; LaSalle, J.M.; Ellegood, J.; et al. Neuronal overexpression of Ube3a isoform 2 causes behavioral impairments and neuroanatomical pathology relevant to 15q11.2-q13.3 duplication syndrome. Hum. Mol. Genet. 2017, 26, 3995–4010. [Google Scholar] [CrossRef]
- Xing, L.; Simon, J.M.; Ptacek, T.S.; Yi, J.J.; Loo, L.; Mao, H.; Wolter, J.M.; McCoy, E.S.; Paranjape, S.R.; Taylor-Blake, B.; et al. Autism-linked UBE3A gain-of-function mutation causes interneuron and behavioral phenotypes when inherited maternally or paternally in mice. Cell Rep. 2023, 42, 112706. [Google Scholar]
- Nuber, U.; Schwarz, S.E.; Scheffner, M. The ubiquitin-protein ligase E6-associated protein (E6-AP) serves as its own substrate. Eur. J. Biochem. 1998, 254, 643–649. [Google Scholar] [CrossRef]
- Sell, G.L.; Xin, W.; Cook, E.K.; Zbinden, M.A.; Schaffer, T.B.; O’Meally, R.N.; Cole, R.N.; Margolis, S.S. Deleting a UBE3A substrate rescues impaired hippocampal physiology and learning in Angelman syndrome mice. Sci. Rep. 2021, 11, 19414. [Google Scholar] [CrossRef] [PubMed]
- Yang, X. Towards an understanding of Angelman syndrome in mice studies. J. Neurosci. Res. 2020, 98, 1162–1173. [Google Scholar] [CrossRef]
- Gardner, Z.; Holbrook, O.; Tian, Y.; Odamah, K.; Man, H.Y. The role of glia in the dysregulation of neuronal spinogenesis in Ube3a-dependent ASD. Exp. Neurol. 2024, 376, 114756. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020370. [Google Scholar] [CrossRef]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef]
- Kim, H.; Kunz, P.A.; Mooney, R.; Philpot, B.D.; Smith, S.L. Maternal Loss of Ube3a Impairs Experience-Driven Dendritic Spine Maintenance in the Developing Visual Cortex. J. Neurosci. 2016, 36, 4888–4894. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, K.; Ishii, K.; Tsuji, M.; Tokumitsu, N.; Hasegawa, E.; Emoto, K. Presynaptic Ube3a E3 ligase promotes synapse elimination through down-regulation of BMP signaling. Science 2023, 381, 1197–1205. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Zhang, Y.; Zheng, X.; Cepeda, C.; Wang, Y.; Duan, S.; Tong, X. Interactions of glial cells with neuronal synapses, from astrocytes to microglia and oligodendrocyte lineage cells. Glia 2023, 71, 1383–1401. [Google Scholar] [CrossRef]
- Furumai, R.; Tamada, K.; Liu, X.; Takumi, T. UBE3A regulates the transcription of IRF, an antiviral immunity. Hum. Mol. Genet. 2019, 28, 1947–1958. [Google Scholar] [CrossRef]
- Dias, C.; Mo, A.; Cai, C.; Sun, L.; Cabral, K.; Brownstein, C.A.; Rockowitz, S.; Walsh, C.A. Cell-type-specific effects of autism-associated 15q duplication syndrome in the human brain. Am. J. Hum. Genet. 2024, 111, 1544–1558. [Google Scholar] [CrossRef]
- Hope, K.A.; LeDoux, M.S.; Reiter, L.T. Glial overexpression of Dube3a causes seizures and synaptic impairments in Drosophila concomitant with down regulation of the Na(+)/K(+) pump ATPalpha. Neurobiol. Dis. 2017, 108, 238–248. [Google Scholar] [PubMed]
- Landaverde, S.; Sleep, M.; Lacoste, A.; Tan, S.; Schuback, R.; Reiter, L.T.; Iyengar, A. Glial expression of Drosophila UBE3A causes spontaneous seizures that can be modulated by 5-HT signaling. Neurobiol. Dis. 2024, 200, 106651. [Google Scholar]
- Sell, G.L.; Margolis, S.S. From UBE3A to Angelman syndrome: A substrate perspective. Front. Neurosci. 2015, 9, 322. [Google Scholar]
- Lopez, S.J.; Segal, D.J.; LaSalle, J.M. UBE3A: An E3 Ubiquitin Ligase With Genome-Wide Impact in Neurodevelopmental Disease. Front. Mol. Neurosci. 2018, 11, 476. [Google Scholar]
- Lopez, S.J.; Laufer, B.I.; Beitnere, U.; Berg, E.L.; Silverman, J.L.; O’Geen, H.; Segal, D.J.; LaSalle, J.M. Imprinting effects of UBE3A loss on synaptic gene networks and Wnt signaling pathways. Hum. Mol. Genet. 2019, 28, 3842–3852. [Google Scholar] [PubMed]
- Martinez-Noel, G.; Luck, K.; Kuhnle, S.; Desbuleux, A.; Szajner, P.; Galligan, J.T.; Rodriguez, D.; Zheng, L.; Boyland, K.; Leclere, F.; et al. Network Analysis of UBE3A/E6AP-Associated Proteins Provides Connections to Several Distinct Cellular Processes. J. Mol. Biol. 2018, 430, 1024–1050. [Google Scholar]
- Singhmar, P.; Kumar, A. Angelman syndrome protein UBE3A interacts with primary microcephaly protein ASPM, localizes to centrosomes and regulates chromosome segregation. PLoS ONE 2011, 6, e20397. [Google Scholar]
- Luo, Y.; Yang, Y.; Yang, C.; Li, C.; Hu, R.; Geng, W.; Kang, X.; Lin, H. UBE3A and MCM6 synergistically regulate the proliferation and migration of lung adenocarcinoma cells. FEBS Open Bio 2023, 13, 1756–1771. [Google Scholar]
- Estridge, R.C.; Yagci, Z.B.; Sen, D.; Ptacek, T.S.; Simon, J.M.; Keung, A.J. Loss of UBE3A impacts both neuronal and non-neuronal cells in human cerebral organoids. bioRxiv, 2024; bioRxiv:2024.2002.2009.579672. [Google Scholar]
- Simchi, L.; Gupta, P.K.; Feuermann, Y.; Kaphzan, H. Elevated ROS levels during the early development of Angelman syndrome alter the apoptotic capacity of the developing neural precursor cells. Mol. Psychiatry 2023, 28, 2382–2397. [Google Scholar]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.L.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B.; et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 2014, 344, 1252304. [Google Scholar]
- Gautier, H.O.; Evans, K.A.; Volbracht, K.; James, R.; Sitnikov, S.; Lundgaard, I.; James, F.; Lao-Peregrin, C.; Reynolds, R.; Franklin, R.J.; et al. Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun. 2015, 6, 8518. [Google Scholar] [PubMed]
- Marisca, R.; Hoche, T.; Agirre, E.; Hoodless, L.J.; Barkey, W.; Auer, F.; Castelo-Branco, G.; Czopka, T. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci. 2020, 23, 363–374. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Huang, Y.-W.A. Unraveling the Roles of UBE3A in Neurodevelopment and Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 2304. https://doi.org/10.3390/ijms26052304
Yang X, Huang Y-WA. Unraveling the Roles of UBE3A in Neurodevelopment and Neurodegeneration. International Journal of Molecular Sciences. 2025; 26(5):2304. https://doi.org/10.3390/ijms26052304
Chicago/Turabian StyleYang, Xin, and Yu-Wen Alvin Huang. 2025. "Unraveling the Roles of UBE3A in Neurodevelopment and Neurodegeneration" International Journal of Molecular Sciences 26, no. 5: 2304. https://doi.org/10.3390/ijms26052304
APA StyleYang, X., & Huang, Y.-W. A. (2025). Unraveling the Roles of UBE3A in Neurodevelopment and Neurodegeneration. International Journal of Molecular Sciences, 26(5), 2304. https://doi.org/10.3390/ijms26052304




