Targeting Ferroptosis in Parkinson’s: Repurposing Diabetes Drugs as a Promising Treatment
Abstract
1. Introduction
2. Antidiabetic Drugs for Parkinson’s Disease
2.1. Metformin
2.2. Incretins and Incretin Mimetics
2.2.1. Preclinical Evidence for GIP and GLP-1R Agonists Usage in Parkinson’s Disease Treatment
2.2.2. Clinical Evidence for GIP and GLP-1R Agonists Usage in Parkinson’s Disease Treatment
2.3. DPP-4 Inhibitors (Gliptins)
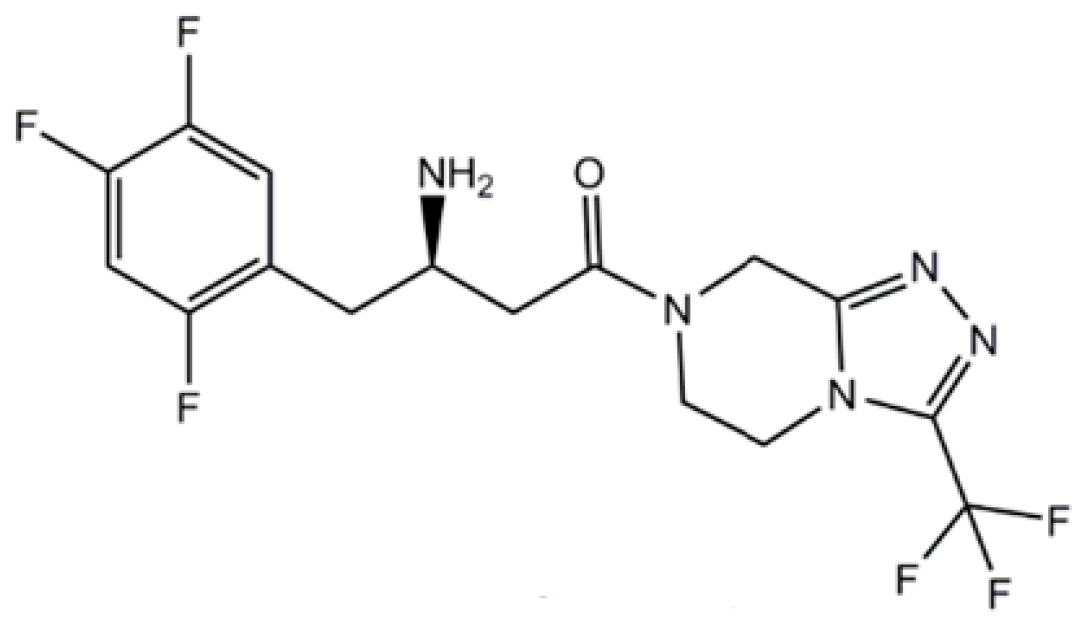
2.4. Peroxisome-Proliferator-Activated Receptor Gamma (PPAR-γ) Agonists
2.5. Sodium–Glucose Cotransporter-2 Inhibitors (SGLT2i)
2.6. Meglitinides (Glinides)
2.7. Alpha-Glucosidase Inhibitors (AGIs)
2.8. T2D Drugs Which Are/Were in Clinical Trials Repurposing for the Treatment of Parkinson’s Disease
- 1.
- Exenatide
- 2.
- Liraglutide
- 3.
- Pioglitazone
- 4.
- Semaglutide
- 5.
- Lixisenatide
- 6.
- Metformin
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maserejian, N.; Vinikoor-Imler, L.; Dilley, A. Estimation of the 2020 Global Population of Parkinson’s Disease (PD). Available online: https://www.mdsabstracts.org/abstract/estimation-of-the-2020-global-population-of-parkinsons-disease-pd/ (accessed on 2 February 2025).
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, C. Brain insulin resistance: Role in neurodegenerative disease and potential for targeting. Expert Opin. Investig. Drugs 2020, 29, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Duță, C.; Muscurel, C.; Dogaru, C.B.; Stoian, I. Ferroptosis-A Shared Mechanism for Parkinson’s Disease and Type 2 Diabetes. Int. J. Mol. Sci. 2024, 25, 8838. [Google Scholar] [CrossRef]
- Das, R.R.; Unger, M.M. Diabetes and Parkinson disease: A sweet spot? Neurology 2018, 90, 869–870. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Sharma, T.; Kaur, D.; Grewal, A.K.; Singh, T.G. Therapies modulating insulin resistance in Parkinson’s disease: A cross talk. Neurosci. Lett. 2021, 749, 135754. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Huerta, M.; González-Usigli, H.A.; Torres-Sánchez, E.D.; Delgado-Lara, D.L.; Pacheco-Moisés, F.P.; Mireles-Ramírez, M.A.; Torres-Mendoza, B.M.; Moreno-Cih, R.I.; Velázquez-Brizuela, I.E. Cognitive disorder and dementia in type 2 diabetes mellitus. World J. Diabetes 2022, 13, 319–337. [Google Scholar] [CrossRef]
- Kleinridders, A.; Ferris, H.A.; Cai, W.; Kahn, C.R. Insulin Action in Brain Regulates Systemic Metabolism and Brain Function. Diabetes 2014, 63, 2232–2243. [Google Scholar] [CrossRef]
- Mielke, J.G.; Wang, Y.-T. Chapter 4-Insulin, Synaptic Function, and Opportunities for Neuroprotection. In Progress in Molecular Biology and Translational Science; Rahman, S., Ed.; The Brain as a Drug Target; Academic Press: Cambridge, MA, USA, 2011; Volume 98, pp. 133–186. [Google Scholar]
- Hong, C.-T.; Chen, K.-Y.; Wang, W.; Chiu, J.-Y.; Wu, D.; Chao, T.-Y.; Hu, C.-J.; Chau, K.-Y.D.; Bamodu, O.A. Insulin Resistance Promotes Parkinson’s Disease through Aberrant Expression of α-Synuclein, Mitochondrial Dysfunction, and Deregulation of the Polo-Like Kinase 2 Signaling. Cells 2020, 9, 740. [Google Scholar] [CrossRef]
- Ruiz-Pozo, V.A.; Tamayo-Trujillo, R.; Cadena-Ullauri, S.; Frias-Toral, E.; Guevara-Ramírez, P.; Paz-Cruz, E.; Chapela, S.; Montalván, M.; Morales-López, T.; Simancas-Racines, D.; et al. The Molecular Mechanisms of the Relationship between Insulin Resistance and Parkinson’s Disease Pathogenesis. Nutrients 2023, 15, 3585. [Google Scholar] [CrossRef] [PubMed]
- Galizzi, G.; Di Carlo, M. Insulin and Its Key Role for Mitochondrial Function/Dysfunction and Quality Control: A Shared Link between Dysmetabolism and Neurodegeneration. Biology 2022, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- de Luca, C.; Olefsky, J.M. Inflammation and Insulin Resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef]
- Tian, Y.; Jing, G.; Ma, M.; Yin, R.; Zhang, M. Microglial activation and polarization in type 2 diabetes-related cognitive impairment: A focused review of pathogenesis. Neurosci. Biobehav. Rev. 2024, 165, 105848. [Google Scholar] [CrossRef]
- Fahn, S. The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov. Disord. 2015, 30, 4–18. [Google Scholar] [CrossRef]
- Hely, M.A.; Morris, J.G.L.; Reid, W.G.J.; Trafficante, R. Sydney Multicenter Study of Parkinson’s disease: Non-L-dopa-responsive problems dominate at 15 years. Mov. Disord. 2005, 20, 190–199. [Google Scholar] [CrossRef]
- Agostini, F.; Masato, A.; Bubacco, L.; Bisaglia, M. Metformin repurposing for parkinson disease therapy: Opportunities and challenges. Int. J. Mol. Sci. 2022, 23, 398. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- Evatt, M.L.; Delong, M.R.; Khazai, N.; Rosen, A.; Triche, S.; Tangpricha, V. Prevalence of vitamin d insufficiency in patients with Parkinson disease and Alzheimer disease. Arch. Neurol. 2008, 65, 1348–1352. [Google Scholar] [CrossRef]
- Kotake, Y.; Ohta, S. MPP+ Analogs Acting on Mitochondria and Inducing Neuro-Degeneration. Curr. Med. Chem. 2003, 10, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. Overcoming Drug Development Bottlenecks with Repurposing: Repurposing biguanides to target energy metabolism for cancer treatment. Nat. Med. 2014, 20, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef] [PubMed]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Natali, A.; Ferrannini, E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: A systematic review. Diabetologia 2006, 49, 434–441. [Google Scholar] [CrossRef]
- Łabuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopień, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Lv, W.-S.; Wen, J.-P.; Li, L.; Sun, R.-X.; Wang, J.; Xian, Y.-X.; Cao, C.-X.; Wang, Y.-L.; Gao, Y.-Y. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res. 2012, 1444, 11–19. [Google Scholar] [CrossRef]
- Wang, D.-S.; Jonker, J.W.; Kato, Y.; Kusuhara, H.; Schinkel, A.H.; Sugiyama, Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 2002, 302, 510–515. [Google Scholar] [CrossRef]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348 Pt 3, 607–614. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- González-Rodríguez, P.; Zampese, E.; Stout, K.A.; Guzman, J.N.; Ilijic, E.; Yang, B.; Tkatch, T.; Stavarache, M.A.; Wokosin, D.L.; Gao, L.; et al. Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 2021, 599, 650. [Google Scholar] [CrossRef] [PubMed]
- Tayara, K.; Espinosa-Oliva, A.M.; García-Domínguez, I.; Ismaiel, A.A.; Boza-Serrano, A.; Deierborg, T.; Machado, A.; Herrera, A.J.; Venero, J.L.; Pablos, R.M. de Divergent Effects of Metformin on an Inflammatory Model of Parkinson’s Disease. Front. Cell. Neurosci. 2018, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.A.K.; Espinosa-Oliva, A.M.; Santiago, M.; García-Quintanilla, A.; Oliva-Martín, M.J.; Herrera, A.J.; Venero, J.L.; de Pablos, R.M. Metformin, besides exhibiting strong in vivo anti-inflammatory properties, increases mptp-induced damage to the nigrostriatal dopaminergic system. Toxicol. Appl. Pharmacol. 2016, 298, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-X.; Chen, A.-D.; Wang, Q.-J.; Xin, Y.-Y.; Yin, J.; Jing, Y.-H. Protective effect of metformin against rotenone-induced parkinsonism in mice. Toxicol. Mech. Methods 2020, 30, 350–357. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Hawley, S.A.; Ross, F.A.; Chevtzoff, C.; Green, K.A.; Evans, A.; Fogarty, S.; Towler, M.C.; Brown, L.J.; Ogunbayo, O.A.; Evans, A.M.; et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010, 11, 554–565. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMP-activated protein kinase (AMPK) signaling pathway coordinates cell growth, autophagy, & metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Curry, D.W.; Stutz, B.; Andrews, Z.B.; Elsworth, J.D. Targeting AMPK Signaling as a Neuroprotective Strategy in Parkinson’s Disease. J. Park. Dis. 2018, 8, 161–181. [Google Scholar] [CrossRef]
- Zhang, C.-S.; Li, M.; Ma, T.; Zong, Y.; Cui, J.; Feng, J.-W.; Wu, Y.-Q.; Lin, S.-Y.; Lin, S.-C. Metformin Activates AMPK through the Lysosomal Pathway. Cell Metab. 2016, 24, 521–522. [Google Scholar] [CrossRef]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.; O’Neill, H.M.; Ford, R.J.; Palanivel, R.; O’Brien, M.; et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Erion, D.M.; Shulman, G.I. Diacylglycerol-mediated insulin resistance. Nat. Med. 2010, 16, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Chibalin, A.V.; Leng, Y.; Vieira, E.; Krook, A.; Björnholm, M.; Long, Y.C.; Kotova, O.; Zhong, Z.; Sakane, F.; Steiler, T.; et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia-induced insulin resistance. Cell 2008, 132, 375–386. [Google Scholar] [CrossRef]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides suppress hepatic glucagon signaling by decreasing production of cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef]
- Johanns, M.; Lai, Y.-C.; Hsu, M.-F.; Jacobs, R.; Vertommen, D.; Van Sande, J.; Dumont, J.E.; Woods, A.; Carling, D.; Hue, L.; et al. AMPK antagonizes hepatic glucagon-stimulated cyclic AMP signalling via phosphorylation-induced activation of cyclic nucleotide phosphodiesterase 4B. Nat. Commun. 2016, 7, 10856. [Google Scholar] [CrossRef]
- Chance, B.; Hollunger, G. The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J. Biol. Chem. 1961, 236, 1534–1543. [Google Scholar] [CrossRef]
- Hinkle, P.C.; Butow, R.A.; Racker, E.; Chance, B. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome beta region of the respiratory chain of beef heart submitochondrial particles. J. Biol. Chem. 1967, 242, 5169–5173. [Google Scholar] [CrossRef]
- Liu, Y.; Fiskum, G.; Schubert, D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002, 80, 780–787. [Google Scholar] [CrossRef]
- Batandier, C.; Guigas, B.; Detaille, D.; El-Mir, M.; Fontaine, E.; Rigoulet, M.; Leverve, X.M. The ROS Production Induced by a Reverse-Electron Flux at Respiratory-Chain Complex 1 is Hampered by Metformin. J. Bioenerg. Biomembr. 2006, 38, 33–42. [Google Scholar] [CrossRef]
- Kukidome, D.; Nishikawa, T.; Sonoda, K.; Imoto, K.; Fujisawa, K.; Yano, M.; Motoshima, H.; Taguchi, T.; Matsumura, T.; Araki, E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 2006, 55, 120–127. [Google Scholar] [CrossRef]
- Batchuluun, B.; Inoguchi, T.; Sonoda, N.; Sasaki, S.; Inoue, T.; Fujimura, Y.; Miura, D.; Takayanagi, R. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis 2014, 232, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D.; Raji, B.; Walrand, S.; Gardès-Albert, M.; Jore, D.; Legrand, A.; Peynet, J.; Vasson, M.P. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism 2003, 52, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Ouslimani, N.; Peynet, J.; Bonnefont-Rousselot, D.; Thérond, P.; Legrand, A.; Beaudeux, J.-L. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism 2005, 54, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, D.; Kocić, R.; Kocić, G.; Jevtović, T.; Radenković, S.; Mikić, D.; Stojanović, M.; Djordjević, P.B. Effect of four-week metformin treatment on plasma and erythrocyte antioxidative defense enzymes in newly diagnosed obese patients with type 2 diabetes. Diabetes Obes. Metab. 2000, 2, 251–256. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Xie, Z.; Zhou, W.; Li, M. Metformin Attenuates Ferroptosis and Promotes Functional Recovery of Spinal Cord Injury. World Neurosurg. 2022, 167, e929–e939. [Google Scholar] [CrossRef]
- Chen, X.; Wu, W.; Gong, B.; Hou, L.; Dong, X.; Xu, C.; Zhao, R.; Yu, Q.; Zhou, Z.; Huang, S.; et al. Metformin attenuates cadmium-induced neuronal apoptosis in vitro via blocking ROS-dependent PP5/AMPK-JNK signaling pathway. Neuropharmacology 2020, 175, 108065. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, Z.; Gaur, U.; Fang, J.; Peng, T.; Li, S.; Zheng, W. Metformin protects PC12 cells and hippocampal neurons from H2 O2 -induced oxidative damage through activation of AMPK pathway. J. Cell. Physiol. 2019, 234, 16619–16629. [Google Scholar] [CrossRef]
- Yue, F.; Shi, Y.; Wu, S.; Xing, L.; He, D.; Wei, L.; Qiu, A.; Russell, R.; Zhang, D. Metformin alleviates hepatic iron overload and ferroptosis through AMPK-ferroportin pathway in HFD-induced NAFLD. iScience 2023, 26, 108560. [Google Scholar] [CrossRef]
- Hsu, S.-K.; Cheng, K.-C.; Mgbeahuruike, M.O.; Lin, Y.-H.; Wu, C.-Y.; Wang, H.-M.D.; Yen, C.-H.; Chiu, C.-C.; Sheu, S.-J. New Insight into the Effects of Metformin on Diabetic Retinopathy, Aging and Cancer: Nonapoptotic Cell Death, Immunosuppression, and Effects beyond the AMPK Pathway. Int. J. Mol. Sci. 2021, 22, 9453. [Google Scholar] [CrossRef]
- Wong, Y.C.; Krainc, D. α-synuclein toxicity in neurodegeneration: Mechanism and therapeutic strategies. Nat. Med. 2017, 23, 1–13. [Google Scholar] [CrossRef]
- Saewanee, N.; Praputpittaya, T.; Malaiwong, N.; Chalorak, P.; Meemon, K. Neuroprotective effect of metformin on dopaminergic neurodegeneration and α-synuclein aggregation in C. elegans model of Parkinson’s disease. Neurosci. Res. 2021, 162, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Revuelta, B.I.; Hettich, M.M.; Ciociaro, A.; Rotermund, C.; Kahle, P.J.; Krauss, S.; Di Monte, D.A. Metformin lowers Ser-129 phosphorylated α-synuclein levels via mTOR-dependent protein phosphatase 2A activation. Cell Death Dis. 2014, 5, e1209. [Google Scholar] [CrossRef]
- Oueslati, A.; Fournier, M.; Lashuel, H.A. Chapter 7-Role of post-translational modifications in modulating the structure, function and toxicity of α-synuclein: Implications for Parkinson’s disease pathogenesis and therapies. In Progress in Brain Research; Björklund, A., Cenci, M.A., Eds.; Recent Advances in Parkinson’s Disease: Basic Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 183, pp. 115–145. [Google Scholar]
- Katila, N.; Bhurtel, S.; Shadfar, S.; Srivastav, S.; Neupane, S.; Ojha, U.; Jeong, G.-S.; Choi, D.-Y. Metformin lowers α-synuclein phosphorylation and upregulates neurotrophic factor in the MPTP mouse model of Parkinson’s disease. Neuropharmacology 2017, 125, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Vicente Miranda, H.; Szegő, É.M.; Oliveira, L.M.A.; Breda, C.; Darendelioglu, E.; de Oliveira, R.M.; Ferreira, D.G.; Gomes, M.A.; Rott, R.; Oliveira, M.; et al. Glycation potentiates α-synuclein-associated neurodegeneration in synucleinopathies. Brain 2017, 140, 1399–1419. [Google Scholar] [CrossRef]
- Ozbey, G.; Nemutlu-Samur, D.; Parlak, H.; Yildirim, S.; Aslan, M.; Tanriover, G.; Agar, A. Metformin protects rotenone-induced dopaminergic neurodegeneration by reducing lipid peroxidation. Pharmacol. Rep. 2020, 72, 1397–1406. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.K.; Touchette, A.D.; Lal, S.; Szwergold, B.S. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 1999, 48, 198–202. [Google Scholar] [CrossRef]
- Patil, S.P.; Jain, P.D.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience 2014, 277, 747–754. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, Y.; Wang, D.; Xing, Y.; Zhang, T.; Wang, W.; Zhou, S.; Cheng, J.; Chang, W.; Kong, X.; et al. Protective effects of metformin on pancreatic β-cell ferroptosis in type 2 diabetes in vivo. Biomed. Pharmacother. 2023, 168, 115835. [Google Scholar] [CrossRef]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 2016, 478, 1138–1143. [Google Scholar] [CrossRef]
- Ping, F.; Jiang, N.; Li, Y. Association between metformin and neurodegenerative diseases of observational studies: Systematic review and meta-analysis. BMJ Open Diabetes Res. Care 2020, 8, e001370. [Google Scholar] [CrossRef]
- Ryu, Y.-K.; Go, J.; Park, H.-Y.; Choi, Y.-K.; Seo, Y.J.; Choi, J.H.; Rhee, M.; Lee, T.G.; Lee, C.-H.; Kim, K.-S. Metformin regulates astrocyte reactivity in Parkinson’s disease and normal aging. Neuropharmacology 2020, 175, 108173. [Google Scholar] [CrossRef] [PubMed]
- Weber, C. Neurogastroenterology: Improving glucose tolerance via the gut-brain axis. Nat. Rev. Gastroeenterol. Hepatol. 2016, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Olmos, I.; Limousin, P.; Lees, A.; Foltynie, T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection. Brain 2013, 136, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Q.; Chung, S.K.; Shen, J. Crosstalk of metabolic factors and neurogenic signaling in adult neurogenesis: Implication of metabolic regulation for mental and neurological diseases. Neurochem. Int. 2017, 106, 24–36. [Google Scholar] [CrossRef]
- Hansen, L.; Deacon, C.F.; Ørskov, C.; Holst, J.J. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 1999, 140, 5356–5363. [Google Scholar] [CrossRef]
- Vrang, N.; Hansen, M.; Larsen, P.J.; Tang-Christensen, M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res. 2007, 1149, 118–126. [Google Scholar] [CrossRef]
- Muscogiuri, G.; DeFronzo, R.A.; Gastaldelli, A.; Holst, J.J. Glucagon-like Peptide-1 and the Central/Peripheral Nervous System: Crosstalk in Diabetes. Trends Endocrinol. Metab. 2017, 28, 88–103. [Google Scholar] [CrossRef]
- Iwai, T.; Ito, S.; Tanimitsu, K.; Udagawa, S.; Oka, J.-I. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci. Res. 2006, 55, 352–360. [Google Scholar] [CrossRef]
- Labandeira, C.; Fraga-Bau, A.; Arias Ron, D.; Alvarez-Rodriguez, E.; Vicente-Alba, P.; Lago-Garma, J.; Rodriguez-Perez, A. Parkinson’s disease and diabetes mellitus: Common mechanisms and treatment repurposing. Neural Regen. Res. 2022, 17, 1652–1658. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Choi, H.I.; Wang, Y.; Luo, Y.; Hoffer, B.J.; Greig, N.H. A New Treatment Strategy for Parkinson’s Disease through the Gut–Brain Axis: The Glucagon-Like Peptide-1 Receptor Pathway. Cell Transpl. 2017, 26, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Mojsov, S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: Brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995, 358, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Holz, G.G.; Kühtreiber, W.M.; Habener, J.F. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37). Nature 1993, 361, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Merchenthaler, I.; Lane, M.; Shughrue, P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J. Comp. Neurol. 1999, 403, 261–280. [Google Scholar] [CrossRef]
- Hölscher, C. Protective properties of GLP-1 and associated peptide hormones in neurodegenerative disorders. Br. J. Pharmacol. 2021, 179, 695–714. [Google Scholar] [CrossRef]
- Nowell, J.; Blunt, E.; Edison, P. Incretin and insulin signaling as novel therapeutic targets for Alzheimer’s and Parkinson’s disease. Mol. Psychiatry 2023, 28, 217–229. [Google Scholar] [CrossRef]
- Hunter, K.; Hölscher, C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012, 13, 33. [Google Scholar] [CrossRef]
- Salameh, T.S.; Rhea, E.M.; Talbot, K.; Banks, W.A. Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer’s and Parkinson’s disease therapeutics. Biochem. Pharmacol. 2020, 180, 114187. [Google Scholar] [CrossRef]
- Mousa, S.A.; Ayoub, B.M. Repositioning of dipeptidyl peptidase-4 inhibitors and glucagon like peptide-1 agonists as potential neuroprotective agents. Neural Regen. Res. 2019, 14, 745–748. [Google Scholar] [CrossRef]
- Filchenko, I.; Simanenkova, A.; Chefu, S.; Kolpakova, M.; Vlasov, T. Neuroprotective effect of glucagon-like peptide-1 receptor agonist is independent of glycaemia normalization in type two diabetic rats. Diab. Vasc. Res. 2018, 15, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.; Patterson, S.; Porter, D.; Gault, V.A.; Holscher, C. Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain. J. Neurosci. Res. 2011, 89, 481–489. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, G.C.; Barker, R.A.; Caldwell, M.A. Dopaminergic modulation of neurogenesis in the subventricular zone of the adult brain. Cell Cycle 2009, 8, 2888–2894. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F.; Nauck, M.A.; Meier, J.; Hücking, K.; Holst, J.J. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J. Clin. Endocrinol. Metab. 2000, 85, 3575–3581. [Google Scholar] [CrossRef]
- Yusta, B.; Baggio, L.L.; Estall, J.L.; Koehler, J.A.; Holland, D.P.; Li, H.; Pipeleers, D.; Ling, Z.; Drucker, D.J. GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006, 4, 391–406. [Google Scholar] [CrossRef]
- Reich, N.; Hölscher, C. The neuroprotective effects of glucagon-like peptide 1 in Alzheimer’s and Parkinson’s disease: An in-depth review. Front. Neurosci. 2022, 16, 970925. [Google Scholar] [CrossRef]
- Li, Y.; Perry, T.; Kindy, M.S.; Harvey, B.K.; Tweedie, D.; Holloway, H.W.; Powers, K.; Shen, H.; Egan, J.M.; Sambamurti, K.; et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl. Acad. Sci. USA 2009, 106, 1285–1290. [Google Scholar] [CrossRef]
- Li, Y.; Tweedie, D.; Mattson, M.P.; Holloway, H.W.; Greig, N.H. Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J. Neurochem. 2010, 113, 1621–1631. [Google Scholar] [CrossRef]
- Li, P.-C.; Liu, L.-F.; Jou, M.-J.; Wang, H.-K. The GLP-1 receptor agonists exendin-4 and liraglutide alleviate oxidative stress and cognitive and micturition deficits induced by middle cerebral artery occlusion in diabetic mice. BMC Neurosci. 2016, 17, 37. [Google Scholar] [CrossRef]
- Briyal, S.; Gulati, K.; Gulati, A. Repeated administration of exendin-4 reduces focal cerebral ischemia-induced infarction in rats. Brain Res. 2012, 1427, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Qin, S.; Lv, Y.; Liu, D.; Ke, Q.; Shi, C.; Jiang, L.; Yang, J.; Zhou, Y. GLP-1 receptor agonist attenuates tubular cell ferroptosis in diabetes via enhancing AMPK-fatty acid metabolism pathway through macropinocytosis. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167060. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kameda, M.; Yasuhara, T.; Agari, T.; Baba, T.; Wang, F.; Shinko, A.; Wakamori, T.; Toyoshima, A.; Takeuchi, H.; et al. Neuroprotective Effects of Liraglutide for Stroke Model of Rats. Int. J. Mol. Sci. 2013, 14, 21513–21524. [Google Scholar] [CrossRef] [PubMed]
- Briyal, S.; Shah, S.; Gulati, A. Neuroprotective and anti-apoptotic effects of liraglutide in the rat brain following focal cerebral ischemia. Neuroscience 2014, 281, 269–281. [Google Scholar] [CrossRef]
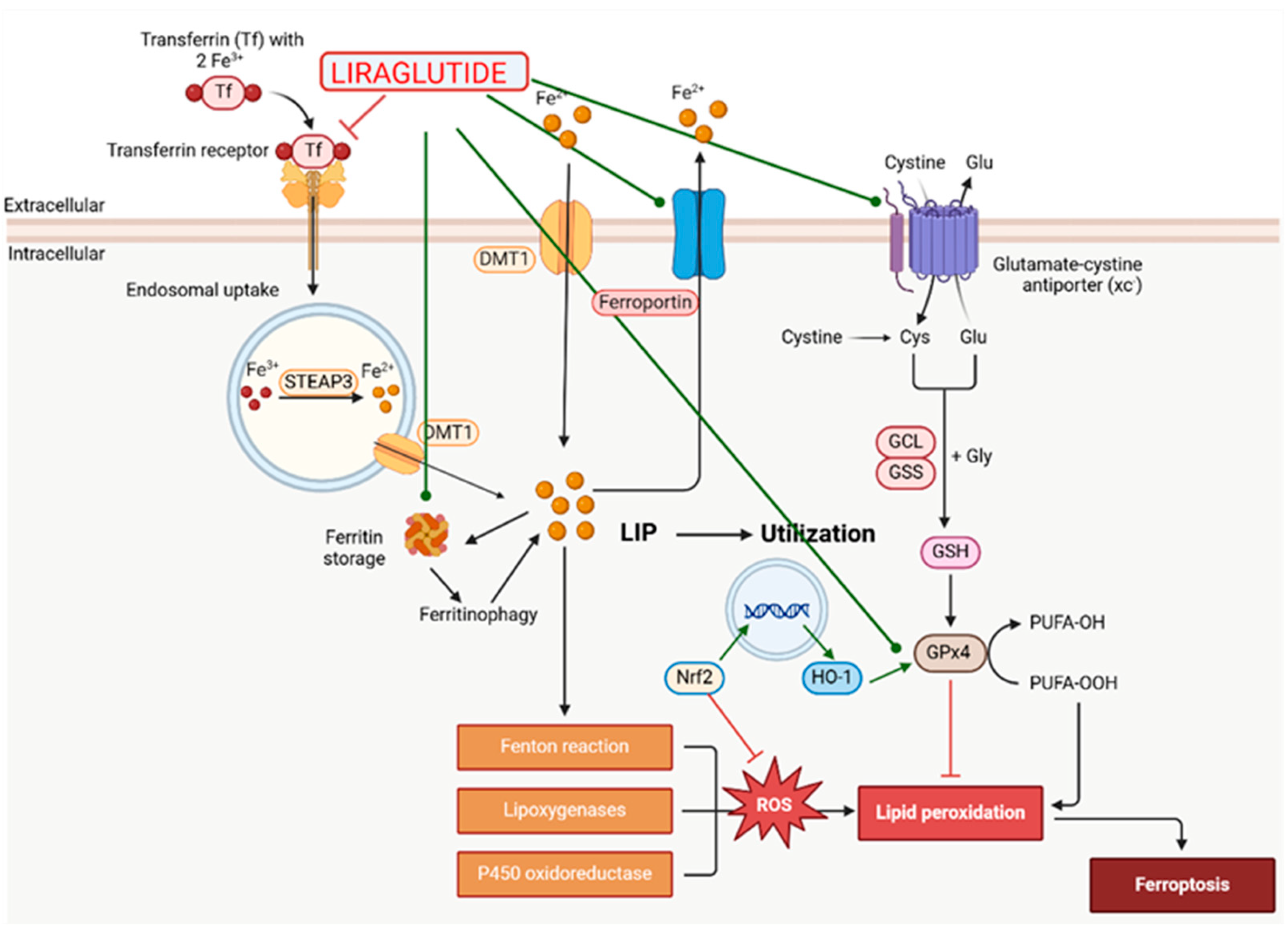
- An, J.R.; Su, J.N.; Sun, G.Y.; Wang, Q.F.; Fan, Y.D.; Jiang, N.; Yang, Y.F.; Shi, Y. Liraglutide Alleviates Cognitive Deficit in db/db Mice: Involvement in Oxidative Stress, Iron Overload, and Ferroptosis. Neurochem. Res. 2022, 47, 279–294. [Google Scholar] [CrossRef]
- Song, J.X.; An, J.R.; Chen, Q.; Yang, X.Y.; Jia, C.L.; Xu, S.; Zhao, Y.-S.; Ji, E.-S. Liraglutide attenuates hepatic iron levels and ferroptosis in db/db mice. Bioengineered 2022, 13, 8334–8348. [Google Scholar] [CrossRef]
- Deng, C.; Cao, J.; Han, J.; Li, J.; Li, Z.; Shi, N.; He, J. Liraglutide Activates the Nrf2/HO-1 Antioxidant Pathway and Protects Brain Nerve Cells against Cerebral Ischemia in Diabetic Rats. Comput. Intell. Neurosci. 2018, 2018, 3094504. [Google Scholar] [CrossRef]
- Duarte, A.I.; Candeias, E.; Alves, I.N.; Mena, D.; Silva, D.F.; Machado, N.J.; Campos, E.J.; Santos, M.S.; Oliveira, C.R.; Moreira, P.I. Liraglutide Protects Against Brain Amyloid-β1-42 Accumulation in Female Mice with Early Alzheimer’s Disease-Like Pathology by Partially Rescuing Oxidative/Nitrosative Stress and Inflammation. Int. J. Mol. Sci. 2020, 21, 1746. [Google Scholar] [CrossRef]
- Wiciński, M.; Socha, M.; Malinowski, B.; Wódkiewicz, E.; Walczak, M.; Górski, K.; Słupski, M.; Pawlak-Osińska, K. Liraglutide and its Neuroprotective Properties—Focus on Possible Biochemical Mechanisms in Alzheimer’s Disease and Cerebral Ischemic Events. Int. J. Mol. Sci. 2019, 20, 1050. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Incretin hormones regulate microglia oxidative stress, survival and expression of trophic factors. Eur. J. Cell Biol. 2017, 96, 240–253. [Google Scholar] [CrossRef]
- Rogers, J.; Mastroeni, D.; Leonard, B.; Joyce, J.; Grover, A. Neuroinflammation in Alzheimer’s Disease and Parkinson’s Disease: Are Microglia Pathogenic in Either Disorder? In International Review of Neurobiology; Neuroinflammation in Neuronal Death and Repair; Academic Press: Cambridge, MA, USA, 2007; Volume 82, pp. 235–246. [Google Scholar]
- Liu, B.; Wang, K.; Gao, H.M.; Mandavilli, B.; Wang, J.Y.; Hong, J.S. Molecular consequences of activated microglia in the brain: Overactivation induces apoptosis. J. Neurochem. 2001, 77, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Ell, P.; Soderlund, T.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A.; et al. Exenatide and the treatment of patients with Parkinson’s disease. J. Clin. Investig. 2013, 123, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Kahan, J.; Ell, P.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A.; et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J. Park. Dis. 2014, 4, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Athauda, D.; Maclagan, K.; Skene, S.S.; Bajwa-Joseph, M.; Letchford, D.; Chowdhury, K.; Hibbert, S.; Budnik, N.; Zampedri, L.; Dickson, J.; et al. Exenatide once weekly versus placebo in Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1664–1675. [Google Scholar] [CrossRef]
- Athauda, D.; Maclagan, K.; Budnik, N.; Zampedri, L.; Hibbert, S.; Aviles-Olmos, I.; Chowdhury, K.; Skene, S.S.; Limousin, P.; Foltynie, T. Post hoc analysis of the Exenatide-PD trial-Factors that predict response. Eur. J. Neurosci. 2019, 49, 410–421. [Google Scholar] [CrossRef]
- Mulvaney, C.A.; Duarte, G.S.; Handley, J.; Evans, D.J.; Menon, S.; Wyse, R.; Emsley, H.C. GLP-1 receptor agonists for Parkinson’s disease. Cochrane Database Syst. Rev. 2020, 7, CD012990. [Google Scholar] [CrossRef]
- Hogg, E.; Wu, T.; Bresee, C.; Wertheimer, J.; Malatt, C.; Tan, E.; Pomeroy, H.; Nuno, M.; Wyse, R.; Tagliati, M. A Phase II, Randomized, Double-Blinded, Placebo-Controlled Trial of Liraglutide in Parkinson’s Disease. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Vijiaratnam, N.; Girges, C.; Auld, G.; Chau, M.; Maclagan, K.; King, A.; Skene, S.; Chowdhury, K.; Hibbert, S.; Morris, H.; et al. Exenatide once weekly over 2 years as a potential disease-modifying treatment for Parkinson’s disease: Protocol for a multicentre, randomised, double blind, parallel group, placebo controlled, phase 3 trial: The “Exenatide-PD3” study. BMJ Open 2021, 11, e047993. [Google Scholar] [CrossRef]
- Finan, B.; Ma, T.; Ottaway, N.; Müller, T.D.; Habegger, K.M.; Heppner, K.M.; Kirchner, H.; Holland, J.; Hembree, J.; Raver, C.; et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 2013, 5, 209ra151. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, X.; Li, D.; Ji, C.; Yuan, Z.; Wang, R.; Xue, G.; Li, G.; Hölscher, C. Two novel dual GLP-1/GIP receptor agonists are neuroprotective in the MPTP mouse model of Parkinson’s disease. Neuropharmacology 2018, 133, 385–394. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Li, Y.; Li, L.; Melchiorsen, J.U.; Rosenkilde, M.; Hölscher, C. The Novel Dual GLP-1/GIP Receptor Agonist DA-CH5 Is Superior to Single GLP-1 Receptor Agonists in the MPTP Model of Parkinson’s Disease. J. Park. Dis. 2020, 10, 523–542. [Google Scholar] [CrossRef]
- Scherrmann, J.M. Drug delivery to brain via the blood-brain barrier. Vascul. Pharmacol. 2002, 38, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Lei, M.; Zhao, J.; Wu, M.; Ren, Z.; Yang, X.; Ouyang, C.; Liu, X.; Liu, C.; Chen, Q. Tirzepatide ameliorates spatial learning and memory impairment through modulation of aberrant insulin resistance and inflammation response in diabetic rats. Front. Pharmacol. 2023, 14, 1146960. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.F.; Ragab, D.; Ibrahim, S.G.; Abd El-Galil, M.M.; Hassan Abd-El-Hamid, A.; Hamed, D.M.; Magdy William, M.; Salem, M.A. The potential role of Tirzepatide as adjuvant therapy in countering colistin-induced nephro and neurotoxicity in rats via modulation of PI3K/p-Akt/GSK3-β/NF-kB p65 hub, shielding against oxidative and endoplasmic reticulum stress, and activation of p-CREB/BDNF/TrkB cascade. Int. Immunopharmacol. 2024, 135, 112308. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, F.; Li, G.; Tao, Y. Novel dual glucagon-like peptide-1/ glucose-dependent insulinotropic polypeptide receptor agonist attenuates diabetes and myocardial injury through inhibiting hyperglycemia, inflammation and oxidative stress in rodent animals. Bioengineered 2022, 13, 9184–9196. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.N.; Calió, M.L.; Hölscher, C.; Pacheco-Soares, C.; Porcionatto, M.; Lobo, A.O. Neuroprotective and restorative properties of the GLP-1/GIP dual agonist DA-JC1 compared with a GLP-1 single agonist in Alzheimer’s disease. Neuropharmacology 2020, 162, 107813. [Google Scholar] [CrossRef]
- Glotfelty, E.J.; Delgado, T.; Tovar-Y-Romo, L.B.; Luo, Y.; Hoffer, B.; Olson, L.; Karlsson, T.; Mattson, M.P.; Harvey, B.; Tweedie, D.; et al. Incretin Mimetics as Rational Candidates for the Treatment of Traumatic Brain Injury. ACS Pharmacol. Transl. Sci. 2019, 2, 66–91. [Google Scholar] [CrossRef]
- Li, Y.; Glotfelty, E.J.; Namdar, I.; Tweedie, D.; Olson, L.; Hoffer, B.J.; DiMarchi, R.D.; Pick, C.G.; Greig, N.H. Neurotrophic and neuroprotective effects of a monomeric GLP-1/GIP/Gcg receptor triagonist in cellular and rodent models of mild traumatic brain injury. Exp. Neurol. 2020, 324, 113113. [Google Scholar] [CrossRef]
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014, 35, 992–1019. [Google Scholar] [CrossRef]
- Huang, K.-H.; Yang, Y.; Gau, S.-Y.; Tsai, T.-H.; Lee, C.-Y. Association between dipeptidyl peptidase-4 inhibitor use and risk of Parkinson’s disease among patients with diabetes mellitus: A retrospective cohort study. Aging 2024, 16, 11994–12007. [Google Scholar] [CrossRef]
- Deacon, C.F. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: A comparative review. Diabetes Obes. Metab. 2011, 13, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, R.M.; Safar, M.M. Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: Role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J. Neurochem. 2015, 133, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wang, X. Profile of vildagliptin in type 2 diabetes: Efficacy, safety, and patient acceptability. Ther. Clin. Risk Manag. 2013, 9, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Nakamura, A.; Shirakawa, J.; Muraoka, T.; Togashi, Y.; Shinoda, K.; Orime, K.; Kubota, N.; Kadowaki, T.; Terauchi, Y. Impact of the dipeptidyl peptidase-4 inhibitor vildagliptin on glucose tolerance and β-cell function and mass in insulin receptor substrate-2-knockout mice fed a high-fat diet. Endocrinology 2012, 153, 1093–1102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamamoto, S.; Kanda, Y.; Shimoda, M.; Tatsumi, F.; Kohara, K.; Tawaramoto, K.; Hashiramoto, M.; Kaku, K. Vildagliptin preserves the mass and function of pancreatic β cells via the developmental regulation and suppression of oxidative and endoplasmic reticulum stress in a mouse model of diabetes. Diabetes Obes. Metab. 2013, 15, 153–163. [Google Scholar] [CrossRef]
- Pintana, H.; Apaijai, N.; Chattipakorn, N.; Chattipakorn, S.C. DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin-resistant rats. J. Endocrinol. 2013, 218, 1–11. [Google Scholar] [CrossRef]
- Pipatpiboon, N.; Pintana, H.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur. J. Neurosci. 2013, 37, 839–849. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, X.; Li, C.; Li, D.; Zhang, J.; Yang, Y.; Kong, Y.; Guo, H.; Liu, D.; Chen, L. Dipeptidyl peptidase-4 inhibitor, vildagliptin, inhibits pancreatic beta cell apoptosis in association with its effects suppressing endoplasmic reticulum stress in db/db mice. Metabolism 2015, 64, 226–235. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Tang, J.; Jiang, J.; Chen, Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim. Biophys. Sin. 2014, 46, 629–640. [Google Scholar] [CrossRef]
- Matsui, T.; Nishino, Y.; Takeuchi, M.; Yamagishi, S. Vildagliptin blocks vascular injury in thoracic aorta of diabetic rats by suppressing advanced glycation end product-receptor axis. Pharmacol. Res. 2011, 63, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Nassar, N.N.; Al-Shorbagy, M.Y.; Arab, H.H.; Abdallah, D.M. Saxagliptin: A novel antiparkinsonian approach. Neuropharmacology 2015, 89, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Badawi, G.A.; Abd El Fattah, M.A.; Zaki, H.F.; El Sayed, M.I. Sitagliptin and liraglutide reversed nigrostriatal degeneration of rodent brain in rotenone-induced Parkinson’s disease. Inflammopharmacology 2017, 25, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Huh, J.-W.; Jang, M.; Suh, J.-H.; Kim, T.-W.; Park, J.-S.; Yoon, S.-Y. Sitagliptin increases tau phosphorylation in the hippocampus of rats with type 2 diabetes and in primary neuron cultures. Neurobiol. Dis. 2012, 46, 52–58. [Google Scholar] [CrossRef]
- Domínguez Avila, J.A.; Rodrigo García, J.; González Aguilar, G.A.; De la Rosa, L.A. The Antidiabetic Mechanisms of Polyphenols Related to Increased Glucagon-Like Peptide-1 (GLP1) and Insulin Signaling. Molecules 2017, 22, 903. [Google Scholar] [CrossRef]
- Ogura, J.; Yamaguchi, H. The Effectiveness of Antidiabetic Drugs in Treating Dementia: A Peek into Pharmacological and Pharmacokinetic Properties. Int. J. Mol. Sci. 2022, 23, 6542. [Google Scholar] [CrossRef]
- Svenningsson, P.; Wirdefeldt, K.; Yin, L.; Fang, F.; Markaki, I.; Efendic, S.; Ludvigsson, J.F. Reduced incidence of Parkinson’s disease after dipeptidyl peptidase-4 inhibitors-A nationwide case-control study. Mov. Disord. 2016, 31, 1422–1423. [Google Scholar] [CrossRef]
- Brauer, R.; Wei, L.; Ma, T.; Athauda, D.; Girges, C.; Vijiaratnam, N.; Auld, G.; Whittlesea, C.; Wong, I.; Foltynie, T. Diabetes medications and risk of Parkinson’s disease: A cohort study of patients with diabetes. Brain 2020, 143, 3067–3076. [Google Scholar] [CrossRef]
- Jeong, S.H.; Chung, S.J.; Yoo, H.S.; Hong, N.; Jung, J.H.; Baik, K.; Lee, Y.H.; Sohn, Y.H.; Lee, P.H. Beneficial effects of dipeptidyl peptidase-4 inhibitors in diabetic Parkinson’s disease. Brain 2021, 144, 1127–1137. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2014, 38, 140–149. [Google Scholar] [CrossRef]
- Cardoso, S.; Moreira, P.I. Antidiabetic drugs for Alzheimer’s and Parkinson’s diseases: Repurposing insulin, metformin, and thiazolidinediones. Int. Rev. Neurobiol. 2020, 155, 37–64. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Baerlocher, M.O.; Silverberg, J. Management of type 2 diabetes mellitus. Role of thiazolidinediones. Can. Fam. Physician 2005, 51, 683–687. [Google Scholar] [PubMed]
- Corona, J.C.; Duchen, M.R. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic. Biol. Med. 2016, 100, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xiong, Z.; Fu, T.; Yang, J.; Zou, J.; Wu, Y.; Kuang, L.; Wang, Q.; Li, S.; Le, A. Regulation of renal ischemia-reperfusion injury and tubular epithelial cell ferroptosis by pparγ m6a methylation: Mechanisms and therapeutic implications. Biol. Direct 2024, 19, 99. [Google Scholar] [CrossRef]
- Lai, W.; Yu, L.; Deng, Y. PPARγ alleviates preeclampsia development by regulating lipid metabolism and ferroptosis. Commun. Biol. 2024, 7, 429. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Y.; Liu, L.; Yang, L.; Li, P.; Liu, X.; Yin, H. Proteomic analysis reveals that ACSL4 activation during reflux esophagitis contributes to ferroptosis-mediated esophageal mucosal damage. Eur. J. Pharmacol. 2022, 931, 175175. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Bi, R.; Su, Y.; Quan, F.; Lin, Y.; Yue, C.; Cui, X.; Zhao, Q.; Liu, S.; et al. ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022, 51, 102262. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Cheng, Y.; Yang, M.; Wang, R. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. FASEB J. 2020, 34, 16262–16275. [Google Scholar] [CrossRef]
- Li, Y.; Feng, D.; Wang, Z.; Zhao, Y.; Sun, R.; Tian, D.; Liu, D.; Zhang, F.; Ning, S.; Yao, J.; et al. Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ 2019, 26, 2284–2299. [Google Scholar] [CrossRef]
- Yang, J.; Shi, X.; Wang, Y.; Ma, M.; Liu, H.; Wang, J.; Xu, Z. Multi-Target Neuroprotection of Thiazolidinediones on Alzheimer’s Disease via Neuroinflammation and Ferroptosis. J. Alzheimers Dis. 2023, 96, 927–945. [Google Scholar] [CrossRef]
- Breidert, T.; Callebert, J.; Heneka, M.T.; Landreth, G.; Launay, J.M.; Hirsch, E.C. Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J. Neurochem. 2002, 82, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Dehmer, T.; Heneka, M.T.; Sastre, M.; Dichgans, J.; Schulz, J.B. Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J. Neurochem. 2004, 88, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L.; Dragicevic, N.; Seifert, K.; Choi, D.Y.; Liu, M.; Kim, H.-C.; Cass, W.A.; Sullivan, P.G.; Bing, G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J. Neurochem. 2007, 100, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kaundal, R.K.; More, S.; Sharma, S.S. Beneficial effects of pioglitazone on cognitive impairment in MPTP model of Parkinson’s disease. Behav. Brain Res. 2009, 197, 398–403. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Liu, J.Z.; Hu, J.X.; Chen, H.L.; Li, W.L.; Hai, C.X. Double antioxidant activities of rosiglitazone against high glucose-induced oxidative stress in hepatocyte. Toxicol. In Vitro 2011, 25, 839–847. [Google Scholar] [CrossRef]
- Jung, T.W.; Lee, J.Y.; Shim, W.S.; Kang, E.S.; Kim, S.K.; Ahn, C.W.; Lee, H.C.; Cha, B.S. Rosiglitazone protects human neuroblastoma SH-SY5Y cells against acetaldehyde-induced cytotoxicity. Biochem. Biophys. Res. Commun. 2006, 340, 221–227. [Google Scholar] [CrossRef]
- Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Gödtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Butler, A.E.; Sahebkar, A. Sodium-glucose cotransporter inhibitors and oxidative stress: An update. J. Cell. Physiol. 2019, 234, 3231–3237. [Google Scholar] [CrossRef]
- Oshima, H.; Miki, T.; Kuno, A.; Mizuno, M.; Sato, T.; Tanno, M.; Yano, T.; Nakata, K.; Kimura, Y.; Abe, K.; et al. Empagliflozin, an SGLT2 Inhibitor, Reduced the Mortality Rate after Acute Myocardial Infarction with Modification of Cardiac Metabolomes and Antioxidants in Diabetic Rats. J. Pharmacol. Exp. Ther. 2019, 368, 524–534. [Google Scholar] [CrossRef]
- Osorio, H.; Coronel, I.; Arellano, A.; Pacheco, U.; Bautista, R.; Franco, M.; Escalante, B. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid. Med. Cell. Longev. 2012, 2012, 542042. [Google Scholar] [CrossRef]
- Terami, N.; Ogawa, D.; Tachibana, H.; Hatanaka, T.; Wada, J.; Nakatsuka, A.; Eguchi, J.; Horiguchi, C.S.; Nishii, N.; Yamada, H.; et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 2014, 9, e100777. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-F.; Chen, Y.-L.; Chiou, T.T.-Y.; Chu, T.-H.; Li, L.-C.; Ng, H.-Y.; Lee, W.-C.; Lee, C.-T. Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases. Antioxidants 2021, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Asil, H.; Demiryürek, A.T.; Düzen, I.V.; Büyükcelebi, O.; Saracaloglu, A.; Demirkiran, C.; Demiryürek, Ş. Effects of empagliflozin and dapagliflozin on serum humanin, MOTS-c levels, nitrosative stress, and ferroptosis parameters in diabetic patients with heart failure. Eur. J. Pharmacol. 2024, 982, 176934. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-M.; Chang, N.-C.; Lin, S.-Z. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic. Biol. Med. 2017, 104, 298–310. [Google Scholar] [CrossRef]
- Sharma, A.; Aruna, D.; Beatrice, A. A Study to Evaluate the Effect of Sodium-Glucose Co-transporter 2 (SGLT2) Inhibitors on Oxidative Stress Parameters in Type 2 Diabetes Mellitus Patients. Cureus 2024, 16, e58536. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Iskander, C.; Wang, C.; Xiong, L.Y.; Shah, B.R.; Edwards, J.D.; Kapral, M.K.; Herrmann, N.; Lanctôt, K.L.; Masellis, M.; et al. Association of Sodium-Glucose Cotransporter 2 Inhibitors with Time to Dementia: A Population-Based Cohort Study. Diabetes Care 2023, 46, 297–304. [Google Scholar] [CrossRef]
- Kim, H.K.; Biessels, G.J.; Yu, M.H.; Hong, N.; Lee, Y.; Lee, B.-W.; Kang, E.S.; Cha, B.-S.; Lee, E.J.; Lee, M. SGLT2 Inhibitor Use and Risk of Dementia and Parkinson Disease Among Patients with Type 2 Diabetes. Neurology 2024, 103, e209805. [Google Scholar] [CrossRef]
- Liu, J.; Shi, X.; Shao, Y. Sodium-glucose cotransporter 1/2 inhibition and risk of neurodegenerative disorders: A Mendelian randomization study. Brain Behav. 2024, 14, e3624. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Han, K.-D.; Kwon, H.; Park, S.-E.; Park, Y.-G.; Kim, Y.-H.; Yoo, S.-J.; Rhee, E.-J.; Lee, W.-Y. Association Between Glycemic Status and the Risk of Parkinson Disease: A Nationwide Population-Based Study. Diabetes Care 2020, 43, 2169–2175. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Jiang, J.; Liu, F.; Zhang, Y. Do oral antidiabetic medications alter the risk of Parkinson’s disease? An updated systematic review and meta-analysis. Neurol. Sci. 2023, 44, 4193–4203. [Google Scholar] [CrossRef]
- Arya, J.K.; Kumar, R.; Singh, A.; Srivastava, P.; Yadawa, A.K.; Rizvi, S.I. Acarbose Mitigates Age-Dependent Alterations in Erythrocyte Membrane Transporters During Aging in Rats. Rejuvenation Res. 2023, 26, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Arya, J.K.; Kumar, R.; Singh, A.; Srivastava, P.; Yadawa, A.K.; Rizvi, S.I. Acarbose, an α-Glucosidase Inhibitor, Maintains Altered Redox Homeostasis During Aging by Targeting Glucose Metabolism in Rat Erythrocytes. Rejuvenation Res. 2023, 26, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-C.; Yu, M.-H.; Lin, M.-C.; Huang, C.-N.; Chung, D.-J.; Lee, Y.-J.; Wu, C.-H.; Wang, C.-J. Pleiotropic effects of acarbose on atherosclerosis development in rabbits are mediated via upregulating AMPK signals. Sci. Rep. 2016, 6, 38642. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Liu, H.; Li, J.; Sunli, Y.; Liu, B.; Liu, D.; Zhang, P.; Meng, X. Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J. Diabetes 2015, 7, 729–739. [Google Scholar] [CrossRef]
- NINDS. Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators Pioglitazone in early Parkinson’s disease: A phase 2, multicentre, double-blind, randomised trial. Lancet Neurol. 2015, 14, 795–803. [Google Scholar] [CrossRef]
- AwadAllah Elgnainy, A.; Hamed, M.I.; Osman Mohamed, W.; Sabri, N.A. Investigation of the Possible Correlation between Idiopathic Parkinson’s Disease and Diabetes Mellitus in Egyptian Patients: A Pilot Study. Neurol. Res. Int. 2021, 2021, 2838669. [Google Scholar] [CrossRef]
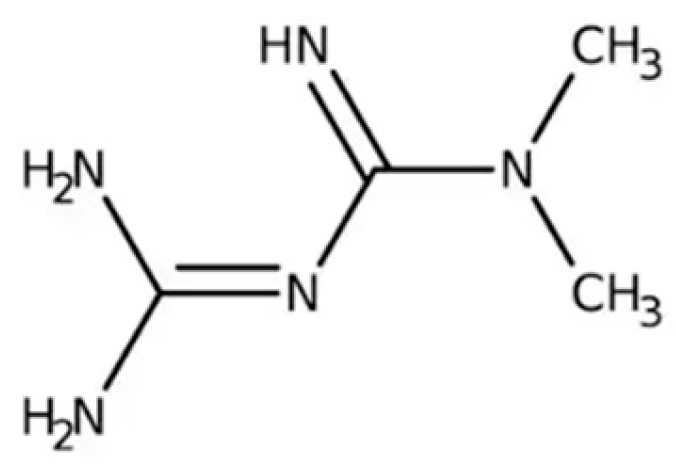
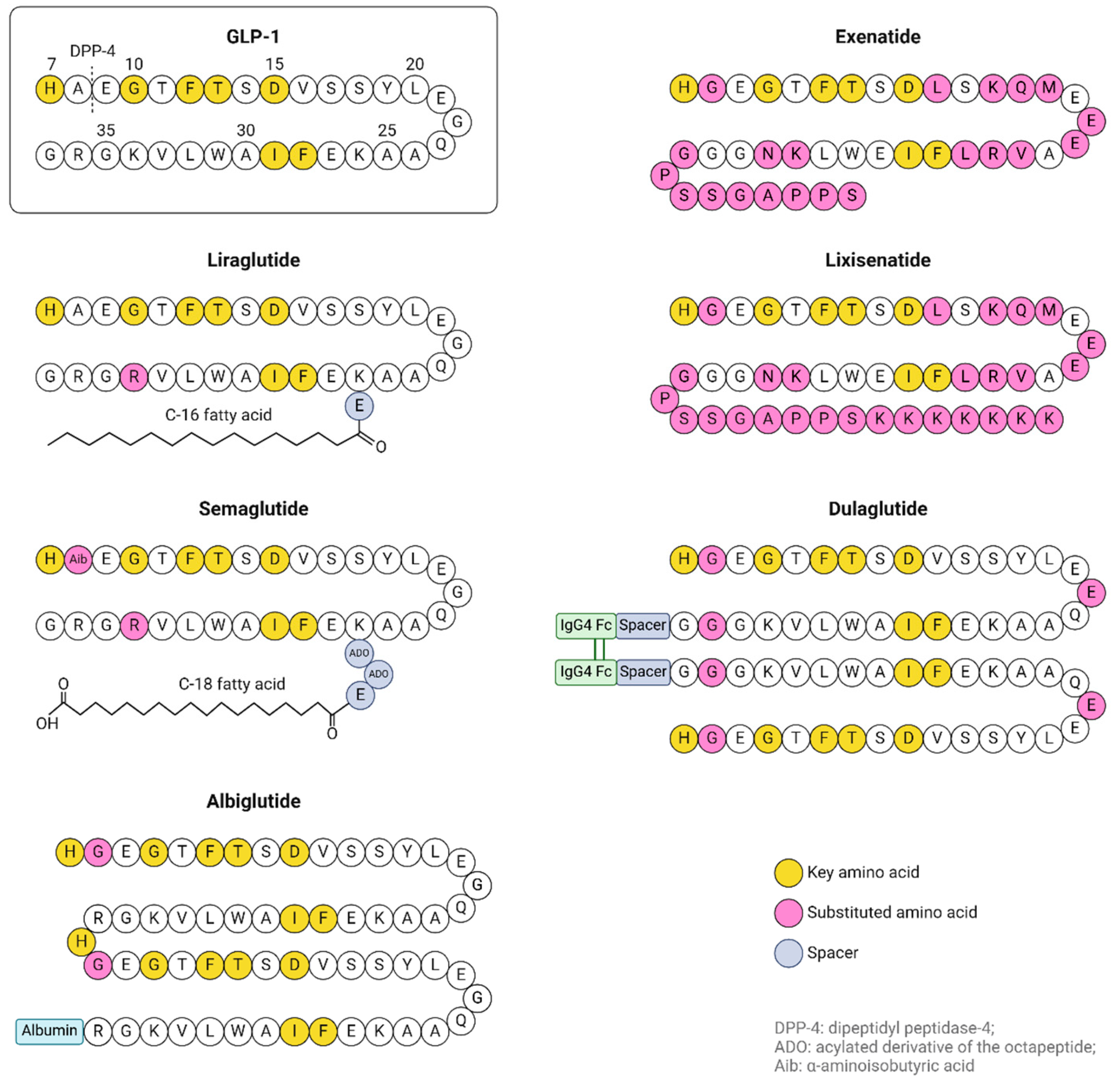

| Key Mechanism | Details |
|---|---|
| Reduction of Oxidative Stress | Decrease in ROS Production: Metformin inhibits reverse electron flux through mitochondrial complex I, reducing ROS production and preventing oxidative stress and cell death [50]. Enhancement of Antioxidant Systems: Increases levels of glutathione (GSH) and superoxide dismutase (SOD), enhancing the cell’s ability to neutralize ROS [56,58]. Scavenging Free Radicals: Directly scavenges hydroxyl free radicals and indirectly decreases ROS through NADPH oxidase or the mitochondrial respiratory chain [53,54]. Induction of MnSOD: Promotes manganese-dependent superoxide dismutase (MnSOD) and mitochondrial biogenesis, reducing mitochondrial ROS [51]. |
| Enhancement of Autophagy and Protein Homeostasis | Activation of Autophagy Pathways: Enhances autophagy, promoting the elimination of α-syn aggregates, which are neurotoxic in PD [65,66,67]. Reduction of α-syn Aggregation: Decreases α-syn aggregation and dopaminergic neuron loss in PD models [65,67]. Regulation of Protein Phosphorylation: Reduces levels of phosphorylated α-syn at serine 129 by activating protein phosphatase 2A (PP2A) via AMPK-dependent and independent pathways [63,64]. |
| Energy Metabolism and Mitochondrial Function | AMPK Activation: Activates AMPK, promoting metabolic balance and neuronal survival. AMPK activation leads to inhibition of lipid biosynthesis and maintenance of ATP levels, crucial for neuronal health [36,37,38,39]. Mitochondrial Protection: Causes mild inhibition of mitochondrial complex I, maintaining ATP production and reducing energy stress. Promotes mitochondrial biogenesis, enhancing mitochondrial function and resilience [30,31,32,33,34,51]. |
| Anti-Inflammatory Effects | Reduction of Inflammation: Regulates changes in astrocytes and microglia, reducing neuroinflammation and protecting dopaminergic neurons [73]. |
| Protection of Dopaminergic Neurons and Improvement in PD Models | Protective Effects in PD Models: Reduces dopaminergic neuron loss and α-syn accumulation in various PD models [65,67,69]. Enhancement of Motor Functions: Improves motor performance in MPTP-induced PD mice by restoring dopamine levels and reducing pathological markers [65,68]. Reduction of Neurotoxic Aggregates: Prevents MGO-induced α-syn oligomerization, mitigating neurodegeneration [19,66,67]. |
| Modulation of Signaling Pathways | Nrf2 Pathway Activation: Activates the Nrf2 signaling pathway, enhancing cellular antioxidant defenses and reducing oxidative damage [56]. AMP-Dependent Pathways: Utilizes alternative AMPK activation routes, such as the lysosomal pathway, stabilizing cellular energy homeostasis and resilience against stress [40]. |
| Miscellaneous Neuroprotective Mechanisms | Indirect Effects on Insulin Sensitivity: Enhances insulin sensitivity in peripheral tissues, potentially supporting overall neuronal health through metabolic regulation [25,26]. Scavenging Advanced Glycation End-Products (AGEs): Acts as a scavenger of methylglyoxal (MGO) and may prevent the accumulation of AGEs, reducing neurotoxicity and protein dysfunction [19,66,67,68]. |
| Inhibition of Ferroptosis | Modulation of Ferroptosis Markers: Increases GPx4 and suppresses ACSL4 levels, preventing lipid peroxidation and ferroptosis [56,70,71]. Regulation of Iron Homeostasis: Upregulates ferroportin (FPN) expression, reducing iron overload and enhancing iron detoxification. Reduces ferritin heavy chain expression, improving iron homeostasis [59]. Enhancement of Antioxidant Defenses: Elevates GSH levels and SOD activity, strengthening defenses against ferroptosis [58,69]. Inhibition of Lipid Peroxidation: Decreases malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) levels, reducing markers of lipid peroxidation [55,67]. |
| Generic Name/Trade Name | Indication | Approval Year |
|---|---|---|
| Exenatide (Byetta®) | T2D | 2005 |
| Liraglutide (Victoza®) | T2D | 2010 |
| Dulaglutide (Trulicity®) | T2D | 2014 |
| Liraglutide (Saxenda®) | Obesity/overweight | 2014 |
| Lixisenatide (Adlyxin®) | T2D | 2016 |
| Liraglutide + insulin degludec (Xultophy®) | T2D | 2016 |
| Lixisenatide + insulin glargine (Soliqua®) | T2D | 2016 |
| Exenatide extended release (Bydureon®) | T2D | 2017 |
| Semaglutide injection (Ozempic®) | T2D | 2017 |
| Semaglutide tablets (Rybelsus®) | T2D | 2019 |
| Semaglutide (Wegovy®) | Obesity/overweight | 2021 |
| Tirzepatide (Mounjaro®) (dual GLP-1/GIP receptor agonist) | T2D | 2022 |
| Tirzepatide (Zepbound®) (dual GLP-1/GIP receptor agonist) | Obesity/overweight | 2023 |
| Key Effects | Details |
|---|---|
| Neuroprotective Efficacy and Neuronal Protection | Synaptic Protection and Synaptogenesis: Protects synapses and promotes the formation of new synapses, enhancing neural connectivity [99]. Enhancement of Synaptic Plasticity: Improves the adaptability of synapses, facilitating learning and memory consolidation [99]. Rescue of Cognitive Decline: Prevents or reverses deterioration in cognitive functions [90,94]. Regulation of Glial Cell Functions: Modulates microglia and astrocyte activation, reducing neuroinflammation [112,113,114]. Prevention of Ca2⁺ Overload: Protects neurons from calcium-induced toxicity [99]. Protection of Nigrostriatal Neurons: Safeguards dopaminergic neurons in the nigrostriatal pathway [100,101,102]. Dopamine Replenishing: Restores dopamine levels in the brain, improving motor functions [100,101,102]. |
| Stress and Inflammation Reduction, Anti-Apoptotic Effects | Suppression of ER Stress: Reduces endoplasmic reticulum stress, preventing protein misfolding and aggregation [104,107]. Anti-Inflammatory Effects: Decreases neuroinflammation by reducing pro-inflammatory cytokines [87,112,113,114,126]. Protection from External Oxidative Stress: Mitigates damage caused by oxidative agents [99,100,101,102,104,107]. Anti-Apoptotic Activities: Prevents programmed cell death in neurons [112,113,114]. |
| Mitochondrial Protection | Mitigation of Mitochondrial Dysfunction: Improves mitochondrial integrity and function, ensuring efficient energy production [110]. Recovery of Mitochondrial Function: Restores normal mitochondrial operations, preventing energy deficits [110]. Reduction of Superoxide Formation: Decreases superoxide radical production, preventing oxidative mitochondrial damage [126,127,128,129]. |
| Ferroptosis Inhibition | Reduction of Iron Overload: Decreases iron accumulation in the brain and other tissues, preventing iron-induced oxidative damage [94,107,125,126,127,128,129,131]. Modulation of Ferroptosis Markers: Increases GPx4 and SLC7A11 expression, and decreases ACSL4 levels, thereby inhibiting ferroptosis [110]. Enhancement of Antioxidant Defenses: Elevates GSH and SOD levels, strengthening cellular defenses against ferroptosis [107,108,126,127,128,129,130,131]. Activation of Antioxidant Signaling Pathways: Activates the Nrf2/HO-1 pathway, enhancing overall antioxidant capacity [107,126]. Regulation of Antioxidant Proteins: Increases Bcl-2 and Bcl-xL, which scavenge free radicals and inhibit superoxide anion formation [111]. Reduction of Lipid Peroxidation: Lowers MDA and HNE levels, reducing markers of lipid peroxidation [99,108,131]. |
| Improvement in Motor and Cognitive Functions | Enhanced Motor Performance: Improves motor functions in PD models and patients through dopamine replenishing and neuronal protection [115,116,117,118,119,120,121]. Cognitive Function Improvement: Enhances cognitive capabilities and reduces cognitive decline through synaptic and neuronal support [90,94,99,120,126,127,128,129,130,131]. |
| Iron Metabolism Regulation | Reduction of Iron Overload in Tissues: Lowers iron levels in the liver and brain, preventing iron-induced oxidative stress [94,107,131]. Upregulation of Iron Exporters: Increases ferroportin (FPN) expression and decreases transferrin receptor (TfR1) expression, improving iron homeostasis and reducing iron import [107,128,129,130,131]. |
| Neurogenesis Enhancement | Promotion of Adult Neurogenesis: Encourages the formation of new neurons in the adult brain, particularly in the hippocampus [90,94]. Increase in Neuronal Progenitor Cells: Boosts the population of neuronal progenitor cells, aiding in neuronal regeneration [94]. |
| Oxidative Stress Reduction | Scavenging of Free Radicals: Directly scavenges hydroxyl radicals and indirectly reduces ROS production through DPP-4 and mitochondrial pathways [52,53,55,87]. Upregulation of Antioxidant Enzymes: Increases the expression of MnSOD, GPx4, and other antioxidant enzymes, enhancing the cellular capacity to neutralize ROS [107,108,126,127,128,129,130,131]. |
| Protection against Dopaminergic Neuron Loss | Reduction of α-Synuclein Aggregation: Prevents the accumulation of toxic α-syn oligomers, mitigating their neurotoxic effects [99,112,113,114,131]. Protection from Dopaminergic Neuron Degeneration: Safeguards neurons that produce dopamine, reducing neuron loss and improving motor functions [100,101,120,121,122,123,124,125,126,127,128,129,130,131]. |
| Generic Name/Trade Name | Approved by | Approval Year |
|---|---|---|
| Sitagliptin (Januvia) | FDA | 2006 |
| Vildagliptin (Galvus) | EU | 2007 |
| Saxagliptin (Onglyza) | FDA | 2009 |
| Linagliptin (Tradjenta) | FDA | 2011 |
| Gemigliptin (Zemiglo) | Korea | 2012 |
| Anagliptin (Suiny) | Japan | 2012 |
| Teneligliptin (Tenelia) | Japan | 2012 |
| Alogliptin (Nesina) | FDA | 2013 |
| Trelagliptin (Zafatek/Wedica) | Japan | 2015 |
| Omarigliptin (Marizev) | Japan | 2015 |
| Sitagliptin (Zituvio) | FDA | 2023 |
| Evogliptin (Suganon/Evodine) | Korea | - |
| Gosogliptin (Saterex) | Rusia | - |
| Key Mechanisms | Details |
|---|---|
| Neuroprotective Efficacy and Neuronal Protection | Promotes neuronal growth and survival [146] Enhances neurotrophic factors, supporting neuronal health and function [146] Prevents Dopamine Reduction and Dopaminergic Neuron Loss: Protects dopaminergic neurons from degeneration, preserving dopamine levels in the brain [135,145,146]. |
| Stress and Inflammation Reduction, Anti-Apoptotic Effects | Downregulation of ER Stress Markers: Reduces ER stress by downregulating CHOP, TRIB3, and activating ATF-4, thereby preventing protein misfolding and apoptosis [141,142,143]. Suppress Inflammatory Pathways: Inhibits inflammatory signaling pathways, reducing the production of pro-inflammatory cytokines [135,145]. Prevention of Apoptosis: Prevents programmed cell death in neurons by reducing oxidative and nitrosative stress [135,145,146]. |
| Mitochondrial Protection | Restoring Mitochondrial Complex I Activity and Bcl-2 Levels: Enhances mitochondrial function and integrity, maintaining ATP synthesis and preventing energy deficits [145]. |
| Ferroptosis Inhibition | Improvement of Iron Metabolism: Regulates iron-related proteins such as TfR1 and FPN1, reducing iron overload and enhancing iron detoxification [141,143]. Reduction of Oxidative and Nitrosative Stress: Decreases iNOS transcription and MPO activity, inhibiting ROS and RNS production [144,145]. Reduction of lipid peroxidation: Decreases TBARS levels [144] Activation of Antioxidant Signaling Pathways (Nrf2): Enhances antioxidant defenses and reduces lipid peroxidation [135,145]. |
| Aspect | Incretin Mimetics | DPP-4 Inhibitors |
|---|---|---|
| Mechanism of Action | Directly activate GLP-1receptors enhancing insulin secretion and neuroprotection | Inhibit DPP-4 enzyme, increasing endogenous GLP-1 and GIP levels |
| Neuroprotective Efficacy | Strong neuroprotective effects through direct activation of GLP-1Rs, promoting neurogenesis, synaptic plasticity, and reducing oxidative stress and inflammation | Moderate neuroprotective effects; primarily reduce inflammation and apoptosis indirectly by increasing GLP-1 levels |
| BBB Permeability | Some (e.g., Exenatide, lixisenatide) can cross the BBB, allowing direct central nervous system effects | Generally limited BBB permeability, restricting central effects |
| Impact on Ferroptosis-related Pathways | Potential impact on ferroptosis through reducing oxidative stress, improving mitochondrial function, and enhancing antioxidant pathways (e.g., Nfr2/HO-1) | Limited direct evidence on ferroptosis; potential indirect effects through reducing oxidative stress and inflammation |
| Clinical Evidence in PD | Demonstrated benefits in motor and cognitive functions in clinical trial; potential disease-modifying effects | Some positive effects in preclinical models; clinical evidence less robust compared to GLP-1RAs |
| Combination Therapy Potential | Can be combined with other therapies for enhanced neuroprotective effects | Potential to enhance GLP-1 activity and complement other PD treatments |
| Generic Name/Trade Name | Indication | Approval Year |
|---|---|---|
| Acarbose (Precose®) | T2D | 1995 |
| Miglitol (Glyset®) | T2D | 1996 |
| Voglibose (Voglib®) | T2D | Only in Japan |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duță, C.; Muscurel, C.; Dogaru, C.B.; Stoian, I. Targeting Ferroptosis in Parkinson’s: Repurposing Diabetes Drugs as a Promising Treatment. Int. J. Mol. Sci. 2025, 26, 1516. https://doi.org/10.3390/ijms26041516
Duță C, Muscurel C, Dogaru CB, Stoian I. Targeting Ferroptosis in Parkinson’s: Repurposing Diabetes Drugs as a Promising Treatment. International Journal of Molecular Sciences. 2025; 26(4):1516. https://doi.org/10.3390/ijms26041516
Chicago/Turabian StyleDuță, Carmen, Corina Muscurel, Carmen Beatrice Dogaru, and Irina Stoian. 2025. "Targeting Ferroptosis in Parkinson’s: Repurposing Diabetes Drugs as a Promising Treatment" International Journal of Molecular Sciences 26, no. 4: 1516. https://doi.org/10.3390/ijms26041516
APA StyleDuță, C., Muscurel, C., Dogaru, C. B., & Stoian, I. (2025). Targeting Ferroptosis in Parkinson’s: Repurposing Diabetes Drugs as a Promising Treatment. International Journal of Molecular Sciences, 26(4), 1516. https://doi.org/10.3390/ijms26041516





