CRISPR/Cas9 Targeting of Aldehyde Dehydrogenase 1A1 Reveals Heterogeneous Roles in Radiation Response and Redox Stress Across Clonal Lines in Triple-Negative Breast Cancer
Abstract
1. Introduction
2. Results
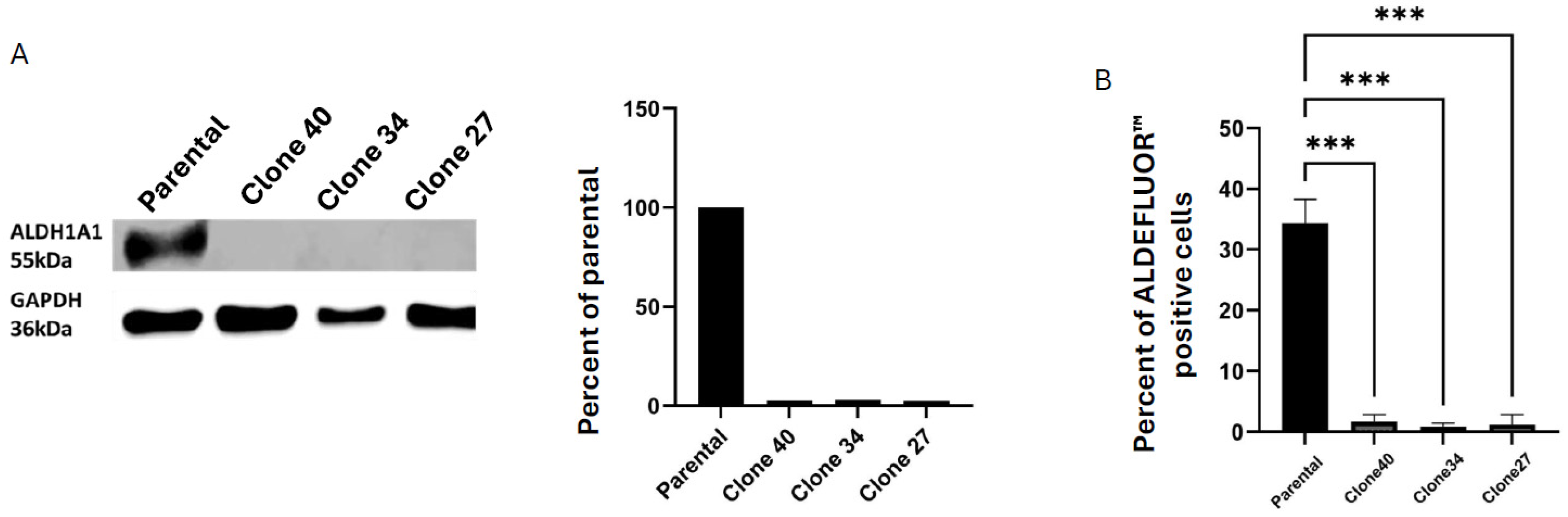
2.1. Effect of CRISPR/Cas9 Knockout (KO) of ALDH1A1 SUM159 Bulk Cells
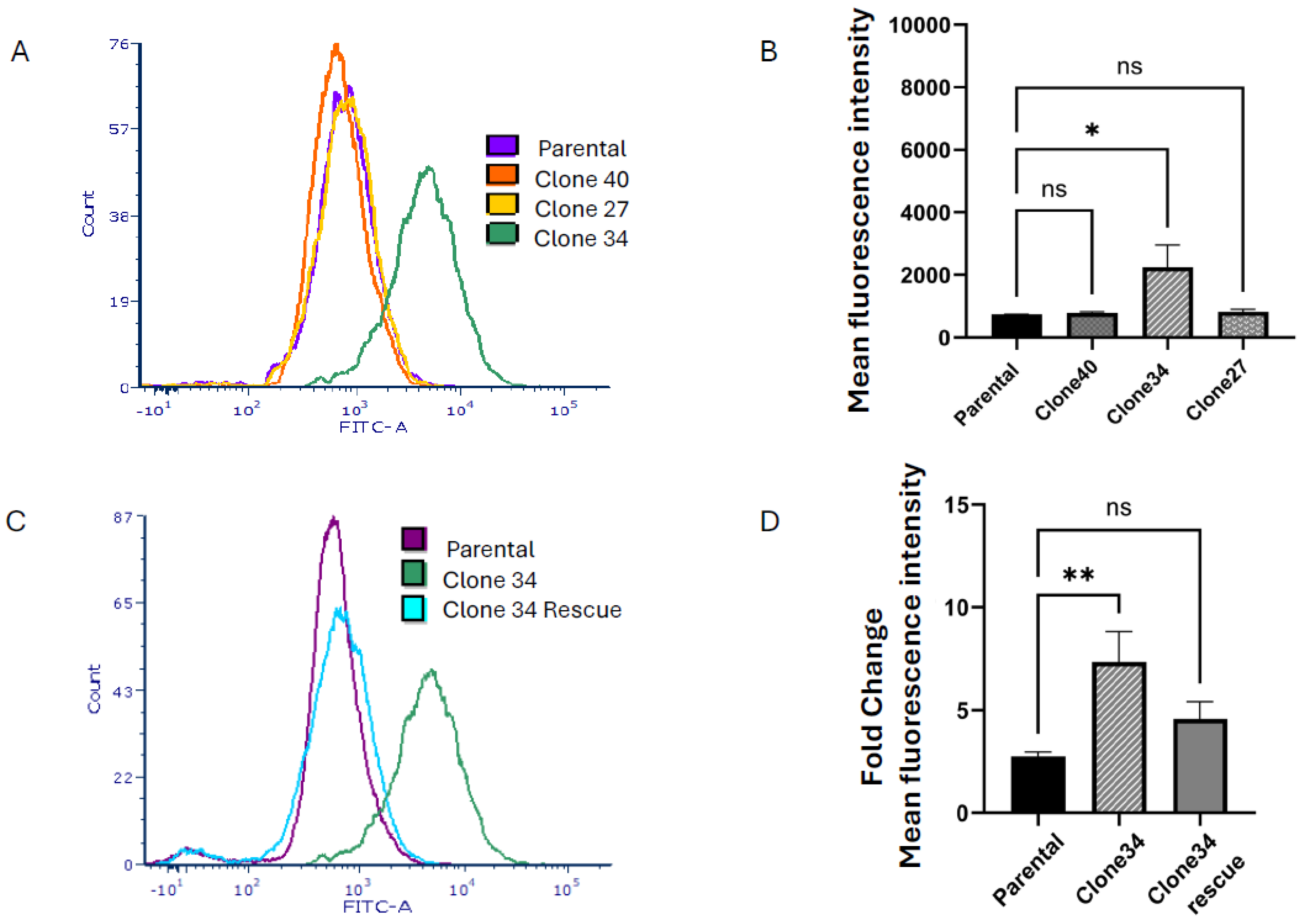
2.2. Clonal Cell Lines Derived from Bulk Knockout Cells Have Decreased ALDEFLUORTM Activity
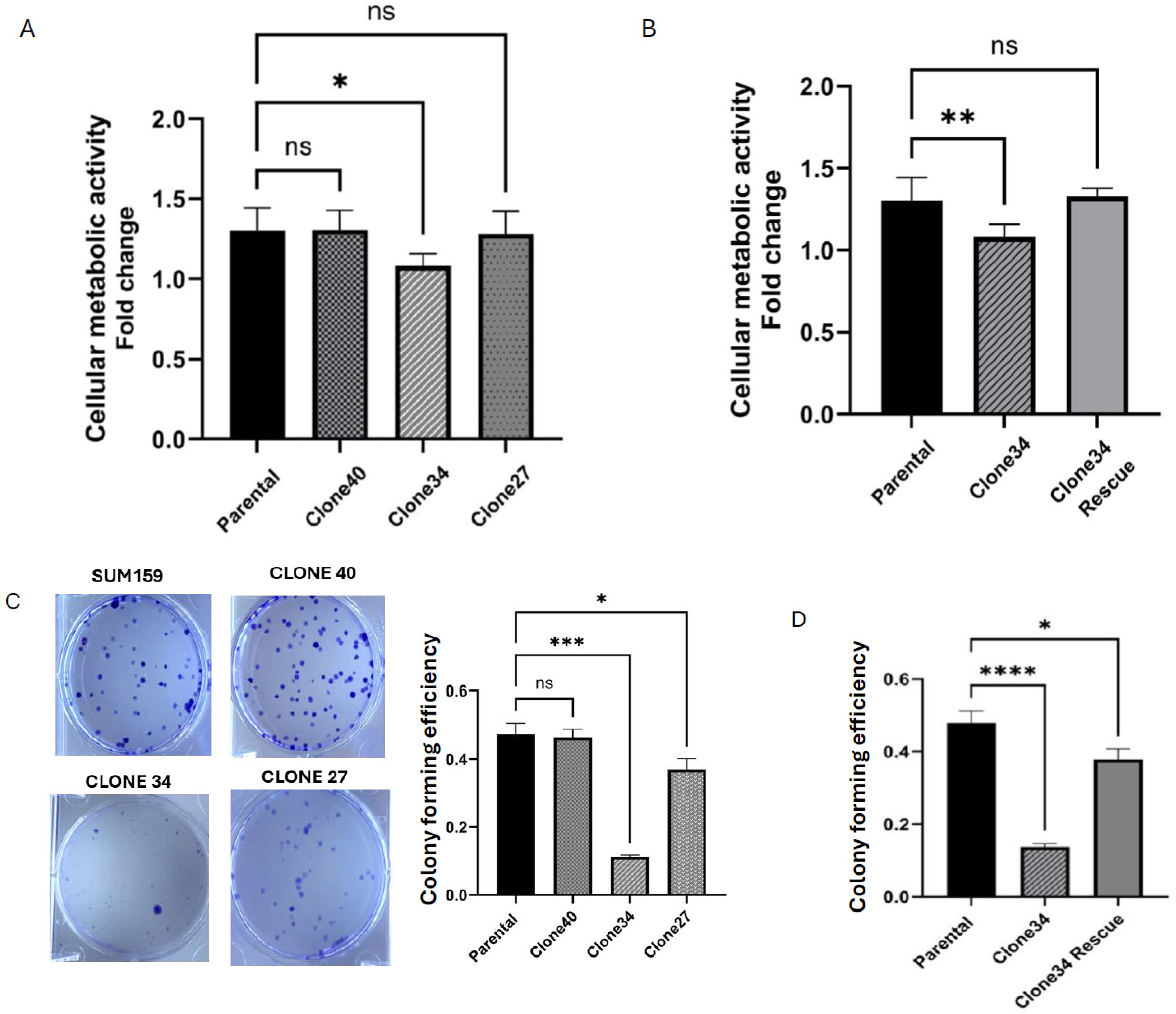
2.3. Clonal Differences in the Effect of ALDH1A1 Knockout on Cellular Metabolic Activity and Colony Formation
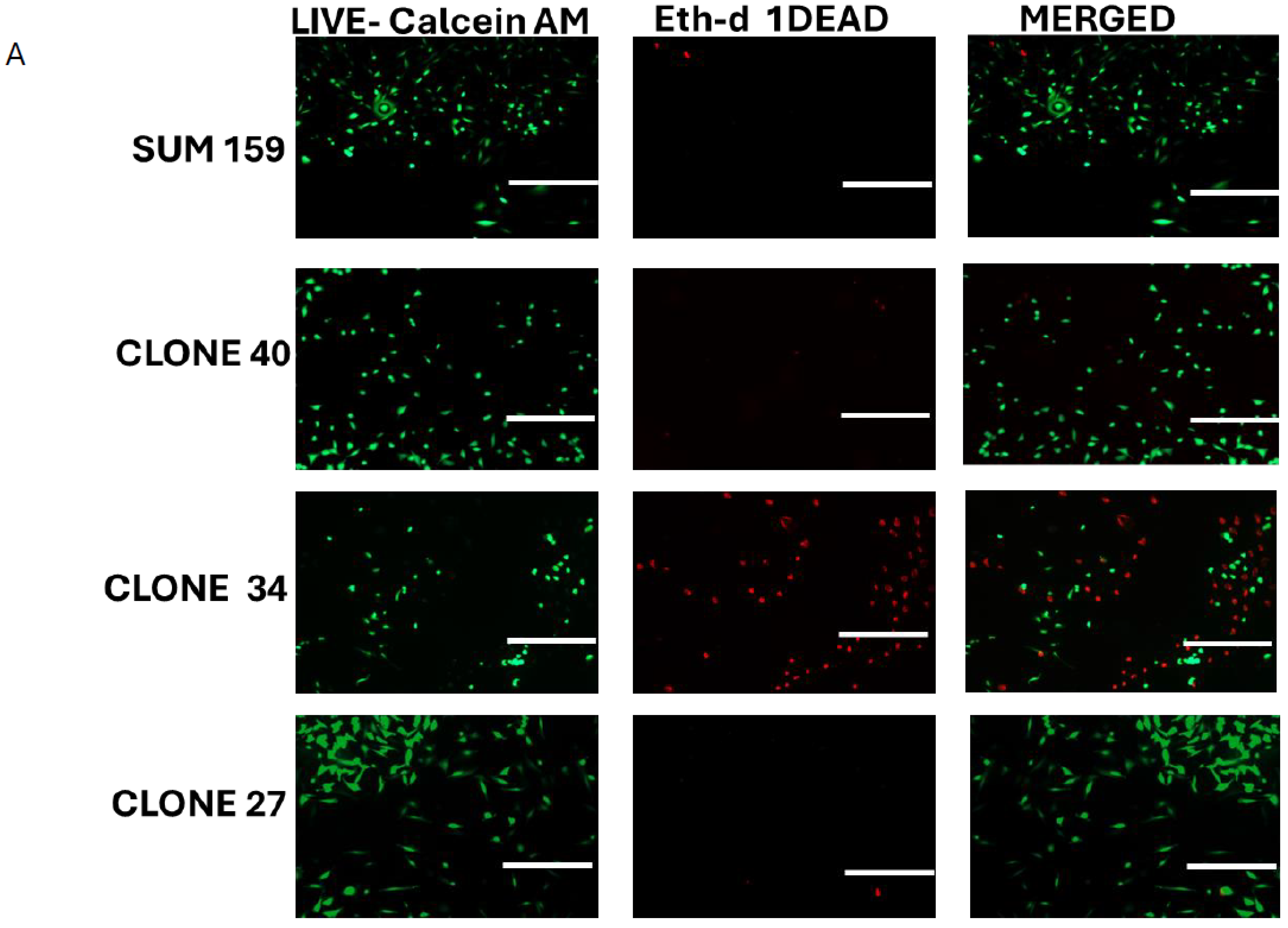
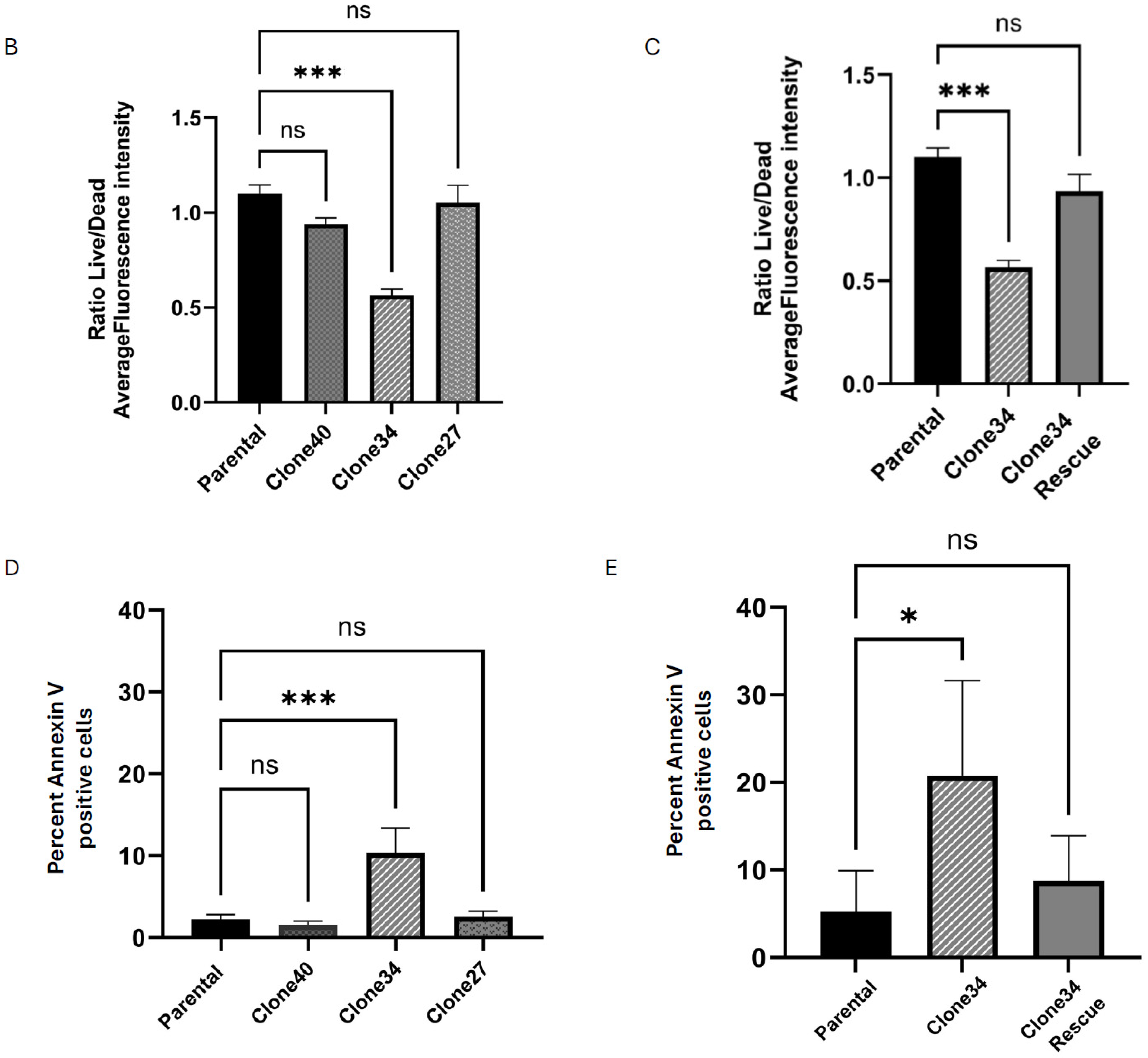
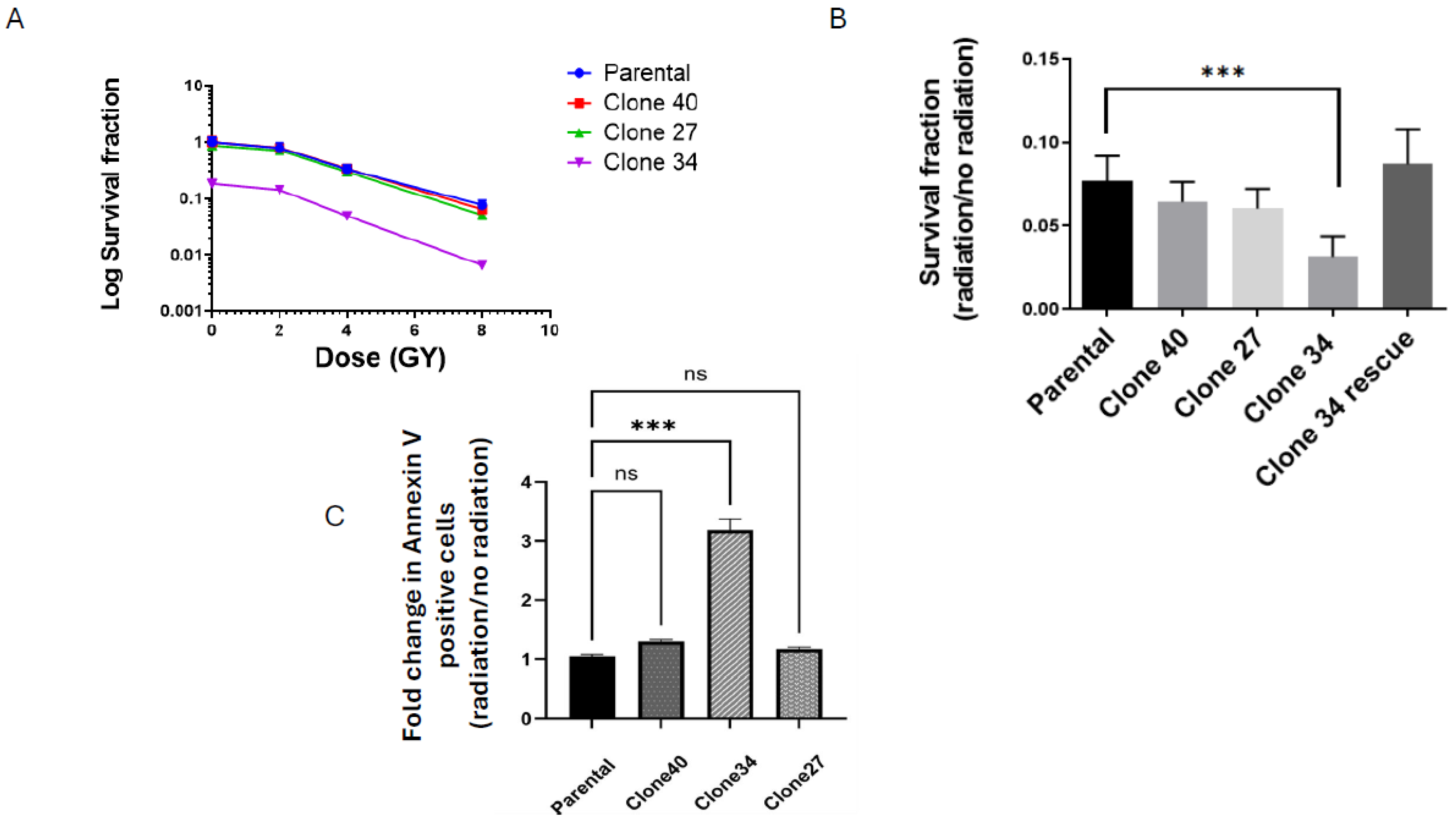
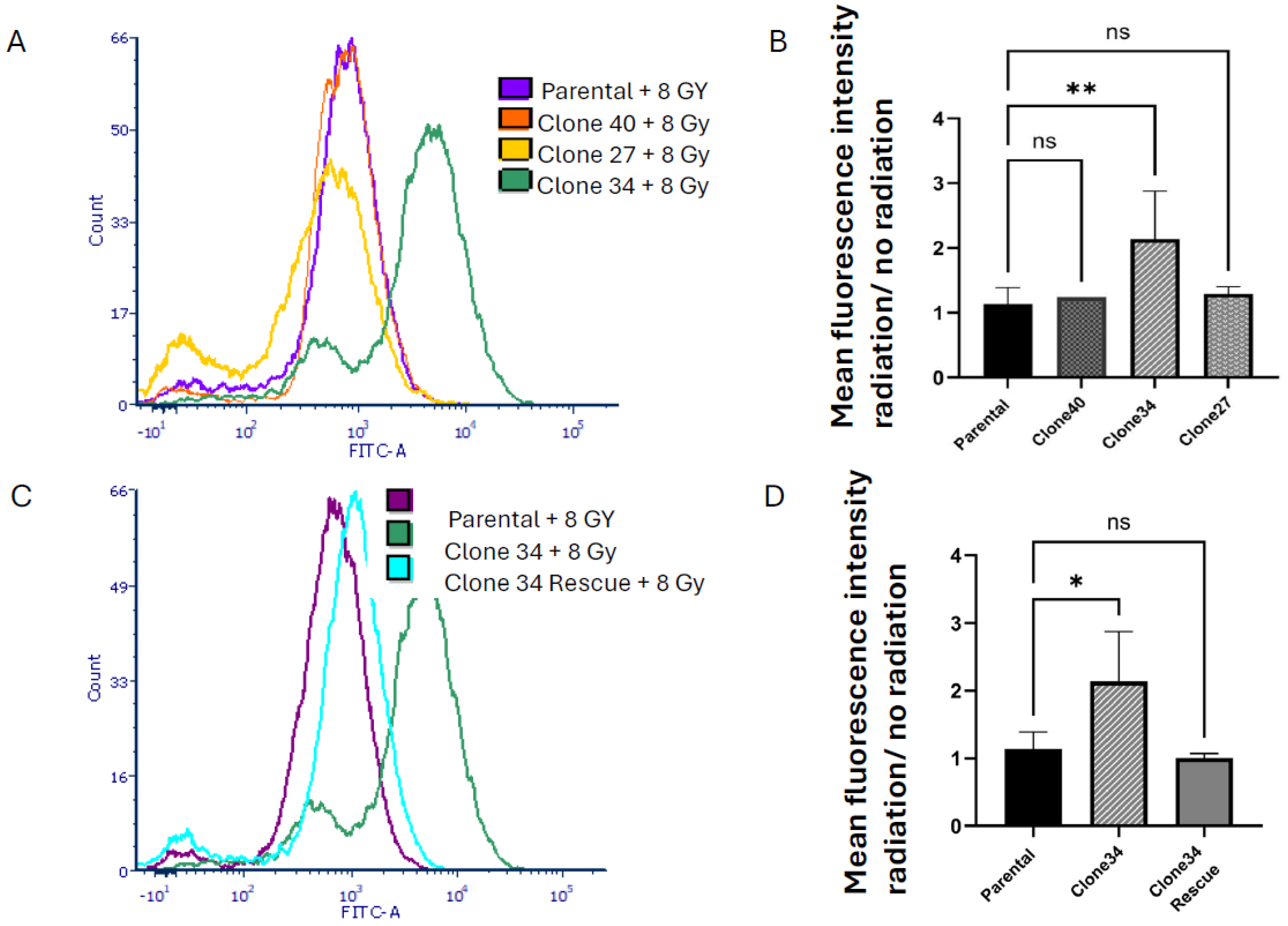
2.4. Clonal Differences in the Effect of ALDH1A1 Knockout on Viability, Apoptosis, and Reactive Oxygen Species
2.5. Radiation Sensitivity in ALDH1A1 Knockout Clones
3. Discussion
4. Materials and Methods
4.1. Cell Culturing
4.2. CRISPR/Cas9 Targeting
4.3. Western Blot Analysis
4.4. Sanger Sequencing
4.5. ALDEFLUOR Assay
4.6. Colony Forming Assay
4.7. Apoptosis Assay
4.8. Live/Dead Assay
4.9. Reactive Oxygen Species
4.10. Alamar Blue Viability Assay
4.11. Lentiviral Transduction
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, X.L.; Li, Z. Incidence trends in triple-negative breast cancer among women in the United States from 2010 to 2019 by race/ethnicity, age and tumor stage. Am. J. Cancer Res. 2023, 13, 678–691. [Google Scholar] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Althobiti, M.; El Ansari, R.; Aleskandarany, M.; Joseph, C.; Toss, M.S.; Green, A.R.; Rakha, E.A. The prognostic significance of ALDH1A1 expression in early invasive breast cancer. Histopathology 2020, 77, 437–448. [Google Scholar] [CrossRef]
- Echeverria, G.V.; Ge, Z.; Seth, S.; Zhang, X.; Jeter-Jones, S.; Zhou, X.; Cai, S.; Tu, Y.; McCoy, A.; Peoples, M.; et al. Resistance to neoadjuvant chemotherapy in triple-negative breast cancer mediated by a reversible drug-tolerant state. Sci. Transl. Med. 2019, 11, eaav0936. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Kumar, D.N.; Dehari, D.; Patil, R.; Singh, S.; Kumar, D.; Agrawal, A.K. Endorsement of TNBC Biomarkers in Precision Therapy by Nanotechnology. Cancers 2023, 15, 2661. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Tanaka, K.; Tanaka, T.; Hara, A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget 2016, 7, 11018–11032. [Google Scholar] [CrossRef] [PubMed]
- Marcato, P.; Dean, C.A.; Giacomantonio, C.A.; Lee, P.W. Aldehyde dehydrogenase: Its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle 2011, 10, 1378–1384. [Google Scholar] [CrossRef]
- Croker, A.K.; Rodriguez-Torres, M.; Xia, Y.; Pardhan, S.; Leong, H.S.; Lewis, J.D.; Allan, A.L. Differential Functional Roles of ALDH1A1 and ALDH1A3 in Mediating Metastatic Behavior and Therapy Resistance of Human Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18, 2039. [Google Scholar] [CrossRef]
- Olsson, M.; Larsson, P.; Johansson, J.; Sah, V.R.; Parris, T.Z. Cancer stem cells are prevalent in the basal-like 2 and mesenchymal triple-negative breast cancer subtypes in vitro. Front. Cell Dev. Biol. 2023, 11, 1237673. [Google Scholar] [CrossRef]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef]
- Liu, Y.; Baglia, M.; Zheng, Y.; Blot, W.; Bao, P.P.; Cai, H.; Nechuta, S.; Zheng, W.; Cai, Q.; Shu, X.O. ALDH1A1 mRNA expression in association with prognosis of triple-negative breast cancer. Oncotarget 2015, 6, 41360–41369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, F.; Li, H.; Li, Y.; Ding, X.; Wang, H.; Fan, Y.; Lin, C.; Qian, H.; Xu, B. Aldehyde dehydrogenase 1 (ALDH1) expression is an independent prognostic factor in triple negative breast cancer (TNBC). Medicine 2017, 96, e6561. [Google Scholar] [CrossRef]
- Maosa, S.F.L.; Schug, Z.; Siegel SSims-Mourtada, J. Strong Tumor Expression of ALDH1A1 is Associated with Black Race, Metabolic Disorders, and Poor Breast Cancer Outcomes. Cancer Health Disparities 2024, 7, 1–14. [Google Scholar]
- Panigoro, S.S.; Kurnia, D.; Kurnia, A.; Haryono, S.J.; Albar, Z.A. ALDH1 Cancer Stem Cell Marker as a Prognostic Factor in Triple-Negative Breast Cancer. Int. J. Surg. Oncol. 2020, 2020, 7863243. [Google Scholar] [CrossRef]
- Ciccone, V.; Terzuoli, E.; Donnini, S.; Giachetti, A.; Morbidelli, L.; Ziche, M. Stemness marker ALDH1A1 promotes tumor angiogenesis via retinoic acid/HIF-1alpha/VEGF signalling in MCF-7 breast cancer cells. J. Exp. Clin. Cancer Res. 2018, 37, 311. [Google Scholar] [CrossRef]
- Allison, S.E.; Chen, Y.; Petrovic, N.; Zhang, J.; Bourget, K.; Mackenzie, P.I.; Murray, M. Activation of ALDH1A1 in MDA-MB-468 breast cancer cells that over-express CYP2J2 protects against paclitaxel-dependent cell death mediated by reactive oxygen species. Biochem. Pharmacol. 2017, 143, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Calleja, L.F.; Yoval-Sanchez, B.; Hernandez-Esquivel, L.; Gallardo-Perez, J.C.; Sosa-Garrocho, M.; Marin-Hernandez, A.; Jasso-Chavez, R.; Macias-Silva, M.; Salud Rodriguez-Zavala, J. Activation of ALDH1A1 by omeprazole reduces cell oxidative stress damage. FEBS J. 2021, 288, 4064–4080. [Google Scholar] [CrossRef]
- Xiao, Z.; Ding, L.; Yu, Y.; Ma, C.; Lei, C.; Liu, Y.; Chang, X.; Chen, Y.; He, Y.; Zhu, Y.; et al. Tanreqing injection inhibits stemness and enhances sensitivity of non-small cell lung cancer models to gefitinib through ROS/STAT3 signaling pathway. J. Cancer 2024, 15, 4259–4274. [Google Scholar] [CrossRef]
- Yue, H.; Hu, Z.; Hu, R.; Guo, Z.; Zheng, Y.; Wang, Y.; Zhou, Y. ALDH1A1 in Cancers: Bidirectional Function, Drug Resistance, and Regulatory Mechanism. Front. Oncol. 2022, 12, 918778. [Google Scholar] [CrossRef]
- Granit Mizrahi, A.; Gugenheim, A.; Hamad, H.; Hamed, R.; Tetro, N.; Maimon, O.; Khutsurauli, S.; Nechushtan, H.; Nisman, B.; Duran, D.; et al. Valproic acid reprograms the metabolic aberration of cisplatin treatment via ALDH modulation in triple-negative breast cancer cells. Front. Cell Dev. Biol. 2023, 11, 1217149. [Google Scholar] [CrossRef]
- Marchitti, S.A.; Chen, Y.; Thompson, D.C.; Vasiliou, V. Ultraviolet radiation: Cellular antioxidant response and the role of ocular aldehyde dehydrogenase enzymes. Eye Contact Lens 2011, 37, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, C.; Yang, L.; Wang, J.; Jn-Simon, N.; Zhou, C.; Bryant, A.; Cao, Q.; Li, C.; Petersen, B.; et al. Liver regeneration and ethanol detoxification: A new link in YAP regulation of ALDH1A1 during alcohol-related hepatocyte damage. FASEB J. 2022, 36, e22224. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Laskar, A.; Younus, H. Aldehyde toxicity and metabolism: The role of aldehyde dehydrogenases in detoxification, drug resistance and carcinogenesis. Drug Metab. Rev. 2019, 51, 42–64. [Google Scholar] [CrossRef]
- Jiang, H.; Zuo, J.; Li, B.; Chen, R.; Luo, K.; Xiang, X.; Lu, S.; Huang, C.; Liu, L.; Tang, J.; et al. Drug-induced oxidative stress in cancer treatments: Angel or devil? Redox. Biol. 2023, 63, 102754. [Google Scholar] [CrossRef]
- Zhang, M.; Shoeb, M.; Goswamy, J.; Liu, P.; Xiao, T.L.; Hogan, D.; Campbell, G.A.; Ansari, N.H. Overexpression of aldehyde dehydrogenase 1A1 reduces oxidation-induced toxicity in SH-SY5Y neuroblastoma cells. J. Neurosci. Res. 2010, 88, 686–694. [Google Scholar] [CrossRef]
- Gorodetska, I.; Offermann, A.; Puschel, J.; Lukiyanchuk, V.; Gaete, D.; Kurzyukova, A.; Freytag, V.; Haider, M.T.; Fjeldbo, C.S.; Di Gaetano, S.; et al. ALDH1A1 drives prostate cancer metastases and radioresistance by interplay with AR- and RAR-dependent transcription. Theranostics 2024, 14, 714–737. [Google Scholar] [CrossRef]
- Arnold, K.M.; Opdenaker, L.M.; Flynn, N.J.; Appeah, D.K.; Sims-Mourtada, J. Radiation induces an inflammatory response that results in STAT3-dependent changes in cellular plasticity and radioresistance of breast cancer stem-like cells. Int. J. Radiat. Biol. 2020, 96, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.J.; Wang, M.F.; Han, C.L.; Wang, S.L. Substrate binding pocket structure of human aldehyde dehydrogenases. A substrate specificity approach. Adv. Exp. Med. Biol. 1995, 372, 9–16. [Google Scholar] [CrossRef]
- Arnold, K.M.; Flynn, N.J.; Raben, A.; Romak, L.; Yu, Y.; Dicker, A.P.; Mourtada, F.; Sims-Mourtada, J. The Impact of Radiation on the Tumor Microenvironment: Effect of Dose and Fractionation Schedules. Cancer Growth Metastasis 2018, 11, 1179064418761639. [Google Scholar] [CrossRef]
- Yang, P.; Luo, X.; Li, J.; Zhang, T.; Gao, X.; Hua, J.; Li, Y.; Ding, N.; He, J.; Zhang, Y.; et al. Ionizing Radiation Upregulates Glutamine Metabolism and Induces Cell Death via Accumulation of Reactive Oxygen Species. Oxid. Med. Cell Longev. 2021, 2021, 5826932. [Google Scholar] [CrossRef]
- Duan, J.J.; Cai, J.; Gao, L.; Yu, S.C. ALDEFLUOR activity, ALDH isoforms, and their clinical significance in cancers. J. Enzyme Inhib. Med. Chem. 2023, 38, 2166035. [Google Scholar] [CrossRef] [PubMed]
- Moreb, J.S.; Ucar-Bilyeu, D.A.; Khan, A. Use of retinoic acid/aldehyde dehydrogenase pathway as potential targeted therapy against cancer stem cells. Cancer Chemother. Pharmacol. 2017, 79, 295–301. [Google Scholar] [CrossRef]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef]
- Cojoc, M.; Peitzsch, C.; Kurth, I.; Trautmann, F.; Kunz-Schughart, L.A.; Telegeev, G.D.; Stakhovsky, E.A.; Walker, J.R.; Simin, K.; Lyle, S.; et al. Aldehyde Dehydrogenase Is Regulated by beta-Catenin/TCF and Promotes Radioresistance in Prostate Cancer Progenitor Cells. Cancer Res. 2015, 75, 1482–1494. [Google Scholar] [CrossRef]
- Lagadec, C.; Vlashi, E.; Alhiyari, Y.; Phillips, T.M.; Bochkur Dratver, M.; Pajonk, F. Radiation-induced Notch signaling in breast cancer stem cells. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, Y.; Zheng, J.; Pan, J. Reactive oxygen species in cancer stem cells. Antioxid. Redox. Signal. 2012, 16, 1215–1228. [Google Scholar] [CrossRef]
- Bo, Y.; Zhou, J.; Cai, K.; Wang, Y.; Feng, Y.; Li, W.; Jiang, Y.; Kuo, S.H.; Roy, J.; Anorma, C.; et al. Leveraging intracellular ALDH1A1 activity for selective cancer stem-like cell labeling and targeted treatment via in vivo click reaction. Proc. Natl. Acad. Sci. USA 2023, 120, e2302342120. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Luo, Y.; Tian, P.; Peng, F.; Lu, J.; Yang, Y.; Su, Q.; Liu, B.; Yu, J.; Luo, X.; et al. Stress-induced epinephrine enhances lactate dehydrogenase A and promotes breast cancer stem-like cells. J. Clin. Investig. 2019, 129, 1030–1046. [Google Scholar] [CrossRef]
- Anorma, C.; Hedhli, J.; Bearrood, T.E.; Pino, N.W.; Gardner, S.H.; Inaba, H.; Zhang, P.; Li, Y.; Feng, D.; Dibrell, S.E.; et al. Surveillance of Cancer Stem Cell Plasticity Using an Isoform-Selective Fluorescent Probe for Aldehyde Dehydrogenase 1A1. ACS Cent. Sci. 2018, 4, 1045–1055. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.H.; Zhang, Y.F.; Zhu, L.; Lei, H.M.; Tang, Y.B. UPLC-MS-based metabolomics reveals metabolic dysregulation in ALDH1A1-overexpressed lung adenocarcinoma cells. Metabolomics 2019, 15, 52. [Google Scholar] [CrossRef]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef] [PubMed]
- Pommier, R.M.; Sanlaville, A.; Tonon, L.; Kielbassa, J.; Thomas, E.; Ferrari, A.; Sertier, A.S.; Hollande, F.; Martinez, P.; Tissier, A.; et al. Comprehensive characterization of claudin-low breast tumors reflects the impact of the cell-of-origin on cancer evolution. Nat. Commun. 2020, 11, 3431. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.; Guarin, J.R.; Le, T.T.; Fatherree, J.; Kelley, C.; Payne, S.; Salhany, K.; McGinn, R.; Henrich, E.; Yui, A.; et al. Cell morphology best predicts tumorigenicity and metastasis in vivo across multiple TNBC cell lines of different metastatic potential. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Fang, C.; Cook, K.C.; Park, N.; Wei, Y.; Spadazzi, C.; Bracha, D.; Gunaratna, R.T.; Laevsky, G.; DeCoste, C.J.; et al. TGF-beta-induced DACT1 biomolecular condensates repress Wnt signalling to promote bone metastasis. Nat. Cell Biol. 2021, 23, 257–267. [Google Scholar] [CrossRef]
- Rizwan, A.; Cheng, M.; Bhujwalla, Z.M.; Krishnamachary, B.; Jiang, L.; Glunde, K. Breast cancer cell adhesome and degradome interact to drive metastasis. NPJ Breast Cancer 2015, 1, 15017. [Google Scholar] [CrossRef]
- Lee, C.T.; Zhou, Y.; Roy-Choudhury, K.; Siamakpour-Reihani, S.; Young, K.; Hoang, P.; Kirkpatrick, J.P.; Chi, J.T.; Dewhirst, M.W.; Horton, J.K. Subtype-Specific Radiation Response and Therapeutic Effect of FAS Death Receptor Modulation in Human Breast Cancer. Radiat. Res. 2017, 188, 169–180. [Google Scholar] [CrossRef]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajayi, G.O.; Ma, A.; Modarai, S.R.; Opdenaker, L.M.; Sims-Mourtada, J. CRISPR/Cas9 Targeting of Aldehyde Dehydrogenase 1A1 Reveals Heterogeneous Roles in Radiation Response and Redox Stress Across Clonal Lines in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2025, 26, 2303. https://doi.org/10.3390/ijms26052303
Ajayi GO, Ma A, Modarai SR, Opdenaker LM, Sims-Mourtada J. CRISPR/Cas9 Targeting of Aldehyde Dehydrogenase 1A1 Reveals Heterogeneous Roles in Radiation Response and Redox Stress Across Clonal Lines in Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2025; 26(5):2303. https://doi.org/10.3390/ijms26052303
Chicago/Turabian StyleAjayi, Grace O., Aihui Ma, Shirin R. Modarai, Lynn M. Opdenaker, and Jennifer Sims-Mourtada. 2025. "CRISPR/Cas9 Targeting of Aldehyde Dehydrogenase 1A1 Reveals Heterogeneous Roles in Radiation Response and Redox Stress Across Clonal Lines in Triple-Negative Breast Cancer" International Journal of Molecular Sciences 26, no. 5: 2303. https://doi.org/10.3390/ijms26052303
APA StyleAjayi, G. O., Ma, A., Modarai, S. R., Opdenaker, L. M., & Sims-Mourtada, J. (2025). CRISPR/Cas9 Targeting of Aldehyde Dehydrogenase 1A1 Reveals Heterogeneous Roles in Radiation Response and Redox Stress Across Clonal Lines in Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 26(5), 2303. https://doi.org/10.3390/ijms26052303







