Alternative Splicing Analysis Reveals Adrenergic Signaling as a Novel Target for Protein Arginine Methyltransferase 5 (PRMT5) in the Heart
Abstract
1. Introduction
2. Results
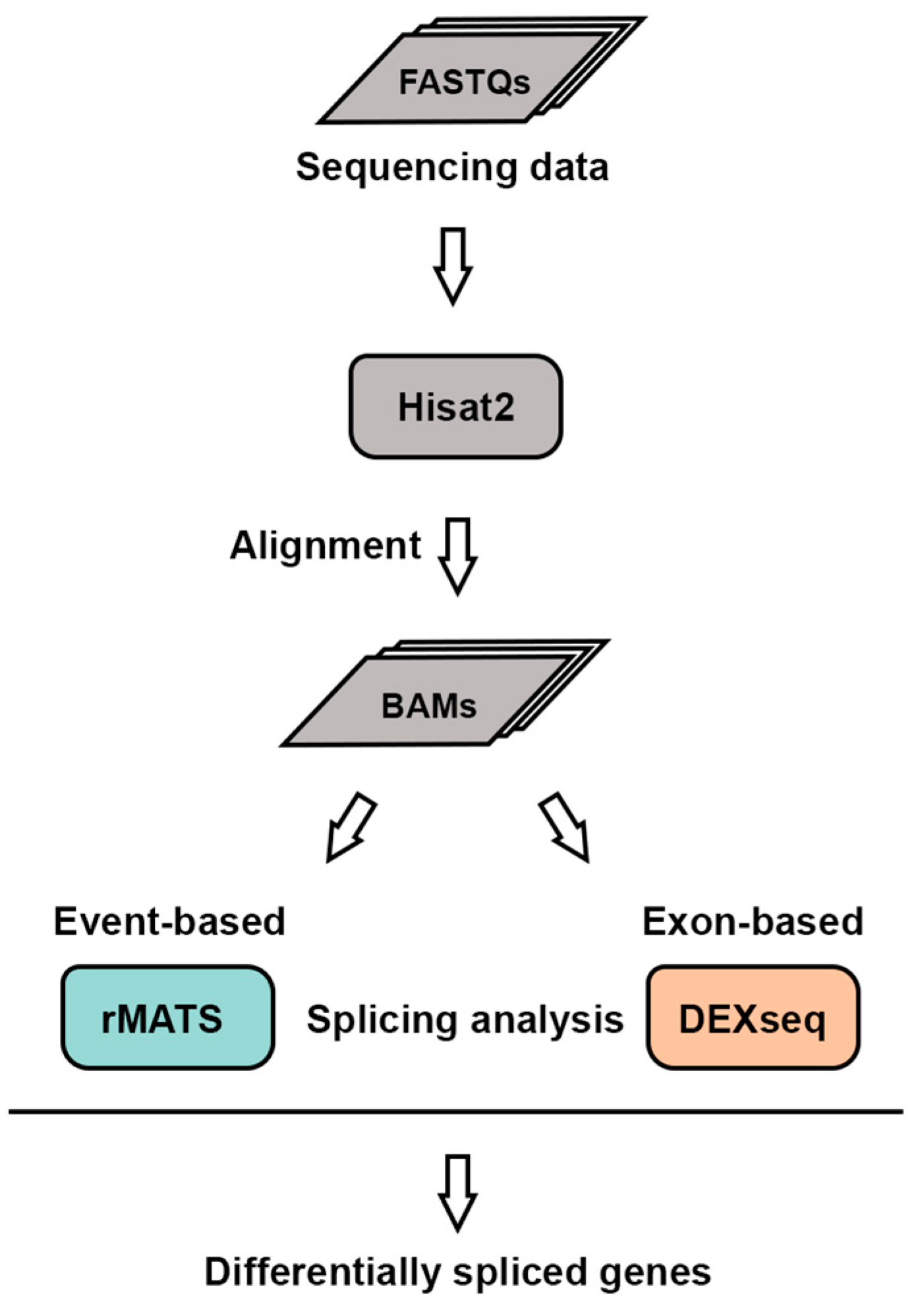
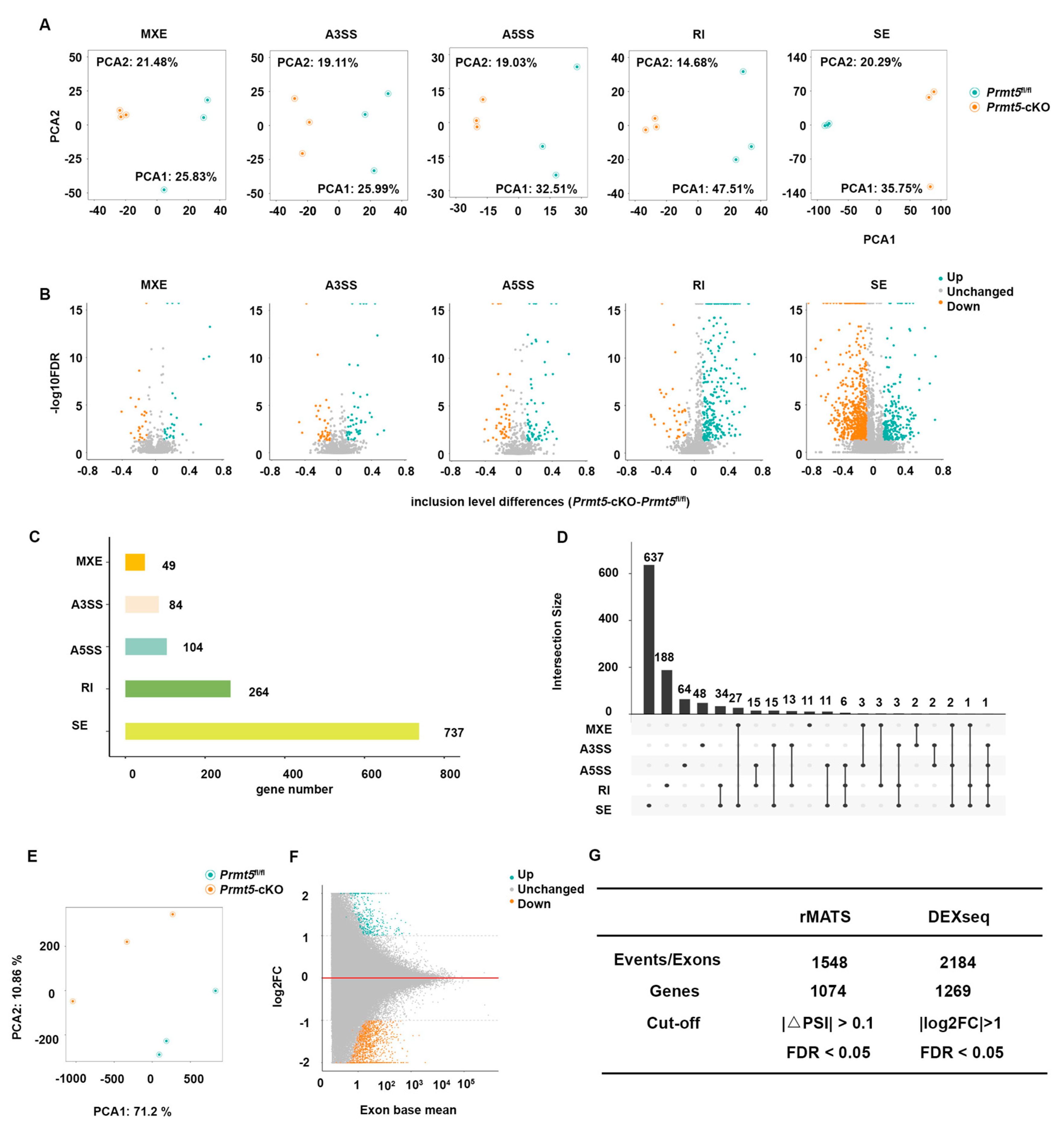
2.1. Analysis of Alternative Splicing in Cardiomyocyte-Specific Prmt5 Knockout Mice
2.2. Identification of Differentially Spliced Genes
2.3. Functional Enrichment Analysis of Differentially Spliced Genes
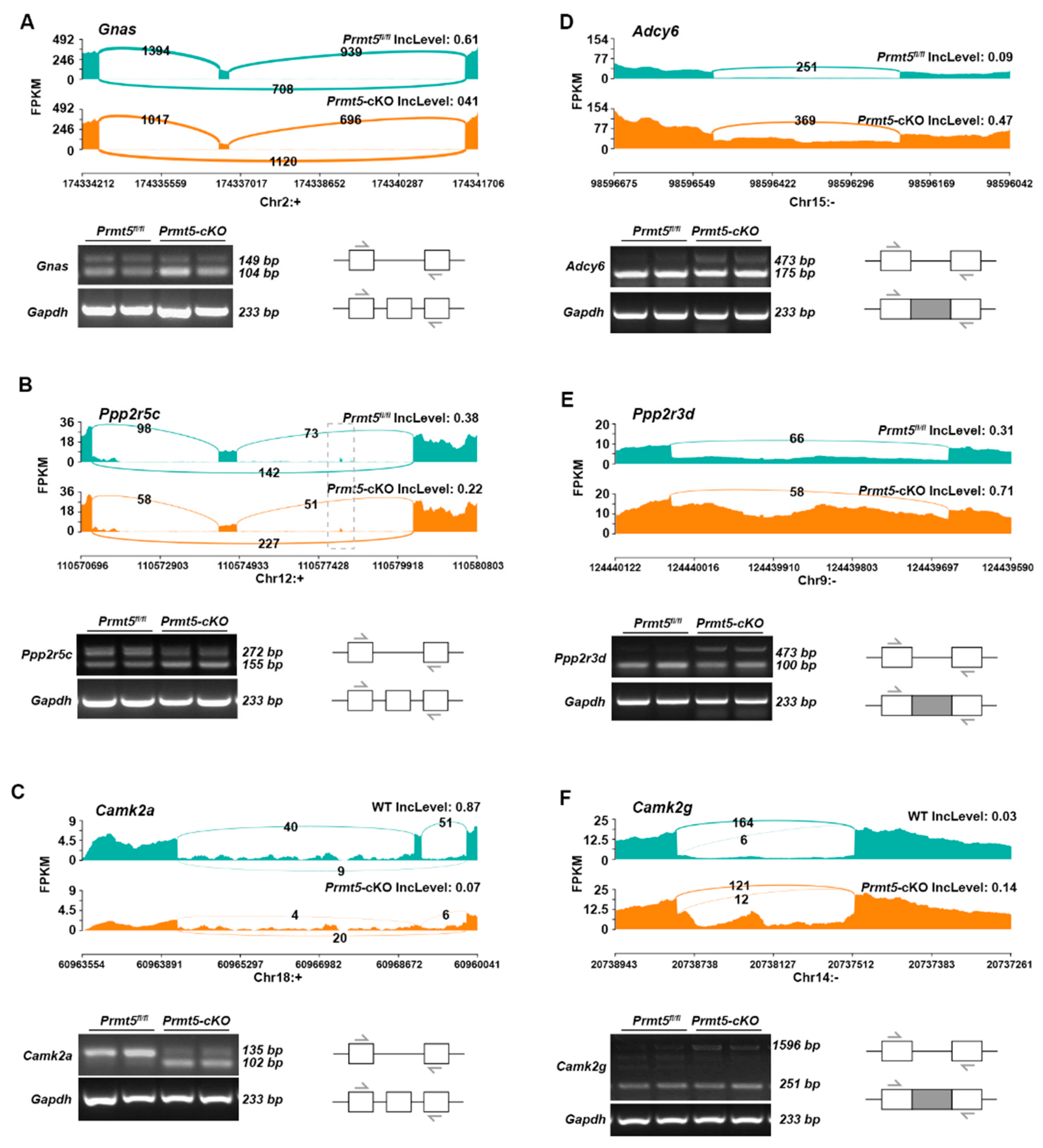
2.4. Validation of DSGs in the Adrenergic Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. RNA Sequencing Data Processing
4.2. Differential Splicing Analysis
4.3. Functional Enrichment Analysis
4.4. RT-PCR Validation
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilman, A.G. G proteins and dual control of adenylate cyclase. Cell 1984, 36, 577–579. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R.; Ginsburg, R.; Minobe, W.; Cubicciotti, R.S.; Sageman, W.S.; Lurie, K.; Billingham, M.E.; Harrison, D.C.; Stinson, E.B. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N. Engl. J. Med. 1982, 307, 205–211. [Google Scholar] [CrossRef]
- Bristow, M.R.; Ginsburg, R.; Umans, V.; Fowler, M.; Minobe, W.; Rasmussen, R.; Zera, P.; Menlove, R.; Shah, P.; Jamieson, S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: Coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ. Res. 1986, 59, 297–309. [Google Scholar] [CrossRef]
- Choi, S.; Cho, N.; Kim, K.K. The implications of alternative pre-mRNA splicing in cell signal transduction. Exp. Mol. Med. 2023, 55, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Briganti, F.; Wang, Z. Alternative Splicing in the Heart: The Therapeutic Potential of Regulating the Regulators. Int. J. Mol. Sci. 2024, 25, 13023. [Google Scholar] [CrossRef]
- Blech-Hermoni, Y.; Ladd, A.N. RNA binding proteins in the regulation of heart development. Int. J. Biochem. Cell Biol. 2013, 45, 2467–2478. [Google Scholar] [CrossRef]
- Khan, M.A.F.; Reckman, Y.J.; Aufiero, S.; van den Hoogenhof, M.M.; van der Made, I.; Beqqali, A.; Koolbergen, D.R.; Rasmussen, T.B.; van der Velden, J.; Creemers, E.E.; et al. RBM20 Regulates Circular RNA Production from the Titin Gene. Circ. Res. 2016, 119, 996–1003. [Google Scholar] [CrossRef]
- Guo, W.; Schafer, S.; Greaser, M.L.; Radke, M.H.; Liss, M.; Govindarajan, T.; Maatz, H.; Schulz, H.; Li, S.; Parrish, A.M.; et al. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat. Med. 2012, 18, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Motolani, A.; Martin, M.; Sun, M.; Lu, T. The Structure and Functions of PRMT5 in Human Diseases. Life 2021, 11, 1074. [Google Scholar] [CrossRef]
- Antonysamy, S.; Bonday, Z.; Campbell, R.M.; Doyle, B.; Druzina, Z.; Gheyi, T.; Han, B.; Jungheim, L.N.; Qian, Y.; Rauch, C.; et al. Crystal structure of the human PRMT5:MEP50 complex. Proc. Natl. Acad. Sci. USA 2012, 109, 17960–17965. [Google Scholar] [CrossRef]
- Gary, J.D.; Clarke, S. RNA and protein interactions modulated by protein arginine methylation. Prog. Nucleic Acid Res. Mol. Biol. 1998, 61, 65–131. [Google Scholar] [CrossRef]
- Stopa, N.; Krebs, J.E.; Shechter, D. The PRMT5 arginine methyltransferase: Many roles in development, cancer and beyond. Cell. Mol. Life Sci. 2015, 72, 2041–2059. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Cao, J.B.; Yang, J.; Liu, T.; Yu, H.L.; He, Z.X.; Bao, S.L.; He, X.X.; Zhu, X.J. PRMT5-mediated homologous recombination repair is essential to maintain genomic integrity of neural progenitor cells. Cell. Mol. Life Sci. 2024, 81, 123. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Nguyen, T.; Song, J.; Zheng, Y.G. Biomedical effects of protein arginine methyltransferase inhibitors. J. Biol. Chem. 2025, 301, 108201. [Google Scholar] [CrossRef]
- Zhu, Y.; Xia, T.; Chen, D.Q.; Xiong, X.; Shi, L.; Zuo, Y.; Xiao, H.; Liu, L. Promising role of protein arginine methyltransferases in overcoming anti-cancer drug resistance. Drug Resist. Update 2023, 72, 101016. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, B.; Wu, Y.; Wu, X.; Wang, Y. Targeting Arginine Methyltransferase PRMT5 for Cancer Therapy: Updated Progress and Novel Strategies. J. Med. Chem. 2023, 66, 8407–8427. [Google Scholar] [CrossRef]
- Quan, X.; Yue, W.; Luo, Y.; Cao, J.; Wang, H.; Wang, Y.; Lu, Z. The protein arginine methyltransferase PRMT5 regulates Aβ-induced toxicity in human cells and Caenorhabditis elegans models of Alzheimer’s disease. J. Neurochem. 2015, 134, 969–977. [Google Scholar] [CrossRef]
- Ratovitski, T.; Arbez, N.; Stewart, J.C.; Chighladze, E.; Ross, C.A. PRMT5- mediated symmetric arginine dimethylation is attenuated by mutant huntingtin and is impaired in Huntington’s disease (HD). Cell Cycle 2015, 14, 1716–1729. [Google Scholar] [CrossRef]
- Nakamura, T.; Sugeno, N.; Hasegawa, T.; Ikeda, K.; Yoshida, S.; Ishiyama, S.; Sato, K.; Takeda, A.; Aoki, M. Alpha-synuclein promotes PRMT5-mediated H4R3me2s histone methylation by interacting with the BAF complex. FEBS J. 2023, 291, 1892–1908. [Google Scholar] [CrossRef]
- Chen, M.; Yi, B.; Sun, J. Inhibition of cardiomyocyte hypertrophy by protein arginine methyltransferase 5. J. Biol. Chem. 2014, 289, 24325–24335. [Google Scholar] [CrossRef]
- Cai, S.; Wang, P.; Xie, T.; Li, Z.; Li, J.; Lan, R.; Ding, Y.; Lu, J.; Ye, J.; Wang, J.; et al. Histone H4R3 symmetric di-methylation by Prmt5 protects against cardiac hypertrophy via regulation of Filip1L/β-catenin. Pharmacol. Res. 2020, 161, 105104. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, C.; Luo, J.; Liang, J. Dysfunctional Network and Mutation Genes of Hypertrophic Cardiomyopathy. J. Heal. Eng. 2022, 2022, 8680178. [Google Scholar] [CrossRef]
- Litzler, L.C.; Zahn, A.; Meli, A.P.; Hébert, S.; Patenaude, A.M.; Methot, S.P.; Sprumont, A.; Bois, T.; Kitamura, D.; Costantino, S.; et al. PRMT5 is essential for B cell development and germinal center dynamics. Nat. Commun. 2019, 10, 22. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Hosohama, L.; Nee, K.; Gkountela, S.; Chaudhari, S.; Cass, A.A.; Xiao, X.; Clark, A.T. The Sm protein methyltransferase PRMT5 is not required for primordial germ cell specification in mice. EMBO J. 2014, 34, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Hamard, P.-J.; Santiago, G.E.; Liu, F.; Karl, D.L.; Martinez, C.; Man, N.; Mookhtiar, A.K.; Duffort, S.; Greenblatt, S.; Verdun, R.E.; et al. PRMT5 Regulates DNA Repair by Controlling the Alternative Splicing of Histone-Modifying Enzymes. Cell Rep. 2018, 24, 2643–2657. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, J.; Song, Y.; Xin, C.; Liu, L.; Hou, N.; Teng, Y.; Cheng, X.; Wang, T.; Yu, Z.; et al. PRMT5 Prevents Dilated Cardiomyopathy via Suppression of Protein O-GlcNAcylation. Circ. Res. 2021, 129, 857–871. [Google Scholar] [CrossRef]
- Xiang, Y.; Kobilka, B.K. Myocyte adrenoceptor signaling pathways. Science 2003, 300, 1530–1532. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, H.; Kaumann, A.J. Regional differences of beta 1- and beta 2-adrenoceptor-mediated functions in feline heart. A beta 2-adrenoceptor-mediated positive inotropic effect possibly unrelated to cyclic AMP. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1991, 344, 56–69. [Google Scholar] [CrossRef]
- Jo, S.H.; Leblais, V.; Wang, P.H.; Crow, M.T.; Xiao, R.P. Phosphatidylinositol 3-kinase functionally compartmentalizes the concurrent G(s) signaling during beta2-adrenergic stimulation. Circ. Res. 2002, 91, 46–53. [Google Scholar] [CrossRef]
- Leblais, V.; Jo, S.H.; Chakir, K.; Maltsev, V.; Zheng, M.; Crow, M.T.; Wang, W.; Lakatta, E.G.; Xiao, R.P. Phosphatidylinositol 3-kinase offsets cAMP-mediated positive inotropic effect via inhibiting Ca2+ influx in cardiomyocytes. Circ. Res. 2004, 95, 1183–1190. [Google Scholar] [CrossRef]
- Xia, B.; Shen, J.; Zhang, H.; Chen, S.; Zhang, X.; Song, M.; Wang, J. The alternative splicing landscape of infarcted mouse heart identifies isoform level therapeutic targets. Sci. Data 2024, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Hoogenhof, M.M.G.v.D.; Beqqali, A.; Amin, A.S.; van der Made, I.; Aufiero, S.; Khan, M.A.F.; Schumacher, C.A.; Jansweijer, J.A.; van Spaendonck-Zwarts, K.Y.; Remme, C.A.; et al. RBM20 Mutations Induce an Arrhythmogenic Dilated Cardiomyopathy Related to Disturbed Calcium Handling. Circulation 2018, 138, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Montañés-Agudo, P.; Aufiero, S.; Schepers, E.N.; van der Made, I.; Cócera-Ortega, L.; Ernault, A.C.; Richard, S.; Kuster, D.W.D.; Christoffels, V.M.; Pinto, Y.M.; et al. The RNA-binding protein QKI governs a muscle-specific alternative splicing program that shapes the contractile function of cardiomyocytes. Cardiovasc. Res. 2023, 119, 1161–1174. [Google Scholar] [CrossRef]
- Gan, P.; Wang, Z.; Bezprozvannaya, S.; McAnally, J.R.; Tan, W.; Li, H.; Bassel-Duby, R.; Liu, N.; Olson, E.N. RBPMS regulates cardiomyocyte contraction and cardiac function through RNA alternative splicing. Cardiovasc. Res. 2023, 120, 56–68. [Google Scholar] [CrossRef]
- Lin, X.; O’Malley, H.; Chen, C.; Auerbach, D.; Foster, M.; Shekhar, A.; Zhang, M.; Coetzee, W.; Jalife, J.; Fishman, G.I.; et al. Scn1b deletion leads to increased tetrodotoxin-sensitive sodium current, altered intracellular calcium homeostasis and arrhythmias in murine hearts. J. Physiol. 2014, 593, 1389–1407. [Google Scholar] [CrossRef]
- Tang, T.; Gao, M.H.; Lai, N.C.; Firth, A.L.; Takahashi, T.; Guo, T.; Yuan, J.X.; Roth, D.M.; Hammond, H.K. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation 2008, 117, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Little, S.C.; Curran, J.; Makara, M.A.; Kline, C.F.; Ho, H.T.; Xu, Z.; Wu, X.; Polina, I.; Musa, H.; Meadows, A.M.; et al. Protein phosphatase 2A regulatory subunit B56α limits phosphatase activity in the heart. Sci. Signal. 2015, 8, ra72. [Google Scholar] [CrossRef]
- Tan, D.Q.; Li, Y.; Yang, C.; Li, J.; Tan, S.H.; Chin, D.W.L.; Nakamura-Ishizu, A.; Yang, H.; Suda, T. PRMT5 Modulates Splicing for Genome Integrity and Preserves Proteostasis of Hematopoietic Stem Cells. Cell Rep. 2019, 26, 2316–2328.e6. [Google Scholar] [CrossRef]
- Muro, R.; Nitta, T.; Nitta, S.; Tsukasaki, M.; Asano, T.; Nakano, K.; Okamura, T.; Nakashima, T.; Okamoto, K.; Takayanagi, H. Transcript splicing optimizes the thymic self-antigen repertoire to suppress autoimmunity. J. Clin. Investig. 2024, 134, e179612. [Google Scholar] [CrossRef]
- Collins, S.; Altschmied, J.; Herbsman, O.; Caron, M.G.; Mellon, P.L.; Lefkowitz, R.J. A cAMP response element in the beta 2-adrenergic receptor gene confers transcriptional autoregulation by cAMP. J. Biol. Chem. 1990, 265, 19330–19335. [Google Scholar] [CrossRef]
- Danner, S.; Frank, M.; Lohse, M.J. Agonist regulation of human beta2-adrenergic receptor mRNA stability occurs via a specific AU-rich element. J. Biol. Chem. 1998, 273, 3223–3229. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Gu, X.; Zhao, R.; Zheng, Q.; Li, L.; Yang, W.; Ding, L.; Xue, F.; Fan, J.; Gong, Y.; et al. Cardiac β2-Adrenergic Receptor Phosphorylation at Ser355/356 Regulates Receptor Internalization and Functional Resensitization. PLoS ONE 2016, 11, e0161373. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, S.; Zhang, Y.; Yang, X.; Wang, J.; Li, Z. Alternative Splicing Analysis Reveals Adrenergic Signaling as a Novel Target for Protein Arginine Methyltransferase 5 (PRMT5) in the Heart. Int. J. Mol. Sci. 2025, 26, 2301. https://doi.org/10.3390/ijms26052301
Jiao S, Zhang Y, Yang X, Wang J, Li Z. Alternative Splicing Analysis Reveals Adrenergic Signaling as a Novel Target for Protein Arginine Methyltransferase 5 (PRMT5) in the Heart. International Journal of Molecular Sciences. 2025; 26(5):2301. https://doi.org/10.3390/ijms26052301
Chicago/Turabian StyleJiao, Shouye, Yimeng Zhang, Xiao Yang, Jian Wang, and Zhenhua Li. 2025. "Alternative Splicing Analysis Reveals Adrenergic Signaling as a Novel Target for Protein Arginine Methyltransferase 5 (PRMT5) in the Heart" International Journal of Molecular Sciences 26, no. 5: 2301. https://doi.org/10.3390/ijms26052301
APA StyleJiao, S., Zhang, Y., Yang, X., Wang, J., & Li, Z. (2025). Alternative Splicing Analysis Reveals Adrenergic Signaling as a Novel Target for Protein Arginine Methyltransferase 5 (PRMT5) in the Heart. International Journal of Molecular Sciences, 26(5), 2301. https://doi.org/10.3390/ijms26052301






