Functional Interactions Between Recombinant Serum Amyloid A1 (SAA1) and Chemokines in Leukocyte Recruitment
Abstract
1. Introduction
2. Isolation and Identification of Chemotactic Factors from Blood Plasma
3. Commercial Recombinant Serum Amyloid A1 (SAA1) Purified to Homogeneity Fails to Induce Inflammatory Mediators
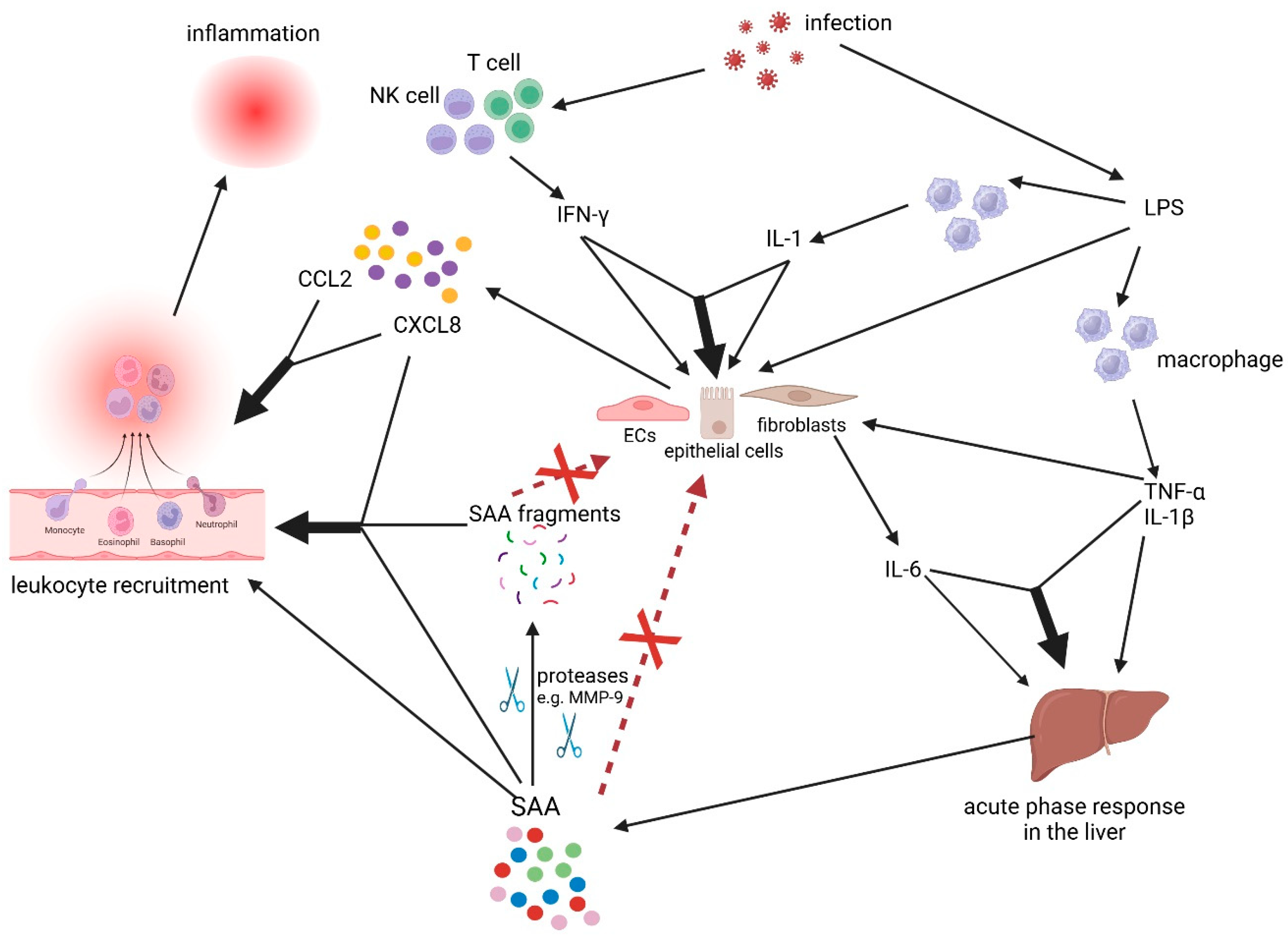
4. The Revised Cytokine-Chemokine-Serum Amyloid A1 (SAA1) Network
5. Synergistic Interaction Between Recombinant Serum Amyloid A1 (SAA1) and Chemokines in Leukocyte Migration
6. Serum Amyloid A1 (SAA1) Fragments: Generation by Matrix Metalloproteinases (MMPs), Chemotactic Properties, and Mode of Action
7. Conclusions: So-Called Recombinant Serum Amyloid A1 (SAA1) Activities Under the Pressure of Contaminants
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mantovani, A. The chemokine system: Redundancy for robust outputs. Immunol. Today 1999, 20, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Gruys, E.; Toussaint, M.J.M.; Niewold, T.A.; Koopmans, S.J. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. 2005, 6, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- De Buck, M.; Gouwy, M.; Wang, J.M.; Van Snick, J.; Opdenakker, G.; Struyf, S.; Van Damme, J. Structure and expression of different serum amyloid A (SAA) variants and their concentration-dependent functions during host insults. Curr. Med. Chem. 2016, 23, 1725–1755. [Google Scholar] [CrossRef]
- Brown, Z.; Gerritsen, M.E.; Carley, W.W.; Strieter, R.M.; Kunkel, S.L.; Westwick, J. Chemokine gene expression and secretion by cytokine-activated human microvascular endothelial cells. Differential regulation of monocyte chemoattractant protein-1 and interleukin-8 in response to interferon-gamma. Am. J. Pathol. 1994, 145, 913–921. [Google Scholar]
- Polentarutti, N.; Introna, M.; Sozzani, S.; Mancinelli, R.; Mantovani, G.; Mantovani, A. Expression of monocyte chemotactic protein-3 in human monocytes and endothelial cells. Eur. Cytokine Netw. 1997, 8, 271–274. [Google Scholar]
- Gouwy, M.; Struyf, S.; Proost, P.; Van Damme, J. Synergy in cytokine and chemokine networks amplifies the inflammatory response. Cytokine Growth Factor Rev. 2005, 16, 561–580. [Google Scholar] [CrossRef]
- Gauldie, J.; Richards, C.; Harnish, D.; Lansdorp, P.; Baumann, H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc. Natl. Acad. Sci. USA 1987, 84, 7251–7255. [Google Scholar] [CrossRef]
- Ramadori, G.; Van Damme, J.; Rieder, H.; Meyer zum Büschenfelde, K.H. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur. J. Immunol. 1988, 18, 1259–1264. [Google Scholar] [CrossRef]
- Larsen, C.G.; Anderson, A.O.; Oppenheim, J.J.; Matsushima, K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology 1989, 68, 31–36. [Google Scholar]
- Van Damme, J.; De Ley, M.; Opdenakker, G.; Billiau, A.; De Somer, P.; Van Beeumen, J. Homogeneous interferon-inducing 22K factor is related to endogenous pyrogen and interleukin-1. Nature 1985, 314, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, J.; Cayphas, S.; Van Snick, J.; Conings, R.; Put, W.; Lenaerts, J.P.; Simpson, R.J.; Billiau, A. Purification and characterization of human fibroblast-derived hybridoma growth factor identical to T-cell-derived B-cell stimulatory factor-2 (interleukin-6). Eur. J. Biochem. 1987, 168, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Jirik, F.R.; Podor, T.J.; Hirano, T.; Kishimoto, T.; Loskutoff, D.J.; Carson, D.A.; Lotz, M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J. Immunol. 1989, 142, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Borré, A.; Wang, J.M.; Tattanelli, M.; Maddalena, F.; Polentarutti, N.; Peri, G.; Mantovani, A. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J. Immunol. 1992, 148, 760–765. [Google Scholar] [CrossRef]
- Struyf, S.; Van Coillie, E.; Paemen, L.; Put, W.; Lenaerts, J.-P.; Proost, P.; Opdenakker, G.; Van Damme, J. Synergistic induction of MCP-1 and -2 by IL-1β and interferons in fibroblasts and epithelial cells. J. Leukoc. Biol. 1998, 63, 364–372. [Google Scholar] [CrossRef]
- Wuyts, A.; Struyf, S.; Gijsbers, K.; Schutyser, E.; Put, W.; Conings, R.; Lenaerts, J.P.; Geboes, K.; Opdenakker, G.; Menten, P.; et al. The CXC chemokine GCP-2/CXCL6 is predominantly induced in mesenchymal cells by interleukin-1β and is down-regulated by interferon-γ: Comparison with interleukin-8/CXCL8. Lab. Investig. 2003, 83, 23–34. [Google Scholar] [CrossRef]
- Gijsbers, K.; Gouwy, M.; Struyf, S.; Wuyts, A.; Proost, P.; Opdenakker, G.; Penninckx, F.; Ectors, N.; Geboes, K.; Van Damme, J. GCP-2/CXCL6 synergizes with other endothelial cell-derived chemokines in neutrophil mobilization and is associated with angiogenesis in gastrointestinal tumors. Exp. Cell Res. 2005, 303, 331–342. [Google Scholar] [CrossRef]
- Loos, T.; Dekeyzer, L.; Struyf, S.; Schutyser, E.; Gijsbers, K.; Gouwy, M.; Fraeyman, A.; Put, W.; Ronsse, I.; Grillet, B.; et al. TLR ligands and cytokines induce CXCR3 ligands in endothelial cells: Enhanced CXCL9 in autoimmune arthritis. Lab. Investig. 2006, 86, 902–916. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 and the pathogenesis of the acute-phase response. N. Engl. J. Med. 1984, 311, 1413–1418. [Google Scholar]
- Edbrooke, M.R.; Foldi, J.; Cheshire, J.K.; Li, F.; Faulkes, D.J.; Woo, P. Constitutive and NF-κB-like proteins in the regulation of the serum amyloid A gene by interleukin-1. Cytokine 1991, 3, 380–388. [Google Scholar] [CrossRef]
- Betts, J.C.; Cheshire, J.K.; Akira, S.; Kishimoto, T.; Woo, P. The role of NF-κB and NF-IL-6 transactivating factors in the synergistic activation of human serum amyloid A gene expression by interleukin-1 and interleukin-6. J. Biol. Chem. 1993, 268, 25624–25631. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.L.; Lozanski, G.; Samols, D.; Kushner, I. Induction of human serum amyloid A in Hep 3B cells by IL-6 and IL-1 beta involves both transcriptional and post-transcriptional mechanisms. J. Immunol. 1995, 154, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, A.; Lindenmann, J. Virus interference. I. The interferon. By A. Isaacs and J. Lindenmann, 1957. J. Interferon Res. 1987, 7, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Vilcek, J. Interferon. Science 1970, 168, 398–399. [Google Scholar] [CrossRef]
- Mier, J.W.; Gallo, R.C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc. Natl. Acad. Sci. USA 1980, 77, 6134–6138. [Google Scholar] [CrossRef]
- Matsushima, K.; Copeland, T.D.; Onozaki, K.; Oppenheim, J.J. Purification and biochemical characteristics of two distinct human interleukins 1 from the myelomonocytic THP-1 cell line. Biochemistry 1986, 25, 3424–3429. [Google Scholar] [CrossRef]
- Williamson, D.J.; Begley, C.G.; Vadas, M.A.; Metcalf, D. The detection and initial characterization of colony-stimulating factors in synovial fluid. Clin. Exp. Immunol. 1988, 72, 67–73. [Google Scholar]
- Nakamura, K.; Okamura, H.; Nagata, K.; Komatsu, T.; Tamura, T. Purification of a factor which provides a costimulatory signal for gamma interferon production. Infect. Immun. 1993, 61, 64–70. [Google Scholar] [CrossRef]
- Struyf, S.; Proost, P.; Lenaerts, J.P.; Stoops, G.; Wuyts, A.; Van Damme, J. Identification of a blood-derived chemoattractant for neutrophils and lymphocytes as a novel CC chemokine, Regakine-1. Blood 2001, 97, 2197–2204. [Google Scholar] [CrossRef]
- De Buck, M.; Gouwy, M.; Proost, P.; Struyf, S.; Van Damme, J. Identification and characterization of MIP-1α/CCL3 isoform 2 from bovine serum as a potent monocyte/dendritic cells chemoattractant. Biochem. Pharmacol. 2013, 85, 789–797. [Google Scholar] [CrossRef]
- Ahmad, S.; Gardner, Q.A.; Shakir, N.A.; Gulzar, S.; Azim, N.; Akhtar, M. Nature of recombinant human serum amyloid A1 in Escherichia coli and its preferable approach for purification. Protein Expr. Purif. 2025, 227, 106620. [Google Scholar] [CrossRef] [PubMed]
- Schindler, R.; Dinarello, C.A. A method for removing interleukin-1 and tumor necrosis factor-inducing substances from bacterial cultures by ultrafiltration with polysulfone. J. Immunol. Methods 1989, 116, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Burgess, E.J.; Hoyt, L.R.; Randall, M.J.; Mank, M.M.; Bivona, J.J.; Eisenhauer, P.L.; Botten, J.W.; Ballif, B.A.; Lam, Y.W.; Wargo, M.J.; et al. Bacterial lipoproteins constitute the TLR2-stimulating activity of serum amyloid A. J. Immunol. 2018, 201, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Abouelasrar Salama, S.; De Bondt, M.; De Buck, M.; Berghmans, N.; Proost, P.; Oliveira, V.L.S.; Amaral, F.A.; Gouwy, M.; Van Damme, J.; Struyf, S. Serum amyloid A1 (SAA1) revisited: Restricted leukocyte-activating properties of homogeneous SAA1. Front. Immunol. 2020, 11, 843–857. [Google Scholar] [CrossRef]
- Gursky, O. Structural Basis for Vital Function and Malfunction of Serum Amyloid A: An Acute-Phase Protein that Wears Hydrophobicity on Its Sleeve. Curr. Atheroscler. Rep. 2020, 22, 69. [Google Scholar] [CrossRef]
- Rollins, B.J. Chemokines. Blood 1997, 90, 909–928. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef]
- Yoshimura, T.; Leonard, E.J. Identification of high affinity receptors for human monocyte chemoattractant protein-1 on human monocytes. Eur. J. Immunol. 1990, 145, 292–297. [Google Scholar] [CrossRef]
- Samson, M.; LaRosa, G.; Libert, F.; Paindavoine, P.; Detheux, M.; Vassart, G.; Parmentier, M. The second extracellular loop of CCR5 is the major determinant of ligand specificity. J. Biol. Chem. 1997, 272, 24934–24941. [Google Scholar] [CrossRef]
- Ludwig, A.; Petersen, F.; Zahn, S.; Götze, O.; Schröder, J.M.; Flad, J.D.; Brandt, E. The CXC-chemokine neutrophil-activating peptide-2 induces two distinct optima of neutrophil chemotaxis by differential interaction with interleukin-8 receptors CXCR-1 and CXCR-2. Blood 1997, 90, 4588–4597. [Google Scholar] [CrossRef]
- Proost, P.; Struyf, S.; Wuyts, A.; Menten, P.; De Meester, I.; Lambeir, A.M.; Scharpé, S.; Schols, D.; De Clercq, E.; Van Damme, J. Isolation and identification of naturally modified C-C chemokines MCP-1, MCP-2 and RANTES: Effects of posttranslational modifications on receptor usage, chemotactic and anti-HIV-1 activity. Eur. Cytokine Netw. 1998, 9, 73–75. [Google Scholar] [PubMed]
- Murphy, P.M.; Baggiolini, M.; Charo, I.F.; Hébert, C.A.; Horuk, R.; Matsushima, K.; Miller, L.H.; Oppenheim, J.J.; Power, C.A. International Union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000, 52, 145–176. [Google Scholar] [CrossRef] [PubMed]
- Thelen, M. Dancing to the tune of chemokines. Nat. Immunol. 2001, 2, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Locati, M.; Vecchi, A.; Sozzani, S.; Allavena, P. Decoy receptors: A strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 2001, 22, 328–336. [Google Scholar] [CrossRef]
- Murphy, P.M. International Union of Pharmacology. XXX. Update on Chemokine Receptor Nomenclature. Pharmacol. Rev. 2002, 54, 227–229. [Google Scholar] [CrossRef]
- Rot, A.; von Andrian, U.H. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004, 22, 891–928. [Google Scholar] [CrossRef]
- Castor, C.W.; Miller, J.W.; Walz, D.A. Structural and biological characteristics of connective tissue activating peptide (CTAP-III), a major human platelet-derived growth factor. Proc. Natl. Acad. Sci. USA 1983, 80, 765–769. [Google Scholar] [CrossRef]
- Schröder, J.M.; Persoon, N.L.; Christophers, E. Lipopolysaccharide-stimulated human monocytes secrete, apart from neutrophil-activating peptide 1/interleukin 8, a second neutrophil-activating protein. NH2-terminal amino acid sequence identity with melanoma growth stimulatory activity. J. Exp. Med. 1990, 171, 1091–1100. [Google Scholar] [CrossRef]
- Ehlert, J.E.; Petersen, F.; Kubbutat, M.H.; Gerdes, J.; Flad, H.D.; Brandt, E. Limited and defined truncation at the C terminus enhances receptor binding and degranulation activity of the neutrophil-activating peptide 2 (NAP-2). Comparison of native and recombinant NAP-2 variants. J. Biol. Chem. 1995, 270, 6338–6344. [Google Scholar] [CrossRef]
- Proost, P.; Wuyts, A.; Van Damme, J. Human monocyte chemotactic proteins-2 and -3: Structural and functional comparison with MCP-1. J. Leukoc. Biol. 1996, 59, 67–74. [Google Scholar] [CrossRef]
- Struyf, S.; Stoops, G.; Van Coillie, E.; Gouwy, M.; Schutyser, E.; Lenaerts, J.P.; Fiten, P.; Van Aelst, I.; Proost, P.; Opdenakker, G.; et al. Gene cloning of a new plasma CC chemokine, activating and attracting myeloid cells in synergy with other chemoattractants. Biochemistry 2001, 40, 11715–11722. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J.; Jongstra, J.; Dyer, B.J.; Jorgensen, J.; Clayberger, C.; Davis, M.M.; Krensky, A.M. A human T-cell specific molecule is a member of a new gene family. J. Immunol. 1988, 141, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Imai, T.; Baba, M.; Ishikawa, I.; Uehira, M.; Nomiyama, H.; Yoshie, O. Selective expression of liver and activation-regulated chemokine (LARC) in intestinal epithelium in mice and humans. Eur. J. Immunol. 1999, 29, 633–642. [Google Scholar] [CrossRef]
- Wakelin, S.J.; Sabroe, I.; Gregory, C.D.; Poxton, I.R.; Forsythe, J.L.; Garden, O.J.; Howie, S.E. “Dirty little secrets”-endotoxin contamination of recombinant proteins. Immunol. Lett. 2006, 106, 1–7. [Google Scholar] [CrossRef]
- Schwarz, H.; Schmittner, M.; Duschl, A.; Horejs-Hoeck, J. Residual endotoxin contaminations in recombinant proteins are sufficient to activate human CD1c+ dendritic cells. PLoS ONE 2014, 9, e113840. [Google Scholar] [CrossRef]
- Abouelasrar Salama, S.; De Bondt, M.; Berghmans, N.; Gouwy, M.; De Oliveira, V.L.S.; Oliveira, S.C.; Amaral, F.A.; Proost, P.; Van Damme, J.; Struyf, S.; et al. Biological characterization of commercial recombinantly expressed immunomodulating proteins contaminated with bacterial products in the year 2020: The SAA3 case. Mediat. Inflamm. 2020, 6, 6087109. [Google Scholar] [CrossRef]
- Freshney, R.I. Culture of Animal Cells. A Manual of Basic Technique; Alan R Liss Inc.: New York, NJ, USA, 1987; pp. 57–84. [Google Scholar]
- Cartwright, T.; Shah, G.P. Culture media. In Basic Cell Culture. A Practical Approach; Davis, J.M., Ed.; Oxford University Press: Oxford, UK, 1998; pp. 57–91. [Google Scholar]
- Helgason, C.D. Culture of primary adherent cells and a continuously growing nonadherent cell line. In Basic Cell Culture Protocols; Humana Press: Totowa, NJ, USA, 2005; pp. 1–12. [Google Scholar]
- De Buck, M.; Gouwy, M.; Berghmans, N.; Opdenakker, G.; Proost, P.; Struyf, S.; Van Damme, J. COOH-terminal SAA1 peptides fail to induce chemokines but synergize with CXCL8 and CCL3 to recruit leukocytes via FPR2. Blood 2018, 131, 439–449. [Google Scholar] [CrossRef]
- Struyf, S.; Burdick, M.D.; Peeters, E.; Van den Broeck, K.; Dillen, C.; Proost, P.; Van Damme, J.; Strieter, R.M. Platelet factor-4 variant chemokine CXCL4L1 inhibits melanoma and lung carcinoma growh and matastasis by preventing angiogenesis. Cancer Res. 2007, 67, 5940–5948. [Google Scholar] [CrossRef]
- Vandercappellen, J.; Van Damme, J.; Struyf, S. The role of the CXC chemokines platelet factor-4 (CXCL4/PF-4) and its variant (CXCL4L1/PF-4var) in inflammation, angiogenesis and cancer. Cytokine Growth Factor Rev. 2001, 22, 1–18. [Google Scholar] [CrossRef]
- Combadière, C.; Ahuja, S.K.; Tiffany, H.L.; Murphy, P.M. Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1 (alpha), MIP-1 (beta), and RANTES. J. Leukoc. Biol. 1996, 60, 147–152. [Google Scholar] [CrossRef]
- Pease, J.E.; Wang, J.; Ponath, P.D.; Murphy, P.M. The N-terminal extracellular segments of the chemokine receptors CCR1 and CCR3 are determinants for MIP-1alpha and eotaxin binding, respectively, but a second domain is essential for efficient receptor activation. J. Biol. Chem. 1998, 273, 19972–19976. [Google Scholar] [CrossRef] [PubMed]
- Gouwy, M.; Struyf, S.; Leutenez, L.; Pörtner, N.; Sozzani, S.; Van Damme, J. Chemokines and other GPCR ligands synergize in receptor-mediated migration of monocyte-derived immature and mature dendritic cells. Immunobiology 2014, 219, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Gouwy, M.; Struyf, S.; Mahieu, F.; Put, W.; Proost, P.; Van Damme, J. The unique property of the CC chemokine regakine-1 to synergize with other plasma-derived inflammatory mediators in neutrophil chemotaxis does not reside in its NH2-terminal structure. Mol. Pharmacol. 2002, 62, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Su, S.B.; Gong, W.; Gao, J.L.; Shen, W.; Murphy, P.M.; Oppenheim, J.J.; Wang, J.M. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J. Exp. Med. 1999, 189, 395–402. [Google Scholar] [CrossRef]
- Gouwy, M.; De Buck, M.; Pörtner, N.; Opdenakker, G.; Proost, P.; Struyf, S.; Van Damme, J. Serum amyloid A chemoattracts immature dendritic cells and indirectly provokes monocyte chemotaxis by induction of cooperating CC and CXC chemokines. Eur. J. Immunol. 2015, 45, 101–112. [Google Scholar] [CrossRef]
- Gouwy, M.; De Buck, M.; Abouelasrar Salama, S.; Vandooren, J.; Knoops, S.; Pörtner, N.; Vanbrabant, L.; Berghmans, N.; Opdenakker, G.; Proost, P.; et al. Matrix metalloproteinase-9-generated COOH-, but not NH2-terminal fragments of serum amyloid A1 retain potentiating activity in neutrophil migration to CXCL8, with loss of direct chemotactic and cytokine-inducing capacity. Front. Immunol. 2018, 9, 1081–1092. [Google Scholar] [CrossRef]
- Davatelis, G.; Tekamp-Olson, P.; Wolpe, S.D.; Hermsen, K.; Luedke, C.; Gallegos, C.; Coit, D.; Merryweather, J.; Cerami, A. Cloning and characterization of a cDNA for murine macrophage inflammatory protein (MIP), a novel monokine with inflammatory and chemokinetic properties. J. Exp. Med. 1988, 167, 1939–1944. [Google Scholar] [CrossRef]
- Baggiolini, M.; Dewald, B.; Moser, B. Interleukin-8 and related chemotactic cytokines- CXC and CC chemokines. Adv. Immunol. 1994, 55, 97–179. [Google Scholar]
- Gouwy, M.; Struyf, S.; Noppen, S.; Schutyser, E.; Springael, J.Y.; Parmentier, M.; Proost, P.; Van Damme, J. Synergy between coproduced CC and CXC chemokines in monocyte chemotaxis through receptor-mediated events. Mol. Pharmacol. 2008, 74, 485–495. [Google Scholar] [CrossRef]
- Kuscher, K.; Danelon, G.; Paoletti, S.; Stefano, L.; Schiraldi, M.; Petkovic, V.; Locati, M.; Gerber, B.O.; Uguccioni, M. Synergy-inducing chemokines enhance CCR2 ligand activities on monocytes. Eur. J. Immunol. 2009, 39, 1118–1128. [Google Scholar] [CrossRef]
- He, R.; Sang, H.; Ye, R.D. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood 2003, 101, 1572–1581. [Google Scholar] [CrossRef]
- Song, C.; Hsu, K.; Yamen, E.; Yan, W.; Fock, J.; Witting, P.K.; Geczy, C.L.; Freedman, S.B. Serum amyloid A induction of cytokines in monocytes/macrophages and lymphocytes. Atherosclerosis 2009, 207, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, S.D.; Shim, J.W.; Kim, H.J.; Yun, J.; Baek, S.H.; Kim, K.; Bae, Y.S. A pertussis toxin sensitive G-protein-independent pathway is involved in serum amyloid A-induced formyl peptide receptor 2-mediated CCL2 production. Exp. Mol. Med. 2010, 42, 302–309. [Google Scholar] [CrossRef] [PubMed]
- De Buck, M.; Berghmans, N.; Pörtner, N.; Vanbrabant, L.; Cockx, M.; Struyf, S.; Opdenakker, G.; Proost, P.; Van Damme, J.; Gouwy, M. Serum amyloid A1α induces paracrine IL-8/CXCL8 via TLR2 and directly synergizes with this chemokine via CXCR2 and formyl peptide receptor 2 to recruit neutrophils. J. Leukoc. Biol. 2015, 98, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.S.; Song, Z.; Willett, M.H.; Heine, S.; Yung, R.C.; Liu, M.C.; Groshong, S.D.; Zhang, Y.; Tuder, R.M.; Moller, D.R. Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2. Am. J. Respir. Crit. Care Med. 2010, 181, 360–373. [Google Scholar] [CrossRef]
- Li, Y.; Cai, L.; Wang, H.; Wu, P.; Gu, W.; Chen, Y.; Hao, H.; Tang, K.; Yi, P.; Liu, M.; et al. Pleiotropic regulation of macrophage polarization and tumorigenesis by formyl peptide receptor-2. Oncogene 2011, 30, 3887–3899. [Google Scholar] [CrossRef]
- Lakota, K.; Mrak-Poljsak, K.; Bozic, B.; Tomsic, M.; Sodin-Semrl, S. Serum amyloid A activation of human coronary artery endothelial cells exhibits a neutrophil promoting molecular profile. Microvasc. Res. 2013, 90, 55–63. [Google Scholar] [CrossRef]
- De Seny, D.; Cobraiville, G.; Charlier, E.; Neuville, S.; Esser, N.; Malaise, D.; Mailaise, O.; Calvo, F.Q.; Relic, B.; Malaise, M.G. Acute-phase serum amyloid A in osteoarthritis: Regulatory mechanism and proinflammatory properties. PLoS ONE 2013, 8, e66769. [Google Scholar] [CrossRef]
- O’Reilly, S.; Cant, R.; Ciechomska, M.; Finnigan, J.; Oakley, F.; Hambleton, S.; van Laar, J.M. Serum amyloid A induces interleukin-6 in dermal fibroblasts via Toll-like receptor 2, interleukin-1 receptor-associated kinase 4 and nuclear factor-ĸB. Immunology 2014, 143, 331–340. [Google Scholar] [CrossRef]
- Yu, N.; Liu, S.; Yi, X.; Zhang, S.; Ding, Y. Serum amyloid A induces interleukin-1beta secretion from keratinocytes via the NACHT, LRR and PYD domains-containing protein 3 inflammasome. Clin. Exp. Immunol. 2015, 179, 344–353. [Google Scholar] [CrossRef]
- Yan, S.D.; Zhu, H.; Zhu, A.; Golabek, A.; Du, H.; Roher, A.; Yu, J.; Soto, C.; Schmidt, A.M.; Stern, D.; et al. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat. Med. 2000, 6, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; de Beer, M.C.; de Beer, F.C.; van der Westhuyzen, D.R. Serum amyloid A is a ligand for scanvenger receptor class B type I and inhibits high density lipoprotein binding and selective lipid uptake. J. Biol. Chem. 2005, 280, 2954–2961. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; He, R.; Tian, J.; Ye, P.P.; Ye, R.D. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J. Immunol. 2008, 181, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Mullan, R.H.; McCormick, J.; Connolly, M.; Bresnihan, B.; Veale, D.J.; Fearon, U. A role for the high-density lipoprotein receptor SR-B1 in synovial inflammation via serum amyloid A. Am. J. Pathol. 2010, 176, 1999–2008. [Google Scholar] [CrossRef]
- Li, W.; Zhu, S.; Li, J.; D’Amore, J.; D’Angelo, J.; Yang, H.; Wang, P.; Tracey, K.J.; Wang, H. Serum amyloid A stimulates PKR expression and HMGB1 release possibly through TLR4/RAGE receptors. Mol. Med. 2015, 21, 515–525. [Google Scholar] [CrossRef]
- Ebert, R.; Benisch, P.; Krug, M.; Zeck, S.; Meißner-Weigl, J.; Steinert, A.; Rauner, M.; Hofbauer, L.; Jakob, F. Acute phase serum amyloid A induces proinflammatory cytokines and mineralization via toll-like receptor 4 in mesenchymal stem cells. Stem Cell Res. 2015, 15, 231–239. [Google Scholar] [CrossRef]
- Connolly, M.; Rooney, P.R.; McGarry, T.; Maratha, A.X.; McCormick, J.; Miggin, S.M.; Veale, D.J.; Fearon, U. Acute serum amyloid A is an endogenous TLR2 ligand that mediates inflammatory and angiogenic mechanisms. Ann. Rheum. Dis. 2016, 75, 1392–1398. [Google Scholar] [CrossRef]
- Han, S.; Jin, S.-P.; Oh, J.-H.; Seo, E.-Y.; Park, C.-H.; Yoon, H.-S.; Lee, D.H.; Chung, J.H. Serum amyloid A1 secreted from UV-irradiated keratinocytes induces matrix metalloproteinase-1 in fibroblasts through toll-like receptor 4. Exp. Dermatol. 2016, 25, 526–531. [Google Scholar] [CrossRef]
- Baranova, I.N.; Souza, A.C.P.; Bocharov, A.V.; Vishnyakova, T.G.; Hu, X.; Vaisman, B.L.; Amar, M.J.; Chen, Z.; Remaley, A.T.; Patterson, A.P.; et al. Human SR-BII mediates SAA uptake and contributes to SAA pro-inflammatory signaling in vitro and in vivo. PLoS ONE 2017, 12, e0175824. [Google Scholar] [CrossRef]
- Abouelasrar Salama, S.; Gouwy, M.; Van Damme, J.; Struyf, S. The turning away of serum amyloid A biological activities and receptor usage. Immunology 2021, 163, 115–127. [Google Scholar] [CrossRef]
- Vallon, R.; Freuler, F.; Desta-Tsedu, N.; Robeva, A.; Dawson, J.; Wenner, P.; Engelhardt, P.; Boes, L.; Schnyder, J.; Tschopp, C.; et al. Serum amyloid A (apoSAA) expression is up-regulated in rheumatoid arthritis and induces transcription of matrix metalloproteinases. J. Immunol. 2001, 166, 2801–2807. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Kim, M.K.; Park, K.S.; Bae, Y.H.; Yun, J.; Park, J.Y.; Kwak, J.Y.; Bae, Y.S. Serum amyloid A stimulates matrix-metalloproteinase-9 upregulation via formyl peptide receptor like-1-mediated signaling in human monocytic cells. Biochem. Biophys. Res. Commun. 2005, 330, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.; Mullan, R.H.; McCormick, J.; Matthews, C.; Sullivan, O.; Kennedy, A.; FitzGerald, O.; Poole, A.R.; Bresnihan, B.; Veale, D.J.; et al. Acute-phase serum amyloid A regulates tumor necrosis factor alpha and matrix turnover and predicts disease progression in patients with inflammatory arthritis before and after biologic therapy. Arthritis Rheum. 2012, 64, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- De Buck, M.; Gouwy, M.; Struyf, S.; Opdenakker, G.; Van Damme, J. The ectoenzyme-side of matrix metalloproteinases (MMPs) makes inflammation by serum amyloid A (SAA) and chemokines go round. Immunol. Lett. 2019, 205, 1–8. [Google Scholar] [CrossRef]
- Van Damme, J.; Proost, P.; Put, W.; Arens, S.; Lenaerts, J.P.; Conings, R.; Opdenakker, G.; Heremans, H.; Billiau, A. Induction of monocyte chemotactic proteins MCP-1 and MCP-2 in human fibroblasts and leukocytes by cytokines and cytokine inducers. Chemical synthesis of MCP-2 and development of specific RIA. J. Immunol. 1994, 152, 5495–5502. [Google Scholar] [CrossRef]
- Ganapathi, M.K.; May, L.T.; Schultz, D.; Brabenec, A.; Weinstein, J.; Sehgal, P.B.; Kushner, I. Role of interleukin-6 in regulating synthesis of C-reactive protein and serum amyloid A in human hepatoma cell lines. Biochem. Biophys. Res. Commun. 1988, 157, 271–277. [Google Scholar] [CrossRef]
- Ganapathi, M.K.; Rzewnicki, D.; Samols, D.; Jiang, S.L.; Kushner, I. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep 3B cells. J. Immunol. 1991, 147, 1261–1265. [Google Scholar] [CrossRef]
- Gouwy, M.; Schiraldi, M.; Struyf, S.; Van Damme, J.; Uguccioni, M. Possible mechanisms involved in chemokine synergy fine tuning the inflammatory response. Immunol. Lett. 2012, 145, 10–14. [Google Scholar] [CrossRef]
- Gouwy, M.; Struyf, S.; Verbeke, H.; Put, W.; Proost, P.; Opdenakker, G.; Van Damme, J. CC chemokine ligand-2 synergizes with the nonchemokine G protein-coupled receptor ligand fMLP in monocyte chemotaxis, and it cooperates with the TLR ligand LPS via induction of CXCL8. J. Leukoc. Biol. 2009, 86, 671–680. [Google Scholar] [CrossRef]
- den Hartigh, L.J.; May, K.S.; Zhang, X.S.; Chait, A.; Blaser, M.J. Serum amyloid A and metabolic disease: Evidence for a critical role in chronic inflammatory conditions. Front. Cardiovasc. Med. 2023, 10, 1197432. [Google Scholar] [CrossRef]
- Li, M.; Kim, Y.M.; Koh, J.H.; Park, J.; Kwon, H.M.; Park, J.H.; Jin, J.; Park, Y.; Kim, D.; Kim, W.U. Serum amyloid A expression in liver promotes synovial macrophage activation and chronic arthritis via NFAT5. J. Clin. Investig. 2024, 134, e167835. [Google Scholar] [CrossRef]
- Garcia-Cortés, C.G.; Parés-Matos, E.I. New regulatory roles for human serum amyloid A. Int. J. Res. Oncol. 2024, 3, 3249. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, Q.; Zheng, J.; Zeng, Z.; Chen, M.; Li, L.; Zhang, S. Serum amyloid protein A in inflammatory bowel disease: From bench to bedside. Cell Death Discov. 2023, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Liepnieks, J.J.; Kluve-Beckerman, B.; Benson, M.D. Cathepsin B generates the most common form of amyloid A (76 residues) as a degradation product from serum amyloid A. Scan. J. Immunol. 1995, 41, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Stix, B.; Kahne, T.; Sletten, K.; Raynes, J.; Roessner, A.; Rocken, C. Proteolysis of AA amyloid fibril proteins by matrix metalloproteinases-1, -2, and -3. Am. J. Pathol. 2001, 159, 561–570. [Google Scholar] [CrossRef]
- Röcken, C.; Menard, R.; Bühling, F.; Vöckler, S.; Raynes, J.; Stix, B.; Krüger, S.; Roessner, A.; Kähne, T. Proteolysis of serum amyloid A and AA amyloid proteins by cysteine proteases: Cathepsin B generates AA amyloid proteins and cathepsin L may prevent their formation. Ann. Rheum. Dis. 2005, 64, 808–815. [Google Scholar] [CrossRef]
- van der Hilst, J.C.; Yamada, T.; Op den Camp, H.J.; van der Meer, J.W.; Drenth, J.P.; Simon, A. Increased susceptibility of serum amyloid A 1.1 to degradation by MMP-1: Potential explanation for higher risk of type AA amyloidosis. Rheumatology 2008, 47, 1651–1654. [Google Scholar] [CrossRef]
- Abouelasrar Salama, S.; Lavie, M.; De Buck, M.; Van Damme, J.; Struyf, S. Cytokines and serum amyloid A in the pathogenesis of hepatitis C virus infection. Cytokine Growth Factor Rev. 2019, 50, 29–42. [Google Scholar] [CrossRef]
| Properties | Chemotactic Factor | |||
|---|---|---|---|---|
| CXCL4/PF-4 | CCL3/MIP-1α | Regakine-1 | SAA1(46-112) | |
| Affinity for heparin | high | moderate | moderate | low |
| Molecular size (kDa) | 7 | 7.7 | 7.5 | 7.3 |
| Receptor usage | ND | CCR1, CCR5 | unknown | FPR2 |
| Target cells | EC | monocytes, iDC | T cells, granulocytes | monocytes, granulocytes |
| Serum concentration (ng/mL) | high | 10 | 100 | ND |
| Angiostatic/Chemotactic activity | potent | very potent | synergy | synergy |
| Synergizing chemokine | ND | CXCL12 | CCL7, CXCL6, CXCL7, CXCL8 | CCL3, CXCL8 |
| Biotest System | Tested Cells | RP-HPLC-Purified rSAA1 | Commercial rSAA1 | ||
|---|---|---|---|---|---|
| MEC (ng/mL) | Result | MEC (ng/mL) | Result | ||
| Chemokine (CXCL8) induction | monocytes | >1000 | neg | 10 | pos |
| Chemokine (CCL3) induction | monocytes | >300 | neg | 10 | pos |
| Proteinase (MMP-9) induction | monocytes | >100 | neg | 10 | pos |
| ROS production | monocytes | >1000 | neg | 100 | pos |
| Leukocyte recruitment after intra-articular injection | mononuclear cells | 100 a | pos | 100 | pos |
| Leukocyte recruitment after intra-articular injection | granulocytes | 100 a | pos | 100 | pos |
| Synergy (CXCL8) in chemotaxis | granulocytes | 3000 | pos | 300 | pos |
| Synergy (CXCL8) in shape change | granulocytes | 3000 | pos | 3000 | pos |
| Synergy (CXCL8) in actin polymerization | granulocytes | 300 | pos | ND | ND |
| Producer Cell | Inducer | Produced Mediators | ||||
|---|---|---|---|---|---|---|
| IL-1 | IL-6 | CXCL8 | CCL2 | SAA1 | ||
| Monocyte | LPS | + | + | + | + | + |
| IL-1 | + | + | + | - | ||
| SAA1 | - | - | - | - | ||
| Fibroblast | LPS | - | - | - | - | - |
| IL-1 | + | + | + | - | ||
| SAA1 | - | - | - | - | ||
| Hepatocyte | IL-1 | + | + | + | + | |
| IL-6 | - | - | - | + | ||
| SAA1 | - | - | - | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Damme, J.; Struyf, S.; Proost, P.; Opdenakker, G.; Gouwy, M. Functional Interactions Between Recombinant Serum Amyloid A1 (SAA1) and Chemokines in Leukocyte Recruitment. Int. J. Mol. Sci. 2025, 26, 2258. https://doi.org/10.3390/ijms26052258
Van Damme J, Struyf S, Proost P, Opdenakker G, Gouwy M. Functional Interactions Between Recombinant Serum Amyloid A1 (SAA1) and Chemokines in Leukocyte Recruitment. International Journal of Molecular Sciences. 2025; 26(5):2258. https://doi.org/10.3390/ijms26052258
Chicago/Turabian StyleVan Damme, Jo, Sofie Struyf, Paul Proost, Ghislain Opdenakker, and Mieke Gouwy. 2025. "Functional Interactions Between Recombinant Serum Amyloid A1 (SAA1) and Chemokines in Leukocyte Recruitment" International Journal of Molecular Sciences 26, no. 5: 2258. https://doi.org/10.3390/ijms26052258
APA StyleVan Damme, J., Struyf, S., Proost, P., Opdenakker, G., & Gouwy, M. (2025). Functional Interactions Between Recombinant Serum Amyloid A1 (SAA1) and Chemokines in Leukocyte Recruitment. International Journal of Molecular Sciences, 26(5), 2258. https://doi.org/10.3390/ijms26052258





