Vitamin D in Reproductive Health Disorders: A Narrative Review Focusing on Infertility, Endometriosis, and Polycystic Ovarian Syndrome
Abstract
1. Introduction
2. Methodology
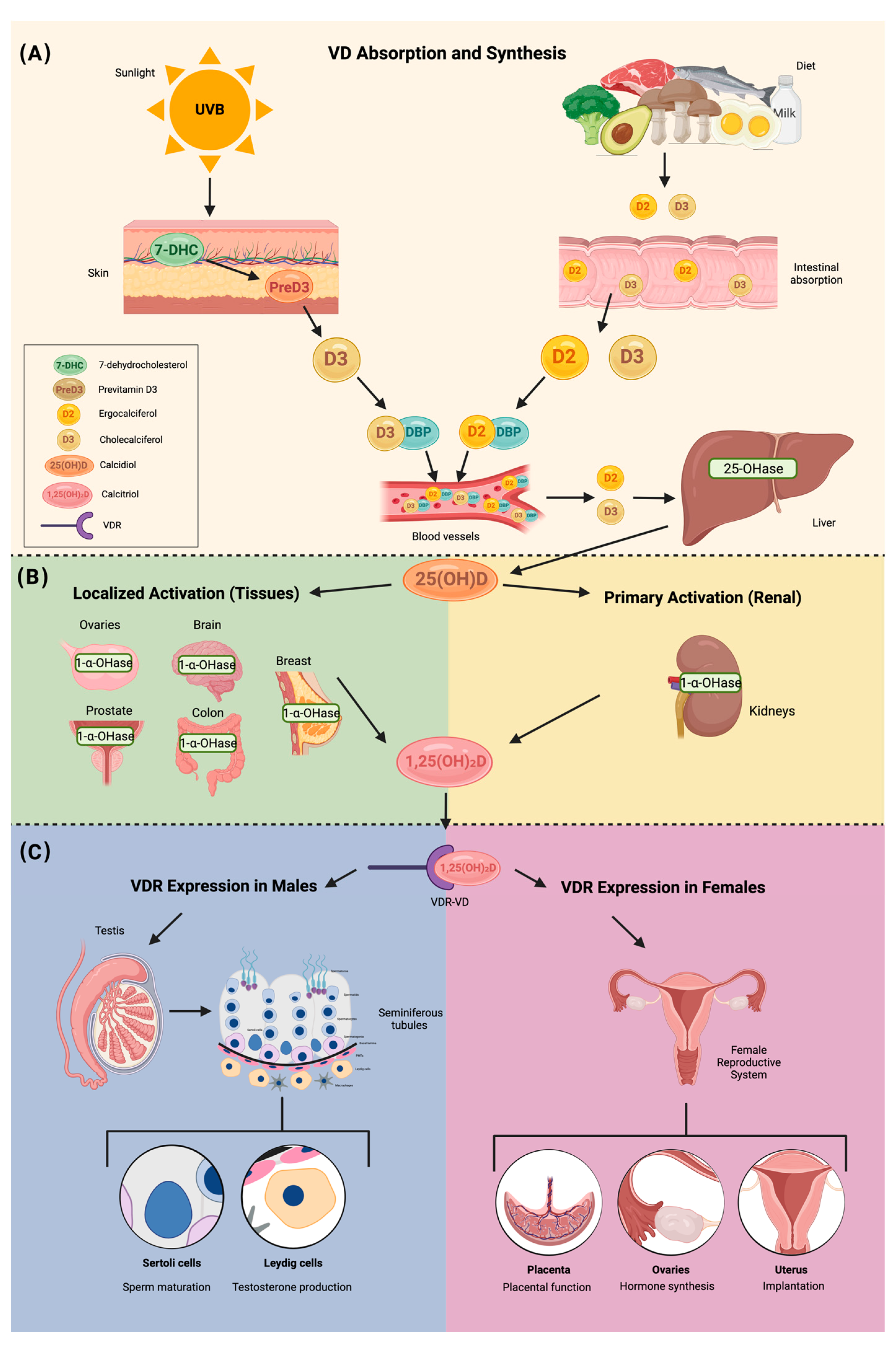
3. Vitamin D Structure and Metabolism
4. Vitamin D Deficiency
5. Correlation Between Vitamin D and Ovarian Reserve, Steroidogenesis, and Follicular Development
6. Vitamin D Receptor in Reproductive-Associated Tissues
7. Vitamin D and Fertility
8. Vitamin D and PCOS
| Study | Country | Total Participants (N) | Cases (n) | Controls (n) | Age | BMI | Baseline 25(OH)D | Type of Study | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Trummer et al. (2019) [100] | Austria | N = 123 | n = 81 | n = 42 | 25.9 ± 4.7 years | 27.5 ± 7.3 kg/m2 | 48.8 ± 16.9 ng/mL | Randomized controlled trial | VD supplementation resulted in a reduction in plasma glucose after 60 min during the OGTT. Aside from this, it had no significant effect on metabolic and endocrine parameters in PCOS. |
| Ostadmohammadi et al. (2019) [105] | Iran | N = 60 | n = 30 | n = 30 | 24.9 ± 4.9 years | 24.7 ± 4.6 kg/m2 | - | Randomize, double-blind, placebo-controlled clinical trial | Supplementation of VD and probiotics, when compared to placebo, significantly improved depression, anxiety, stress, general health, and overall well-being in women with PCOS. It also contributed to a decrease in total testosterone, hirsutism, inflammation, and oxidative stress. Furthermore, there was a notable increase in total antioxidant capacity and glutathione levels. |
| Lerchbaum et al. (2021) [102] | Austria | N = 330 | n = 180 | n = 150 | 30.5 ± 6.9 years | 26.5 ± 6.7 kg/m2 | 50.4 ± 19.0 ng/mL | Double-blind randomized controlled trial | VD treatment in women with PCOS significantly affected FSH and the LH/FSH ratio after 24 weeks, but did not impact AMH levels. No significant effects were observed in non-PCOS women. |
| Dastorani et al. (2018) [106] | Iran | N = 40 | n = 20 | n = 20 | 30.0 ± 3.93 years | 28.05 ± 3.31 kg/m2 | 10.75 ± 2.45 ng/mL | Randomize, double-blind, placebo-controlled trial | VD supplementation significantly reduced serum AMH, insulin levels, and insulin resistance (HOMA-IR) while increasing insulin sensitivity (QUICKI) compared to the placebo. Additionally, it led to a significant decrease in total cholesterol and LDL cholesterol levels. |
| Javed et al. (2019) [107] | United Kingdom | N = 37 | n = 18 | n = 19 | 28.6 ± 6.4 years | 34.58 ± 9.01 kg/m2 | 28.32 ± 11.25 ng/mL | Randomized, double-blind, placebo-controlled study | This study shows that VD supplementation has beneficial effects on liver injury and fibrosis markers (ALT levels), along with modest improvements in insulin resistance (HOMA-IR) in overweight and obese VD-deficient women with PCOS. However, no significant changes were seen in other cardiovascular risk factors or hormones. |
| Azhar et al. (2024) [108] | Pakistan | N = 180 | n = 120 | n = 60 | 33.2 ± 6.7 years | 28.1 ± 5.7 kg/m2 | - | Comparative descriptive study | Women with PCOS had lower VD levels. Both women with PCOS and VD deficiency exhibited lower HDL levels and higher total cholesterol, LDL, VLDL, and triglyceride levels compared to women without PCOS or those with sufficient or insufficient VD levels. |
| Irani et al. (2015) [109] | United States | N= 53 | n = 35 | n = 18 | 30.2 ± 1.3 years | 29.3 ± 1.6 kg/m2 | - | Randomized placebo-controlled trial | VD supplementation in women with PCOS increased VD levels and led to shorter menstrual cycles, reduced hirsutism (Ferriman–Gallwey score), lower triglycerides, and a decreased TGF-β1-to-sENG ratio, highlighting VD’s potential role in improving lipid metabolism and inflammation in PCOS. |
| Wen et al. (2024) [110] | China | N = 57 | n = 28 | n = 29 | 26.0 ± 8.9 years | 25.0 ± 7.6 kg/m2 | 12.7 ± 4.7 ng/mL | Randomized controlled trial | VD supplementation increased serum 25(OH)D levels. After 12 weeks, women in the VD group had lower BMI, WHR, insulin, HOMA-IR, triglycerides, total cholesterol, and LDL-C compared to the control group in women with obesity or insulin resistance. |
| Krul-Poel et al. (2018) [111] | Netherlands | N = 1088 | n = 639 | n = 449 | 33.2 ± 5.1 years | 24.7 ± 5.4 kg/m2 | 55.4 ± 35.5 ng/mL | Cross-sectional comparison study | VD deficiency in PCOS may contribute to metabolic issues, especially insulin resistance and lipid abnormalities. Women with PCOS had lower VD levels, which were associated with higher insulin resistance (HOMA-IR) and poorer lipid profiles, including reduced HDL-cholesterol and apolipoprotein A1. |
9. Vitamin D and Endometriosis
10. Discussion
11. Conclusions
Funding
Conflicts of Interest
References
- Huhtakangas, J.A.; Olivera, C.J.; Bishop, J.E.; Zanello, L.P.; Norman, A.W. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1α,25(OH)2-vitamin D3 in vivo and in vitro. Mol. Endocrinol. 2004, 18, 2660–2671. [Google Scholar] [CrossRef] [PubMed]
- Kahlon Simon-Collins, M.; Nylander, E.; Segars, J.; Singh, B.B.K. A systematic review of vitamin D and endometriosis: Role in pathophysiology, diagnosis, treatment, and prevention. FS Rev. 2022, 4, 1–14. [Google Scholar]
- Grundmann, M.; von Versen-Höynck, F. Vitamin D—Roles in women’s reproductive health? Reprod. Biol. Endocrinol. 2011, 9, 146. [Google Scholar] [CrossRef]
- Costanzo, P.R.; Knoblovits, P. Vitamin D and male reproductive system. Horm. Mol. Biol. Clin. Investig. 2016, 28, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Seth, T.; Wei, R.; Veldurthy, V.; Sun, C.; Kim, K.I.; Kim, K.Y.; Tariq, U.; Dhawan, P. Vitamin D and health: Beyond bone. MD Advis. 2014, 7, 28–32. [Google Scholar]
- Miao, C.Y.; Fang, X.J.; Chen, Y.; Zhang, Q. Effect of vitamin D supplementation on polycystic ovary syndrome: A meta-analysis. Exp. Ther. Med. 2020, 19, 2641–2649. [Google Scholar] [CrossRef]
- Nodler, J.L.; DiVasta, A.D.; Vitonis, A.F.; Karevicius, S.; Malsch, M.; Sarda, V.; Fadayomi, A.; Harris, H.R.; Missmer, S.A. Supplementation with vitamin D or ω-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2020, 112, 229–236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mousa, A.; Abell, S.; Scragg, R.; de Courten, B. Vitamin D in reproductive health and pregnancy. Semin. Reprod. Med. 2016, 34, e1–e13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, X.; Luo, X.; Shao, J.; Guo, D.; Deng, B.; Wu, Z. Effect of vitamin D supplementation on in vitro fertilization outcomes: A trial sequential meta-analysis of 5 randomized controlled trials. Front. Endocrinol. 2022, 13, 852428. [Google Scholar] [CrossRef]
- Bednarska-Czerwińska, A.; Olszak-Wąsik, K.; Olejek, A.; Czerwiński, M.; Tukiendorf, A. Vitamin D and Anti-Müllerian Hormone Levels in Infertility Treatment: The Change-Point Problem. Nutrients 2019, 11, 1053. [Google Scholar] [CrossRef]
- Mohan, A.; Haider, R.; Fakhor, H.; Hina, F.; Kumar, V.; Jawed, A.; Majumder, K.M.; Ayaz, A.M.; Lal, P.M.M.; Tejwaney, U.P.D.; et al. Vitamin D and polycystic ovary syndrome (PCOS): A review. Ann. Med. Surg. 2023, 85, 3506–3511. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.L.; Spedding, S.; Buckley, J.D. Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin. Endocrinol. 2012, 77, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Bishop, E.L.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-inflammatory. JBMR Plus. 2021, 5, e10405. [Google Scholar] [CrossRef]
- Lerchbaum, E.; Rabe, T. Vitamin D and female fertility. Curr. Opin. Obstet. Gynecol. 2014, 26, 145–150. [Google Scholar] [CrossRef]
- Lagana, A.S.; Vitale, S.G.; Ban Frangež, H.; Vrtačnik-Bokal, E.; D’Anna, R. Vitamin D in human reproduction: The more, the better? An evidence-based critical appraisal. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4243–4251. [Google Scholar]
- Pilz, S.; Zittermann, A.; Obeid, R.; Hahn, A.; Pludowski, P.; Trummer, C.; Lerchbaum, E.; Pérez-López, F.R.; Karras, S.N.; März, W. The role of vitamin D in fertility and during pregnancy and lactation: A review of clinical data. Int. J. Environ. Res. Public Health 2018, 15, 2241. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; Lee, H.C. Vitamin D and health—The missing vitamin in humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef]
- Fantini, C.; Corinaldesi, C.; Lenzi, A.; Migliaccio, S.; Crescioli, C. Vitamin D as a shield against aging. Int. J. Mol. Sci. 2023, 24, 4546. [Google Scholar] [CrossRef]
- Martínez-Zavala, N.; López-Sánchez, G.N.; Vergara-Lopez, A.; Chávez-Tapia, N.C.; Uribe, M.; Nuño-Lámbarri, N. Vitamin D deficiency in Mexicans has a high prevalence: A cross-sectional analysis of patients from the Centro Médico Nacional 20 de noviembre. Arch. Osteoporos. 2020, 15, 88. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for preventing and treating vitamin D deficiency: A 2023 update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Merhi, Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil. Steril. 2014, 102, 460–468.e3. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Silva, I.C.J.; Lazzaretti-Castro, M. Vitamin D metabolism and extraskeletal outcomes: An update. Arch. Endocrinol. Metab. 2022, 66, 748–755. [Google Scholar] [CrossRef]
- Várbíró, S.; Takács, I.; Tűű, L.; Nas, K.; Sziva, R.E.; Hetthéssy, J.R.; Török, M. Effects of Vitamin D on fertility, pregnancy, and polycystic ovary syndrome—A review. Nutrients 2022, 14, 1649. [Google Scholar] [CrossRef] [PubMed]
- Parikh, G.; Varadinova, M.; Suwandhi, P.; Araki, T.; Rosenwaks, Z.; Poretsky, L.; Seto-Young, D. Vitamin D regulates steroidogenesis and insulin-like growth factor binding protein-1 (IGFBP-1) production in human ovarian cells. Horm. Metab. Res. 2010, 42, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Sinha, N.; Ong, E.; Sonmez, H.; Poretsky, L. Is there a role for vitamin D in human reproduction? Horm. Mol. Biol. Clin. Investig. 2016, 25, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Durazo-Arvizu, R.A.; Tian, L.; Brooks, S.P.J.; Sarafin, K.; Cashman, K.D.; Kiely, M.; Merkel, J.; Myers, G.L.; Coates, P.M.; Sempos, C.T. The Vitamin D Standardization Program (VDSP) Manual for Retrospective Laboratory Standardization of Serum 25-Hydroxyvitamin D Data. J. AOAC Int. 2017, 100, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Stoecklin, E.; Eggersdorfer, M. A glimpse of vitamin D status in Mainland China. Nutrition 2013, 29, 953–957. [Google Scholar] [CrossRef]
- Jiang, Z.; Pu, R.; Li, N.; Chen, C.; Li, J.; Dai, W.; Wang, Y.; Hu, J.; Zhu, D.; Yang, G. High prevalence of vitamin D deficiency in Asia: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 63, 3602–3611. [Google Scholar] [CrossRef]
- Fernando, M.; Ellery, S.J.; Marquina, C.; Lim, S.; Naderpoor, N.; Mousa, A. Vitamin D-Binding Protein in Pregnancy and Reproductive Health. Nutrients 2020, 12, 1489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Babić Leko, M.; Jureško, I.; Rozić, I.; Pleić, N.; Gunjača, I.; Zemunik, T. Vitamin D and the Thyroid: A Critical Review of the Current Evidence. Int. J. Mol. Sci. 2023, 24, 3586. [Google Scholar] [CrossRef] [PubMed]
- Collin, P.; Kaukinen, K.; Välimäki, M.; Salmi, J. Endocrinological Disorders and Celiac Disease. Endocr. Rev. 2002, 23, 464–483. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Litzow, M.R.; Murray, J.A. Hematologic manifestations of celiac disease. Blood 2007, 109, 412–421. [Google Scholar] [CrossRef]
- González, G. Vitamin D status among healthy postmenopausal women in South America. Dermato-Endocrinology 2013, 5, 117–120. [Google Scholar] [CrossRef]
- Contreras-Manzano, A.; Mejía-Rodríguez, F.; Villalpando, S.; Rebollar, R.; Flores-Aldana, M. Vitamin D status in Mexican women at reproductive age, Ensanut 2018–19. Salud Publica Mex. 2021, 63, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.R.; Anthias, C.; Madrigal, A.; Snowden, J.A. Insights Into the Role of Vitamin D as a Biomarker in Stem Cell Transplantation. Front. Immunol. 2020, 11, 500910. [Google Scholar] [CrossRef]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Bacanakgil, B.H.; İlhan, G.; Ohanoğlu, K. Effects of vitamin D supplementation on ovarian reserve markers in infertile women with diminished ovarian reserve. Medicine 2022, 101, e28796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moridi, I.; Chen, A.; Tal, O.; Tal, R. The Association between Vitamin D and Anti-Müllerian Hormone: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1567. [Google Scholar] [CrossRef] [PubMed]
- Safaei, Z.; Bakhshalizadeh, S.H.; Nasr Esfahani, M.H.; Akbari Sene, A.; Najafzadeh, V.; Soleimani, M.; Shirazi, R. Effect of Vitamin D3 on Mitochondrial Biogenesis in Granulosa Cells Derived from Polycystic Ovary Syndrome. Int. J. Fertil. Steril. 2020, 14, 143–149. [Google Scholar] [CrossRef]
- Ling, Y.; Xu, F.; Xia, X.; Dai, D.; Xiong, A.; Sun, R.; Qiu, L.; Xie, Z. Vitamin D supplementation reduces the risk of fall in the vitamin D deficient elderly: An updated meta-analysis. Clin. Nutr. 2021, 40, 5531–5537. [Google Scholar] [CrossRef] [PubMed]
- Mumford, S.L.; Garbose, R.A.; Kim, K.; Kissell, K.; Kuhr, D.L.; Omosigho, U.R.; Perkins, N.J.; Galai, N.; Silver, R.M.; Sjaarda, L.A.; et al. Association of preconception serum 25-hydroxyvitamin D concentrations with livebirth and pregnancy loss: A prospective cohort study. Lancet Diabetes Endocrinol. 2018, 6, 725–732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, J.; Gallos, I.; Tobias, A.; Tan, B.; Eapen, A.; Coomarasamy, A. Vitamin D and assisted reproductive treatment outcome: A systematic review and meta-analysis. Hum. Reprod. 2018, 33, 65–80. [Google Scholar] [CrossRef]
- Iliuta, F.; Pijoan, J.I.; Lainz, L.; Exposito, A.; Matorras, R. Women’s vitamin D levels and IVF results: A systematic review of the literature and meta-analysis, considering three categories of vitamin status (replete, insufficient, and deficient). Hum. Fertil. 2022, 25, 228–246. [Google Scholar] [CrossRef] [PubMed]
- Rudick, B.J.; Ingles, S.A.; Chung, K.; Stanczyk, F.Z.; Paulson, R.J.; Bendikson, K.A. Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil. Steril. 2014, 101, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake, D.L.; Małopolska, M.M.; Schwarz, T.; Tuz, R.; Bartlewski, P.M. The roles and expression of HOXA/Hoxa10 gene: A prospective marker of mammalian female fertility? Reprod Biol. 2022, 22, 100647. [Google Scholar] [CrossRef] [PubMed]
- Shilpasree, A.S.; Kulkarni, V.B.; Shetty, P.; Bargale, A.; Goni, M.; Oli, A.; Sarathkumar, E.; Patil, V.S.; Desai, R.M. Induction of Endometrial HOXA 10 Gene Expression by Vitamin D and its Possible Influence on Reproductive Outcome of PCOS Patients Undergoing Ovulation Induction Procedure. Indian J. Endocrinol. Metab. 2022, 26, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Ersahin, S.S.; Ersahin, A. Serum 25-hydroxyvitamin D correlates with endometrial HOXA10 mRNA expression. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3483–3486. [Google Scholar] [CrossRef]
- Ozkan, S.; Jindal, S.; Greenseid, K.; Shu, J.; Zeitlian, G.; Hickmon, C.; Pal, L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil. Steril. 2010, 94, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- McGovern, P.G. Is Vitamin D important for in vitro fertilization success? Fertil. Steril. 2020, 114, 962. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Chitale, R.; O’Callaghan, K.M.; Sudfeld, C.R.; Smith, E.R. The Effects of Vitamin D Supplementation During Pregnancy on Maternal, Neonatal, and Infant Health: A Systematic Review and Meta-analysis. Nutr. Rev. 2025, 83, e892–e903. [Google Scholar] [CrossRef]
- Fu, G.K.; Lin, D.; Zhang, M.Y.; Bikle, D.D.; Shackleton, C.H.; Miller, W.L.; Portale, A.A. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol. Endocrinol. 1997, 11, 1961–1970. [Google Scholar]
- Shahbazi, M.; Jeddi-Tehrani, M.; Zareie, M.; Salek-Moghaddam, A.; Akhondi, M.M.; Bahmanpoor, M.; Sadeghi, M.R.; Zarnani, A.H. Expression profiling of vitamin D receptor in placenta, decidua, and ovary of pregnant mice. Placenta 2011, 32, 657–664. [Google Scholar] [CrossRef] [PubMed]
- de Angelis, C.; Galdiero, M.; Pivonello, C.; Garifalos, F.; Menafra, D.; Cariati, F.; Cirillo, F.; Lombardi, G.; Colao, A. The role of vitamin D in male fertility: A focus on the testis. Rev. Endocr. Metab. Disord. 2017, 18, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Bollen, S.E.; Bass, J.J.; Fujita, S.; Wilkinson, D.; Hewison, M.; Atherton, P.J. The vitamin D/vitamin D receptor (VDR) axis in muscle atrophy and sarcopenia. Cell Signal 2022, 96, 110355. [Google Scholar] [CrossRef]
- Blomberg Jensen, M.; Jørgensen, A.; Meyts, E.R.-D.; Kristensen, D.M.; Jørgensen, N.; Skakkebaek, N.E.; Juul, A.; Leffers, H. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum. Reprod. 2010, 25, 1303–1311. [Google Scholar] [CrossRef]
- Vienonen, A.; Miettinen, S.; Bläuer, M.; Martikainen, P.M.; Tomás, E.; Heinonen, P.K.; Ylikomi, T. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J. Soc. Gynecol. 2004, 11, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Cordes, T.; Diesing, D.; Diedrich, K.; Friedrich, M. Expression of 25 hydroxyvitamin D3-1α-hydroxylase in human endometrial tissue. J. Steroid Biochem. Mol. Biol. 2007, 103, 771–775. [Google Scholar] [CrossRef]
- Bergadà, L.; Pallares, J.; Maria Vittoria, A.; Cardus, A.; Santacana, M.; Valls, J.; Cao, G.; Fernàndez, E.; Dolcet, X.; Dusso, A.S.; et al. Role of local bioactivation of vitamin D by CYP27A1 and CYP2R1 in the control of cell growth in normal endometrium and endometrial carcinoma. Lab. Invest. 2014, 94, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Cermisoni, G.C.; Alteri, A.; Corti, L.; Rabellotti, E.; Papaleo, E.; Viganò, P.; Sanchez, A.M. Vitamin D and Endometrium: A Systematic Review of a Neglected Area of Research. Int. J. Mol. Sci. 2018, 19, 2320. [Google Scholar] [CrossRef]
- Pal, L.; Zhang, H.; Williams, J.; Santoro, N.F.; Diamond, M.P.; Schlaff, W.D.; Coutifaris, C.; Carson, S.A.; Steinkampf, M.P.; Carr, B.R.; et al. Reproductive Medicine Network. Vitamin D Status Relates to Reproductive Outcome in Women with Polycystic Ovary Syndrome: Secondary Analysis of a Multicenter Randomized Controlled Trial. J. Clin. Endocrinol. Metab. 2016, 101, 3027–3035. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Hum Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Farhangnia, P.; Noormohammadi, M.; Delbandi, A.A. Vitamin D and reproductive disorders: A comprehensive review with a focus on endometriosis. Reprod. Health 2024, 21, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jukic, A.M.Z.; Baird, D.D.; Weinberg, C.R.; Wilcox, A.J.; McConnaughey, D.R.; Steiner, A.Z. Pre-conception 25-hydroxyvitamin D (25(OH)D) and fecundability. Hum. Reprod. 2019, 34, 2163–2172. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Asp. Med. 2013, 34, 939–980. [Google Scholar] [CrossRef]
- Aflatoonian, A.; Karimzadeh Maybodi, M.A.; Aflatoonian, N.; Tabibnejad, N.; Amir-Arjmand, M.H.; Soleimani, M.; Aflatoonian, B.; Aflatoonian, A. Perinatal outcome in fresh versus frozen embryo transfer in ART cycles. Int. J. Reprod. Biomed. 2016, 14, 167–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abedi, S.; Taebi, M.; Nasr Esfahani, M.H. Effect of Vitamin D Supplementation on Intracytoplasmic Sperm Injection Outcomes: A Randomized Double-Blind Placebo-Controlled Trial. Int. J. Fertil. Steril. 2019, 13, 18–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roth, D.E.; Leung, M.; Mesfin, E.; Qamar, H.; Watterworth, J.; Papp, E. Vitamin D Supplementation During Pregnancy: State of the Evidence from a Systematic Review of Randomized Trials. BMJ 2017, 359, j5237. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. 25-Hydroxyvitamin D concentration is key to analyzing vitamin D’s effects. J. Fam. Pract. 2021, 70, 289–292. [Google Scholar]
- Liu, Y.; Ding, C.; Xu, R.; Wang, K.; Zhang, D.; Pang, W.; Tu, W.; Chen, Y. Effects of Vitamin D Supplementation During Pregnancy on Offspring Health at Birth: A Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2022, 41, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef]
- Dennis, N.A.; Houghton, L.A.; Jones, G.T.; van Rij, A.M.; Morgan, K.; McLennan, I.S. The level of serum anti-Müllerian hormone correlates with vitamin D status in men and women but not in boys. J. Clin. Endocrinol. Metab. 2012, 97, 2450–2455. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, J.; Wan, Q.; Huang, J.; Han, T.; Qu, T.; Yu, L.-L. Influence of Vitamin D supplementation on reproductive outcomes of infertile patients: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 17. [Google Scholar] [CrossRef]
- Aşır, F.; Duran, S.Ç.; Afşin, M.; Duran, E.; Korak, T.; Şahin, F. Investigation of vitamin D levels in men with suspected infertility. Life 2024, 14, 273. [Google Scholar] [CrossRef]
- Subramanian, A.; Steiner, A.Z.; Weinberg, C.R.; Doss, G.L.; Jukic, A.M.Z. Preconception vitamin D and miscarriage in a prospective cohort study. Hum. Reprod. 2022, 37, 2465–2473. [Google Scholar] [CrossRef]
- Paffoni, A.; Somigliana, E.; Sarais, V.; Ferrari, S.; Reschini, M.; Makieva, S.; Papaleo, E.; Viganò, P. Effect of vitamin D supplementation on assisted reproduction technology (ART) outcomes and underlying biological mechanisms: Protocol of a randomized clinical controlled trial. The “supplementation of vitamin D and reproductive outcome” (SUNDRO) study. BMC Pregnancy Childbirth 2019, 19, 395. [Google Scholar] [CrossRef] [PubMed]
- Somigliana, E.; Panina-Bordignon, P.; Murone, S.; Di Lucia, P.; Vercellini, P.; Vigano, P. Vitamin D reserve is higher in women with endometriosis. Hum. Reprod. 2007, 22, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Gallos, I.; Tobias, A.; Robinson, L.; Kirkman-Brown, J.; Dhillon-Smith, R.; Harb, H.; Eapen, A.; Rajkhowa, M.; Coomarasamy, A. Vitamin D and assisted reproductive treatment outcome: A prospective cohort study. Reprod. Health 2019, 16, 106. [Google Scholar] [CrossRef] [PubMed]
- Aflatoonian, A.; Arabjahvani, F.; Eftekhar, M.; Sayadi, M. Effect of vitamin D insufficiency treatment on fertility outcomes in frozen-thawed embryo transfer cycles: A randomized clinical trial. Iran. J. Reprod. Med. 2014, 12, 595–600, Erratum in: Int. J. Reprod. Biomed. 2022, 20, 68. [Google Scholar] [CrossRef]
- Bezerra Espinola, M.S.; Bilotta, G.; Aragona, C. Positive effect of a new supplementation of vitamin D3 with myo-inositol, folic acid and melatonin on IVF outcomes: A prospective randomized and controlled pilot study. Gynecol. Endocrinol. 2021, 37, 251–254. [Google Scholar] [CrossRef]
- Doryanizadeh, L.; Morshed-Behbahani, B.; Parsanezhad, M.E.; Dabbaghmanesh, M.H.; Jokar, A. Calcitriol effect on outcomes of in vitro fertilization in infertile women with vitamin D deficiency: A double-blind randomized clinical trial. Z. Geburtshilfe Neonatol. 2021, 225, 226–231. [Google Scholar] [CrossRef]
- Somigliana, E.; Sarais, V.; Reschini, M.; Ferrari, S.; Makieva, S.; Cermisoni, G.C.; Paffoni, A.; Papaleo, E.; Vigano, P. Single oral dose of vitamin D3 supplementation prior to in vitro fertilization and embryo transfer in normal weight women: The SUNDRO randomized controlled trial. Am. J. Obstet. Gynecol. 2021, 225, 283.e1–283.e10. [Google Scholar] [CrossRef]
- Anifandis, G.M.; Dafopoulos, K.; Messini, C.I.; Chalvatzas, N.; Liakos, N.; Pournaras, S.; Messinis, I.E. Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod. Biol. Endocrinol. 2010, 8, 91. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Molinaro, T.A.; Dubell, E.K.; Scott, K.L.; Ruiz, A.R.; Forman, E.J.; Werner, M.D.; Hong, K.H.; Scott, R.T., Jr. Vitamin D levels do not affect IVF outcomes following the transfer of euploid blastocysts. Am. J. Obstet. Gynecol. 2015, 212, 315.e1–315.e6. [Google Scholar] [CrossRef]
- Fabris, A.; Pacheco, A.; Cruz, M.; Puente, J.M.; Fatemi, H.; Garcia-Velasco, J.A. Impact of circulating levels of total and bioavailable serum vitamin D on pregnancy rate in egg donation recipients. Fertil. Steril. 2014, 102, 1608–1612. [Google Scholar] [CrossRef]
- Gupta, K.; Thakur, R.; Sharma, P.; Kamra, P.; Khetarpal, P. Polycystic Ovarian Syndrome (PCOS) and its association with VDR gene variants Cdx2 (rs11568820) and ApaI (rs7975232): Systematic review, meta-analysis and in silico analysis. Hum. Gene 2024, 40, 201293. [Google Scholar] [CrossRef]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The prevalence and phenotypic features of polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 194: Polycystic Ovary Syndrome. Obstet. Gynecol. 2020, 136, 638. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; on behalf of theInternational PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Kuyucu, Y.; Çelik, L.S.; Kendirlinan, Ö.; Tap, Ö.; Mete, U.Ö. Investigation of the uterine structural changes in the experimental model with polycystic ovary syndrome and effects of vitamin D treatment: An ultrastructural and immunohistochemical study. Reprod. Biol. 2018, 18, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Karras, S.; Goulis, D.G. Vitamin D in human reproduction: A narrative review. Int. J. Clin. Pract. 2013, 67, 225–235. [Google Scholar] [CrossRef]
- Morgante, G.; Darino, I.; Spanò, A.; Luisi, S.; Luddi, A.; Piomboni, P.; Governini, L.; De Leo, V. PCOS Physiopathology and Vitamin D Deficiency: Biological Insights and Perspectives for Treatment. J. Clin. Med. 2022, 11, 4509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maktabi, M.; Chamani, M.; Asemi, Z. The Effects of Vitamin D Supplementation on Metabolic Status of Patients with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm. Metab. Res. 2017, 49, 493–498. [Google Scholar] [CrossRef]
- Trummer, C.; Schwetz, V.; Kollmann, M.; Wölfler, M.; Münzker, J.; Pieber, T.R.; Pilz, S.; Heijboer, A.C.; Obermayer-Pietsch, B.; Lerchbaum, E. Effects of vitamin D supplementation on metabolic and endocrine parameters in PCOS: A randomized-controlled trial. Eur. J. Nutr. 2019, 58, 2019–2028. [Google Scholar] [CrossRef]
- Davis, E.M.; Peck, J.D.; Hansen, K.R.; Neas, B.R.; Craig, L.B. Associations between vitamin D levels and polycystic ovary syndrome phenotypes. Minerva Endocrinol. 2019, 44, 176–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lerchbaum, E.; Theiler-Schwetz, V.; Kollmann, M.; Wölfler, M.; Pilz, S.; Obermayer-Pietsch, B.; Trummer, C. Effects of Vitamin D Supplementation on Surrogate Markers of Fertility in PCOS Women: A Randomized Controlled Trial. Nutrients 2021, 13, 547. [Google Scholar] [CrossRef] [PubMed]
- Wehr, E.; Trummer, O.; Giuliani, A.; Gruber, H.J.; Pieber, T.R.; Obermayer-Pietsch, B. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur. J. Endocrinol. 2011, 164, 741–749. [Google Scholar] [CrossRef]
- Ota, K.; Mitsui, J.; Katsumata, S.; Takayanagi, Y.; Nako, Y.; Tajima, M.; Komiya, A.; Takahashi, T.; Kawai, K. Seasonal serum 25(OH) vitamin D level and reproductive or immune markers in reproductive-aged women with infertility: A cross-sectional observational study in East Japan. Nutrients 2023, 15, 5059. [Google Scholar] [CrossRef]
- Ostadmohammadi, V.; Jamilian, M.; Bahmani, F.; Asemi, Z. Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J. Ovarian Res. 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Dastorani, M.; Aghadavod, E.; Mirhosseini, N.; Foroozanfard, F.; Zadeh Modarres, S.; Amiri Siavashani, M.; Asemi, Z. The effects of vitamin D supplementation on metabolic profiles and gene expression of insulin and lipid metabolism in infertile polycystic ovary syndrome candidates for in vitro fertilization. Reprod. Biol. Endocrinol. 2018, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Papageorgiou, M.; Deshmukh, H.; Kilpatrick, E.S.; Mann, V.; Corless, L.; Abouda, G.; Rigby, A.S.; Atkin, S.L.; Sathyapalan, T. A Randomized, Controlled Trial of Vitamin D Supplementation on Cardiovascular Risk Factors, Hormones, and Liver Markers in Women with Polycystic Ovary Syndrome. Nutrients 2019, 11, 188. [Google Scholar] [CrossRef]
- Azhar, A.; Alam, S.M.; Rehman, R. Vitamin D and Lipid Profiles in Infertile PCOS and Non-PCOS Females. J. Coll. Physicians Surg. Pak. 2024, 34, 767–770. [Google Scholar] [CrossRef]
- Irani, M.; Seifer, D.B.; Grazi, R.V.; Julka, N.; Bhatt, D.; Kalgi, B.; Irani, S.; Tal, O.; Lambert-Messerlian, G.; Tal, R. Vitamin D Supplementation Decreases TGF-β1 Bioavailability in PCOS: A Randomized Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2015, 100, 4307–4314. [Google Scholar] [CrossRef]
- Wen, X.; Wang, L.; Li, F.; Yu, X. Effects of vitamin D supplementation on metabolic parameters in women with polycystic ovary syndrome: A randomized controlled trial. J. Ovarian Res. 2024, 17, 147. [Google Scholar] [CrossRef]
- Krul-Poel, Y.H.M.; Koenders, P.P.; Steegers-Theunissen, R.P.; Ten Boekel, E.; Wee, M.M.T.; Louwers, Y.; Lips, P.; Laven, J.S.E.; Simsek, S. Vitamin D and metabolic disturbances in polycystic ovary syndrome (PCOS): A cross-sectional study. PLoS ONE 2018, 13, e0204748. [Google Scholar] [CrossRef]
- Edi, R.; Cheng, T. Endometriosis: Evaluation and Treatment. Am. Fam. Physician 2022, 106, 397–404. [Google Scholar] [PubMed]
- Cousins, F.L.; McKinnon, B.D.; Mortlock, S.; Fitzgerald, H.C.; Zhang, C.; Montgomery, G.W.; Gargett, C.E. New concepts on the etiology of endometriosis. J. Obstet. Gynaecol. Res. 2023, 49, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Cano-Herrera, G.; Salmun Nehmad, S.; Ruiz de Chávez Gascón, J.; Méndez Vionet, A.; van Tienhoven, X.A.; Osorio Martínez, M.F.; Muleiro Alvarez, M.; Vasco Rivero, M.X.; López Torres, M.F.; Barroso Valverde, M.J.; et al. Endometriosis: A Comprehensive Analysis of the Pathophysiology, Treatment, and Nutritional Aspects, and Its Repercussions on the Quality of Life of Patients. Biomedicines 2024, 12, 1476. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C.; Oskotsky, T.T.; Falako, S.; Opoku-Anane, J.; Sirota, M. Endometriosis in the era of precision medicine and impact on sexual and reproductive health across the lifespan and in diverse populations. FASEB J. 2023, 37, e23130. [Google Scholar] [CrossRef]
- Esfandiari, F.; Chitsazian, F.; Jahromi, M.G.; Favaedi, R.; Bazrgar, M.; Aflatoonian, R.; Afsharian, P.; Aflatoonian, A.; Shahhoseini, M. HOX cluster and their cofactors showed an altered expression pattern in eutopic and ectopic endometriosis tissues. Reprod. Biol. Endocrinol. 2021, 19, 132. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef]
- Smolarz, B.; Szyłło, K.; Romanowicz, H. Endometriosis: Epidemiology, Classification, Pathogenesis, Treatment and Genetics (Review of Literature). Int. J. Mol. Sci. 2021, 22, 10554. [Google Scholar] [CrossRef]
- Ocampo Hernández, D.M.; Gallardo Valencia, L.E.; Guzmán-Valdivia Gómez, G. Evaluación de la calidad de vida en pacientes con endometriosis mediante una escala original. Acta méd. Grupo Ángeles 2023, 21, 349–355. [Google Scholar] [CrossRef]
- Salvador, J.; Lorente, E.; Ripollés, T.; Martínez, M.J.; Vizuete del Río, J. Infiltrating endometriosis: Diagnostic keys in abdominal ultrasonography. Radiología 2021, 63, 32–41. [Google Scholar] [CrossRef]
- Maciel, C.; Ferreira, H.; Djokovic, D.; Kyaw Tun, J.; Keckstein, J.; Rizzo, S.; Manganaro, L. MRI of endometriosis in correlation with the #Enzian classification: Applicability and structured report. Insights Imaging 2023, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- França, P.R.C.; Lontra, A.C.P.; Fernandes, P.D. Endometriosis: A Disease with Few Direct Treatment Options. Molecules 2022, 27, 4034. [Google Scholar] [CrossRef]
- Kalaitzopoulos, D.R.; Samartzis, N.; Daniilidis, A.; Leeners, B.; Makieva, S.; Nirgianakis, K.; Dedes, I.; Metzler, J.M.; Imesch, P.; Lempesis, I.G. Effects of vitamin D supplementation in endometriosis: A systematic review. Reprod. Biol. Endocrinol. 2022, 20, 176. [Google Scholar] [CrossRef]
- Delbandi, A.A.; Torab, M.; Abdollahi, E.; Khodaverdi, S.; Rokhgireh, S.; Moradi, Z.; Heidari, S.; Mohammadi, T. Vitamin D deficiency as a risk factor for endometriosis in Iranian women. J. Reprod. Immunol. 2021, 143, 103266. [Google Scholar] [CrossRef] [PubMed]
- Jennings, B.S.; Hewison, M. Vitamin D and Endometriosis: Is There a Mechanistic Link? Cell Biochem. Funct. 2025, 43, e70037. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.H.; Chen, X.X.; Chen, Y.; Wu, Z.C.; Chen, X.Q.; Li, X.L. Antioxidant vitamins supplementation reduce endometriosis related pelvic pain in humans: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2023, 21, 79. [Google Scholar] [CrossRef]
- Chamgordani, N.S.; Esmaeil, N.; Hashemi, M.; Amari, A.; Seyedtabib, M.; Ghafourian, M. Evaluation of the natural killer cell subsets and their relationship with serum interferon gamma and vitamin D levels in women with stages III and IV endometriosis: A case-control study. Int. J. Reprod. Biomed. 2024, 22, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Koga, K.; Izumi, G.; Sue, F.; Makabe, T.; Taguchi, A.; Nagai, M.; Urata, Y.; Takamura, M.; Harada, M.; et al. Effects of 1,25-Dihydroxy Vitamin D3 on Endometriosis. J. Clin. Endocrinol. Metab. 2016, 101, 2371–2379. [Google Scholar] [CrossRef]
- Mehdizadehkashi, A.; Rokhgireh, S.; Tahermanesh, K.; Eslahi, N.; Minaeian, S.; Samimi, M. The effect of vitamin D supplementation on clinical symptoms and metabolic profiles in patients with endometriosis. Gynecol. Endocrinol. 2021, 37, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Chavarro, J.E.; Malspeis, S.; Willett, W.C.; Missmer, S.A. Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: A prospective cohort study. Am. J. Epidemiol. 2013, 177, 420–430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, B.; Liao, M.; Huang, Y.; Hang, F.; Ma, N.; Hu, Q.; Wang, J.; Jin, Y.; Qin, A. Association between vitamin D and endometriosis among American women: National Health and Nutrition Examination Survey. PLoS ONE 2024, 19, e0296190. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Holtz, D.N.; Schmidt, N.; Kolipaka, S.; Hata, E.; Sutton, M.; Znayenko-Miller, T.; Hazen, N.D.; Cobb, C.; Kahleova, H. Nutrition in the prevention and treatment of endometriosis: A review. Front. Nutr. 2023, 10, 1089891. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shen, X.; Lu, D.; Peng, J.; Zhou, S.; Xu, L.; Zhang, J. Effects of vitamin D supplementation on ovulation and pregnancy in women with polycystic ovary syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1148556. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Total Participants (N) | Cases (n) | Controls (n) | Age | BMI | Baseline 25(OH)D | Type of Study | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Aflatoonian et al. (2014) [84] | Iran | N = 114 | n = 57 | n = 57 | 29.0 ± 4.3 years | 26.58 ± 1.72 kg/m2 | 15.02 ± 6.14 ng/mL | Randomized clinical trial | VD insufficiency treatment did not significantly improve pregnancy rates in frozen-thawed embryo transfer cycles. |
| Bezerra Espinola et al. (2020) [85] | Italy | N = 100 | n = 50 | n = 50 | 35.30 ± 5.41 years | 21.95 ± 2.20 kg/m2 | 23.40 ± 6.65 ng/mL | Prospective randomized controlled pilot study | Vitamin D3 supplementation (2000 IU/day) improved implantation rates in IVF patients (37.1% vs. 19.2%, p = 0.0151), particularly in those with baseline VD ≥ 20 ng/mL. No significant difference in ongoing pregnancy or miscarriage rates. |
| Doryanizadeh et al. (2020) [86] | Iran | N = 95 | n = 51 | n = 44 | 32.08 ± 4.90 years | 25.11 ± 3.29 kg/m2 | 27.55 ± 1.80 ng/mL | Double-blind randomized clinical trial | Calcitriol significantly increased chemical pregnancy rates (31.4% vs. 18.2%, p < 0.05), but had no significant effect on clinical pregnancy (25.5% vs. 13.6%) or ongoing pregnancy at 20 weeks (9.8% vs. 11.6%). |
| Somigliana et al. (2021) [87] | Italy | N = 630 | n = 308 | n = 322 | 35.0 ± 3.9 years | 21.5 ± 2.3 kg/m2 | 20.0 ± 5.1 ng/mL | Double-blind randomized controlled trial | A single high dose (600,000 IU) of vitamin D3 before IVF did not improve clinical pregnancy rates (37% vs. 40%, p = 0.37) or IVF outcomes. No specific subgroup benefited from supplementation. Further studies are needed. |
| Anifandis et al. (2010) [88] | Greece | N = 101 | n = 21 | n = 80 | 36.16 ± 5.29 years | 24.10 ± 2.99 kg/m2 | 23.37 ± 8.45 ng/mL | Prospective observational study | Higher follicular fluid VD levels (>30 ng/mL) were associated with lower embryo quality and reduced pregnancy rates (14.3% vs. 32.3–32.7%, p = 0.047). Increased VD levels correlated with lower follicular fluid glucose levels, possibly affecting oocyte quality and IVF outcomes. |
| Franasiak et al. (2015) [89] | United States | N = 517 | n = 208 | n = 309 | 35.25 ± 4.27 years | 25.04 ± 5.42 kg/m2 | 24.20 ± 4.65 ng/mL | Retrospective cohort study | Vitamin D levels were not associated with IVF success after euploid blastocyst transfer. No significant differences in implantation or pregnancy rates across VD categories. Measuring VD does not predict implantation likelihood in this population. |
| Fabris et al. (2014) [90] | Spain | N = 267 | n = 41 | n = 226 | 40.78 ± 0.76 years | 22.89 ± 0.76 kg/m2 | >30 ng/mL | Retrospective cohort study | VD levels did not correlate with pregnancy rates in egg donation recipients. No significant differences in implantation (61% vs. 63.4% vs. 65.2%), pregnancy (70% vs. 69.9% vs. 73.9%), or ongoing pregnancy rates (55.9% vs. 52.7% vs. 60.7%). No evidence to recommend VD screening in egg donation patients. |
| Abedi et al. (2019) [72] | Iran | N = 85 | n = 42 | n = 43 | 31.34 ± 4.30 years | 23.85 ± 2.00 kg/m2 | 13.54 ± 6.50 ng/mL | Randomized double-blind placebo-controlled trial | VD supplementation (50,000 IU weekly for 6 weeks before ICSI) significantly improved endometrial quality (81% vs. 55.8%, p = 0.015), chemical pregnancy rate (47.6% vs. 25.5%, p = 0.013), and clinical pregnancy rate (38.1% vs. 20.9%, p = 0.019). No significant effect on oocyte retrieval, fertilization rate, or embryo quality. |
| Study | Country | Total Participants (N) | Cases (n) | Controls (n) | Age | BMI | Baseline 25(OH)D | Type of Study | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Chamgordani et al. (2024) [127] | Iran | N = 59 | n= 29 | n= 30 | 36.5 ± 5.5 years | 26.4 ± 3.9 kg/m2 | 27.3 ± 3.2 ng/mL | Case Control Study | Women with advanced endometriosis had higher NK cell percentages than controls, but no significant correlation was found between NK cells and vitamin D levels. Serum vitamin D levels were slightly higher in endometriosis cases, but the difference was not significant (p = 0.12). |
| Somigliana E et al. (2007) [82] | Italy | N = 140 | n = 87 | n = 53 | 33.7 ± 6.0 years | 21.9 ± 3.4 | 24.9 ± 14.8 ng/mL | Prospective cohort study | Women with endometriosis had higher serum levels of 25-hydroxyvitamin D3. A positive gradient was observed according to disease severity. |
| Miyashita M et al. (2016) [128] | Japan | N = 76 | n = 39 | n = 37 | 34.3 ± 1.4 years | - | 20.2 ± 1.3 ng/mL | Case–control study with in vitro experiments | Lower serum 25(OH)D3 levels were found in patients with severe endometriosis. VD suppressed inflammatory markers and reduced ESC proliferation, suggesting a potential therapeutic role. |
| Nodler et al. (2020) [7] | United States | N = 149 | n = 47 | n = 22 | 19.67 ± 3.1 years | 26.07 ± 5.46 kg/m2 | 35.3 ± 13.9 ng/mL | Randomize, double-blind, placebo-controlled clinical trial | In young women with endometriosis, neither VD nor fish oil significantly reduced pain compared to placebo. The improvement observed in the placebo group highlights the need for further research to understand this effect. |
| Zheng et al. (2023) [126] | Chinese | N = 589 | n = 315 | n = 274 | 31.47 ± 4.24 years | - | - | Randomized controlled trial | A randomized controlled trial showed that supplementation with antioxidant vitamins, including VD, significantly reduced dysmenorrhea, dyspareunia, and chronic pelvic pain in women with endometriosis. It also improved the overall quality of life. |
| Mehdizadehkashi et al. (2021) [129] | Iran | N = 60 | n = 30 | n = 30 | 35.2 ± 7.05 years | 24.7 ± 3.5 kg/m2 | - | Randomize, double-blind, placebo-controlled clinical trial | A 12-week supplementation with VD improved pelvic pain, cholesterol ratio, hs-CRP, and TAC levels in endometriosis patients but did not affect other symptoms. Findings on VD-binding protein were inconsistent. Further research is needed to fully understand its effects on endometriosis. |
| Harris et al. (2013) [130] | United States | N = 70,556 | n = 1385 | n = 69,171 | 35.92 ± 4.25 years | - | - | Prospective cohort study | This cohort showed that plasma 25(OH)D levels were inversely associated with endometriosis. Women with the highest levels of VD had a 24% lower risk of endometriosis than women with the lowest levels. |
| Xie et al. (2024) [131] | Germany | N = 3232 | n = 257 | n = 2975 | 38.43 ± 9.57 years | 27.32 ± 4.5 kg/m2 | 21.36 ± 10.01 ng/mL | Observational cross-sectional study | Findings were that higher serum 25(OH)D concentrations were associated with a decreased incidence of endometriosis, with data suggesting that adequate sun exposure may lower risk for VD deficiency. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Tienhoven, X.A.; Ruiz de Chávez Gascón, J.; Cano-Herrera, G.; Sarkis Nehme, J.A.; Souroujon Torun, A.A.; Bautista Gonzalez, M.F.; Esparza Salazar, F.; Sierra Brozon, A.; Rivera Rosas, E.G.; Carbajal Ocampo, D.; et al. Vitamin D in Reproductive Health Disorders: A Narrative Review Focusing on Infertility, Endometriosis, and Polycystic Ovarian Syndrome. Int. J. Mol. Sci. 2025, 26, 2256. https://doi.org/10.3390/ijms26052256
van Tienhoven XA, Ruiz de Chávez Gascón J, Cano-Herrera G, Sarkis Nehme JA, Souroujon Torun AA, Bautista Gonzalez MF, Esparza Salazar F, Sierra Brozon A, Rivera Rosas EG, Carbajal Ocampo D, et al. Vitamin D in Reproductive Health Disorders: A Narrative Review Focusing on Infertility, Endometriosis, and Polycystic Ovarian Syndrome. International Journal of Molecular Sciences. 2025; 26(5):2256. https://doi.org/10.3390/ijms26052256
Chicago/Turabian Stylevan Tienhoven, Ximena A., Jimena Ruiz de Chávez Gascón, Gabriela Cano-Herrera, José Antonio Sarkis Nehme, Ariela A. Souroujon Torun, Maria Fernanda Bautista Gonzalez, Felipe Esparza Salazar, Ana Sierra Brozon, Eder Gabriel Rivera Rosas, Dante Carbajal Ocampo, and et al. 2025. "Vitamin D in Reproductive Health Disorders: A Narrative Review Focusing on Infertility, Endometriosis, and Polycystic Ovarian Syndrome" International Journal of Molecular Sciences 26, no. 5: 2256. https://doi.org/10.3390/ijms26052256
APA Stylevan Tienhoven, X. A., Ruiz de Chávez Gascón, J., Cano-Herrera, G., Sarkis Nehme, J. A., Souroujon Torun, A. A., Bautista Gonzalez, M. F., Esparza Salazar, F., Sierra Brozon, A., Rivera Rosas, E. G., Carbajal Ocampo, D., & Cabrera Carranco, R. (2025). Vitamin D in Reproductive Health Disorders: A Narrative Review Focusing on Infertility, Endometriosis, and Polycystic Ovarian Syndrome. International Journal of Molecular Sciences, 26(5), 2256. https://doi.org/10.3390/ijms26052256








