Lactobacillus plantae Expressing Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Single-Chain Antibody Can Inhibit PRRSV Replication and Change the Intestinal Flora Structure of Piglets
Abstract
1. Introduction
2. Results
2.1. Lactobacillus plantarum Anchors and Expresses PRRSV Single-Chain Antibody and Prokaryotic Expression
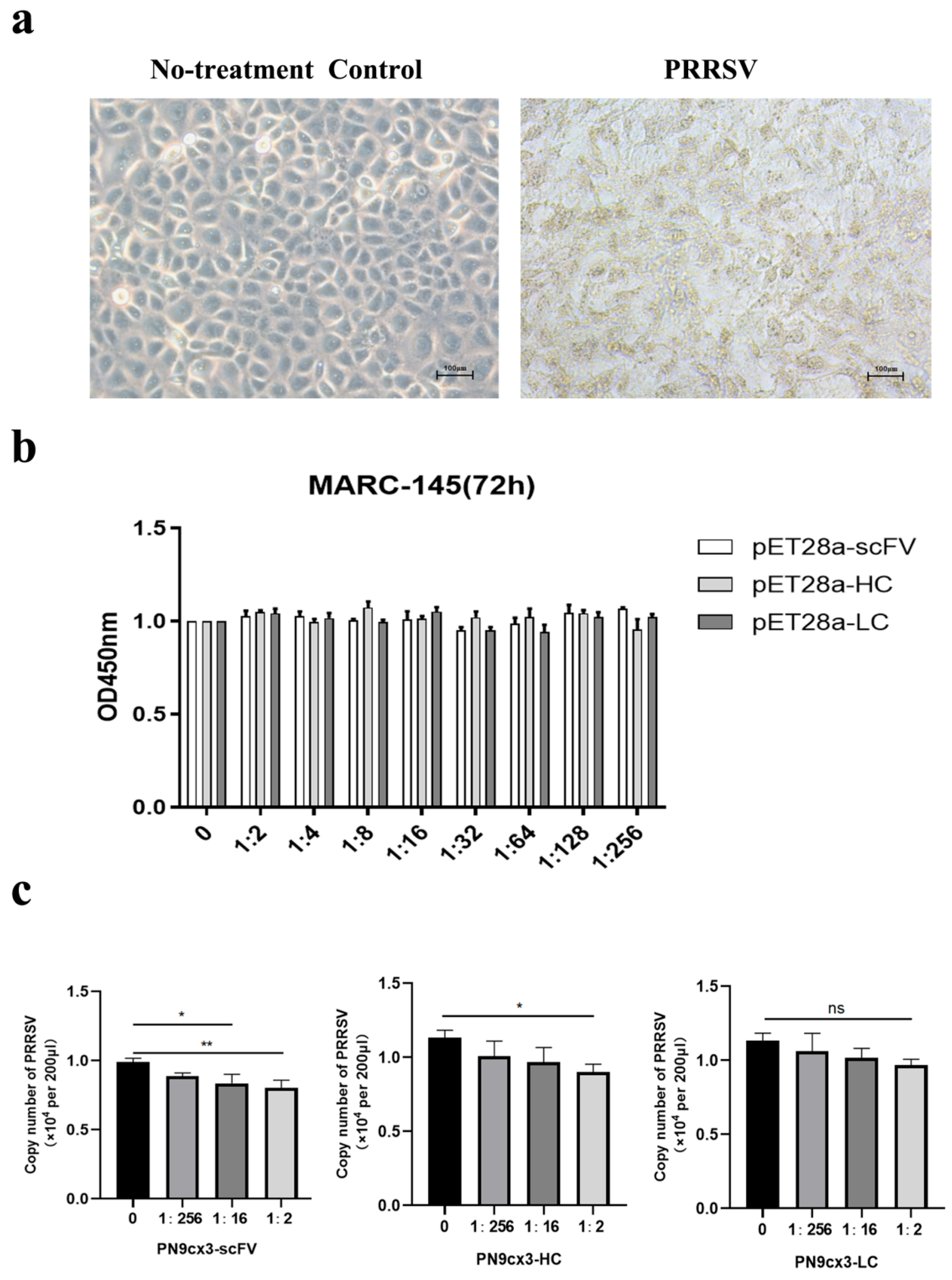
2.2. Antiviral Effect in Vitro
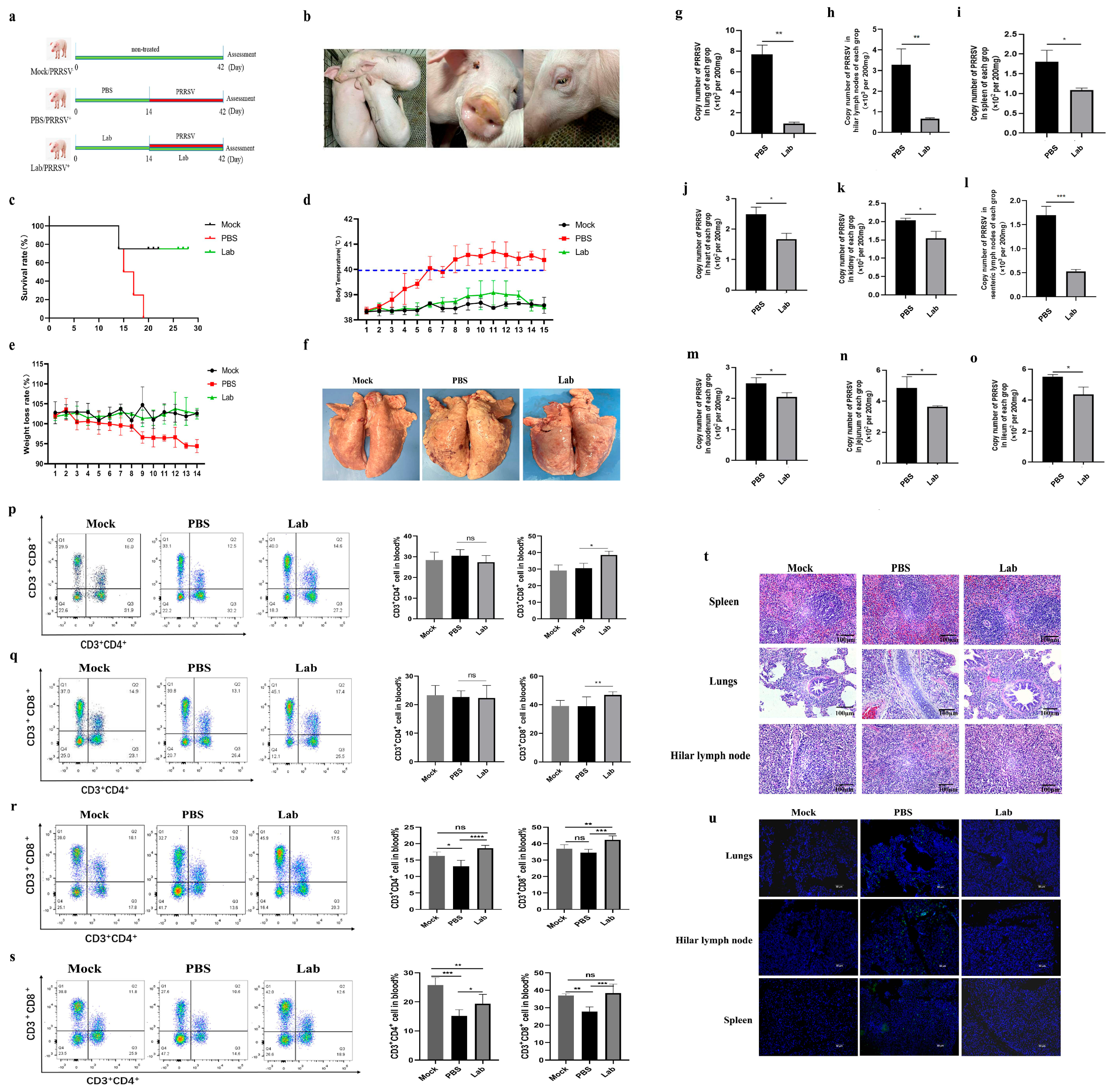
2.3. Changes in Clinical Characterization of Experimental Pigs After Challenge
2.4. Poison Attack Pigs’ Autopsy Results After the Experiment
2.5. Virus Quantification Results of Each Tissue and Organ
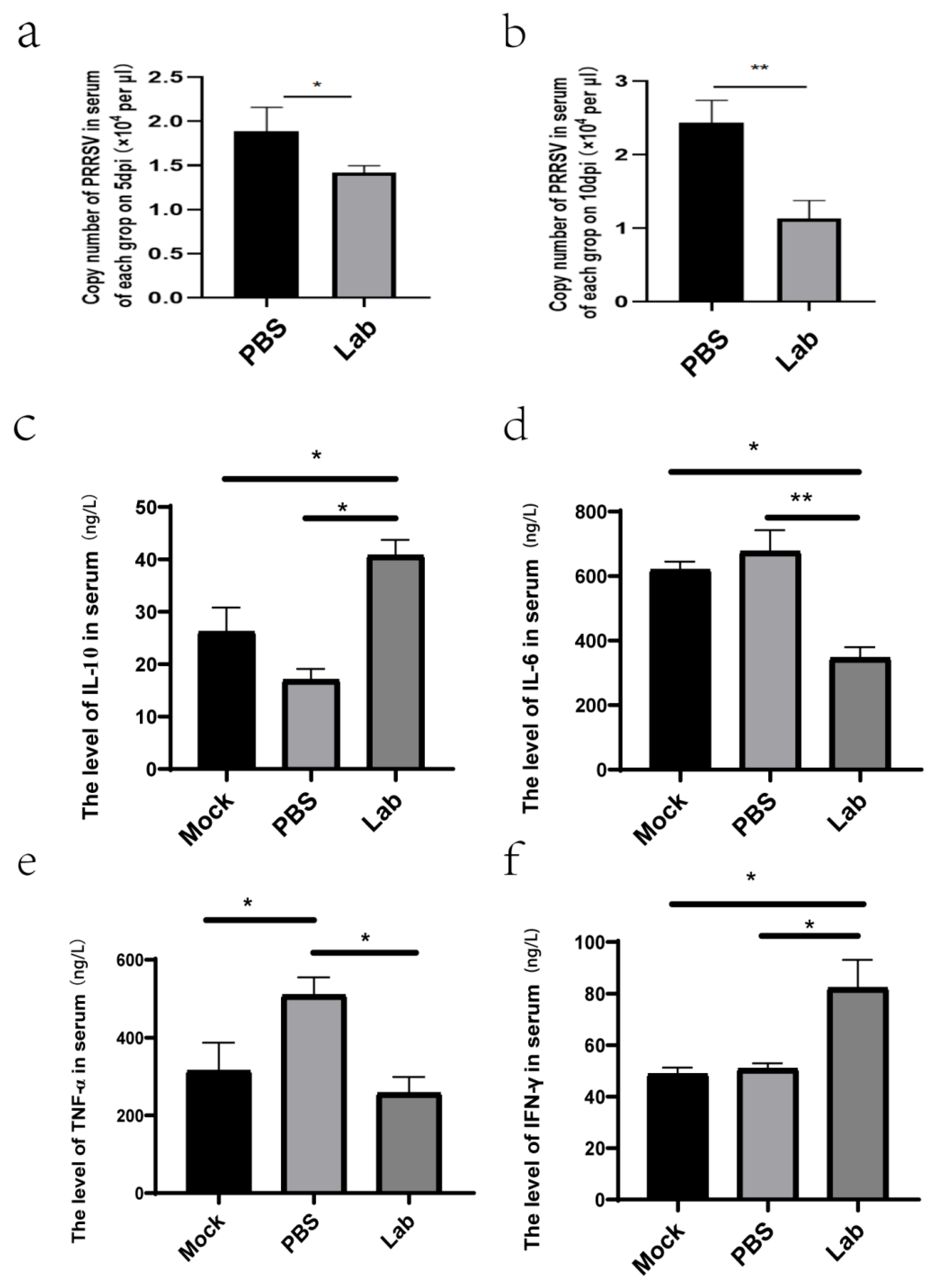
2.6. Results of Viral Load Determination in Serum After Challenge
2.7. ELISA Analysis
2.8. Localization of Virus in Tissues via Immunofluorescence
2.9. Histopathological Observation
2.10. Flow Cytometry of Peripheral Blood T Lymphocytes After Challenge
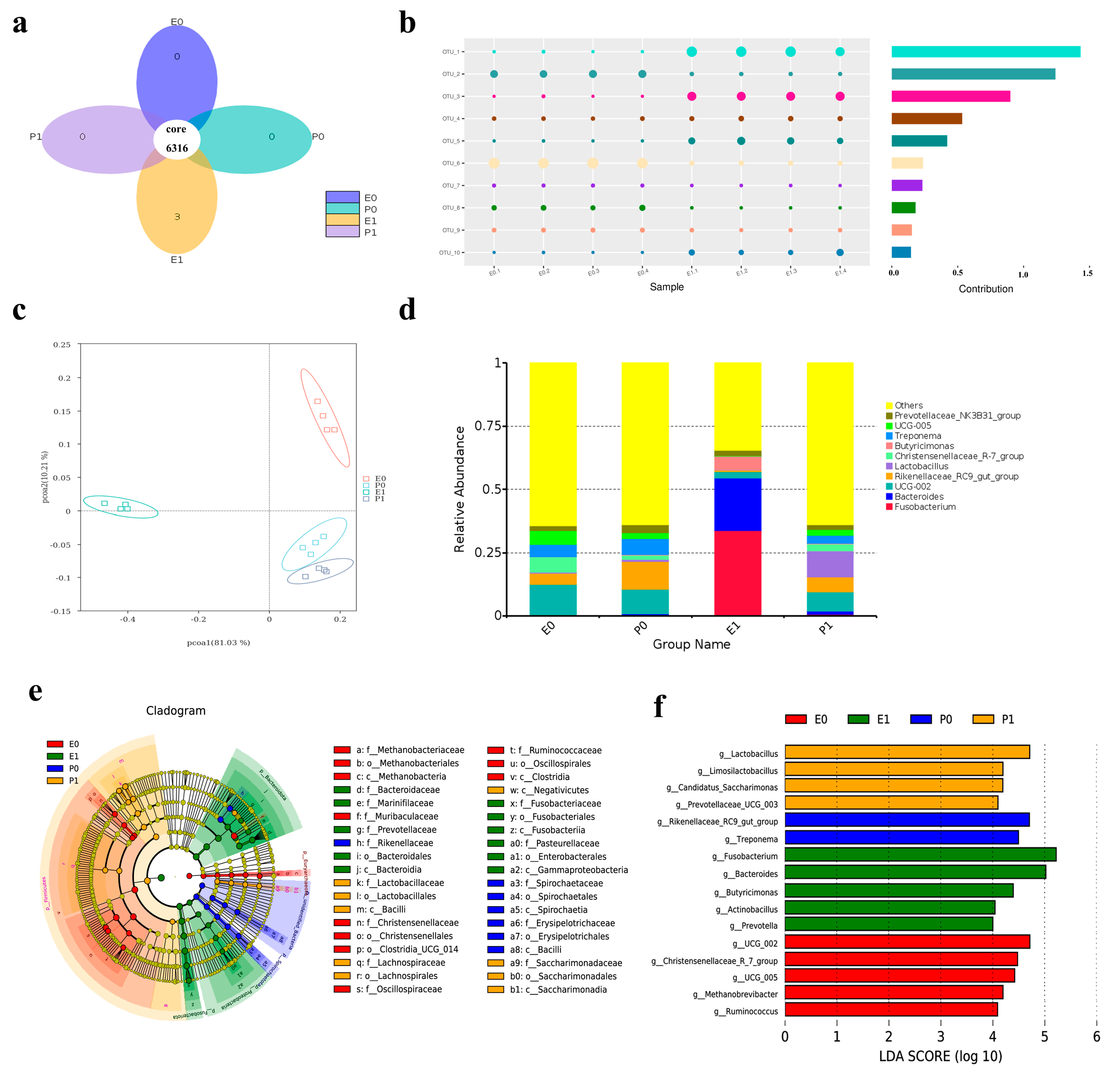
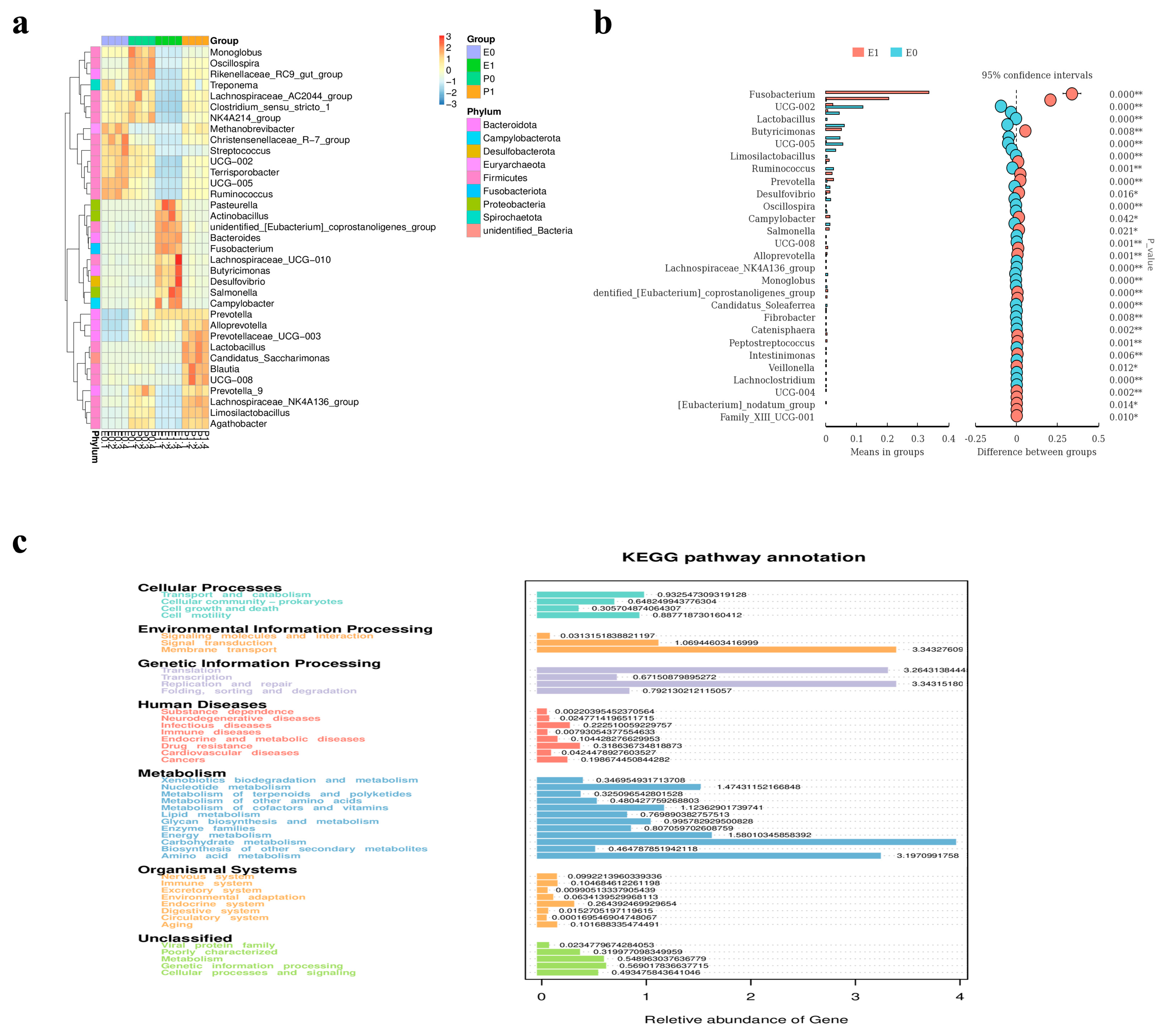
2.11. Analysis of Comparative Results of Intestinal Flora
2.12. Analysis of Transcriptome Comparison Results
3. Discussion
4. Materials and Methods
4.1. Strains and Preservation
4.2. Construction of Recombinant Lactobacillus plantarum
4.3. Western Blot
4.4. PRRSV Titer Determination
4.5. Cytotoxicity Test
4.6. Three Groups of Proteins Against PRRSV Effect Research
4.7. Quantification of Virions in Cells
4.8. Design and Grouping of Animal Experiments
4.9. Flow Cytometry
4.10. Pathological Anatomical Observation
4.11. Hematoxylin–Eosin Staining
4.12. Enzyme-Linked Immunosorbent Assay
4.13. Indirect Immunofluorescence
4.14. Quantification of Virus Particles in Various Tissues and Organs
4.15. Intestinal Microbiota Sequencing
4.16. Transcriptome Sequencing
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.; Li, J.; Zhang, Y.; Xue, C.; Cao, Y. Tandem 3′ UTR Patterns and Gene Expression Profiles of Marc-145 Cells During PRRSV Infection. Virol. Sin. 2018, 33, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Ladreyt, H.; Durand, B.; Dussart, P.; Chevalier, V. How Central Is the Domestic Pig in the Epidemiological Cycle of Japanese Encephalitis Virus? A Review of Scientific Evidence and Implications for Disease Control. Viruses 2019, 11, 949. [Google Scholar] [CrossRef]
- He, L.; Tai, W.; Li, J.; Chen, Y.; Gao, Y.; Li, J.; Sun, S.; Zhou, Y.; Du, L.; Zhao, G. Enhanced Ability of Oligomeric Nanobodies Targeting MERS Coronavirus Receptor-Binding Domain. Viruses 2019, 11, 166. [Google Scholar] [CrossRef]
- Luo, H.Z.; Jiang, H.; Huang, X.S.; Jia, A.Q. New Sesquiterpenoids from Plant-Associated Irpex lacteus. Front. Chem. 2022, 10, 905108. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shi, B.; Yang, D.; Zhang, K.; Li, J.; Wang, J.; Liu, H.; Zhao, Q.; Zhou, E.M.; Nan, Y. Porcine Reproductive and Respiratory Syndrome Virus Promotes SLA-DR-Mediated Antigen Presentation of Nonstructural Proteins to Evoke a Nonneutralizing Antibody Response In Vivo. J. Virol. 2020, 94, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Vuono, E.A.; Ramirez-Medina, E.; Azzinaro, P.; Berggren, K.A.; Rai, A.; Pruitt, S.; Silva, E.; Velazquez-Salinas, L.; Borca, M.V.; Gladue, D. SERTA Domain Containing Protein 1 (SERTAD1) Interacts with Classical Swine Fever Virus Structural Glycoprotein E2, Which Is Involved in Virus Virulence in Swine. Viruses 2020, 12, 421. [Google Scholar] [CrossRef]
- Sutherland, H.; Conley, M.J.; Emmott, E.; Streetley, J.; Goodfellow, I.G.; Bhella, D. The Cryo-EM Structure of Vesivirus 2117 Highlights Functional Variations in Entry Pathways for Viruses in Different Clades of the Vesivirus Genus. J. Virol. 2021, 95, e0028221. [Google Scholar] [CrossRef]
- Jin, Y.B.; Yang, W.T.; Shi, C.W.; Feng, B.; Huang, K.Y.; Zhao, G.X.; Li, Q.Y.; Xie, J.; Huang, H.B.; Jiang, Y.L.; et al. Immune responses induced by recombinant Lactobacillus plantarum expressing the spike protein derived from transmissible gastroenteritis virus in piglets. Appl. Microbiol. Biotechnol. 2018, 102, 8403–8417. [Google Scholar] [CrossRef]
- Seelbinder, B.; Chen, J.; Brunke, S.; Vazquez-Uribe, R.; Santhaman, R.; Meyer, A.C.; de Oliveira Lino, F.S.; Chan, K.F.; Loos, D.; Imamovic, L.; et al. Antibiotics create a shift from mutualism to competition in human gut communities with a longer-lasting impact on fungi than bacteria. Microbiome 2020, 8, 133. [Google Scholar] [CrossRef]
- Yang, L.; Li, W.; Ujiroghene, O.J.; Yang, Y.; Lu, J.; Zhang, S.; Pang, X.; Lv, J. Occurrence and Diversity of CRISPR Loci in Lactobacillus casei Group. Front. Microbiol. 2020, 11, 624. [Google Scholar] [CrossRef]
- Kang, S.; Lin, Z.; Xu, Y.; Park, M.; Ji, G.E.; Johnston, T.V.; Ku, S.; Park, M.S. A recombinant Bifidobacterium bifidum BGN4 strain expressing the streptococcal superoxide dismutase gene ameliorates inflammatory bowel disease. Microb. Cell Factories 2022, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.T.; Li, Q.Y.; Ata, E.B.; Jiang, Y.L.; Huang, H.B.; Shi, C.W.; Wang, J.Z.; Wang, G.; Kang, Y.H.; Liu, J.; et al. Immune response characterization of mice immunized with Lactobacillus plantarum expressing spike antigen of transmissible gastroenteritis virus. Appl. Microbiol. Biotechnol. 2018, 102, 8307–8318. [Google Scholar] [CrossRef]
- Selimović, A.; Miličević, D.; Selimović, A.; Žuljević, S.O.; Jašića, A.; Vranac, A. Properties of crackers with buckwheat sourdough. Acta Chim. Slovaca 2017, 10, 152–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Wang, C.; Zhang, C.; Wang, H.; Feng, Z.; Gu, Y.; Wang, Z. Design and characterization of small-diameter tissue-engineered blood vessels constructed by electrospun polyurethane-core and gelatin-shell coaxial fiber. Bioengineered 2021, 12, 5769–5788. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ma, B.; Wang, Z.; Chen, Y.; Tian, W.; Dong, Y. Royal Jelly Protected against Dextran-Sulfate-Sodium-Induced Colitis by Improving the Colonic Mucosal Barrier and Gut Microbiota. Nutrients 2022, 14, 2069. [Google Scholar] [CrossRef]
- Wang, S.; Geng, N.; Zhou, D.; Qu, Y.; Shi, M.; Xu, Y.; Liu, K.; Liu, Y.; Liu, J. Oral Immunization of Chickens with Recombinant Lactobacillus plantarum Vaccine Against Early ALV-J Infection. Front. Immunol. 2019, 10, 2299. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, Y.; Xia, Q.; Guan, Z.; Zhang, J.; Li, B.; Qiu, Y.; Liu, K.; Shao, D.; Ma, Z.; et al. Genetic characterization of porcine reproductive and respiratory syndrome virus from Eastern China during 2017–2022. Front. Microbiol. 2022, 13, 971817. [Google Scholar] [CrossRef]
- Berke, K.; Sun, P.; Ong, E.; Sanati, N.; Huffman, A.; Brunson, T.; Loney, F.; Ostrow, J.; Racz, R.; Zhao, B.; et al. VaximmutorDB: A Web-Based Vaccine Immune Factor Database and Its Application for Understanding Vaccine-Induced Immune Mechanisms. Front. Immunol. 2021, 12, 639491. [Google Scholar] [CrossRef]
- Jones, A.T.; Shen, X.; Walter, K.L.; LaBranche, C.C.; Wyatt, L.S.; Tomaras, G.D.; Montefiori, D.C.; Moss, B.; Barouch, D.H.; Clements, J.D.; et al. HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge. Nat. Commun. 2019, 10, 798. [Google Scholar] [CrossRef]
- Lv, J.; Jiang, Y.; Feng, Q.; Fan, Z.; Sun, Y.; Xu, P.; Hou, Y.; Zhang, X.; Fan, Y.; Xu, X.; et al. Porcine Circovirus Type 2 ORF5 Protein Induces Autophagy to Promote Viral Replication via the PERK-eIF2α-ATF4 and mTOR-ERK1/2-AMPK Signaling Pathways in PK-15 Cells. Front. Microbiol. 2020, 11, 320. [Google Scholar] [CrossRef]
- Wayah, S.B.; Philip, K. Pentocin MQ1: A Novel, Broad-Spectrum, Pore-Forming Bacteriocin from Lactobacillus pentosus CS2 With Quorum Sensing Regulatory Mechanism and Biopreservative Potential. Front. Microbiol. 2018, 9, 564. [Google Scholar] [CrossRef]
- Tanweer, F.A.; Rafii, M.Y.; Sijam, K.; Rahim, H.A.; Ahmed, F.; Ashkani, S.; Latif, M.A. Introgression of Blast Resistance Genes (Putative Pi-b and Pi-kh) into Elite Rice Cultivar MR219 through Marker-Assisted Selection. Front. Plant Sci. 2015, 6, 1002. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Yi, H.Y.; Ma, J.; Wei, Y.F.; Cai, M.K.; Li, Q.; Qin, C.X.; Chen, Y.J.; Han, X.L.; Zhong, R.T.; et al. Ginsenoside Rg1 Suppresses Type 2 PRRSV Infection via NF-κB Signaling Pathway In Vitro, and Provides Partial Protection against HP-PRRSV in Piglet. Viruses 2019, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Hao, X.; Hu, Y.; Du, R.; Lang, S.; Bian, L.; Gao, F.; Yang, C.; Cui, B.; Zhu, F.; et al. A neonatal mouse model of central nervous system infections caused by Coxsackievirus B5. Emerg. Microbes Infect. 2018, 7, 185. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, N.; Cheng, Q.; Deng, F.; Liu, M.; Zhu, A.; Min, Y.Q.; Zhu, D.; Huang, W.; Feng, X.; et al. IFP35 as a promising biomarker and therapeutic target for the syndromes induced by SARS-CoV-2 or influenza virus. Cell Rep. 2021, 37, 110126. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Monaco, M.H.; Wang, M.; Comstock, S.S.; Kuhlenschmidt, T.B.; Fahey, G.C.; Miller, M.J.; Kuhlenschmidt, M.S.; Donovan, S.M. Human milk oligosaccharides shorten rotavirus-induced diarrhea and modulate piglet mucosal immunity and colonic microbiota. ISME J. 2014, 8, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, X.; Dong, W.; Wang, X.; He, S.; Zhang, H.; Wang, X.; Wei, R.; Chen, Y.; Liu, X.; et al. TREM2 suppresses the proinflammatory response to facilitate PRRSV infection via PI3K/NF-κB signaling. PLoS Pathog. 2020, 16, e1008543. [Google Scholar] [CrossRef]
- Geng, G.; Xu, C.; Peng, N.; Li, Y.; Liu, J.; Wu, J.; Liang, J.; Zhu, Y.; Shi, L. PTBP1 is necessary for dendritic cells to regulate T-cell homeostasis and antitumour immunity. Immunology 2021, 163, 74–85. [Google Scholar] [CrossRef]
- Chen, J.; Kang, B.; Jiang, Q.; Han, M.; Zhao, Y.; Long, L.; Fu, C.; Yao, K. Alpha-Ketoglutarate in Low-Protein Diets for Growing Pigs: Effects on Cecal Microbial Communities and Parameters of Microbial Metabolism. Front. Microbiol. 2018, 9, 1057. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, H.; He, T.; Sun, Z.; Gifty, Z.B.; Hu, P.; Rao, Z.; Tang, Z. Effects of Dietary Protein Levels on Fecal Amino Acids Excretion and Apparent Digestibility, and Fecal and Ileal Microbial Amino Acids Composition in Weaned Piglets. Front. Nutr. 2021, 8, 738707. [Google Scholar] [CrossRef]
- Su, J.; Li, D.; Chen, Q.; Li, M.; Su, L.; Luo, T.; Liang, D.; Lai, G.; Shuai, O.; Jiao, C.; et al. Anti-breast Cancer Enhancement of a Polysaccharide from Spore of Ganoderma lucidum With Paclitaxel: Suppression on Tumor Metabolism with Gut Microbiota Reshaping. Front. Microbiol. 2018, 9, 3099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, R.; Xiang, C.; Jia, Q.; Wu, H.; Yang, H. Huangbai Liniment Accelerated Wound Healing by Activating Nrf2 Signaling in Diabetes. Oxidative Med. Cell. Longev. 2020, 2020, 4951820. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.; Zhang, X.; Luo, Y.; Ma, L.; Lu, J.; Liang, Q.; Xu, C.; Zhao, C.; Pan, C.Q. IP-10 Interferes with the Antiviral Response of Direct-Acting Antiviral Agents for Hepatitis C Virus Infection. Front. Public Health 2022, 10, 911551. [Google Scholar] [CrossRef]
- Wu, Q.; Han, Y.; Wu, X.; Wang, Y.; Su, Q.; Shen, Y.; Guan, K.; Michal, J.J.; Jiang, Z.; Liu, B.; et al. Integrated time-series transcriptomic and metabolomic analyses reveal different inflammatory and adaptive immune responses contributing to host resistance to PRRSV. Front. Immunol. 2022, 13, 960709. [Google Scholar] [CrossRef]
- Fulzele, S.; Sahay, B.; Yusufu, I.; Lee, T.J.; Sharma, A.; Kolhe, R.; Isales, C.M. COVID-19 Virulence in Aged Patients Might Be Impacted by the Host Cellular MicroRNAs Abundance/Profile. Aging Dis. 2020, 11, 509–522. [Google Scholar] [CrossRef]
- Sung, P.Y.; Wang, Y.T.; Yu, Y.W.; Chung, R.H. An efficient gene-gene interaction test for genome-wide association studies in trio families. Bioinformatics 2016, 32, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, A.; Gao, Y.; Xie, Y.; Liu, Z.; Sun, M.; Mao, H.; Wang, X. Graphene oxide exacerbates dextran sodium sulfate-induced colitis via ROS/AMPK/p53 signaling to mediate apoptosis. J. Nanobiotechnol. 2021, 19, 85. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Wang, K.; Ni, Q.; Li, H.; Su, Z.; Xu, Y. Is Immune Suppression Involved in the Ischemic Stroke? A Study Based on Computational Biology. Front. Aging Neurosci. 2022, 14, 830494. [Google Scholar] [CrossRef]
- Prakash, O.; Singh, D.D.; Mishra, G.; Prakash, S.; Singh, A.; Gupta, S.; Singh, J.; Khan, D.N.; Jain, P.; Vishal, A.; et al. Observation on dengue cases from a virus diagnostic laboratory of a tertiary care hospital in North India. Indian J. Med. Res. 2015, 142, S7–S11. [Google Scholar] [CrossRef]
- Ai, J.W.; Zhou, X.; Xu, T.; Yang, M.; Chen, Y.; He, G.Q.; Pan, N.; Cai, Y.; Li, Y.; Wang, X.; et al. CRISPR-based rapid and ultra-sensitive diagnostic test for Mycobacterium tuberculosis. Emerg. Microbes Infect. 2019, 8, 1361–1369. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hsu, W.T.; Chao, Y.C.; Chang, H.W. Display of Porcine Epidemic Diarrhea Virus Spike Protein on Baculovirus to Improve Immunogenicity and Protective Efficacy. Viruses 2018, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, C.W.; Zhang, Y.T.; Huang, H.B.; Jiang, Y.L.; Wang, J.Z.; Cao, X.; Wang, N.; Zeng, Y.; Yang, G.L.; et al. Riboflavin Attenuates Influenza Virus Through Cytokine-Mediated Effects on the Diversity of the Gut Microbiota in MAIT Cell Deficiency Mice. Front. Microbiol. 2022, 13, 916580. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Chen, C.; Hu, Y.; Gong, Q.; Gan, L.; Xu, Y. Identify Function of WASL in Prognosis of Cervical Cancer Based on Omics Data. Front. Cell Dev. Biol. 2021, 9, 670890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, K.; Wang, Y.; Ma, J.; Song, B. Analysis of the Function of LncRNA-MSTRG.16919.1 in BHV-1-Infected Bovine Kidney Subculture Cells by Transcriptome Sequencing. Viruses 2022, 14, 2104. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Li, Y.; Xu, T.; Zhao, H.; Zeng, M.; Liu, Z. Dissecting of the AI-2/LuxS Mediated Growth Characteristics and Bacteriostatic Ability of Lactiplantibacillus plantarum SS-128 by Integration of Transcriptomics and Metabolomics. Foods 2022, 11, 638. [Google Scholar] [CrossRef]
- Huang, R.; Zeng, Z.; Yan, P.; Yin, H.; Zhu, X.; Hu, P.; Zhuang, J.; Li, J.; Li, S.; Song, D.; et al. Targeting Lymphotoxin Beta and Paired Box 5: A potential therapeutic strategy for soft tissue sarcoma metastasis. Cancer Cell Int. 2021, 21, 3. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, T.; Fan, T.; Wang, Y.; Gao, K.; Zhao, J.; Wang, R.; Chen, X.; Xing, J.; Qiu, J.; Zou, B.; et al. Lactobacillus plantae Expressing Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Single-Chain Antibody Can Inhibit PRRSV Replication and Change the Intestinal Flora Structure of Piglets. Int. J. Mol. Sci. 2025, 26, 2257. https://doi.org/10.3390/ijms26052257
Niu T, Fan T, Wang Y, Gao K, Zhao J, Wang R, Chen X, Xing J, Qiu J, Zou B, et al. Lactobacillus plantae Expressing Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Single-Chain Antibody Can Inhibit PRRSV Replication and Change the Intestinal Flora Structure of Piglets. International Journal of Molecular Sciences. 2025; 26(5):2257. https://doi.org/10.3390/ijms26052257
Chicago/Turabian StyleNiu, Tianming, Tianqi Fan, Yingjie Wang, Kuipeng Gao, Jinhui Zhao, Ruyu Wang, Xiaolei Chen, Junhong Xing, Jingjing Qiu, Boshi Zou, and et al. 2025. "Lactobacillus plantae Expressing Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Single-Chain Antibody Can Inhibit PRRSV Replication and Change the Intestinal Flora Structure of Piglets" International Journal of Molecular Sciences 26, no. 5: 2257. https://doi.org/10.3390/ijms26052257
APA StyleNiu, T., Fan, T., Wang, Y., Gao, K., Zhao, J., Wang, R., Chen, X., Xing, J., Qiu, J., Zou, B., Fan, S., Zhang, S., Wu, Q., Yang, G., Wang, N., Zeng, Y., Cao, X., Jiang, Y., Wang, J., ... Wang, C. (2025). Lactobacillus plantae Expressing Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Single-Chain Antibody Can Inhibit PRRSV Replication and Change the Intestinal Flora Structure of Piglets. International Journal of Molecular Sciences, 26(5), 2257. https://doi.org/10.3390/ijms26052257





