Immunotherapy for Elderly Patients with Advanced Non-Small Cell Lung Cancer: Challenges and Perspectives
Abstract
1. Introduction
2. Materials and Methods
3. Older Patients
4. The Particularity of the Elderly Subject
5. Immunosenescence
6. Efficacy of Immunotherapy in Older Patients
6.1. Results of Phase 3 Studies. Subgroup Analysis in Those > 65 Years Old
6.2. Results of Phase 3 Studies. Pooled Analysis in Those > 75 Years Old
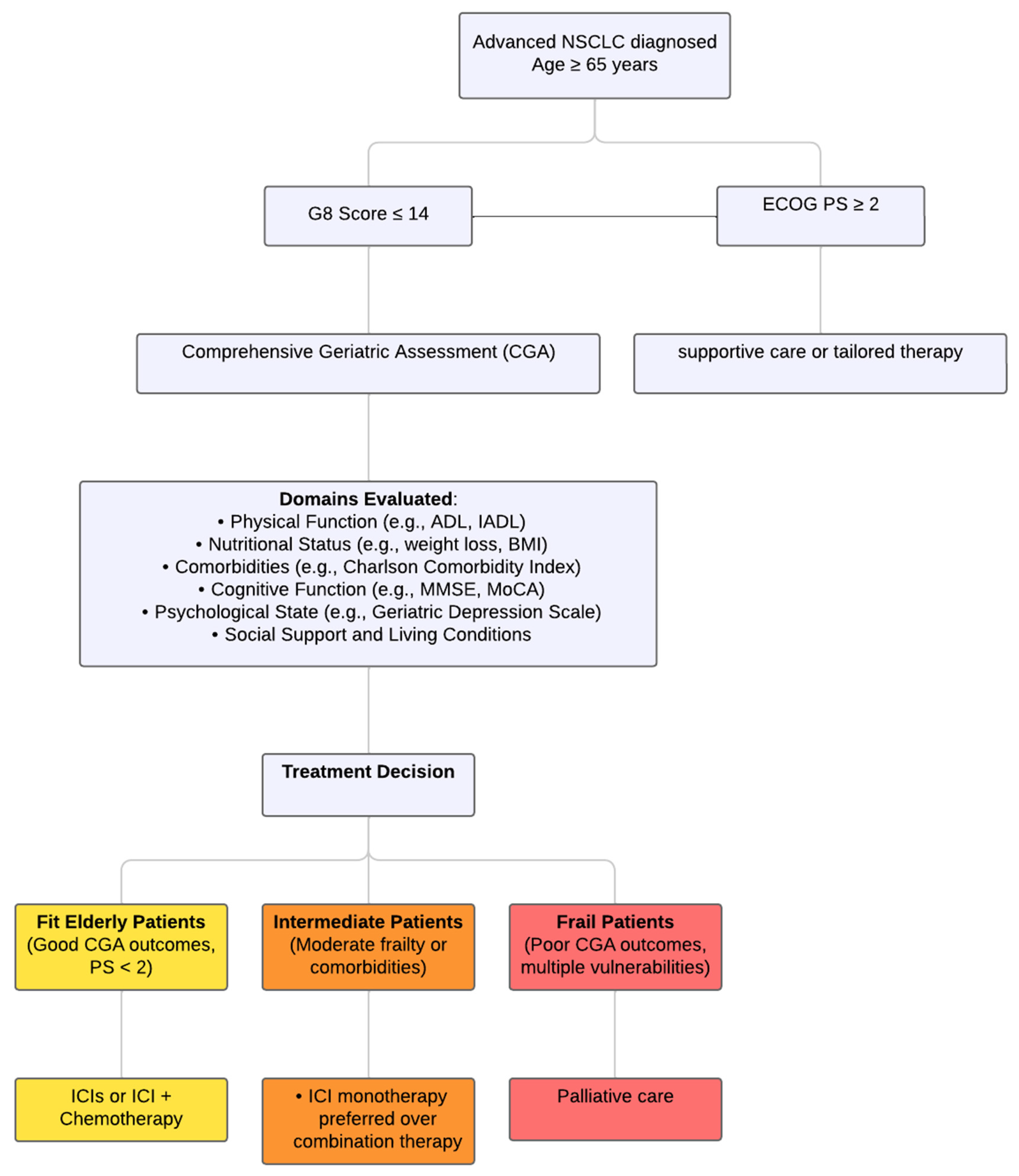
7. Current Decision-Making Tools
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICIs | Immune Checkpoint Inhibitors |
| NSCLC | Non-Small Cell Lung Cancer |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression-Free Survival |
| OS | Overall Survival |
| PS | Performance Status |
| G8 | Geriatric Assessment Tool |
| ECOG | Eastern Cooperative Oncology Group |
| irAEs | Immune-Related Adverse Events |
| mGPS | Modified Glasgow Prognostic Score |
| SASP | Senescence-Associated Secretory Phenotype |
| CGA | Comprehensive Geriatric Assessment |
| ADL | Activities of Daily Living |
| IADL | Instrumental Activities of Daily Living |
| BMI | Body Mass Index |
| MMSE | Mini-Mental State Examination for Cognitive Function |
| MoCA | Montreal Cognitive Assessment for Cognitive Impairment |
References
- Li, C.; Lei, S.; Ding, L.; Xu, Y.; Wu, X.; Wang, H.; Zhang, Z.; Gao, T.; Zhang, Y.; Li, L. Global burden and trends of lung cancer incidence and mortality. Chin. Med. J. 2023, 136, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Zhang, Y.; Etxeberria, J.; Arnold, M.; Cai, X.; Hao, Y.; Zou, H. Projections of Lung Cancer Incidence by 2035 in 40 Countries Worldwide: Population-Based Study. JMIR Public Health Surveill. 2023, 9, e43651. [Google Scholar] [CrossRef] [PubMed]
- Cranford, H.M.; Koru-Sengul, T.; Lopes, G.; Pinheiro, P.S. Lung Cancer Incidence by Detailed Race–Ethnicity. Cancers 2023, 15, 2164. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.T.; Janes, S.M.; Hackshaw, A.; Dur, C.A.C.; Buist, D.S.; Hubbell, E.A. Overall and non-lung cancer incidence in the national lung screening trial (NLST) as indicators of potential for multi-cancer screening. J. Clin. Oncol. 2023, 41, 10633. [Google Scholar] [CrossRef]
- Guarga, L.; Ameijide, A.; Marcos-Gragera, R.; Carulla, M.; Delgadillo, J.; Borràs, J.M.; Galceran, J. Trends in lung cancer incidence by age, sex and histology from 2012 to 2025 in Catalonia (Spain). Sci. Rep. 2021, 11, 23274. [Google Scholar] [CrossRef]
- Zenke, Y.; Hakozaki, T.; Nakahara, Y.; Horinouchi, H.; Ohe, Y.; The Lung Cancer Study Group of the Japan Clinical Oncology Group (JCOG). Medical management of older patients with lung cancer. Jpn. J. Clin. Oncol. 2022, 52, 1082–1088. [Google Scholar] [CrossRef]
- Kharagezov, D.A.; Lazutin, Y.N.; Pyltsin, S.P.; Milakin, A.G.; Stateshny, O.N.; Leyman, I.A.; Mirzoyan, E.A. Lung cancer in older patients. Innov. Med. Kuban 2021, 22, 65–71. [Google Scholar] [CrossRef]
- Kosacka, M.; Jankowska, R. The epidemiology of lung cancer. Pneumonol. Alergol. Pol. 2007, 75, 76–80. [Google Scholar] [CrossRef]
- Alberg, A.J.; Gupta, R.D.; Akonde, M. Cancer: Epidemiology of lung cancer. In Encyclopedia of Human Nutrition; Elsevier: Amsterdam, The Netherlands, 2023; pp. 154–161. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128218488001876 (accessed on 17 March 2024).
- Repana, D.; Spicer, J. Epidemiology of Lung Cancer. In Pathology and Epidemiology of Cancer; Loda, M., Mucci, L.A., Mittelstadt, M.L., Van Hemelrijck, M., Cotter, M.B., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 347–366. Available online: http://link.springer.com/10.1007/978-3-319-35153-7_19 (accessed on 17 March 2024).
- October 2008—Volume 3—Issue Suppl 2: Annals of Thoracic Medicine. 2008. Available online: https://journals.lww.com/aotm/toc/2008/03002 (accessed on 17 March 2024).
- Voruganti, T.; Soulos, P.R.; Mamtani, R.; Presley, C.J.; Gross, C.P. Association Between Age and Survival Trends in Advanced Non–Small Cell Lung Cancer After Adoption of Immunotherapy. JAMA Oncol. 2023, 9, 334–341. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Lyu, J.; Shi, F.; Kong, Y.; Sheng, C.; Wang, S.; Wang, Q. An aging-related signature predicts favorable outcome and immunogenicity in lung adenocarcinoma. Cancer Sci. 2022, 113, 891–903. [Google Scholar] [CrossRef]
- Isik, D.; Alan, Ö.; Akdağ, G.; Yildirim, S.; Kınıkoğlu, O.; Altintas, Y.E.; Turkoglu, E.; Surmeli, H.; Basoglu, T.; Sever, O.N. Evaluating the Effectiveness of Nivolumab in Metastatic Lung Cancer Among Patients Aged 65 and Older. J. Clin. Med. 2024, 13, 6263. Available online: https://www.preprints.org/manuscript/202409.0812/v1 (accessed on 29 January 2025). [CrossRef] [PubMed]
- Orillard, E.; Adhikari, A.; Malouf, R.S.; Calais, F.; Marchal, C.; Westeel, V. Immune checkpoint inhibitors plus platinum-based chemotherapy compared to platinum-based chemotherapy with or without bevacizumab for first-line treatment of older people with advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2024, 2024, CD015495. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, H.; Pan, X.; Hong, J.; Wong, J.W.H.; Chen, H.; Zhang, Y. Abstract 6102: Single-cell RNA sequencing of lung adenocarcinoma patients reveals age-dependent tumour microenvironment alterations that facilitate response to immunotherapy. Cancer Res. 2022, 82 (Suppl. 12), 6102. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Wang, D.; Xuan, Y.; Yu, Y.; Qi, Y.; Ge, M.; Luo, N.; Zhu, X.; Li, M. Better tumor immune microenvironment in patients with older lung adenocarcinoma. J. Clin. Oncol. 2022, 40, e20544. [Google Scholar] [CrossRef]
- Gridelli, C.; Peters, S.; Velcheti, V.; Attili, I.; de Marinis, F. Immunotherapy in the first-line treatment of elderly patients with advanced non-small-cell lung cancer: Results of an International Experts Panel Meeting by the Italian Association of Thoracic Oncology (AIOT). ESMO Open 2023, 8, 101192. [Google Scholar] [CrossRef]
- Essai Clinique Cancer Appareil Respiratoire/Poumon, Type Non à Petites Cellules Clos Aux Inclusions. 2025. Available online: https://www.e-cancer.fr/Professionnels-de-sante/Le-registre-des-essais-cliniques/Le-registre-des-essais-cliniques/Etudes-cliniques/Etude-ELDERLY-etude-de-phase-3-randomisee-evaluant-l-efficacite-et-la-tolerance-de-l-atezolizumab-chez-des-patients-ages-ayant-un-cancer-du-poumon-non-a-petites-cellules-CPNPC-de-stade-avance-et-recevant-une-chimiotherapie-a-base-de-carboplatine-en (accessed on 27 April 2024).
- Hossain, C.M.; Maitra, S.; Lyle, N.; Gera, M.; Paul, S.; Dutta, D. Immunotherapy as Novel Treatment of Lung Cancer: A Systematic Review. Asian J. Pharm. Clin. Res. 2022, 15, 9–17. [Google Scholar] [CrossRef]
- Wahyudin, H.U.C.; Afriani, A.; Anggrainy, F.; Ermayanti, S. Immunotherapy in Lung Cancer: A Narrative Literature Review. Biosci. Med. J. Biomed. Transl. Res. 2023, 7, 3024–3030. [Google Scholar] [CrossRef]
- Wael, M.; Hosam, M.; Youssef, A.; Ellackany, R.; Alzahabi, A. Immunotherapy, Immunobiomarkers and Gene Analysis Role in the Improvement of Lung Cancer Treatment. Radiother. Clin. Oncol. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Ertorun, I.; Huseynli, A.; Ertekın, S.N.; Çiftçi, G.A. The role of immunotherapy in lung cancer: Actual scenery. Eur. J. Life Sci. 2023, 2, 45–51. [Google Scholar] [CrossRef]
- Hsieh, K.; Dickstein, D.R.; Runnels, J.; Lehrer, E.J.; Rosenzweig, K.; Hirsch, F.R.; Samstein, R.M. Radiotherapy and Immunotherapy in Lung Cancer. Biomedicines 2023, 11, 1642. [Google Scholar] [CrossRef]
- Mclean, L.; Lim, A.; Pizzolla, A.; Solomon, B.; Rischin, D. 1259 Real world experience of immunotherapy in an elderly trial-ineligible cohort of patients with advanced cutaneous squamous cell carcinoma. J. ImmunoTher. Cancer 2022, 10, A1308. [Google Scholar]
- Araujo Vargas, T.P.; Al-Humiqani, A.; Giffoni De Mello Morais Mata, D.; Menjak, I.B. Immunotherapy for older patients with cancer. Curr. Opin. Support. Palliat. Care 2023, 17, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.F.; Tejedor, J.R.; Fernández, A.F.; Fraga, M.F. Aging and cancer epigenetics: Where do the paths fork? Aging Cell 2022, 21, e13709. [Google Scholar] [CrossRef] [PubMed]
- Chatsirisupachai, K.; Lagger, C.; de Magalhães, J.P. Age-associated differences in the cancer molecular landscape. Trends Cancer 2022, 8, 962–971. [Google Scholar] [CrossRef]
- Minteer, C.J.; Thrush, K.; Gonzalez, J.; Niimi, P.; Rozenblit, M.; Rozowsky, J.; Liu, J.; Frank, M.; McCabe, T.; Sehgal, R.; et al. More than bad luck: Cancer and aging are linked to replication-driven changes to the epigenome. Sci. Adv. 2023, 9, eadf4163. [Google Scholar] [CrossRef]
- Clough-Gorr, K.M.; Silliman, R.A. Epidemiology of Cancer and Aging. In The Epidemiology of Aging; Newman, A.B., Cauley, J.A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 377–399. Available online: http://link.springer.com/10.1007/978-94-007-5061-6_22 (accessed on 17 March 2024).
- DeGregori, J. Aging and Cancer: A new forum for research that spans disciplines and seeks new answers. Aging Cancer 2020, 1, 3–4. [Google Scholar] [CrossRef]
- Sood, A. Connection Between Ageing and Cancer. 2014. Available online: https://www.semanticscholar.org/paper/CONNECTION-BETWEEN-AGEING-AND-CANCER-Sood/363e8e0f892fd492ace3fc6e7005c201667b0234 (accessed on 17 March 2024).
- Chauhan, M.; Dulloo, S.; Jaitly, J.; Arif, Z.; Thomson, J.; Sharma, S.; Anees, I.; Aleem, B.; Vijay, A. Indicators of frailty in newly diagnosed lung cancer patients: A retrospective review. J. Clin. Oncol. 2022, 40, e24017. [Google Scholar] [CrossRef]
- Chupryna, O.G. Dynamics of the Concept Elderly Person American Institutional Discourse. Foreign Lang. High. Educ. 2022, 2, 31–40. [Google Scholar]
- Dzhuhan, V.; Dzhuhan, R. Definition of the Concept of «Elderly People» as Categories of Social Work. Sci. Bull. Uzhhorod. Univ. Ser. Pedagog. Soc. Work 2022, 1, 77–80. [Google Scholar] [CrossRef]
- Peretz, L.; Rappoport, N. Deviation of Physiological from Chronological Age Is Associated with Health. In Studies in Health Technology and Informatics; Séroussi, B., Weber, P., Dhombres, F., Grouin, C., Liebe, J.D., Pelayo, S., Pinna, A., Wang, Y., Zhang, G., Braunstein, M.L., et al., Eds.; IOS Press: Amsterdam, The Netherlands, 2022; Available online: https://ebooks.iospress.nl/doi/10.3233/SHTI220442 (accessed on 17 March 2024).
- Galliera, G.E.O.O.; Rollandi, G.A.; Chiesa, A.; Sacchi, N.; Castagnetta, M.; Puntoni, M.; Amaro, A.; Martino, G.I.O.P.S.; Banelli, B.; Pfeffer, U. Biological Age versus Chronological Age in the Prevention of Age Associated Diseases. OBM Geriatr. 2019, 3, 051. [Google Scholar]
- Dharmarajan, T.S.; Dharmarajan, T.S.; Dharmarajan, T.S.; Dharmarajan, T.S. The Physiology of Aging. In Geriatric Gastroenterology; Pitchumoni, C.S., Dharmarajan, T.S., Eds.; Springer: New York, NY, USA, 2012; pp. 17–31. Available online: https://link.springer.com/10.1007/978-1-4419-1623-5_4 (accessed on 17 March 2024).
- Bond, S.M. Physiological Aging in Older Adults with Cancer: Implications for Treatment Decision Making and Toxicity Management. J. Gerontol. Nurs. 2010, 36, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Park, D.I.; Park, M.R.; Jung, S.S.; Kim, J.O.; Kim, S.Y.; Lee, J.E. Clinical Characteristics of Patients Older than 76 with Lung Cancer. Korean J. Med. 2012, 82, 562–568. [Google Scholar] [CrossRef]
- Gridelli, C.; Langer, C.; Maione, P.; Rossi, A.; Schild, S.E. Lung Cancer in the Elderly. J. Clin. Oncol. 2007, 25, 1898–1907. [Google Scholar] [CrossRef] [PubMed]
- Montella, M.; Gridelli, C.; Crispo, A.; Scognamiglio, F.; Ruffolo, P.; Gatani, T.; Boccia, V.; Maione, P.; Fabbrocini, G. Has lung cancer in the elderly different characteristics at presentation? Oncol. Rep. 2002, 9, 1093–1096. [Google Scholar] [CrossRef]
- Rossi, A.; Cova, D.; Leo, S.; Repetto, L. Impact of the Physiological Effects of Aging on the Pharmacokinetics and Pharmacodynamics of Systemic Lung Cancer Treatment. In Management of Lung Cancer in Older People; Gridelli, C., Audisio, R.A., Eds.; Springer: London, UK, 2013; pp. 65–87. Available online: https://link.springer.com/10.1007/978-0-85729-793-8_5 (accessed on 17 March 2024).
- Casaluce, F.; Sgambato, A.; Maione, P.; Spagnuolo, A.; Gridelli, C. Lung cancer, elderly and immune checkpoint inhibitors. J. Thorac. Dis. 2018, 10 (Suppl. 13), S1474–S1481. [Google Scholar] [CrossRef]
- Sarrió, R.G.; Torregrosa, M.D.; López, P.; Gómez-Codina, J.; Rosell, R. Smoking habits in elderly lung cancer patients: Still no changes in epidemiology? A single-center experience. Clin. Transl. Oncol. 2010, 12, 686–691. [Google Scholar] [CrossRef]
- Dagnault, A.; Archambault, J. Lung Cancer in Elderly. In Topics in Cancer Survivorship; Mohan, R., Ed.; InTech: Rijeka, Croatia, 2012; Available online: http://www.intechopen.com/books/topics-in-cancer-survivorship/approach-to-lung-cancer-in-the-elderly-population (accessed on 17 March 2024).
- Wu, G.; Gu, X.; Yuan, D.; Yao, Y.; Yang, W.; Lv, T.; Song, Y. Diagnosis status and pathological diagnosis derived treatment of elderly lung cancer patients over 75 years old. Transl. Cancer Res. 2019, 8, 87–95. [Google Scholar] [CrossRef]
- Weiss, J.; Langer, C. NSCLC in the Elderly—The Legacy of Therapeutic Neglect. Curr. Treat. Options Oncol. 2009, 10, 180–194. [Google Scholar] [CrossRef]
- Fasano, M.; Cantile, F.; Morgillo, F.; Ciardiello, F. Basic Science of Lung Cancer in Older Patients. In Management of Lung Cancer in Older People; Gridelli, C., Audisio, R.A., Eds.; Springer: London, UK, 2013; pp. 3–12. Available online: https://link.springer.com/10.1007/978-0-85729-793-8_1 (accessed on 17 March 2024).
- Gettinger, S.; Tanoue, L. Lung Cancer in Older Patients. In Aging and Lung Disease; Pisani, M., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 89–109. Available online: http://link.springer.com/10.1007/978-1-60761-727-3_5 (accessed on 17 March 2024).
- Naltet, C.; Besse, B. Immune checkpoint inhibitors in elderly patients treated for a lung cancer: A narrative review. Transl. Lung Cancer Res. 2021, 10, 3014–3028. [Google Scholar] [CrossRef]
- Castelo-Branco, C.; Soveral, I. The immune system and aging: A review. Gynecol. Endocrinol. 2013, 30, 16–22. [Google Scholar] [CrossRef]
- Grubeck-Loebenstein, B.; Della Bella, S.; Iorio, A.M.; Michel, J.-P.; Pawelec, G.; Solana, R. Immunosenescence and vaccine failure in the elderly. Aging Clin. Exp. Res. 2009, 21, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-A.; Flores, R.R.; Jang, I.H.; Saathoff, A.; Robbins, P.D. Immune Senescence, Immunosenescence and Aging. Front. Aging 2022, 3, 900028. [Google Scholar] [CrossRef] [PubMed]
- Ongrádi, J.; Stercz, B.; Kövesdi, V.; Vértes, L. Immunosenescence and vaccination of the elderly I. Age-related immune impairment. Acta Microbiol. Immunol. Hung. 2009, 56, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Ongrádi, J.; Kövesdi, V. Factors that may impact on immunosenescence: An appraisal. Immun. Ageing 2010, 7, 7. [Google Scholar] [CrossRef]
- Hong, H.; Wang, Q.; Li, J.; Liu, H.; Meng, X.; Zhang, H. Aging, Cancer and Immunity. J. Cancer 2019, 10, 3021–3027. [Google Scholar] [CrossRef]
- Puca, A.A.; Ciaglia, E. Editorial: Immunosenescence in the cancer microenvironment. Front. Immunol. 2023, 14, 1161110. [Google Scholar] [CrossRef]
- Naigeon, M.; Dugage, M.R.; Danlos, F.-X.; De Oliveira, C.; Boselli, L.; Jouniaux, J.-M.; Griscelli, F.; Marabelle, A.; Cassard, L.; Roman, G.; et al. 20P Human virome epitope-level antiviral antibody profiling identified the cytomegalovirus (CMV) as the main driver of senescent immune phenotype (SIP) in patients with advanced non-small cell lung cancer (aNSCLC). Immuno-Oncol. Technol. 2022, 16, 100125. [Google Scholar] [CrossRef]
- Tanaka, T.; Yoshida, T.; Masuda, K.; Takeyasu, Y.; Shinno, Y.; Matsumoto, Y.; Okuma, Y.; Goto, Y.; Horinouchi, H.; Yamamoto, N.; et al. Prognostic role of modified Glasgow Prognostic score in elderly non-small cell lung cancer patients treated with anti-PD-1 antibodies. Respir. Investig. 2023, 61, 74–81. [Google Scholar] [CrossRef]
- Yao, J.; Lin, X.; Zhang, X.; Xie, M.; Ma, X.; Bao, X.; Song, J.; Liang, Y.; Wang, Q.; Xue, X. Predictive biomarkers for immune checkpoint inhibitors therapy in lung cancer. Hum. Vaccines Immunother. 2024, 20, 2406063. [Google Scholar] [CrossRef]
- Tostes, K.; Siqueira, A.P.; Reis, R.M.; Leal, L.F.; Arantes, L.M.R.B. Biomarkers for Immune Checkpoint Inhibitor Response in NSCLC: Current Developments and Applicability. Int. J. Mol. Sci. 2023, 24, 11887. [Google Scholar] [CrossRef]
- Spagnolo, C.C.; Pepe, F.; Ciappina, G.; Nucera, F.; Ruggeri, P.; Squeri, A.; Speranza, D.; Silvestris, N.; Malapelle, U.; Santarpia, M. Circulating biomarkers as predictors of response to immune checkpoint inhibitors in NSCLC: Are we on the right path? Crit. Rev. Oncol. Hematol. 2024, 197, 104332. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.; Ramos, C.; Senechal, V.; Ruez, R.; Delebecq, S. Integrative multimodality spatial biomarker analysis for tailoring checkpoint inhibitor therapies in non-small cell lung cancer. J. Clin. Oncol. 2024, 42, e14664. [Google Scholar] [CrossRef]
- Jain, K.; Mehra, D.; Ganguly, N.K.; Rana, R.; Ganguly, S.; Aggarwal, S. Comprehensive overview of biomarkers to predict response to immune checkpoint therapy in lung cancer. Curr. Med. Res. Pract. 2023, 13, 232–242. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet Lond. Engl. 2016, 387, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab Versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): A multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol. 2017, 18, 1600–1609. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Gomes, F.; Wong, M.; Battisti, N.M.L.; Kordbacheh, T.; Kiderlen, M.; Greystoke, A.; Luciani, A. Immunotherapy in Older Patients with Non-Small Cell Lung Cancer: Young International Society of Geriatric Oncology position paper. Br. J. Cancer 2020, 123, 874–884. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Spigel, D.R.; Vokes, E.E.; Holgado, E.; Ready, N.; Steins, M.; Poddubskaya, E.; Borghaei, H.; Felip, E.; Paz-Ares, L.; et al. Nivolumab Versus Docetaxel in Previously Treated Patients with Advanced Non–Small-Cell Lung Cancer: Two-Year Outcomes from Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J. Clin. Oncol. 2017, 35, 3924–3933. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kim, G.; Maher, V.E.; Beaver, J.A.; Pai-Scherf, L.H.; Balasubramaniam, S.; Theoret, M.R.; Blumenthal, G.M.; Pazdur, R. FDA subset analysis of the safety of nivolumab in elderly patients with advanced cancers. J. Clin. Oncol. 2016, 34, 10010. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.-E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Grossi, F.; Crinò, L.; Misino, A.; Bidoli, P.; Delmonte, A.; Gelsomino, F.; Proto, C.; Mancini, M.; Landi, L.; Turci, D.; et al. Efficacy and safety of nivolumab in elderly patients (pts) with advanced squamous non small cell lung cancer (Sq-NSCLC) participating in the expanded access programme (EAP) in Italy. Ann. Oncol. 2016, 27, vi369. [Google Scholar] [CrossRef][Green Version]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Popat, S.; Ardizzoni, A.; Ciuleanu, T.; Dols, M.C.; Laktionov, K.; Szilasi, M.; Califano, R.; Costa, E.C.; Griffiths, R.; Paz-Ares, L.; et al. Nivolumab in previously treated patients with metastatic squamous NSCLC: Results of a European single-arm, phase 2 trial (CheckMate 171) including patients aged ≥70 years and with poor performance status. Ann. Oncol. 2017, 28, v463. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet Lond. Engl. 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Peters, S.; Gettinger, S.; Johnson, M.L.; Jänne, P.A.; Garassino, M.C.; Christoph, D.; Toh, C.K.; Rizvi, N.A.; Chaft, J.E.; Costa, E.C.; et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1–Selected Advanced Non–Small-Cell Lung Cancer (BIRCH). J. Clin. Oncol. 2017, 35, 2781–2789. [Google Scholar] [CrossRef]
- Nosaki, K.; Saka, H.; Hosomi, Y.; Baas, P.; De Castro, G.; Reck, M.; Wu, Y.; Brahmer, J.R.; Felip, E.; Sawada, T.; et al. Safety and efficacy of pembrolizumab monotherapy in elderly patients with PD-L1–positive advanced non–small-cell lung cancer: Pooled analysis from the KEYNOTE-010, KEYNOTE-024, and KEYNOTE-042 studies. Lung Cancer 2019, 135, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Korthagen, I.; Larsen, C.F.; Nielsen, R.Ø. Non-binding Decision-Making. In European E-Democracy in Practice; Hennen, L., Van Keulen, I., Korthagen, I., Aichholzer, G., Lindner, R., Nielsen, R.Ø., Eds.; Studies in Digital Politics and Governance; Springer International Publishing: Cham, Switzerland, 2020; pp. 237–271. Available online: http://link.springer.com/10.1007/978-3-030-27184-8_10 (accessed on 17 March 2024).
- Belhouari, M.; Khalfi, S.; Khalfi, C.; Benchakroune, N.; Bourhafour, M.; Chekrine, T.; Bouchbika, Z.; Tawfik, N.; Jouhadi, H.; Alami, Z.; et al. Complete remission with Immunotherapy in second-Line in metastatic lung cancer without targetable oncogenic addiction: About a case and revue of the literature. World J. Adv. Res. Rev. 2023, 17, 598–601. [Google Scholar] [CrossRef]
- Sela, I.; Christopoulos, P.; Puzanov, I.; Bar, J.; Zer, A.; Moskovitz, M.; Reinmuth, N.; Lotem, M.; Katzenelson, R.; Agbarya, A.; et al. A decision-making tool for first-line treatment in advanced non–small-cell lung cancer based on plasma proteome biomarkers. J. Clin. Oncol. 2023, 41, 9122. [Google Scholar] [CrossRef]
- Sehgal, K.; Gill, R.R.; Widick, P.; Bindal, P.; McDonald, D.C.; Shea, M.; Rangachari, D.; Costa, D.B. Association of Performance Status with Survival in Patients with Advanced Non–Small Cell Lung Cancer Treated with Pembrolizumab Monotherapy. JAMA Netw. Open 2021, 4, e2037120. [Google Scholar] [CrossRef] [PubMed]
- Meyers, D.E.; Pasternak, M.; Dolter, S.; Grosjean, H.A.; Lim, C.A.; Stukalin, I.; Goutam, S.; Navani, V.; Heng, D.Y.; Cheung, W.Y.; et al. Impact of Performance Status on Survival Outcomes and Health Care Utilization in Patients with Advanced NSCLC Treated with Immune Checkpoint Inhibitors. JTO Clin. Res. Rep. 2023, 4, 100482. [Google Scholar] [CrossRef]
- Hickam, D.H. Decision Aids: Evolving from Novelties to Effective Communication Tools. Med. Decis. Mak. 2010, 30, 699–700. [Google Scholar] [CrossRef]
- Cortellini, A.; Cannita, K.; Tiseo, M.; Cortinovis, D.L.; Aerts, J.G.; Baldessari, C.; Giusti, R.; Ferrara, M.G.; D’Argento, E.; Grossi, F.; et al. Post-progression outcomes of NSCLC patients with PD-L1 expression ≥ 50% receiving first-line single-agent pembrolizumab in a large multicentre real-world study. Eur. J. Cancer 2021, 148, 24–35. [Google Scholar] [CrossRef]
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chrétien, B.; Da-Silva, A.; Plane, A.F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef]
- A Kazarinova, L.; Matveichuk, N.V.; Lukin, N.S. Study of inosine transformation into 5′-inosinic acid by the culture of Pseudomonas trifoli. Prikl. Biokhim. Mikrobiol. 1975, 11, 195–202. [Google Scholar] [PubMed]
- Ramos-Casals, M.; Brito-Zeron’, P.; Lopez-Soto, A.; Lopez-Soto, P. Systemic autoimmune diseases in elderly patients. Autoimmun. Rev. 2004, 3, 376–382. [Google Scholar] [CrossRef]
- E Dobretsov, G.; Kharitonenkov, I.G.; E Mishiev, V.; A Vladimirov, I. Relation between fluorescence and circular dichroism of the complex of the fluorescence probe 4-dimethylaminochalcone with serum albumin. Biofizika 1975, 20, 581–585. [Google Scholar]
- Morimoto, K.; Yamada, T.; Yokoi, T.; Kijima, T.; Goto, Y.; Nakao, A.; Hibino, M.; Takeda, T.; Yamaguchi, H.; Takumi, C.; et al. Clinical impact of pembrolizumab combined with chemotherapy in elderly patients with advanced non-small-cell lung cancer. Lung Cancer Amst. Neth. 2021, 161, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, D.; Miura, S.; Yoshimura, K.; Wakuda, K.; Oya, Y.; Haratani, K.; Itoh, S.; Uemura, T.; Morinaga, R.; Takahama, T.; et al. A Real-World Study on the Effectiveness and Safety of Pembrolizumab Plus Chemotherapy for Nonsquamous NSCLC. JTO Clin. Res. Rep. 2021, 3, 100265. [Google Scholar] [CrossRef] [PubMed]
- Kanesvaran, R.; Mohile, S.; Soto-Perez-De-Celis, E.; Singh, H. The Globalization of Geriatric Oncology: From Data to Practice. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, e107–e115. [Google Scholar] [CrossRef] [PubMed]
- Galvin, A.; Bertrand, N.; Boulahssass, R.; De Decker, L.; Dorval, É.; Clairaz, B.; Castaignède, M.; Mourey, L.; Baldini, C.; Bauvin, E.; et al. Repenser la prise en charge des sujets âgés atteints d’un cancer: Propositions du groupe Priorités Âge Cancer. Bull. Cancer 2022, 109, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Molina Garrido, M.J. Oncogeriatría: Una forma de optimizar la atención global del paciente anciano con cáncer. Nutr. Hosp. 2016, 33, 31–39. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-16112016000700005 (accessed on 17 March 2024). [CrossRef] [PubMed]
- Verma, S.P.; Mahour, P. Aging and Cancer: Intervention Strategies. In Research Anthology on Supporting Healthy Aging in a Digital Society; IGI Global: Hershey, PA, USA, 2022; pp. 1100–1116. Available online: https://services.igi-global.com/resolvedoi/resolve.aspx?doi=10.4018/978-1-6684-5295-0.ch060 (accessed on 17 March 2024).
- Monfardini, S.; Perrone, F.; Balducci, L. Pitfalls in Oncogeriatrics. Cancers 2023, 15, 2910. [Google Scholar] [CrossRef]
- Panagopoulos, N.; Grapatsas, K.; Leivaditis, V.; Galanis, M.; Dougenis, D. Are Extensive Open Lung Resections for Elderly Patients with Lung Cancer Justified? Curr. Oncol. 2023, 30, 5470–5484. [Google Scholar] [CrossRef]
- Scheffold, A.; Eul, B.; Degen, M.; Witte, B. Adjuvante Therapie betagter Lungenkrebspatienten: Komparative Analyse von Indikationsstellung, Therapieadhärenz und Langzeitüberleben. Pneumologie 2022, 76, 488–493. [Google Scholar] [CrossRef]
- Sheth, H.; Kumar, P.; Limaye, S. Management of Metastatic Nonsmall Cell Lung Cancer in Elderly. Indian J. Med. Paediatr. Oncol. 2021, 42, 229–239. [Google Scholar] [CrossRef]
- Marmor, H.N.; Zorn, J.T.; Deppen, S.A.; Massion, P.P.; Grogan, E.L. Biomarkers in lung cancer screening: A narrative review. Curr. Chall. Thorac. Surg. 2023, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Kharagezov, D.A.; Lazutin, Y.N.; Mirzoyan, E.A.; Milakin, A.G.; Stateshny, O.N.; Leiman, I.A.; Iozefi, K.D. Lung cancers biomarkers. Res. Pract. Med. J. 2022, 9, 103–116. [Google Scholar] [CrossRef]
- Miao, R.; Ge, C.; Zhang, X.; He, Y.; Ma, X.; Xiang, X.; Gu, J.; Fu, Y.; Qu, K.; Liu, C.; et al. Combined eight-long noncoding RNA signature: A new risk score predicting prognosis in elderly non-small cell lung cancer patients. Aging 2019, 11, 467–479. [Google Scholar] [CrossRef] [PubMed]
| Trial | Population | Intervention | Efficacy Outcomes | Safety Outcomes | Key Findings for Elderly Patients |
|---|---|---|---|---|---|
| KEYNOTE-010 | Relapsed PD-L1-positive (≥1%) NSCLC (41% ≥65 years; no patients >70 years) | Pembrolizumab (2 or 10 mg/kg) vs. Docetaxel | Median OS: 10.4−12.7 vs. 8.5 months (HR 0.71, p = 0.0008). ORR: 18% vs. 9%. PFS benefit only for PD-L1 ≥50%. | Grade 3−5 AEs: 13–16% (Pembrolizumab) vs. 35% (Docetaxel). No age-specific safety data. | Similar survival benefit for older (HR 0.76) and younger (HR 0.63) patients, with lower toxicity for Pembrolizumab. |
| KEYNOTE-024 | Advanced NSCLC, PD-L1 ≥50% (subgroup for older patients analyzed) | Pembrolizumab (200 mg) vs. Chemotherapy | Median OS: 30 vs. 14.2 months (HR 0.63, p = 0.002). Median PFS: 10.3 vs. 6.0 months (HR 0.5, p < 0.001). | Grade 3–5 AEs: 26.6% (Pembrolizumab) vs. 53.3% (Chemotherapy). Better QoL with Pembrolizumab (p = 0.002). | Stronger PFS benefit for older patients (HR 0.45) than younger (HR 0.61), but confounders may affect interpretation. |
| KEYNOTE-042 | Advanced NSCLC, PD-L1 ≥1% (33% ≥65 years) | Pembrolizumab vs. Chemotherapy | Median OS: 16.7 vs. 12.1 months (HR 0.81, p = 0.0018). Subgroup with PD-L1 ≥50%: HR 0.53. | Lower incidence of grade ≥3 AEs with Pembrolizumab (17.8% vs. 41% for chemotherapy). | Survival benefit consistent across older (HR 0.77) and younger patients (HR 0.81). |
| KEYNOTE-189 | Treatment-naïve advanced non-squamous NSCLC (median age: 65 years) | Platinum–pemetrexed + Pembrolizumab vs. Placebo | OS (1-year): 69.2% vs. 49.4% (HR 0.49, p < 0.001). Survival benefit across all age groups. | Grade 3–5 AEs: ~66% for both arms. Discontinuation rates: 13.8% (Pembrolizumab) vs. 7.9% (Placebo). | Less pronounced OS benefit in older patients (HR 0.64) vs. younger patients (HR 0.43). High AE rates in both groups. |
| IMpower150 | Advanced NSCLC (median age: 63 years) | Atezolizumab + Bevacizumab + Chemotherapy vs. Bevacizumab + Chemotherapy | Median OS: 19.2 vs. 14.7 months (HR 0.78). Survival benefit across all age groups, including elderly. | Grade 3–4 AEs: 42% (Atezolizumab arm) vs. 36% (control). No age-specific data provided. | Consistent OS benefit across age groups, including those ≥65 years. |
| CheckMate-017 | Relapsed squamous NSCLC (44% ≥65 years) | Nivolumab vs. Docetaxel | Median OS: 9.2 vs. 6.0 months (HR 0.59, p < 0.001). Median PFS: 3.5 vs. 2.8 months (HR 0.62, p < 0.001). ORR: 20% vs. 9%. | Grade 3–4 AEs: 7% (Nivolumab) vs. 55% (Docetaxel). Significant QoL improvement for Nivolumab. | OS benefit consistent for patients up to 74 years but absent in ≥75 subgroup (HR 1.85). Small subgroup limits conclusions. |
| CheckMate-026 | PD-L1-positive (≥1%) NSCLC (48% ≥65 years) | Nivolumab vs. Platinum-based chemotherapy | Median OS: 14.4 vs. 13.2 months (HR 1.09, p = 0.25). Median PFS: 5.9 vs. 4.2 months. | Grade 3–4 AEs: 18% (Nivolumab) vs. 51% (Chemotherapy). Discontinuation rates lower for Nivolumab. | OS and PFS outcomes similar across age groups. Real-world data indicate modest ORR and median OS for patients ≥75 years. |
| CheckMate-227 | Advanced NSCLC (43% ≥65 years) | Nivolumab + Ipilimumab vs. Chemotherapy | Median OS: 17.1 vs. 14.9 months (HR 0.73). Durable response observed in PD-L1 ≥1%. | Grade 3–4 AEs: 31% (Nivolumab + Ipilimumab) vs. 36% (Chemotherapy). | Consistent OS benefit in older (HR 0.78) and younger (HR 0.71) patients, though toxicity was higher in combination therapy. |
| OAK | Pre-treated advanced NSCLC (47% ≥65 years) | Atezolizumab vs. Docetaxel | Median OS: 13.8 vs. 9.6 months (HR 0.73, p = 0.0003). PD-L1-independent OS benefit. | Grade 3–5 AEs: 15% (Atezolizumab) vs. 43% (Docetaxel). Favorable safety profile for Atezolizumab. | OS benefit seen across all age groups, with greater benefit in older patients (HR 0.66 vs. HR 0.80 for younger patients). |
| Pooled Analysis | Advanced NSCLC, PD-L1 ≥50% | Chemo-ICI vs. ICI Monotherapy | OS/PFS comparable for ICI monotherapy vs. chemo-ICI in patients ≥75 years. | Chemo-ICI associated with higher toxicity in older patients. | Supports ICI monotherapy for patients ≥75 years due to reduced toxicity without loss of efficacy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baladi, A.; Tafenzi, H.A.; Zouiten, O.; Afani, L.; Essaadi, I.; El Fadli, M.; Belbaraka, R. Immunotherapy for Elderly Patients with Advanced Non-Small Cell Lung Cancer: Challenges and Perspectives. Int. J. Mol. Sci. 2025, 26, 2120. https://doi.org/10.3390/ijms26052120
Baladi A, Tafenzi HA, Zouiten O, Afani L, Essaadi I, El Fadli M, Belbaraka R. Immunotherapy for Elderly Patients with Advanced Non-Small Cell Lung Cancer: Challenges and Perspectives. International Journal of Molecular Sciences. 2025; 26(5):2120. https://doi.org/10.3390/ijms26052120
Chicago/Turabian StyleBaladi, Anass, Hassan Abdelilah Tafenzi, Othmane Zouiten, Leila Afani, Ismail Essaadi, Mohammed El Fadli, and Rhizlane Belbaraka. 2025. "Immunotherapy for Elderly Patients with Advanced Non-Small Cell Lung Cancer: Challenges and Perspectives" International Journal of Molecular Sciences 26, no. 5: 2120. https://doi.org/10.3390/ijms26052120
APA StyleBaladi, A., Tafenzi, H. A., Zouiten, O., Afani, L., Essaadi, I., El Fadli, M., & Belbaraka, R. (2025). Immunotherapy for Elderly Patients with Advanced Non-Small Cell Lung Cancer: Challenges and Perspectives. International Journal of Molecular Sciences, 26(5), 2120. https://doi.org/10.3390/ijms26052120








