Evaluation of Physicochemical Properties of Polymeric Systems for Potential Applications in Cartilage Tissue Engineering
Abstract
1. Introduction
2. Results
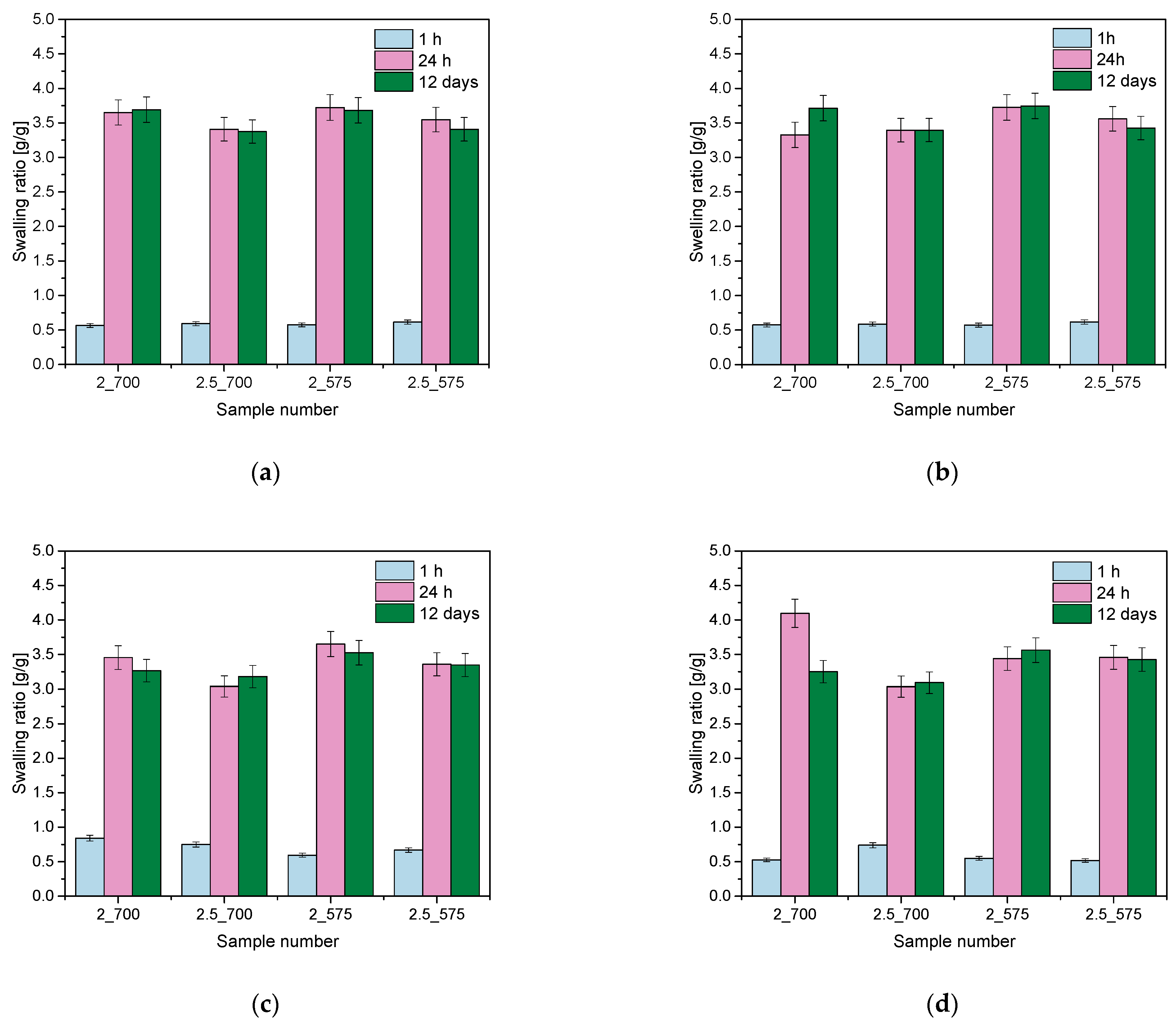
2.1. Analysis of Sorption Capacity
2.2. Microscopic Observations with Determination of Roughness Profile
2.3. Results of the Incubation Study
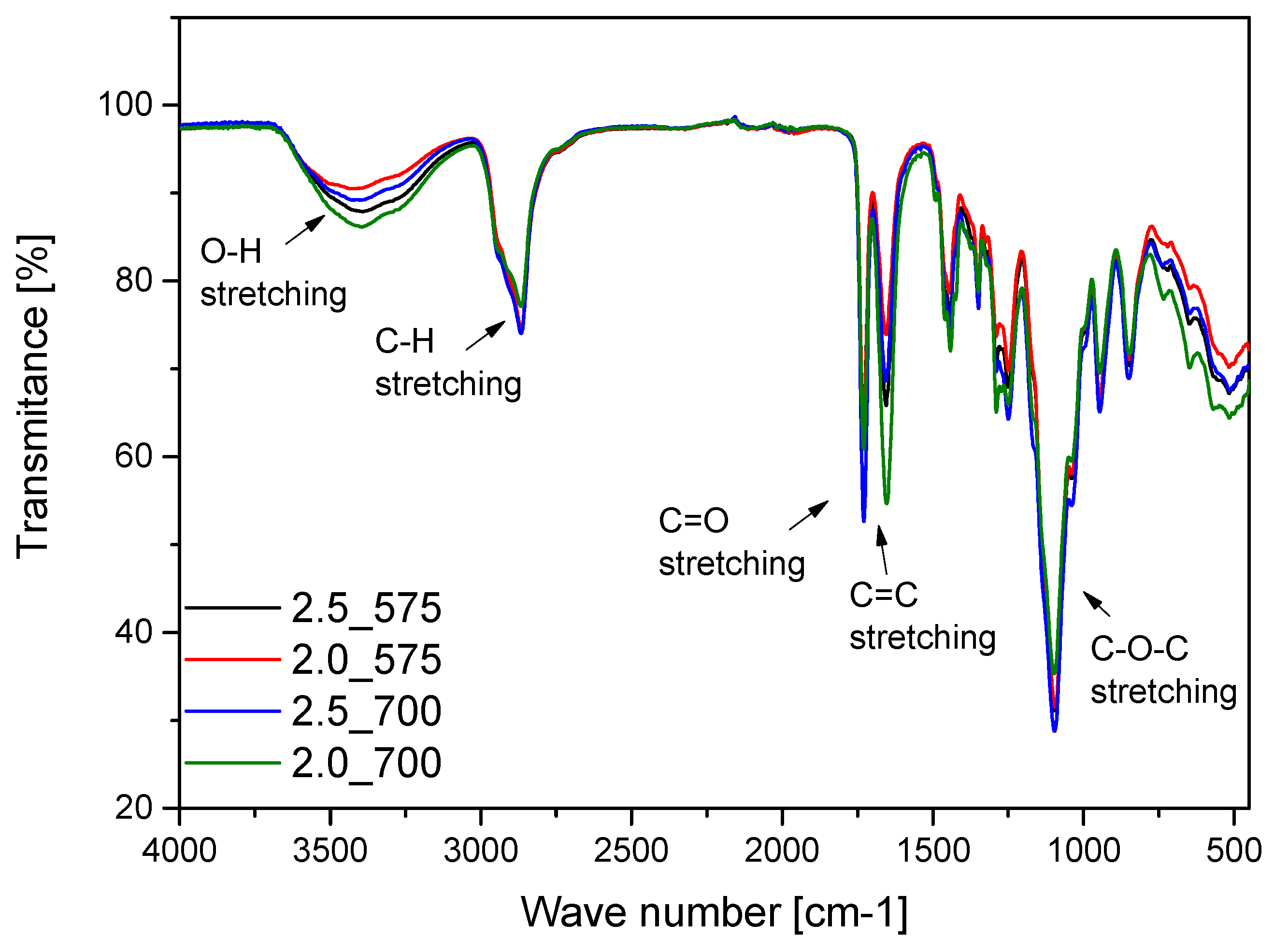
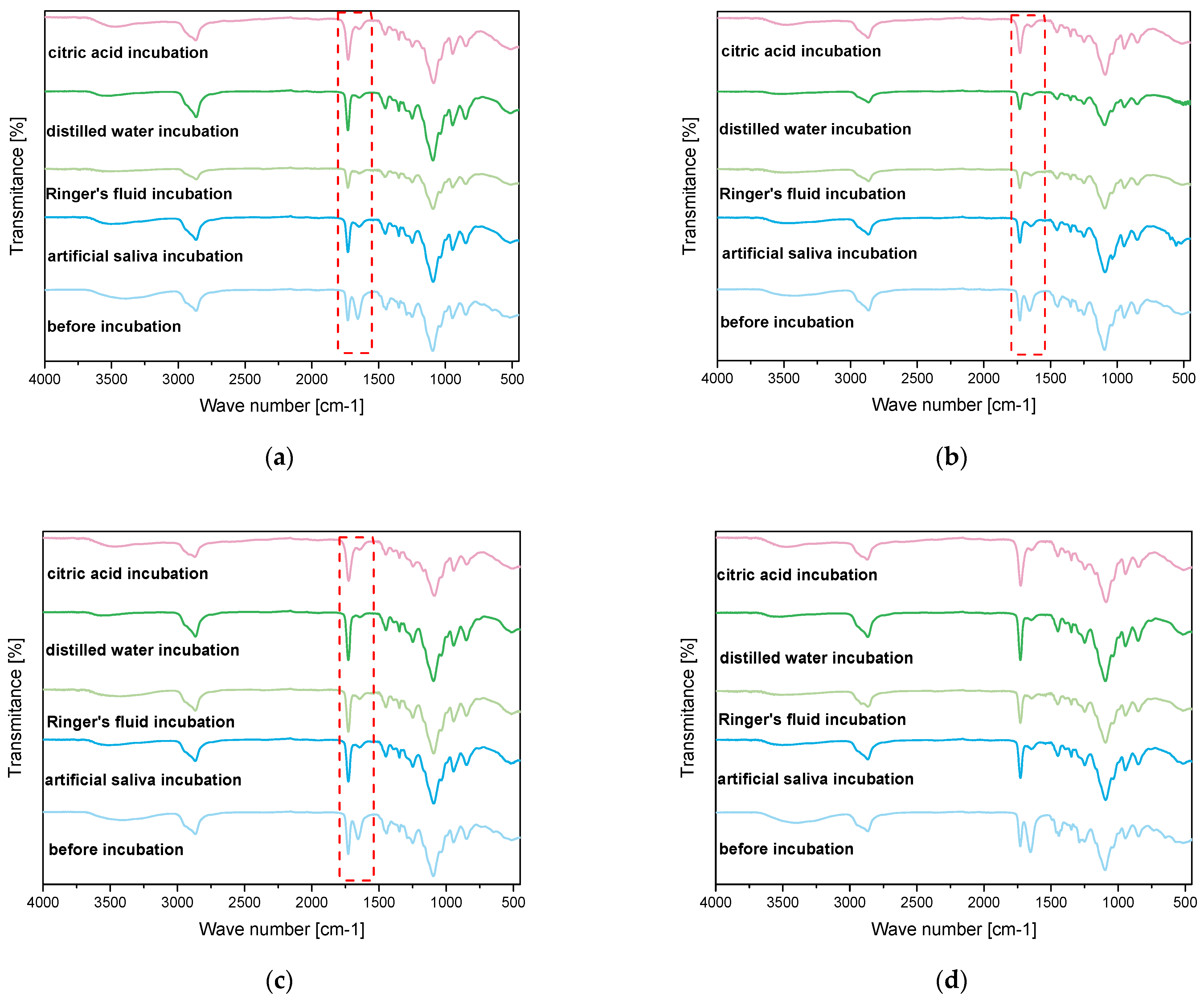
2.4. Results of Infrared Spectroscopy Analysis
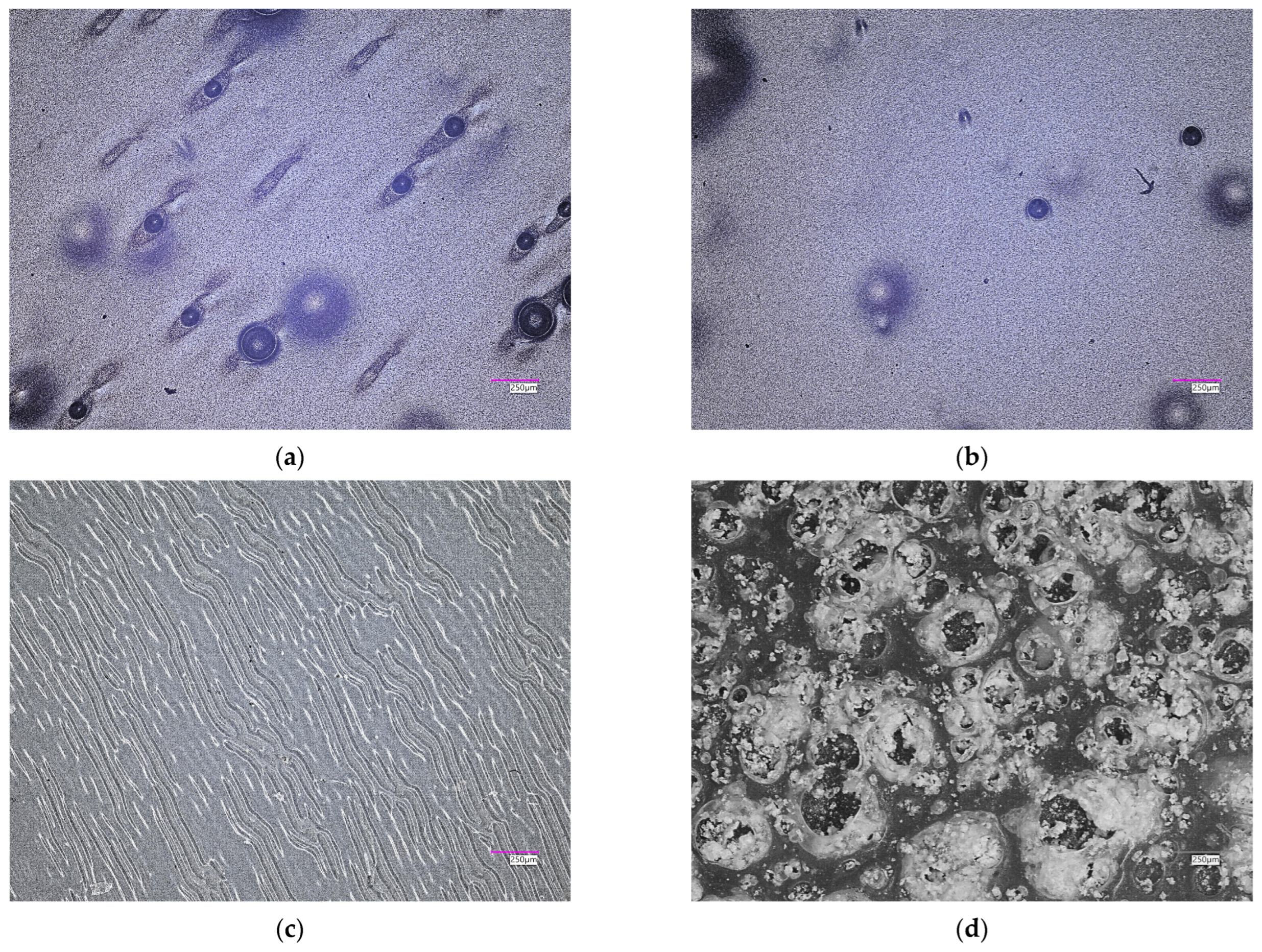
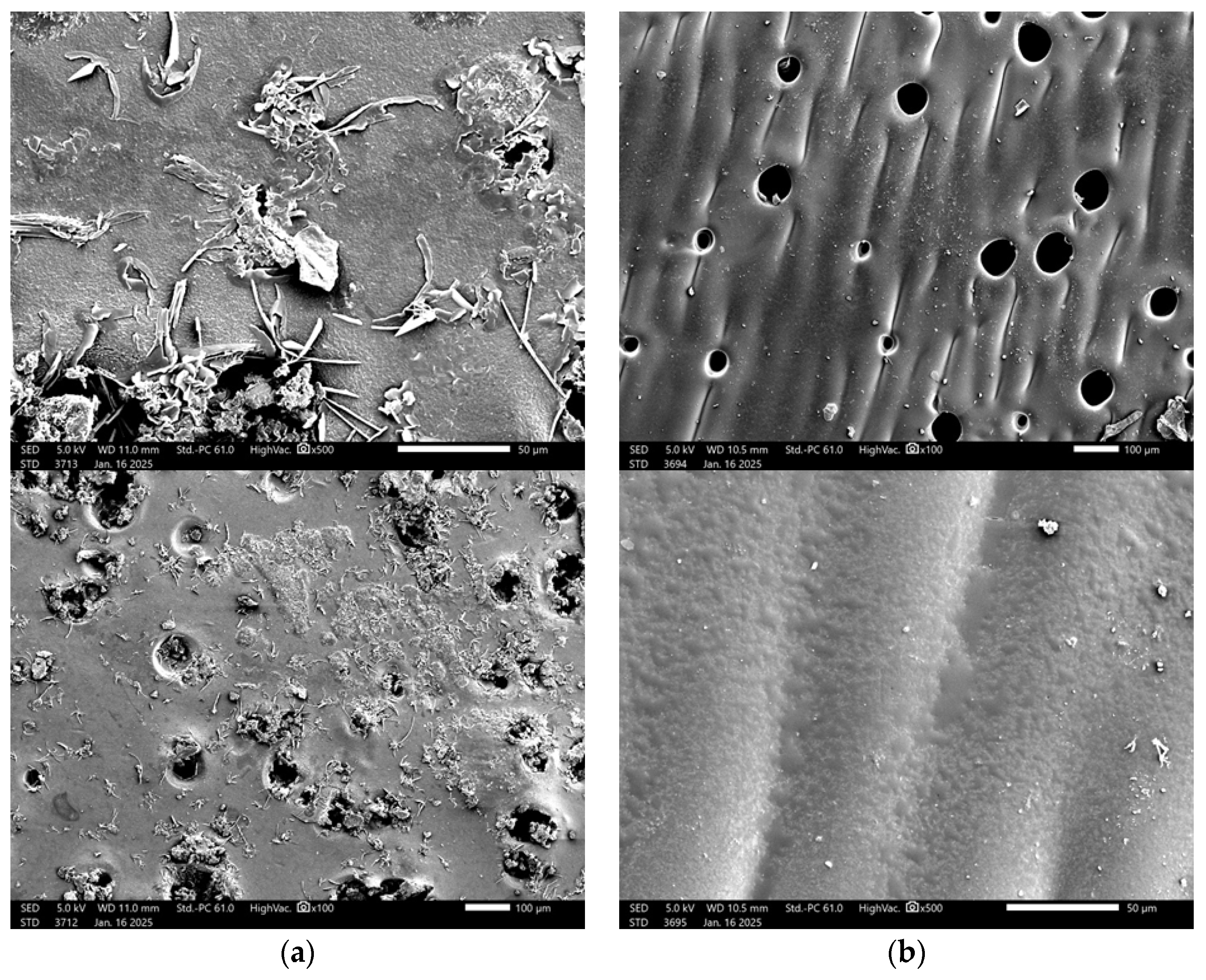
2.5. Characterization of Hydrogels via Microscopic Techniques
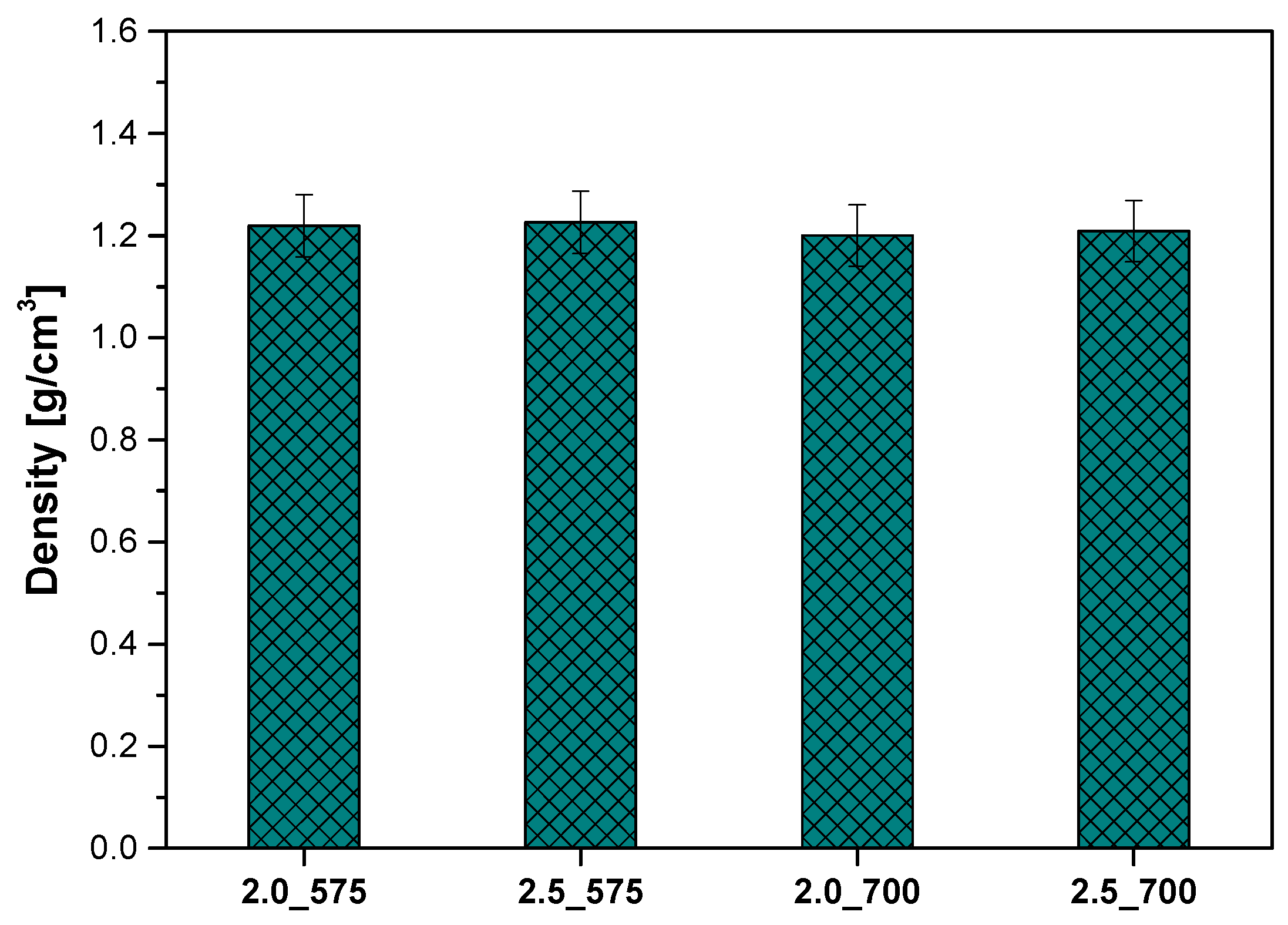
2.6. Analysis of the Density of Polymeric Materials
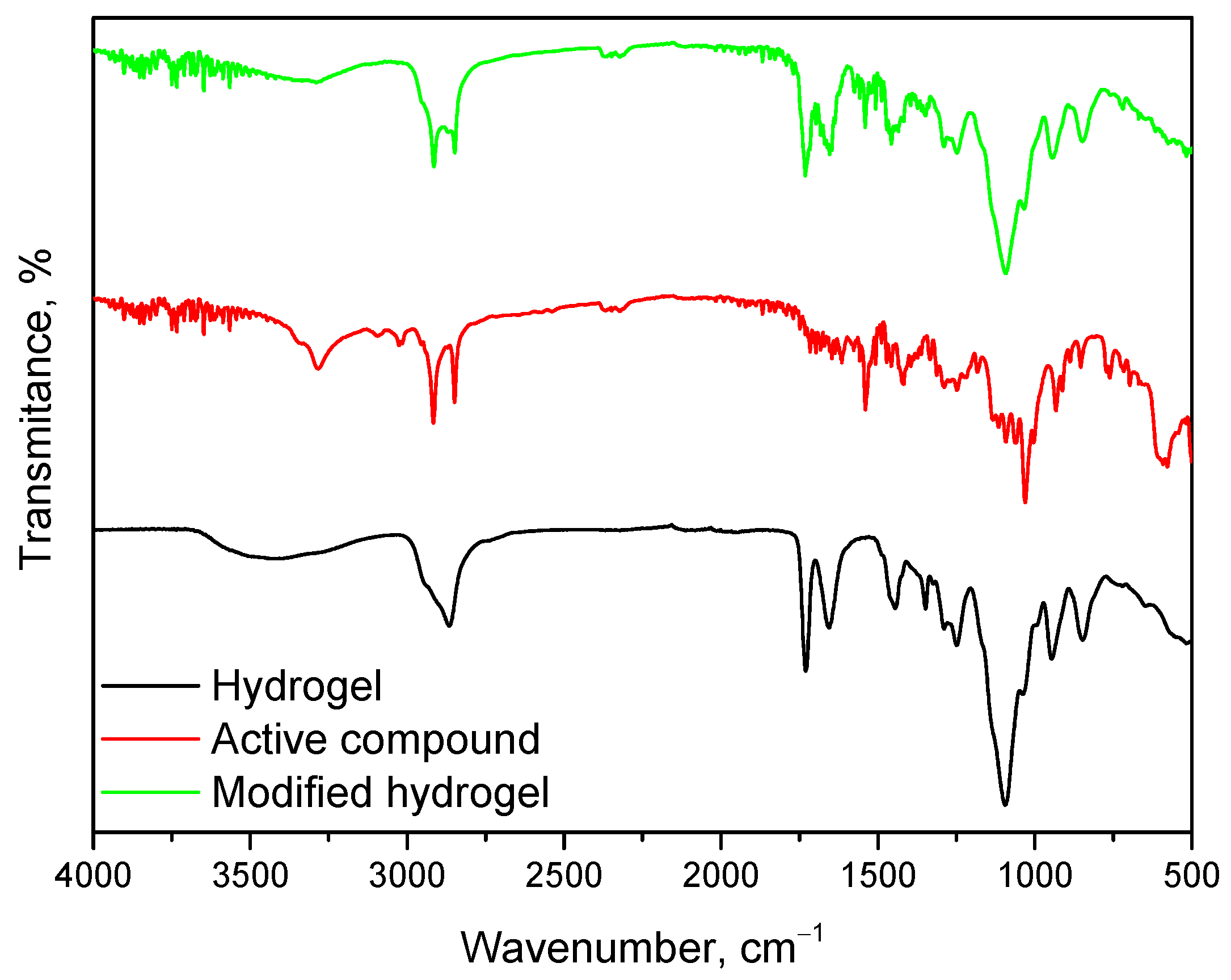
2.7. Synthesis and Characterization of Polymeric Material Modified with Mixture of Bioactive Compounds
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Hydrogel Materials
3.3. Sorption Capacity Analysis
3.4. Incubation Studies
3.5. Microscopic Observations and Surface Roughness Profile Analysis
3.6. Infrared Spectroscopy Analysis
3.7. Analysis of the Density of Polymeric Materials
3.8. Characterization of Hydrogels via Microscopic Techniques
3.9. Synthesis and Characterization of Polymeric Material Modified with Mixture of Bioactive Compounds
4. Conclusions
- The swelling capacity of hydrogels is significantly influenced by the amount and molecular weight of PEGDA. Lower amounts of PEGDA and lower molecular weight (575 g/mol) resulted in higher swelling coefficients due to a looser network structure, while higher amounts of PEGDA with higher molecular weight (700 g/mol) produced denser networks with reduced water absorption.
- Increased amounts of PEGDA lead to higher surface roughness both before and after incubation, with significant changes observed in samples containing PEGDA 575 g/mol, highlighting their susceptibility to structural alterations. PEGDA 700 g/mol provided better stability and resistance to degradation.
- The pH stability of the hydrogels was largely unaffected by incubation in distilled water and citric acid, confirming the absence of degradation. However, a significant pH jump was observed in artificial saliva, likely due to interactions with its complex composition, without evidence of hydrogel degradation. Such interactions underscore the importance of the chemical environment in determining hydrogel performance.
- FT-IR spectroscopy confirmed the presence of characteristic functional groups of both the hydrogel matrix and bioactive mixture. The integration of bioactive components (glucosamine, chondroitin, MSM) was successful, as indicated by new and intensified absorption bands. These modifications suggest potential improved bioactivity and compatibility.
- The study demonstrates that the developed hydrogels, particularly those modified with bioactive compounds, have significant potential for applications in regenerative medicine, such as joint disease treatment and cartilage repair, due to their customizable properties, biocompatibility, and chemical stability. Further studies are recommended to optimize their properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartolomeo, D.; Feng, J.; Cao, P.; Yang, T.; Ao, H.; Xing, B. Fabrication of Microgel-Modified Hydrogel Flexible Strain Sensors Using Electrohydrodynamic Direct Printing Method. Sensors 2024, 24, 3038. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yu, W.; Guo, X.; Wang, C.; Wang, Q.; Chen, B.; Hu, Y.; Dai, H. Development and Evaluation of a Novel Antibacterial Wound Dressing: A Powder Preparation Based on Cross-Linked Pullulan with Polyhexamethylene Biguanide for Hydrogel-Transition in Advanced Wound Management and Infection Control. Polymers 2024, 16, 1352. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.-Y.; Zhang, Y.-S.; Cheng, J.-K.; Chen, Y.-H.; Lin, H.-C.; Yeh, M.-Y. Lysine-Triggered Polymeric Hydrogels with Self-Adhesion, Stretchability, and Supportive Properties. Polymers 2024, 16, 1388. [Google Scholar] [CrossRef] [PubMed]
- Arabpour, Z.; Abedi, F.; Salehi, M.; Baharnoori, S.M.; Soleimani, M.; Djalilian, A.R. Hydrogel-Based Skin Regeneration. Int. J. Mol. Sci. 2024, 25, 1982. [Google Scholar] [CrossRef]
- Malka, E.; Margel, S. Engineering of PVA/PVP Hydrogels for Agricultural Applications. Gels 2023, 9, 895. [Google Scholar] [CrossRef]
- Muresan-Pop, M.; Magyari, K.; Vulpoi, A. PVA and PVP Hydrogel Blends for Wound Dressing: Synthesis and Characterisation. Adv. Mater. Res. 2019, 1151, 9–14. [Google Scholar] [CrossRef]
- Jalageri, M.B.; Mohan Kumar, G.C. Hydroxyapatite Reinforced Polyvinyl Alcohol/Polyvinyl Pyrrolidone Based Hydrogel for Cartilage Replacement. Gels 2022, 8, 555. [Google Scholar] [CrossRef]
- Himawan, A.; Anjani, Q.K.; Detamornrat, U.; Vora, L.K.; Permana, A.D.; Ghanma, R.; Naser, Y.; Rahmawanty, D.; Scott, C.J.; Donnelly, R.F. Multifunctional Low Temperature-Cured PVA/PVP/Citric Acid-Based Hydrogel Forming Microarray Patches: Physicochemical Characteristics and Hydrophilic Drug Interaction. Eur. Polym. J. 2023, 186, 111836. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in Medical and Pharmaceutical Applications: Perspectives and Challenges. Biotechnol. Adv. 2019, 37, 109–131. [Google Scholar] [CrossRef]
- Sun, Z.; Yin, Y.; Liu, B.; Xue, T.; Zou, Q. Amphibious Multifunctional Hydrogel Flexible Haptic Sensor with Self-Compensation Mechanism. Sensors 2024, 24, 3232. [Google Scholar] [CrossRef]
- Burratti, L.; Bertelà, F.; Sisani, M.; Di Guida, I.; Battocchio, C.; Iucci, G.; Prosposito, P.; Venditti, I. Three-Dimensional Printed Filters Based on Poly(Ethylene Glycol) Diacrylate Hydrogels Doped with Silver Nanoparticles for Removing Hg(II) Ions from Water. Polymers 2024, 16, 1034. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Pastuszka, D.; Stawicka, K.; Musiał, W. Biopolymer-Based Hydrogel Incorporated with Naproxen Sodium and Lidocaine Hydrochloride for Controlled Drug Delivery. Polymers 2024, 16, 1353. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Tao, S.; Wang, Q.; Ma, P.Q.; Li, Z.B.; Wu, Y.L.; Li, D.W. Research Advances in Smart Responsive-Hydrogel Dressings with Potential Clinical Diabetic Wound Healing Properties. Mil. Med. Res. 2023, 10, 37. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Volpi, M.; Paradiso, A.; Costantini, M.; Świȩszkowski, W. Hydrogel-Based Fiber Biofabrication Techniques for Skeletal Muscle Tissue Engineering. ACS Biomater. Sci. Eng. 2022, 8, 379–405. [Google Scholar] [CrossRef]
- McBath, R.A.; Shipp, D.A. Swelling and Degradation of Hydrogels Synthesized with Degradable Poly(β-Amino Ester) Crosslinkers. Polym. Chem. 2010, 1, 860–865. [Google Scholar] [CrossRef]
- Hakim Khalili, M.; Zhang, R.; Wilson, S.; Goel, S.; Impey, S.A.; Aria, A.I. Additive Manufacturing and Physicomechanical Characteristics of PEGDA Hydrogels: Recent Advances and Perspective for Tissue Engineering. Polymers 2023, 15, 2341. [Google Scholar] [CrossRef]
- Schüler, T.; Guder, C.; Alt, F.; Lorenz, K.; Sterzenbach, T.; Hannig, C.; Wiesmann, H.-P.; Kruppke, B. Degradable Polycaprolactone/Buffer Composites as pH Regulating Carrier Materials for Drug Delivery and 3D Printed Biomaterials. Materialia 2024, 34, 102087. [Google Scholar] [CrossRef]
- Fan, Y.; Wen, Z.T.; Liao, S.; Lallier, T.; Hagan, J.L.; Twomley, J.T.; Zhang, J.-F.; Sun, Z.; Xu, X. Novel Amelogenin-Releasing Hydrogel for Remineralization of Enamel Artificial Caries. J. Bioact. Compat. Polym. 2012, 27, 585–603. [Google Scholar] [CrossRef]
- Han, Y.; Xu, J.; Chopra, H.; Zhang, Z.; Dubey, N.; Dissanayaka, W.L.; Nör, J.E.; Bottino, M.C. Injectable Tissue-Specific Hydrogel System for Pulp-Dentin Regeneration. J. Dent. Res. 2024, 103, 398–408. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Li, L.; Feng, L.; Luo, Y.; Xu, M.; Leong, K.W.; Yao, R. Biomaterial-Assisted Scalable Cell Production for Cell Therapy. Biomaterials 2019, 230, 119627. [Google Scholar] [CrossRef]
- Gan, X.; Wang, X.; Huang, Y.; Li, G.; Kang, H. Applications of Hydrogels in Osteoarthritis Treatment. Biomedicines 2024, 12, 923. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and Interactions in Covalently and Ionically Crosslinked Chitosan Hydrogels for Biomedical Applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, X.; He, X.; Tian, N.; Ding, P.; Guo, W.; Okoro, O.V.; Sun, Y.; Jiang, G.; Liu, Z.; et al. Fabrication and Properties of Hydrogel Dressings Based on Genipin Crosslinked Chondroitin Sulfate and Chitosan. Polymers 2024, 16, 2876. [Google Scholar] [CrossRef]
- Melnyk, Y.; Stetsyshyn, Y.; Skorokhoda, V.; Nastishin, Y. Polyvinylpyrrolidone-graft-poly(2-hydroxyethylmethacrylate) hydrogel membranes for encapsulated forms of drugs. J. Polym. Res. 2020, 27, 354. [Google Scholar] [CrossRef]
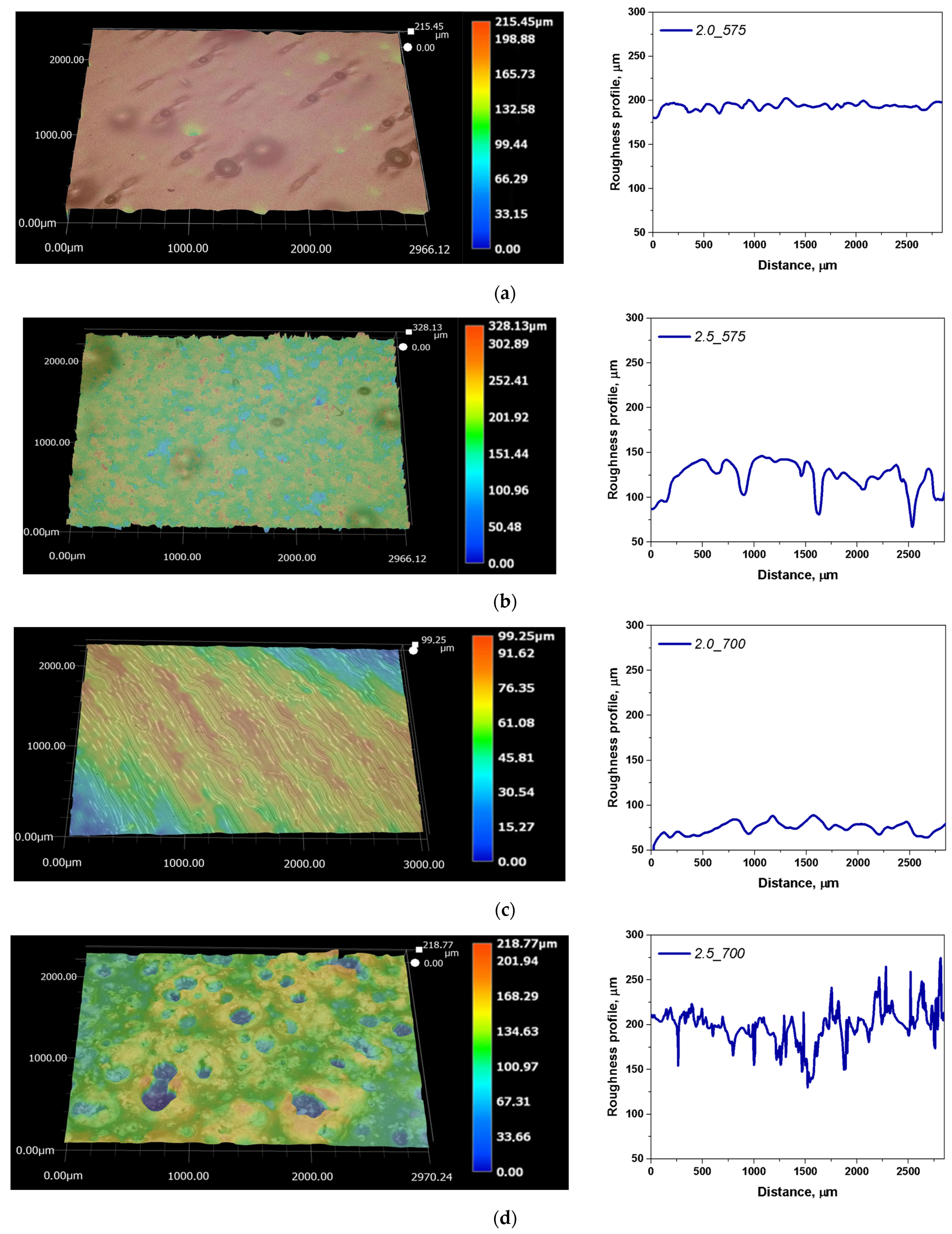



| Sample Name | Ra [µm] | Rz [µm] |
|---|---|---|
| 2_575 | 2.43 | 17.42 |
| 2.5_575 | 14.32 | 153.47 |
| 2_700 | 5.12 | 25.52 |
| 2.5_700 | 13.42 | 80.20 |
| 15% PVP 10 k [mL] | 5% PVA 13–23 k [mL] | PEGDA [mL] | Photoinitiator [µL] | Sample Name * |
|---|---|---|---|---|
| 3 | 7 | 2 (700) | 50 | 2_700 |
| 2.5 (700) | 2.5_700 | |||
| 2 (575) | 2_575 | |||
| 2.5 (575) | 2.5_575 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanat, D.; Garbowska, C.; Wrzesińska, W.; Grzywacz, O.; Sala, K.; Zapotoczny, K.; Bańkosz, M.; Jampilek, J.; Walter, J.; Tyliszczak, B. Evaluation of Physicochemical Properties of Polymeric Systems for Potential Applications in Cartilage Tissue Engineering. Int. J. Mol. Sci. 2025, 26, 2057. https://doi.org/10.3390/ijms26052057
Wanat D, Garbowska C, Wrzesińska W, Grzywacz O, Sala K, Zapotoczny K, Bańkosz M, Jampilek J, Walter J, Tyliszczak B. Evaluation of Physicochemical Properties of Polymeric Systems for Potential Applications in Cartilage Tissue Engineering. International Journal of Molecular Sciences. 2025; 26(5):2057. https://doi.org/10.3390/ijms26052057
Chicago/Turabian StyleWanat, Dominika, Claudia Garbowska, Wiktoria Wrzesińska, Oliwia Grzywacz, Katarzyna Sala, Kacper Zapotoczny, Magdalena Bańkosz, Josef Jampilek, Janusz Walter, and Bożena Tyliszczak. 2025. "Evaluation of Physicochemical Properties of Polymeric Systems for Potential Applications in Cartilage Tissue Engineering" International Journal of Molecular Sciences 26, no. 5: 2057. https://doi.org/10.3390/ijms26052057
APA StyleWanat, D., Garbowska, C., Wrzesińska, W., Grzywacz, O., Sala, K., Zapotoczny, K., Bańkosz, M., Jampilek, J., Walter, J., & Tyliszczak, B. (2025). Evaluation of Physicochemical Properties of Polymeric Systems for Potential Applications in Cartilage Tissue Engineering. International Journal of Molecular Sciences, 26(5), 2057. https://doi.org/10.3390/ijms26052057








