Thromboinflammatory Biomarkers in Lymphomas: Linking Inflammation to Thrombosis Risk
Abstract
1. Introduction
2. Results
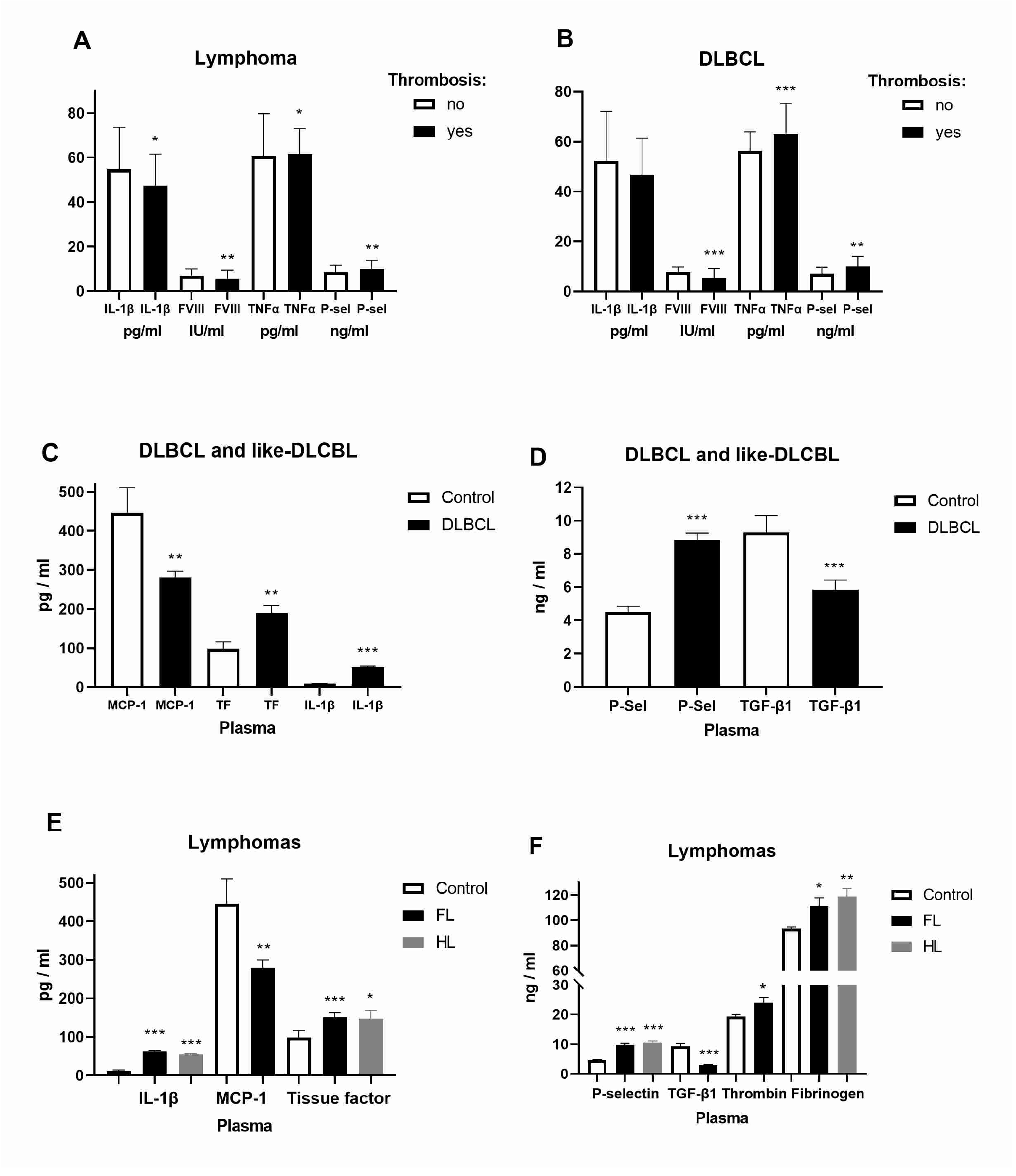
2.1. Expression of Inflammatory and Coagulation Factors in Lymphomas
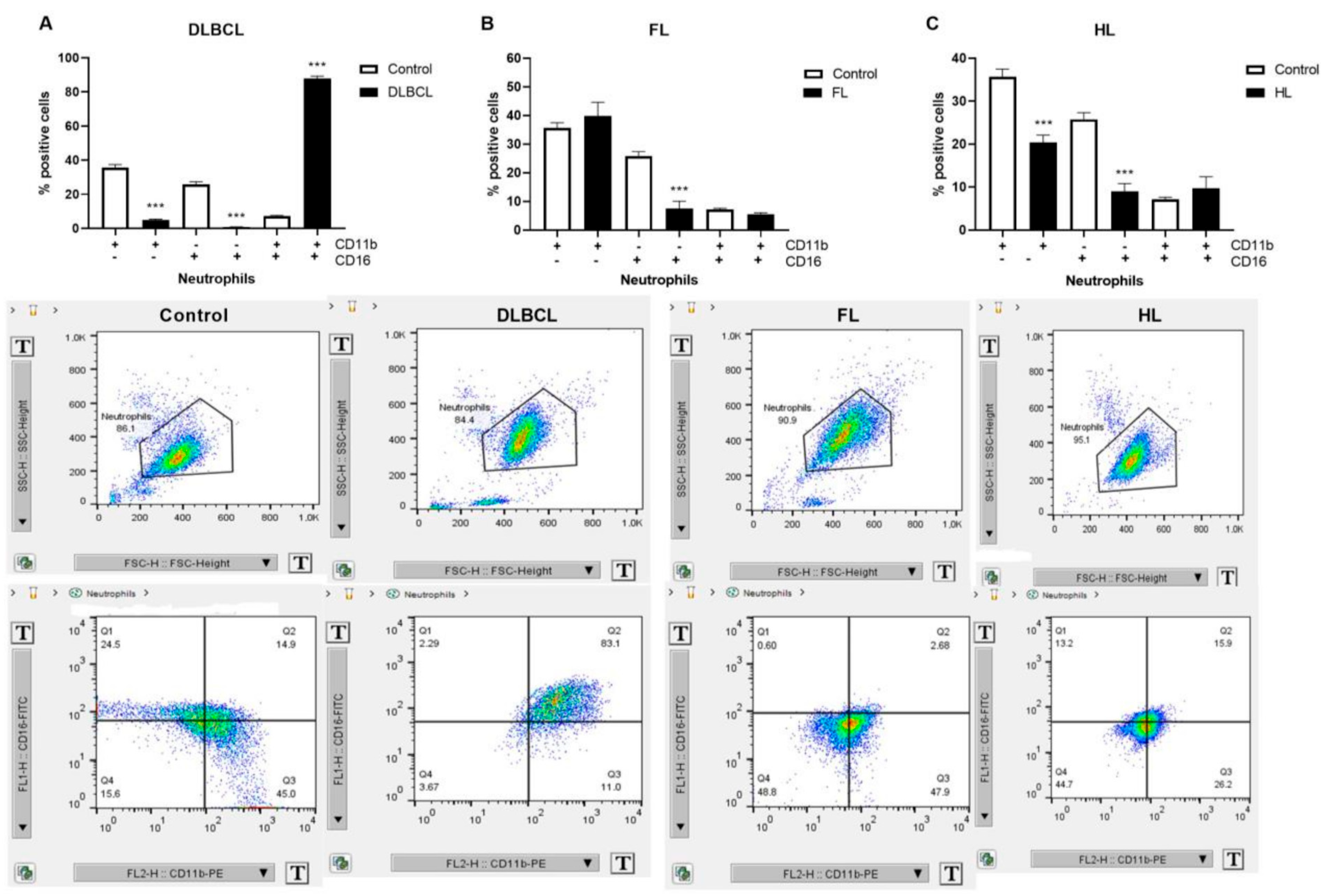
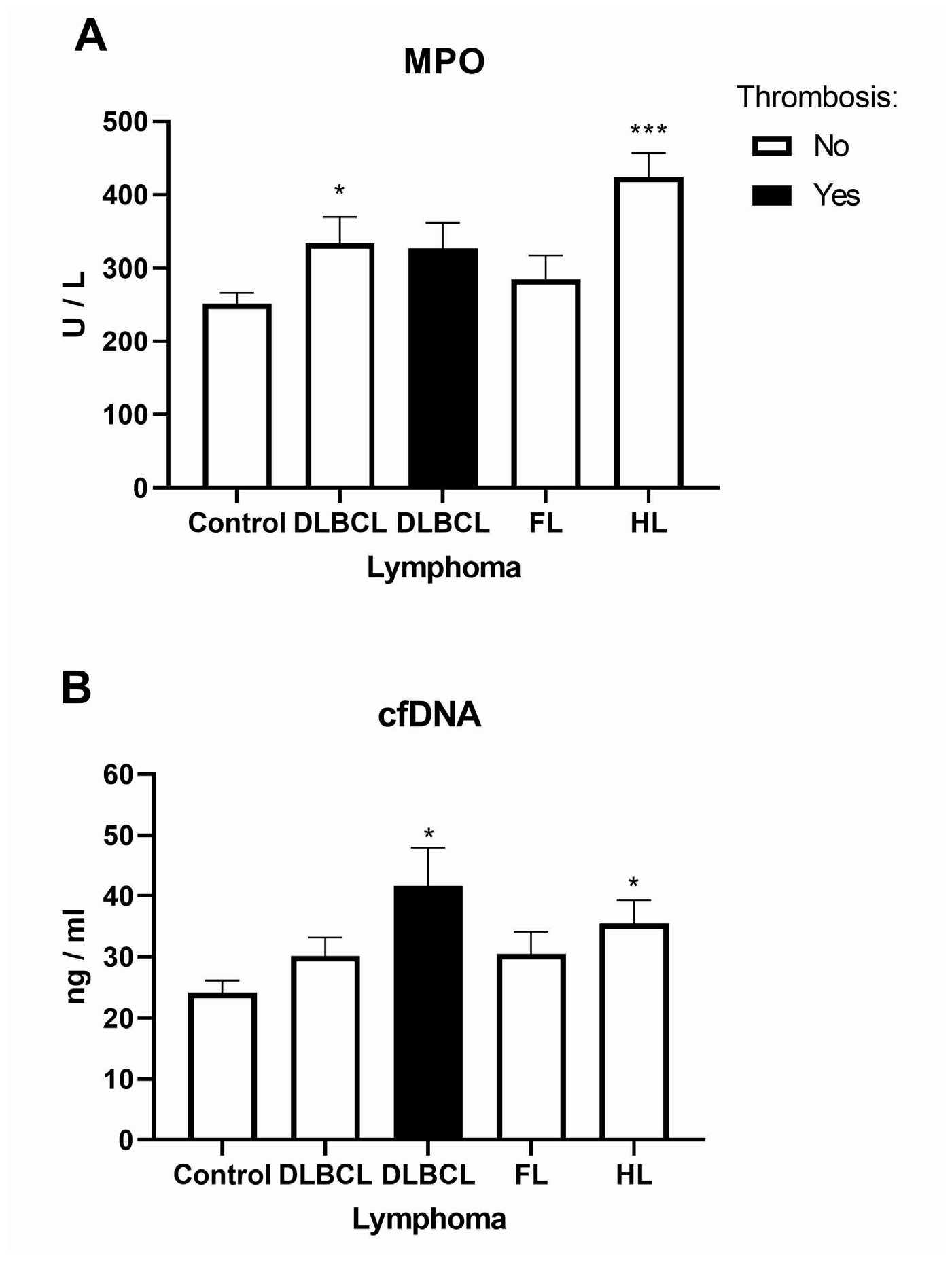
2.2. Inflammatory Response Through Neutrophil Extracellular Traps in Lymphomas
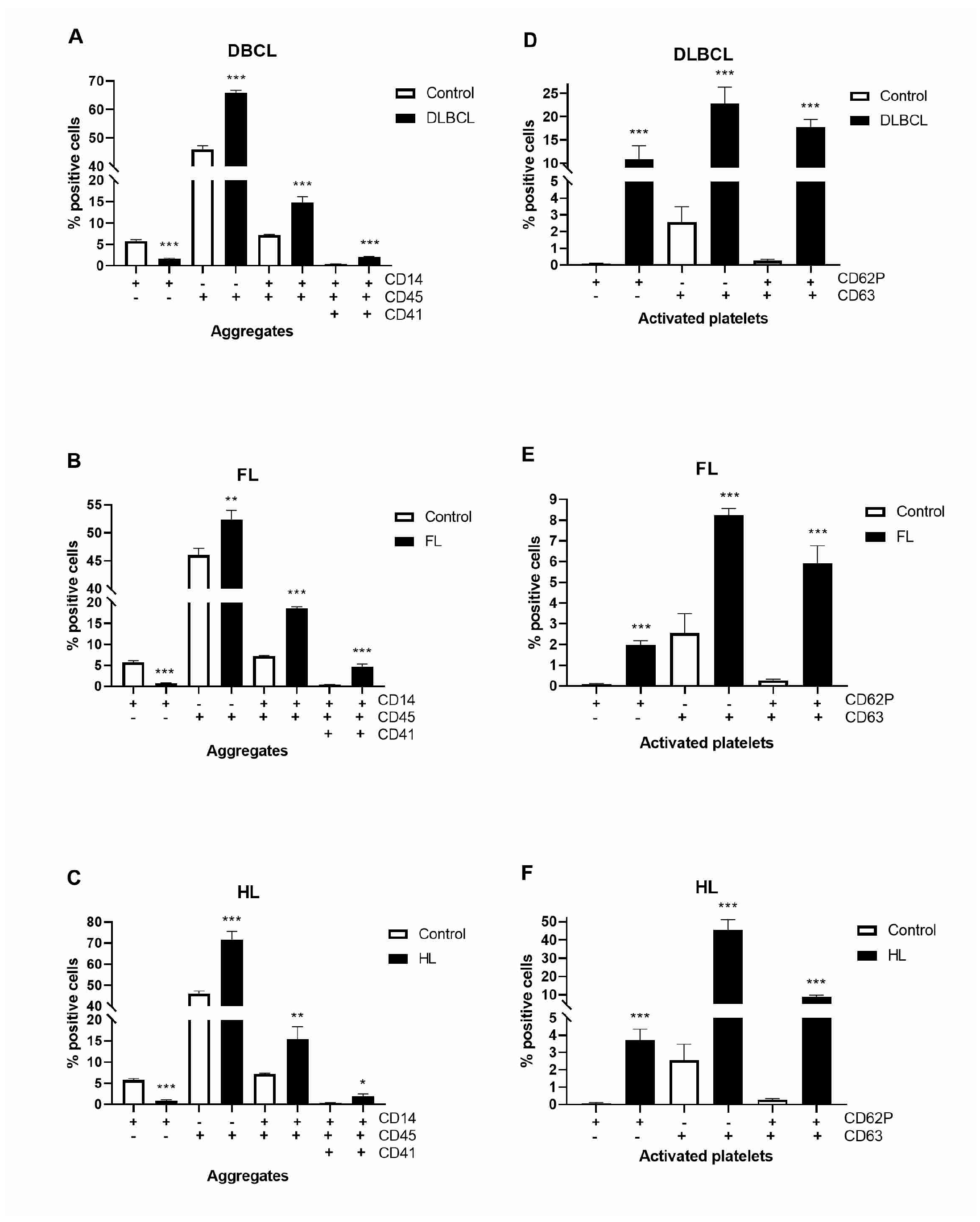
2.3. The Platelet and Monocyte as Carriers of Circulating Tissue Factor Microparticles in Lymphomas
2.4. Platelet–Monocyte Aggregates and Platelets Activation in Lymphomas
3. Discussion
4. Materials and Methods
4.1. Patients’ Characteristics
4.2. Isolation and Preparation Cells from Peripheral Blood
4.3. ELISA Assay
4.4. Determination of NET Quantity from the Plasma of the Patients and Healthy Controls
4.5. Flow Cytometry
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of Non-Hodgkin’s Lymphoma. Med. Sci. 2021, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Hohaus, S.; Bartolomei, F.; Cuccaro, A.; Maiolo, E.; Alma, E.; D’Alò, F.; Bellesi, S.; Rossi, E.; De Stefano, V. Venous Thromboembolism in Lymphoma: Risk Stratification and Antithrombotic Prophylaxis. Cancers 2020, 12, 1291. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.M.; Ihorst, G.; Mertelsmann, R.; Engelhardt, M. Epidemiology of non-Hodgkin’s lymphoma (NHL): Trends, geographic distribution, and etiology. Ann. Hematol. 2005, 84, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kanas, G.; Ge, W.; Quek, R.G.W.; Keeven, K.; Nersesyan, K.; Arnason, J.E. Epidemiology of diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) in the United States and Western Europe: Population-level projections for 2020–2025. Leuk. Lymphoma 2022, 63, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, K.M.; Wang, T.F.; Gage, B.F.; Luo, S.; Riedell, P.; Carson, K.R. Incidence of venous thromboembolism in patients with non-Hodgkin lymphoma. Thromb. Res. 2016, 143, 86–90. [Google Scholar] [CrossRef]
- Borg, I.H.; Bendtsen, M.D.; Bøgsted, M.; Madsen, J.; Severinsen, M.T. Incidence of venous thromboembolism in patients with diffuse large B-cell lymphoma. Leuk. Lymphoma 2016, 57, 2771–2776. [Google Scholar] [CrossRef]
- Lim, S.H.; Woo, S.Y.; Kim, S.; Ko, Y.H.; Kim, W.S.; Kim, S.J. Cross-sectional Study of Patients with Diffuse Large B-Cell Lymphoma: Assessing the Effect of Host Status, Tumor Burden, and Inflammatory Activity on Venous Thromboembolism. Cancer Res. Treat. 2016, 48, 312–321. [Google Scholar] [CrossRef]
- Caruso, V.; di Castelnuovo, A.; Meschengieser, S.; Lazzari, M.A.; de Gaetano, G.; Storti, S.; Iacoviello, L.; Donati, M.B. Thrombotic complications in adult patients with lymphoma: A meta-analysis of 29 independent cohorts including 18 018 patients and 1149 events. Blood 2010, 115, 5322–5328. [Google Scholar] [CrossRef]
- Borchmann, S.; Müller, H.; Hude, I.; Fuchs, M.; Borchmann, P.; Engert, A. Thrombosis as a treatment complication in Hodgkin lymphoma patients: A comprehensive analysis of three prospective randomized German Hodgkin Study Group (GHSG) trials. Ann. Oncol. 2019, 30, 1329–1334. [Google Scholar] [CrossRef]
- Jaradeh, M.; Baig, N.; Bontekoe, E.; Mitrovic, M.; Antic, D.; Hoppensteadt, D.; Kantarcioglu, B.; Fareed, J. The Relationship Between Thrombo-Inflammatory Biomarkers and Cellular Indices of Inflammation in Lymphoma Patients. Clin. Appl. Thromb./Hemost. 2021, 27, 10760296211050358. [Google Scholar] [CrossRef]
- Francischetti, I.M.B.; Alejo, J.C.; Sivanandham, R.; Davies-Hill, T.; Fetsch, P.; Pandrea, I.; Jaffe, E.S.; Pittaluga, S. Neutrophil and Eosinophil Extracellular Traps in Hodgkin Lymphoma. Hemasphere 2021, 5, e633. [Google Scholar] [CrossRef] [PubMed]
- Conroy, S.M.; Maskarinec, G.; Morimoto, Y.; Franke, A.A.; Cooney, R.V.; Wilkens, L.R.; Goodman, M.T.; Hernadez, B.Y.; Le Marchand, L.; Henderson, B.E.; et al. Non-hodgkin lymphoma and circulating markers of inflammation and adiposity in a nested case-control study: The multiethnic cohort. Cancer Epidemiol. Biomark. Prev. 2013, 22, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Cerhan, J.R.; Hartge, P.; Davis, S.; Cozen, W.; Severson, R.K.; Chatterjee, N.; Yeager, M.; Chanock, S.J.; Rothman, N. Common genetic variants in proinflammatory and other immunoregulatory genes and risk for non-Hodgkin lymphoma. Cancer Res. 2006, 66, 9771–9780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, Z.G.; Zhu, J.H.; Chen, M.B.; Wang, T.M.; Shen, W.X.; He, J. Association of Interleukin-10 -3575T>A and -1082A>G polymorphisms with non-Hodgkin lymphoma susceptibility: A comprehensive review and meta-analysis. Mol. Genet. Genom. 2015, 290, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Loong, F.; Chan, A.C.; Ho, B.C.; Chau, Y.P.; Lee, H.Y.; Cheuk, W.; Yuen, W.K.; Ng, W.S.; Cheung, H.L.; Chan, J.K. Diffuse large B-cell lymphoma associated with chronic inflammation as an incidental finding and new clinical scenarios. Mod. Pathol. 2010, 23, 493–501. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Rolling, C.C.; Barrett, T.J.; Berger, J.S. Platelet-monocyte aggregates: Molecular mediators of thromboinflammation. Front. Cardiovasc. Med. 2023, 10, 960398. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Laake, K.; Myhre, P.; Bratseth, V.; Arnesen, H.; Solheim, S.; Badimon, L.; Seljeflot, I. Platelet-, monocyte-derived and tissue factor-carrying circulating microparticles are related to acute myocardial infarction severity. PLoS ONE 2017, 12, e0172558. [Google Scholar] [CrossRef]
- Kiss, M.; Caro, A.A.; Raes, G.; Laoui, D. Systemic Reprogramming of Monocytes in Cancer. Front. Oncol. 2020, 10, 1399. [Google Scholar] [CrossRef]
- Park, L.C.; Woo, S.Y.; Kim, S.; Jeon, H.; Ko, Y.H.; Kim, S.J.; Kim, W.S. Incidence, risk factors and clinical features of venous thromboembolism in newly diagnosed lymphoma patients: Results from a prospective cohort study with Asian population. Thromb. Res. 2012, 130, e6–e12. [Google Scholar] [CrossRef]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta-A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018, 19, 2155. [Google Scholar] [CrossRef]
- Sarani, H.; Mollashahi, B.; Taheri, M.; Bahari, G.; Hashemi, S.M.; Hashemi, M.; Ghavami, S. Association between the IL-1A, IL-1B and IL-1R polymorphisms and lymphoma. Nucleosides Nucleotides Nucleic Acids 2021, 40, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Hamed Anber, N.; El-Sebaie, A.H.; Darwish, N.H.E.; Mousa, S.A.; Shamaa, S.S. Prognostic value of some inflammatory markers in patients with lymphoma. Biosci. Rep. 2019, 39, BSR20182174. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Chen, Z.; Huang, Y.; Shao, S.; Yu, H.; Wang, Y.; He, S. β-TrCP1 promotes cell proliferation via TNF-dependent NF-κB activation in diffuse large B cell lymphoma. Cancer Biol. Ther. 2020, 21, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Hofbauer, T.M.; Ondracek, A.S.; Chausheva, S.; Alimohammadi, A.; Artner, T.; Panzenboeck, A.; Rinderer, J.; Shafran, I.; Mangold, A.; et al. Neutrophil extracellular traps promote fibrous vascular occlusions in chronic thrombosis. Blood 2021, 137, 1104–1116. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; del Vecchio, S.; Carriero, M.V. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front. Immunol. 2020, 11, 1749. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, W.; Shen, F.; Du, W.; Xu, Z.; Liu, Z. The Emerging Role of Neutrophil Extracellular Traps in Arterial, Venous and Cancer-Associated Thrombosis. Front. Cardiovasc. Med. 2021, 8, 786387. [Google Scholar] [CrossRef]
- Shi, H.; Pan, Y.; Xiang, G.; Wang, M.; Huang, Y.; He, L.; Wang, J.; Fang, Q.L.L.; Liu, Z. A novel NET-related gene signature for predicting DLBCL prognosis. J. Transl. Med. 2023, 21, 630. [Google Scholar] [CrossRef]
- Nie, M.; Yang, L.; Bi, X.; Wang, Y.; Sun, P.; Yang, H.; Liu, P.; Li, Z.; Xia, Y.; Jiang, W. Neutrophil Extracellular Traps Induced by IL8 Promote Diffuse Large B-cell Lymphoma Progression via the TLR9 Signaling. Clin. Cancer Res. 2019, 25, 1867–1879. [Google Scholar] [CrossRef]
- Rondon, A.M.R.; Kroone, C.; Kapteijn, M.Y.; Versteeg, H.H.; Buijs, J.T. Role of Tissue Factor in Tumor Progression and Cancer-Associated Thrombosis. Semin. Thromb. Hemost. 2019, 45, 396–412. [Google Scholar] [CrossRef]
- Shehata, A.M.F.; Aldesoky, A.I.; Gohar, S.F. Plasma fibrinogen level as possible prognostic biomarker in diffuse large B-cell lymphoma. Hematology 2019, 24, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Troppan, K.T.; Melchardt, T.; Wenzl, K.; Schlick, K.; Deutsch, A.; Bullock, M.D.; Reitz, D.; Beham-Schmid, C.; Weiss, L.; Neureiter, D.; et al. The clinical significance of fibrinogen plasma levels in patients with diffuse large B cell lymphoma. J. Clin. Pathol. 2016, 69, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.; Antic, D.; Tafur, A.; Bontekoe, E.; Hoppensteadt, D.; Gerotziafas, G.; Elalamy, I.; Fareed, J. Thrombin Generation Profile in Various Lymphoma Sub-Groups and Its Augmentation by Andexanet Alfa. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620983466. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.F.B.; Fregnani, J.H.T.G.; Strunz, C.M.C.; de Andrade Ramos, N.A.; Longatto-Filho, A. Role of P-selectin in thromboembolic events in patients with cancer. Mol. Clin. Oncol. 2018, 8, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Haznedaroglu, I.C.; Benekli, M.; Ozcebe, O.; Savaş, M.C.; Güllü, I.H.; Dündar, S.V.; Kirazli, S. Serum L-selectin and P-selectin levels in lymphomas. Haematologia 2000, 30, 27–30. [Google Scholar] [CrossRef]


| Spearman or Pearson | HGB/ PT-s | MCV/ aPTT | MCH/ β2M | MCHC/MPV | RDW/ PDW | PLT/ ECOG PS | PCT/ Bulky | INR/ Khorana Score | PT%/ ECOG PS | Fibrinogen | ESR/ThroLy Score | NLR/ CRP | PLR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-1β | ρ/r | 0.203 | 0.17 | 0.259 | 0.324 | −0.256 | −0.217 | −0.231 | −0.213 | −0.214 | −0.246 | −0.175 | −0.22 | |
| 95% CI | 0.032–0.36 | −0.002–0.33 | 0.09–0.41 | 0.16–0.47 | −0.41–−0.09 | −0.37–−0.05 | −0.39–−0.06 | −0.37–−0.04 | −0.37–−0.04 | −0.4–−0.076 | −0.34–−0.003 | −0.38–−0.05 | ||
| p value | 0.017 | 0.046 | 0.002 | 0.0001 | 0.002 | 0.011 | 0.006 | 0.013 | 0.012 | 0.004 | 0.04 | 0.01 | ||
| FVIII | ρ/r | 0.175 | 0.214 | 0.267 | 0.254 | −0.278 | 0.335 | |||||||
| 95% CI | 0.003–0.337 | 0.043–0.37 | 0.1–0.42 | 0.086–0.41 | −0.43–−0.11 | 0.029–0.58 | ||||||||
| p value | 0.04 | 0.012 | 0.002 | 0.003 | 0.001 | 0.028 | ||||||||
| IL-8 | ρ/r | −0.214 | 0.245 | 0.237 | 0.204 | −0.301 | −0.213 | −0.302 | −0.216 | −0.181 | −0.235 | |||
| 95% CI | −0.37–−0.04 | 0.07–0.4 | 0.07–0.39 | 0.03–0.36 | −0.45–−0.14 | −0.37–−0.04 | −0.559–0.007 | −0.37–−0.045 | −0.34–−0.009 | −0.39–−0.07 | ||||
| p value | 0.012 | 0.004 | 0.005 | 0.017 | 0.0003 | 0.012 | 0.049 | 0.0111 | 0.0336 | 0.0055 | ||||
| Fibrinogen | ρ/r | −0.24 | −0.236 | 0.1977 | −0.468 * | |||||||||
| 95% CI | −0.4–−0.07 | −0.39–−0.066 | 0.03–0.36 | −0.744–−0.057 | ||||||||||
| p value | 0.0045 | 0.0054 | 0.0201 | 0.0242 | ||||||||||
| TNF-α | ρ/r | −0.185 | −0.173 | −0.204 | 0.2617 | 0.2699 | −0.624 * | |||||||
| 95% CI | −0.35–−0.013 | −0.34–−0.001 | −0.36–−0.032 | 0.09–0.42 | 0.1–0.42 | −0.828–−0.274 | ||||||||
| p value | 0.0301 | 0.0422 | 0.017 | 0.0019 | 0.0014 | 0.0014 | ||||||||
| P-selectin | ρ/r | 0.168 | −0.192 | −0.275 | −0.288 | 0.272 | −0.424 | 0.169 | −0.211 | 0.184 | ||||
| 95% CI | −0.01–0.33 | −0.35–−0.021 | −0.43–−0.11 | −0.44–−0.12 | 0.1–0.42 | −0.718–−0.001 | −0.003–0.33 | −0.37–−0.04 | 0.012–0.35 | |||||
| p value | 0.0496 | 0.024 | 0.0011 | 0.0006 | 0.0013 | 0.043 | 0.047 | 0.0145 | 0.0314 | |||||
| MCP-1 | ρ/r | −0.191 | −0.178 | 0.1775 | ||||||||||
| 95% CI | −0.35–−0.02 | −0.34–−0.006 | 0.004–0.34 | |||||||||||
| p value | 0.0249 | 0.0366 | 0.0394 | |||||||||||
| Thrombin | ρ/r | −0.24 | 0.222 | 0.2391 | −0.245 | −0.21 | −0.216 | 0.244 | −0.222 | −0.214 | −0.305 | −0.313 | ||
| 95% CI | −0.4–−0.07 | 0.05–0.38 | 0.0–0.39 | −0.4–−0.08 | −0.37–−0.04 | −0.37–−0.05 | 0.07–0.4 | −0.380.05 | −0.37–−0.04 | −0.45–−0.14 | −0.46–−0.15 | |||
| p value | 0.0045 | 0.0089 | 0.0047 | 0.0038 | 0.0137 | 0.01 | 0.0044 | 0.0087 | 0.0128 | 0.0003 | 0.0002 | |||
| TGF-β1 | ρ/r | 0.264 | 0.261 | 0.23 | ||||||||||
| 95% CI | 0.05–0.46 | 0.045–0.45 | 0.013–0.43 | |||||||||||
| p value | 0.0142 | 0.0157 | 0.033 | |||||||||||
| MPO | ρ/r | 0.55 * | −0.56 * | |||||||||||
| 95% CI | 0.05–0.83 | −0.84–−0.05 | ||||||||||||
| p value | 0.035 | 0.032 | ||||||||||||
| cfDNA | ρ/r | −0.46 | −0.44 | 0.447 | 0.355 | −0.588 | 0.429 | |||||||
| 95% CI | −0.68–−0.16 | −0.67–−0.122 | 0.13–0.679 | 0.02–0.615 | −0.85–−0.108 | 0.11–0.667 | ||||||||
| p value | 0.004 | 0.007 | 0.006 | 0.031 | 0.021 | 0.008 | ||||||||
| Spearman | P-Selectin | Tissue Factor | Fibrinogen | TNF-α | MPO | |
|---|---|---|---|---|---|---|
| Lymphoma (no thrombosis n = 106 and with thrombosis n = 33) | ||||||
| Clinical stage | ρ | 0.205 | 0.228 | −0.372 | ||
| 95% CI | 0.01–0.39 | 0.03–0.41 | −0.65–−0.009 | |||
| p value | 0.035 | 0.018 | 0.039 | |||
| International Prognostic Index (IPI) | ρ | −0.357 | −0.242 | −0.448 | ||
| 95% CI | −0.64–0.008 | −0.42–−0.05 | −0.698–−0.1 | |||
| p value | 0.049 | 0.012 | 0.012 | |||
| DLBCL (no thrombosis n = 43 and with thrombosis n = 24) | ||||||
| Clinical stage | ρ | −0.34 | 0.466 | |||
| 95% CI | −0.587–−0.035 | 0.12–0.71 | ||||
| p value | 0.026 | 0.008 | ||||
| International Prognostic Index (IPI) | ρ | −0.426 | ||||
| 95% CI | −0.714–−0.014 | |||||
| p value | 0.038 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Živković, E.; Mitrović-Ajtić, O.; Subotički, T.; Ivanović, J.; Otašević, V.; Đikić, D.; Diklić, M.; Vukotić, M.; Dragojević, T.; Stanisavljević, D.; et al. Thromboinflammatory Biomarkers in Lymphomas: Linking Inflammation to Thrombosis Risk. Int. J. Mol. Sci. 2025, 26, 2058. https://doi.org/10.3390/ijms26052058
Živković E, Mitrović-Ajtić O, Subotički T, Ivanović J, Otašević V, Đikić D, Diklić M, Vukotić M, Dragojević T, Stanisavljević D, et al. Thromboinflammatory Biomarkers in Lymphomas: Linking Inflammation to Thrombosis Risk. International Journal of Molecular Sciences. 2025; 26(5):2058. https://doi.org/10.3390/ijms26052058
Chicago/Turabian StyleŽivković, Emilija, Olivera Mitrović-Ajtić, Tijana Subotički, Jelena Ivanović, Vladimir Otašević, Dragoslava Đikić, Miloš Diklić, Milica Vukotić, Teodora Dragojević, Dejana Stanisavljević, and et al. 2025. "Thromboinflammatory Biomarkers in Lymphomas: Linking Inflammation to Thrombosis Risk" International Journal of Molecular Sciences 26, no. 5: 2058. https://doi.org/10.3390/ijms26052058
APA StyleŽivković, E., Mitrović-Ajtić, O., Subotički, T., Ivanović, J., Otašević, V., Đikić, D., Diklić, M., Vukotić, M., Dragojević, T., Stanisavljević, D., Antić, D., & Čokić, V. P. (2025). Thromboinflammatory Biomarkers in Lymphomas: Linking Inflammation to Thrombosis Risk. International Journal of Molecular Sciences, 26(5), 2058. https://doi.org/10.3390/ijms26052058







