Synthesis, Physicochemical Properties and Anti-Fungal Activities of New Meso-Arylporphyrins
Abstract
1. Introduction
2. Results and Discussion
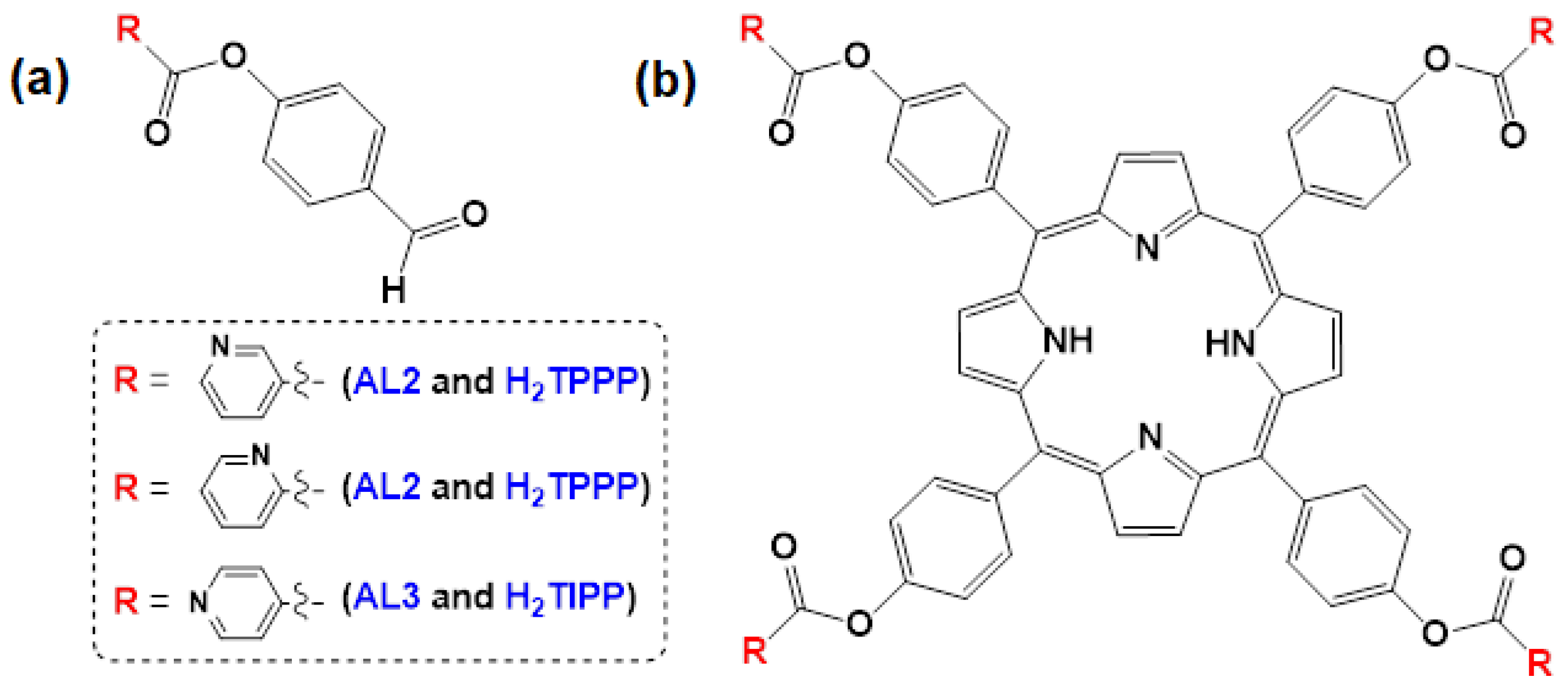
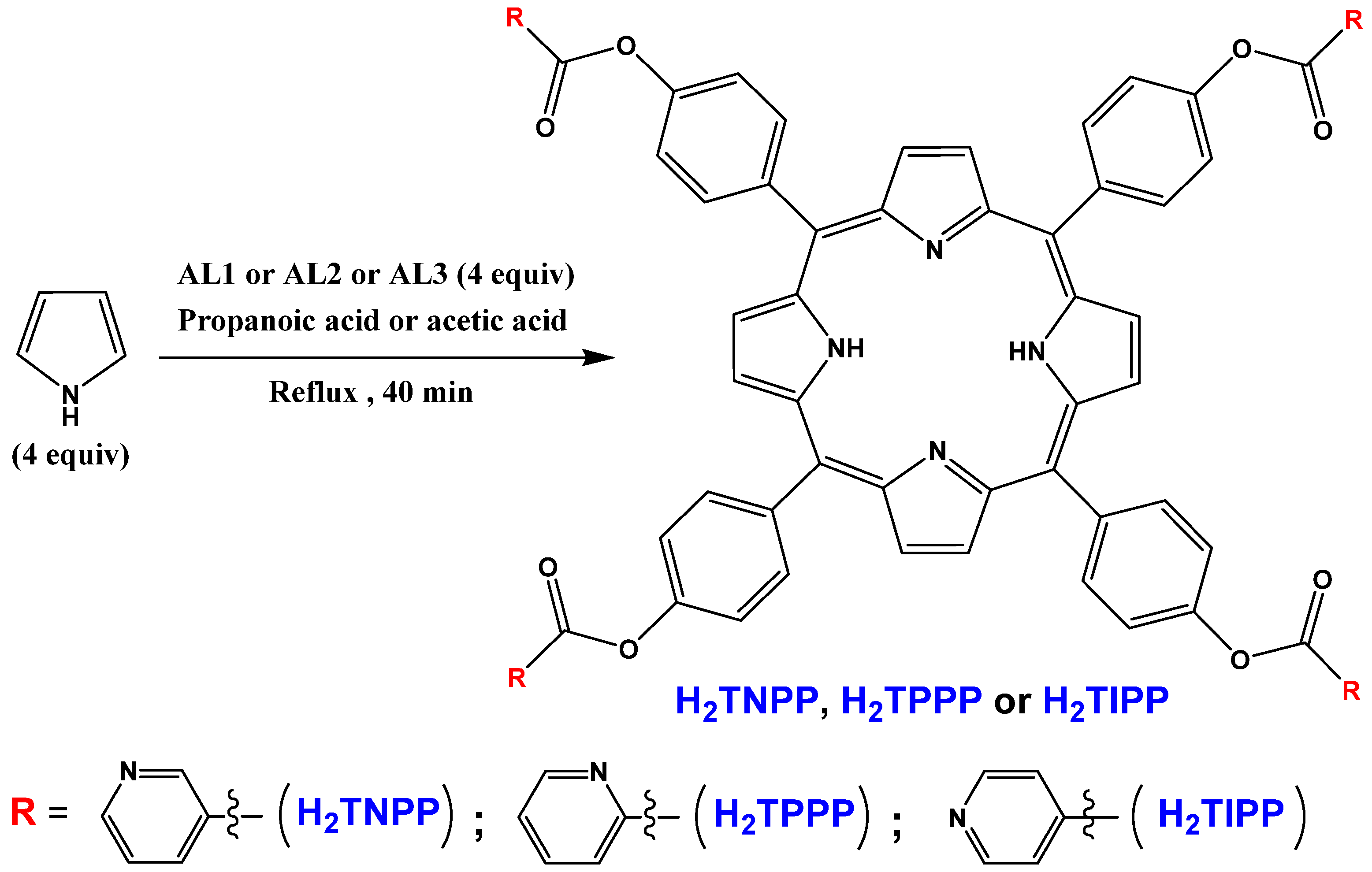
2.1. Synthesis
2.2. Characterization
2.2.1. Proton (1H) and Carbon (13C) J-Modulated Spin-Echo (JMOD) NMR Spectroscopy
2.2.2. IR Spectroscopy
2.2.3. UV–Visible Spectroscopy
2.2.4. Fluorescence Spectroscopy
2.2.5. Singlet Oxygen
2.3. Anti-Fungal Activity
2.3.1. Anti-Candidal Activity
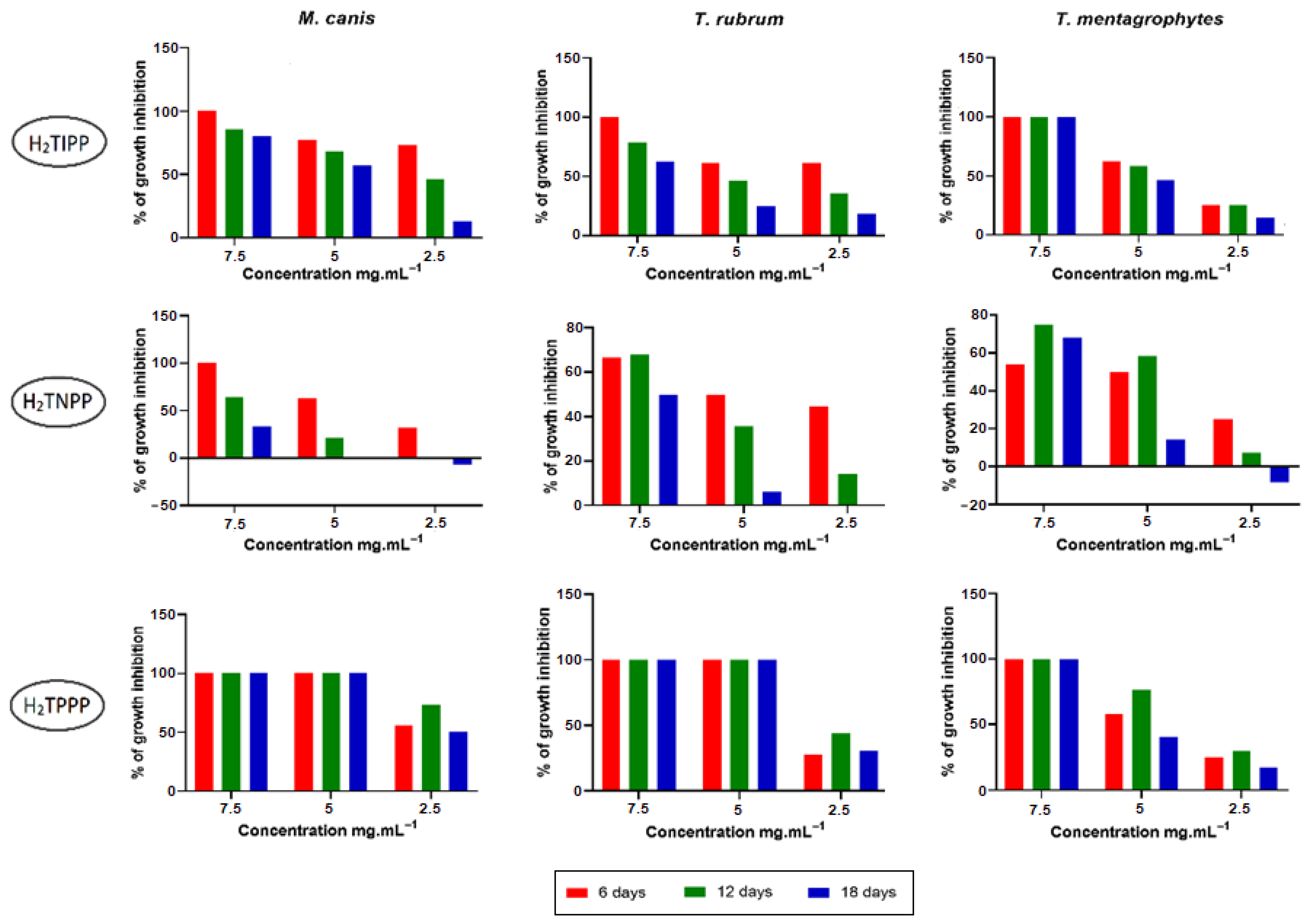
2.3.2. Anti-Dermatophyte Activity
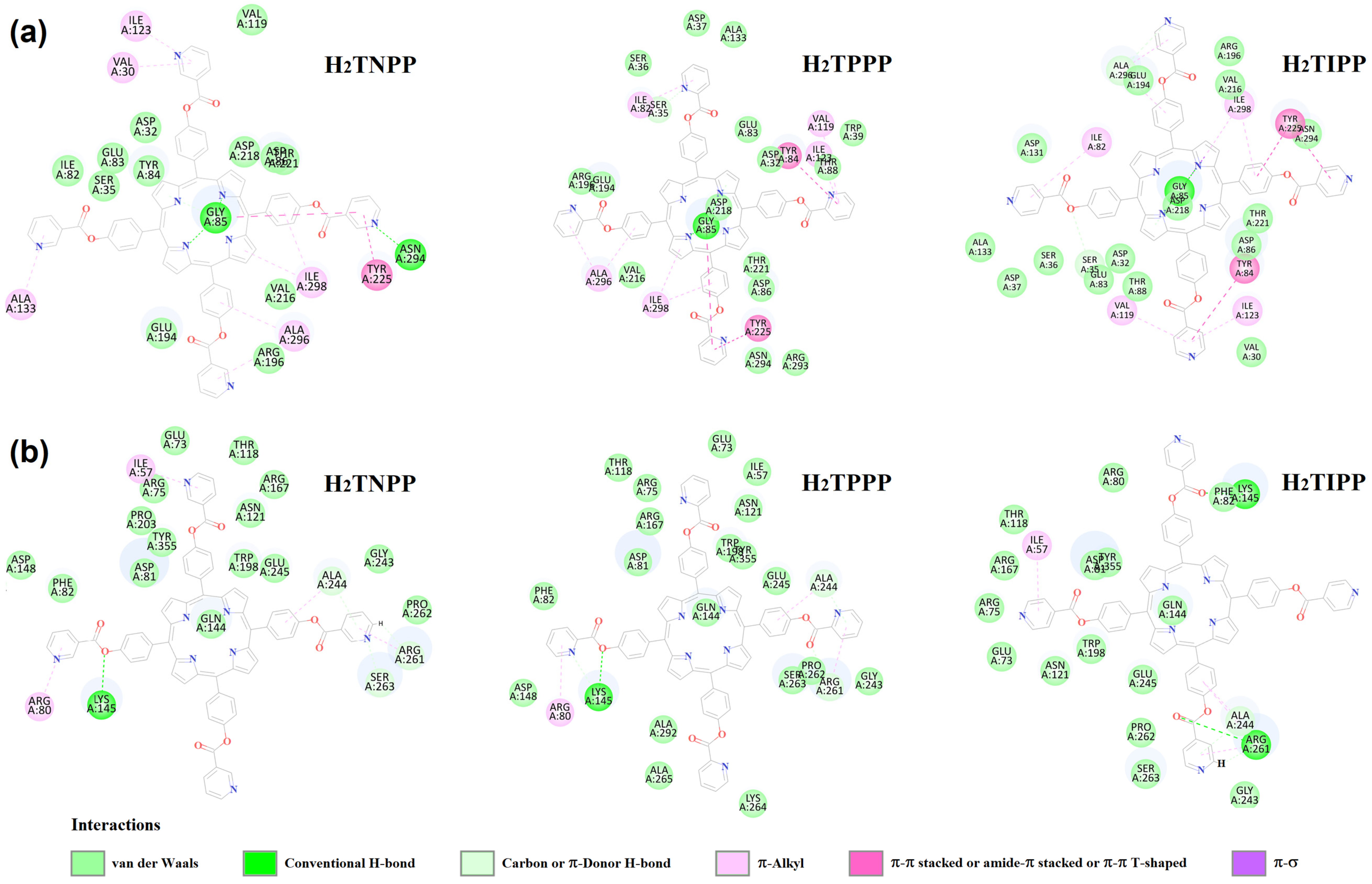
2.4. Molecular Docking Study
2.4.1. Candida tropicalis
2.4.2. Trichophyton rubrum
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Materials Instrumentation
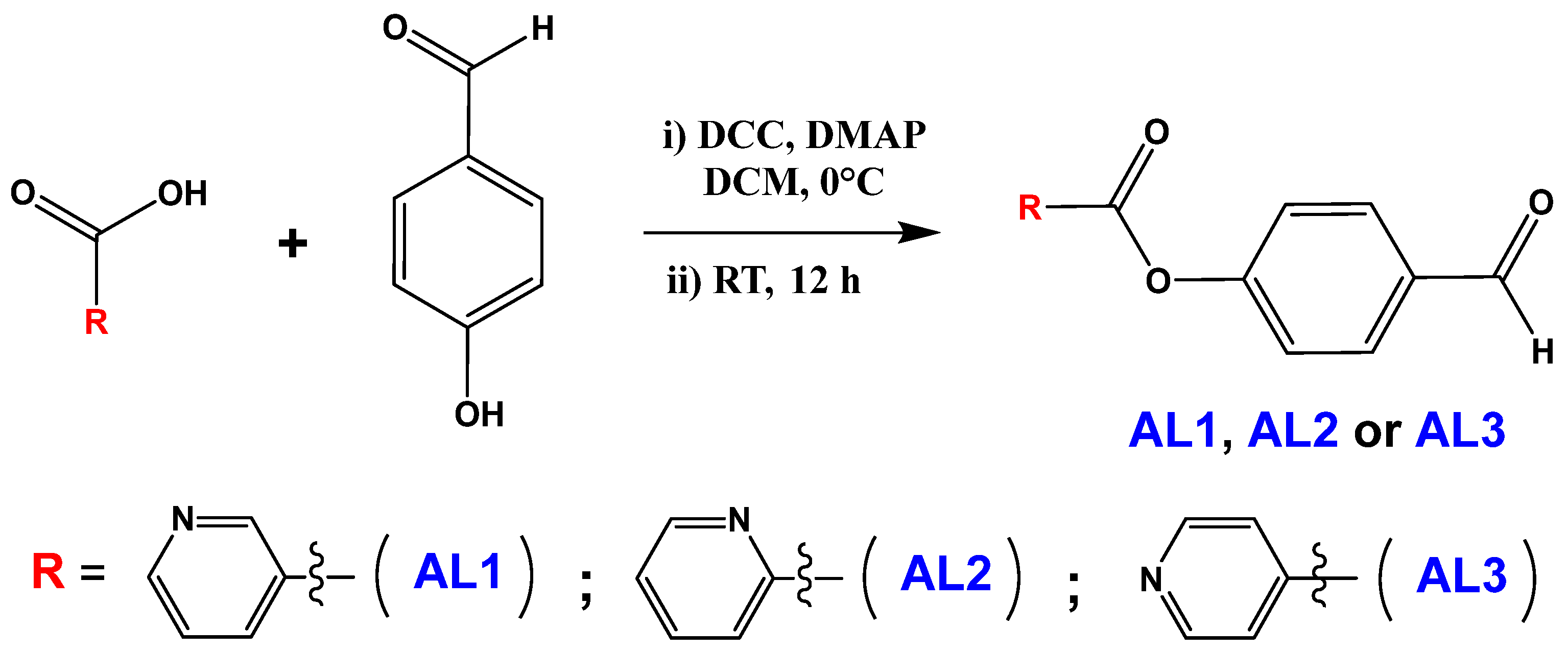
3.3. Aldehydes AL1–AL3
3.3.1. Synthesis
3.3.2. Characterization
3.4. Meso-Arylporphyrins H2TNPP, H2TPPP and H2TIPP
3.4.1. Synthesis
3.4.2. Characterization
3.5. Biological Evaluation
3.5.1. Anti-Candidal Activity
3.5.2. Anti-Dermatophyte Activity
3.6. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denning, D.W. Global Incidence and Mortality of Severe Fungal Disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 2 September 2024).
- O’Neill, J. Infection Prevention, Control and Surveillance: Limiting the Development and Spread of Drug Resistance. In The Review on Antimicrobial Resistance; London, UK, 2016; Available online: https://amr-review.org/sites/default/files/Health%20infrastructure%20and%20surveillance%20final%20version_LR_NO%20CROPS.pdf (accessed on 2 September 2024).
- Ventola, C.L. The Antibiotic Resistance Crisis. P T 2015, 40, 277–283. [Google Scholar]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 2 September 2024).
- Kohler, J.R.; Casadevall, A.; Perfect, J. The Spectrum of Fungi That Infects Humans. Cold Spring Harb. Perspect. Med. 2015, 5, a019273. [Google Scholar] [CrossRef]
- Rokas, A. Evolution of the Human Pathogenic Lifestyle in Fungi. Nat. Microbiol. 2022, 7, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Voit, T.; Cieplik, F.; Regensburger, J.; Hiller, K.-A.; Gollmer, A.; Buchalla, W.; Maisch, T. Spatial Distribution of a Porphyrin-Based Photosensitizer Reveals Mechanism of Photodynamic Inactivation of Candida Albicans. Front. Med. 2021, 8, 641244. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, F.; Linhardt, R.J. Porphyrin-Based Compounds and Their Applications in Materials and Medicine. Dye. Pigment. 2021, 188, 109136. [Google Scholar] [CrossRef]
- Saulle, C.C.; Claus, A.; Sales, L.D.A.; Gonçalves, A.G.; Ducatti, D.R.B.; Noseda, M.D.; May De Mio, L.L. Photoinactivation of Colletotrichum truncatum, Corynespora cassiicola, Sclerotinia sclerotiorum and Rhizoctonia solani in Soybean Seeds by Cationic Porphyrins. Plant Pathol. 2023, 72, 67–75. [Google Scholar] [CrossRef]
- Kolyada, M.N.; Osipova, V.P.; Berberova, N.T.; Shpakovsky, D.B.; Milaeva, E.R. Porphyrins with Phenolic Fragments at the Periphery of the Macrocycle as Perspective Antioxidants, Cytoprotectors and Heavy Metal Scavengers. Chem. Heterocycl. Compd. 2021, 57, 875–888. [Google Scholar] [CrossRef]
- Mihai, D.P.; Boscencu, R.; Manda, G.; Burloiu, A.M.; Vasiliu, G.; Neagoe, I.V.; Socoteanu, R.P.; Lupuliasa, D. Interaction of Some Asymmetrical Porphyrins with U937 Cell Membranes–In Vitro and In Silico Studies. Molecules 2023, 28, 1640. [Google Scholar] [CrossRef]
- Han, R.; Kim, S.; Janda, K.J.; Fleischer, E.B. Structural Study of Fluoro-Tetraphenylporphyrins Relating to Large Variability in Solubilities of the Para-F-TPP, Ortho-F-TPP and Meta-F-TPP. J. Porphyrins Phthalocyanines 2018, 22, 355–358. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Effects of Substituents on the Photophysical Properties of Symmetrical Porphyrins. Dye. Pigment. 2013, 96, 440–448. [Google Scholar] [CrossRef]
- Nasri, S.; Zahou, I.; Turowska-Tyrk, I.; Roisnel, T.; Loiseau, F.; Saint-Amant, E.; Nasri, H. Synthesis, Electronic Spectroscopy, Cyclic Voltammetry, Photophysics, Electrical Properties and X-ray Molecular Structures of Meso-{Tetrakis[4-(Benzoyloxy)Phenyl]Porphyrinato}zinc(II) Complexes with Aza Ligands. Eur. J. Inorg. Chem. 2016, 2016, 5004–5019. [Google Scholar] [CrossRef]
- Amiri, N.; Guergueb, M.; Al-Fakeh, M.S.; Bourguiba, M.; Nasri, H. A New Cobalt(II) Meso-Porphyrin: Synthesis, Characterization, Electric Properties and Application in the Catalytic Degradation of Dyes. RSC Adv. 2020, 10, 44920–44932. [Google Scholar] [CrossRef]
- Dp, L.-J. The Candida Genus Complex: Biology, Evolution, Pathogenicity Virulence and One Health Aspects, Beyond the Candida Albicans Paradigm. A Comprehensive Review. Virol. Immunol. J. 2023, 7, 1–38. [Google Scholar] [CrossRef]
- Ghannoum, M. Azole Resistance in Dermatophytes. J. Am. Podiatr. Med. Assoc. 2016, 106, 79–86. [Google Scholar] [CrossRef]
- Mohammadifard, H.; Amini, K.; Bayat, M.; Hashemi, S.J.; Noorbakhsh, F. Molecular Study and Antifungal Susceptibility Profile of Trichophyton Rubrum and Trichophyton Mentagrophytes Strains Isolated from Lesions of Humans and Cattle. Iran. J. Microbiol. 2022, 14, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Aided-Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Bras, G.; Satala, D.; Juszczak, M.; Kulig, K.; Wronowska, E.; Bednarek, A.; Zawrotniak, M.; Rapala-Kozik, M.; Karkowska-Kuleta, J. Secreted Aspartic Proteinases: Key Factors in Candida Infections and Host-Pathogen Interactions. Int. J. Mol. Sci. 2024, 25, 4775. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A Simplified Synthesis for Meso-Tetraphenylporphine. J. Org. Chem. 1967, 32, 476. [Google Scholar] [CrossRef]
- Mkacher, H. Préparation et Caractérisations Spectrale et Structurale de Nouvelles Méso-Arylporphyrines et Métalloporphyrines de Manganèse(III). Ph.D Thesis, Faculty of Sciences of Monastir, University of Monastir, Monastir, Tunisia, 2023. [Google Scholar]
- Amiri, N.; Taheur, F.B.; Chevreux, S.; Wenger, E.; Lemercier, G.; Nasri, H. Synthesis, Crystal Structure and Spectroscopic Characterizations of Porphyrin-Based Mg(II) Complexes–Potential Application as Antibacterial Agent. Tetrahedron 2017, 73, 7011–7016. [Google Scholar] [CrossRef]
- Mkacher, H.; Gassoumi, B.; Dardouri, N.E.; Nasri, S.; Loiseau, F.; Molton, F.; Roisnel, T.; Turowska-Tyrk, I.; Ghala, H.; Acherar, S.; et al. Photophysical, Cyclic Voltammetry, Electron Paramagnetic Resonance, X-Ray Molecular Structure, DFT Calculations and Molecular Docking Study of a New Mn(III) Metalloporphyrin. J. Mol. Struct. 2025, 1319, 139455. [Google Scholar] [CrossRef]
- Mkacher, H.; Taheur, F.B.; Amiri, N.; Almahri, A.; Loiseau, F.; Molton, F.; Vollbert, E.M.; Roisnel, T.; Turowska-Tyrk, I.; Nasri, H. DMAP and HMTA Manganese(III) Meso-Tetraphenylporphyrin-Based Coordination Complexes: Syntheses, Physicochemical Properties, Structural and Biological Activities. Inorg. Chim. Acta 2023, 545, 121278. [Google Scholar] [CrossRef]
- Amiri, N.; Ben Taheur, F.; Chevreux, S.; Rodrigues, C.M.; Dorcet, V.; Lemercier, G.; Nasri, H. Syntheses, Crystal Structures, Photo-Physical Properties, Antioxidant and Antifungal Activities of Mg(II) 4,4′-Bipyridine and Mg(II) Pyrazine Complexes of the 5,10,15,20 Tetrakis(4–Bromophenyl)Porphyrin. Inorg. Chim. Acta 2021, 525, 120466. [Google Scholar] [CrossRef]
- Amiri, N.; Bourguiba, M.; Guergueb, M.; Chevreux, S.; Nasri, H. Synthesis, Molecular Structure, Spectroscopic Characterization and Dielectric Properties of New Cobalt(II) Meso-Tetraphenylporphyrin-Based Coordination Complex. Inorg. Chem. Commun. 2020, 118, 107995. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Kanofsky, J.R.; Hoogland, H.; Wever, R.; Weiss, S.J. Singlet Oxygen Production by Human Eosinophils. J. Biol. Chem. 1988, 263, 9692–9696. [Google Scholar] [CrossRef]
- Agnez-Lima, L.F.; Melo, J.T.A.; Silva, A.E.; Oliveira, A.H.S.; Timoteo, A.R.S.; Lima-Bessa, K.M.; Martinez, G.R.; Medeiros, M.H.G.; Di Mascio, P.; Galhardo, R.S.; et al. DNA Damage by Singlet Oxygen and Cellular Protective Mechanisms. Mutat. Res. Mol. Mech. Mutagen. 2012, 751, 15–28. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Lebrun, V.; Tron, A.; Scarpantonio, L.; Lebrun, C.; Ravanat, J.; Latour, J.; McClenaghan, N.D.; Sénèque, O. Efficient Oxidation and Destabilization of Zn(Cys)4 Zinc Fingers by Singlet Oxygen. Angew. Chem. 2014, 126, 9519–9522. [Google Scholar] [CrossRef]
- Sadiq, F.; Zhao, J.; Hussain, M.; Wang, Z. Effect of Thiophene Substitution on the Intersystem Crossing of Arene Photosensitizers. Photochem. Photobiol. Sci. 2018, 17, 1794–1803. [Google Scholar] [CrossRef]
- Jabli, S.; Hrichi, S.; Chaabane-Banaoues, R.; Molton, F.; Loiseau, F.; Roisnel, T.; Turowska-Tyrk, I.; Babba, H.; Nasri, H. Study on the Synthesis, Physicochemical, Electrochemical Properties, Molecular Structure and Antifungal Activities of the 4-Pyrrolidinopyridine Mg(II) Meso-Tetratolylporphyrin Complex. J. Mol. Struct. 2022, 1261, 132882. [Google Scholar] [CrossRef]
- Eliaš, D.; Tóth Hervay, N.; Gbelská, Y. Ergosterol Biosynthesis and Regulation Impact the Antifungal Resistance and Virulence of Candida spp. Stresses 2024, 4, 641–662. [Google Scholar] [CrossRef]
- Martinez-Rossi, N.M.; Bitencourt, T.A.; Peres, N.T.A.; Lang, E.A.S.; Gomes, E.V.; Quaresemin, N.R.; Martins, M.P.; Lopes, L.; Rossi, A. Dermatophyte Resistance to Antifungal Drugs: Mechanisms and Prospectus. Front. Microbiol. 2018, 9, 1108. [Google Scholar] [CrossRef]
- Arooj, M.; Sakkiah, S.; Cao, G.P.; Lee, K.W. An Innovative Strategy for Dual Inhibitor Design and Its Application in Dual Inhibition of Human Thymidylate Synthase and Dihydrofolate Reductase Enzymes. PLoS ONE 2013, 8, e60470. [Google Scholar] [CrossRef]
- Tanwar, O.; Deora, G.S.; Tanwar, L.; Kumar, G.; Janardhan, S.; Alam, M.M.; Shaquiquzzaman, M.; Akhter, M. Novel Hydrazine Derivatives as Selective DPP-IV Inhibitors: Findings from Virtual Screening and Validation through Molecular Dynamics Simulations. J. Mol. Model. 2014, 20, 2118. [Google Scholar] [CrossRef]
- Sapundzhi, F.; Prodanova, K.; Lazarova, M. Survey of the Scoring Functions for Protein-Ligand Docking. AIP Conf. Proc. 2019, 2172, 100008. [Google Scholar]
- Symersky, J.; Monod, M.; Foundling, S.I. High-Resolution Structure of the Extracellular Aspartic Proteinase from Candida Tropicalis Yeast. Biochemistry 1997, 36, 12700–12710. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDBe): The Single Global Macromolecular Structure Archive. In Protein Crystallography: Methods and Protocols; Wlodawer, A., Dauter, Z., Jaskolski, M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; pp. 627–641. ISBN 978-1-4939-7000-1. [Google Scholar]
- Nesbitt, J.R.; Steves, E.Y.; Schonhofer, C.R.; Cait, A.; Manku, S.S.; Yeung, J.H.F.; Bennet, A.J.; McNagny, K.M.; Choy, J.C.; Hughes, M.R.; et al. The Aspergillus Fumigatus Sialidase (Kdnase) Contributes to Cell Wall Integrity and Virulence in Amphotericin B-Treated Mice. Front. Microbiol. 2018, 8, 2706. [Google Scholar] [CrossRef]
- Listvan, V.N.; Listvan, V.V.; Shekel’, A.N. Synthesis of Cholesteryl Esters of Heterocyclic Analogs of Cinnamic Acid and Hetaroyloxycinnamic Acids by the Wittig Reaction. Chem. Heterocycl. Compd. 2002, 38, 1480–1483. [Google Scholar] [CrossRef]
- Zhu, D.; Luo, Y.; Shuai, L.; Xie, W.; Yan, X.; Duan, Z.; Cai, W. A Hemicyanine-Based Selective and Sensitive Colorimetric and Fluorescent Turn-on Probe for Cu2+. Tetrahedron Lett. 2016, 57, 5326–5329. [Google Scholar] [CrossRef]
- Kandasamy, P.; Keerthiga, R.; Vijayalakshmi, S.; Kaliyappan, T. Synthesis and Liquid Crystal Properties of Supramolecular Side-Chain Liquid-Crystalline Polymers Containing Poly(Acrylic Acid) Intermolecular Hydrogen Bonds. Mol. Cryst. Liq. Cryst. 2015, 606, 1–11. [Google Scholar] [CrossRef]
- Hrichi, S.; Chaabane-Banaoues, R.; Giuffrida, D.; Mangraviti, D.; Oulad El Majdoub, Y.; Rigano, F.; Mondello, L.; Babba, H.; Mighri, Z.; Cacciola, F. Effect of Seasonal Variation on the Chemical Composition and Antioxidant and Antifungal Activities of Convolvulus Althaeoides L. Leaf Extracts. Arab. J. Chem. 2020, 13, 5651–5668. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and Validation of a Genetic Algorithm for Flexible Docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Gholam, G.M.; Firdausy, I.A.; Artika, I.M.; Abdillah, R.M.; Firmansyah, R.P. Molecular Docking: Bioactive Compounds of Mimosa pudica as an Inhibitor of Candida albicans Sap 3. bioRxiv 2022. [Google Scholar] [CrossRef]

| Attribution of Bands | IR Bands (cm−1) | ||
|---|---|---|---|
| H2TNPP | H2TPPP | H2TIPP | |
| υ(NH) pyrrolic | 3310 | 3310 | 3310 |
| υ(CH) porphyrin | 3099–2970 | 3066–2971 | 3067–2973 |
| υ(C=O) ester | 1732 | 1739 | 1743 |
| υ(C-O) ester | 1270 | 1268 | 1266 |
| δ(CCH) porphyrin | 966 | 965 | 965 |
| Compounds | UV–Visible Absorption Data | Fluorescence Emission Data (λmax = 420 nm) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bands λmax (nm) (log ε) | Egap (eV) | Emission (nm) | ϕf | |||||||
| Soret | QIV | QIII | QII | QI | Q(0,0) | Q(0,1) | ||||
| H2TPP 1 | 419 (5.48) | 516 (4.70) | 551 (4.33) | 591 (4.19) | 648 (4.17) | 1.877 | 652 | 715 | 0.20 | [25,26] |
| H2TTP 2 | 420 (5.96) | 518 (4.82) | 553 (4.27) | 593 (4.16) | 650 (4.09) | 1.859 | 653 | 720 | 0.20 | [25] |
| H2TClPP 3 | 420 (5.49) | 516 (4.93) | 551 (4.54) | 591 (4.48) | 649 (4.40) | 1.921 | 650 | 715 | 0.13 | [25,27] |
| H2TMPP 4 | 423 (5.55) | 519 (4.38) | 557 (4.31) | 595 (4.26) | 652 (4.20) | 1.842 | 657 | 724 | 0.39 | [28] |
| H2TBrPP 5 | 419 (6.65) | 515 (5.31) | 549 (4.93) | 590 (4.77) | 648 (4.68) | - | 654 | 720 | 0.04 | [29] |
| H2TPBP 6 | 419 (5.90) | 515 (4.46) | 551 (4.13) | 590 (3.94) | 646 (3.84) | - | 653 | 715 | 0.04 | [17,30] |
| H2TMAPP 7 | 425 (5.92) | 522 (5.67) | 550 (4.32) | 597 (4.15) | 653 (3.98) | 655 | 719 | 0.05 | [18] | |
| H2TNPP | 419 (5.97) | 516 (4.51) | 551 (4.06) | 591 (3.73) | 647 (3.44) | 1.875 | 650 | 716 | 0.13 | t.w. 8 |
| H2TPPP | 419 (5.86) | 516 (4.40) | 551 (3.93) | 591 (3.61) | 647 (3.32) | 1.874 | 650 | 716 | 0.16 | t.w. 8 |
| H2TIPP | 419 (5.75) | 516 (4.29) | 551 (3.80) | 591 (3.48) | 647 (3.20) | 1.876 | 650 | 716 | 0.19 | t.w. 8 |
| Compounds | Excitation (nm) | Emission (nm) | ϕΔ | Ref. |
|---|---|---|---|---|
| H2TPP 1 | 420 | 1270 | 0.62 | [36] |
| H2TTP 2 | 420 | 1275 | 0.60 | [25] |
| H2TClPP 3 | 420 | 1275 | 0.77 | [25] |
| H2TMPP 4 | 420 | 1275 | 0.59 | [25] |
| H2TNPP | 420 | 1275 | 0.51 | t.w. 5 |
| H2TPPP | 420 | 1275 | 0.52 | t.w. 5 |
| H2TIPP | 420 | 1275 | 0.53 | t.w. 5 |
| Compounds | C. albicans (ATCC 90028) | C. glabrata (ATCC 64677) | C. tropicalis (ATCC 66029) | |||
|---|---|---|---|---|---|---|
| MIC (mg/mL) | MFC (mg/mL) | MIC (mg/mL) | MFC (mg/mL) | MIC (mg/mL) | MFC (mg/mL) | |
| H2TIPP | 1.25 | 2.5 | 2.5 | >10 | 5 | 5 |
| H2TNPP | 5 | 5 | 5 | >10 | >10 | - |
| H2TPPP | 2.5 | 2.5 | 1.25 | 2.5 | - | - |
| Compounds | C. tropicalis | T. rubrum | ||
|---|---|---|---|---|
| Binding Energy (PLP Fitness) | H-Bond and Short Contact Interactions | Binding Energy (PLP Fitness) | H-Bond and Short Contact Interactions | |
| H2TNPP | 146.578 | G85-Y225-I123-V30-I298 A296-A133 | 96.761 | K145-R261-A244-S263-R80 |
| H2TPPP | 141.545 | G85-Y84-Y225-I298-A296 | 89.578 | K145-R261-A244-R80 |
| H2TIPP | 148.964 | G85-Y225-A296-I123-Y84 V119-I82-S35-I298 | 88.6343 | K145-R261-A244-I57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mkacher, H.; Chaâbane-Banaoues, R.; Hrichi, S.; Arnoux, P.; Babba, H.; Frochot, C.; Nasri, H.; Acherar, S. Synthesis, Physicochemical Properties and Anti-Fungal Activities of New Meso-Arylporphyrins. Int. J. Mol. Sci. 2025, 26, 1991. https://doi.org/10.3390/ijms26051991
Mkacher H, Chaâbane-Banaoues R, Hrichi S, Arnoux P, Babba H, Frochot C, Nasri H, Acherar S. Synthesis, Physicochemical Properties and Anti-Fungal Activities of New Meso-Arylporphyrins. International Journal of Molecular Sciences. 2025; 26(5):1991. https://doi.org/10.3390/ijms26051991
Chicago/Turabian StyleMkacher, Hayfa, Raja Chaâbane-Banaoues, Soukaina Hrichi, Philippe Arnoux, Hamouda Babba, Céline Frochot, Habib Nasri, and Samir Acherar. 2025. "Synthesis, Physicochemical Properties and Anti-Fungal Activities of New Meso-Arylporphyrins" International Journal of Molecular Sciences 26, no. 5: 1991. https://doi.org/10.3390/ijms26051991
APA StyleMkacher, H., Chaâbane-Banaoues, R., Hrichi, S., Arnoux, P., Babba, H., Frochot, C., Nasri, H., & Acherar, S. (2025). Synthesis, Physicochemical Properties and Anti-Fungal Activities of New Meso-Arylporphyrins. International Journal of Molecular Sciences, 26(5), 1991. https://doi.org/10.3390/ijms26051991









