Significant Impact of Growth Medium on Itraconazole Susceptibility in Azole-Resistant Versus Wild-Type Trichophyton indotineae, rubrum, and quinckeanum Isolates
Abstract
1. Introduction
2. Results
2.1. Characterization of Dermatophyte Isolates and Overview of Experimental Conditions
2.2. Genomic Copy Number Analysis Reveals Erg11B Amplification Is Specific to T. indotineae Isolates and Absent in T. rubrum
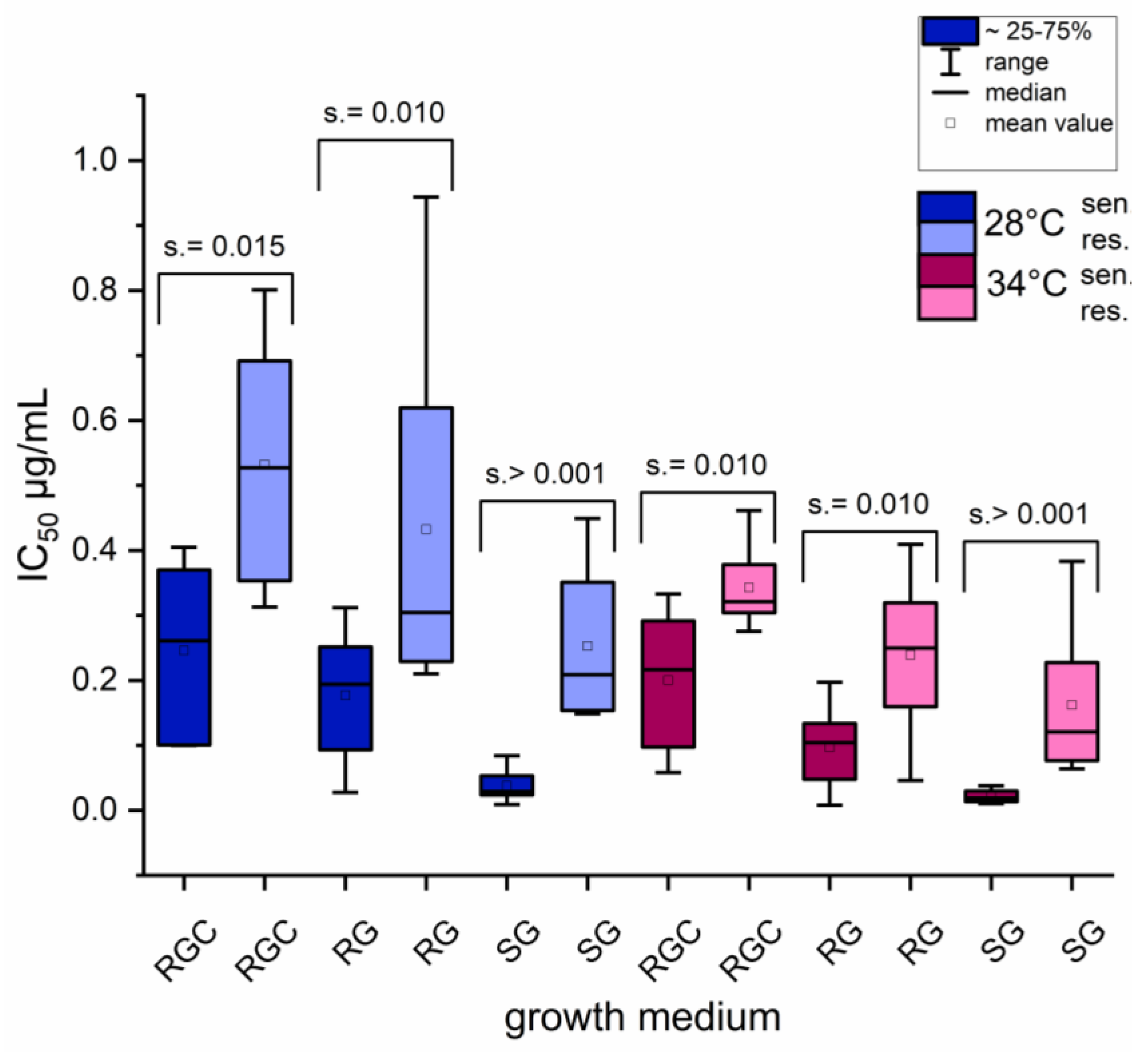
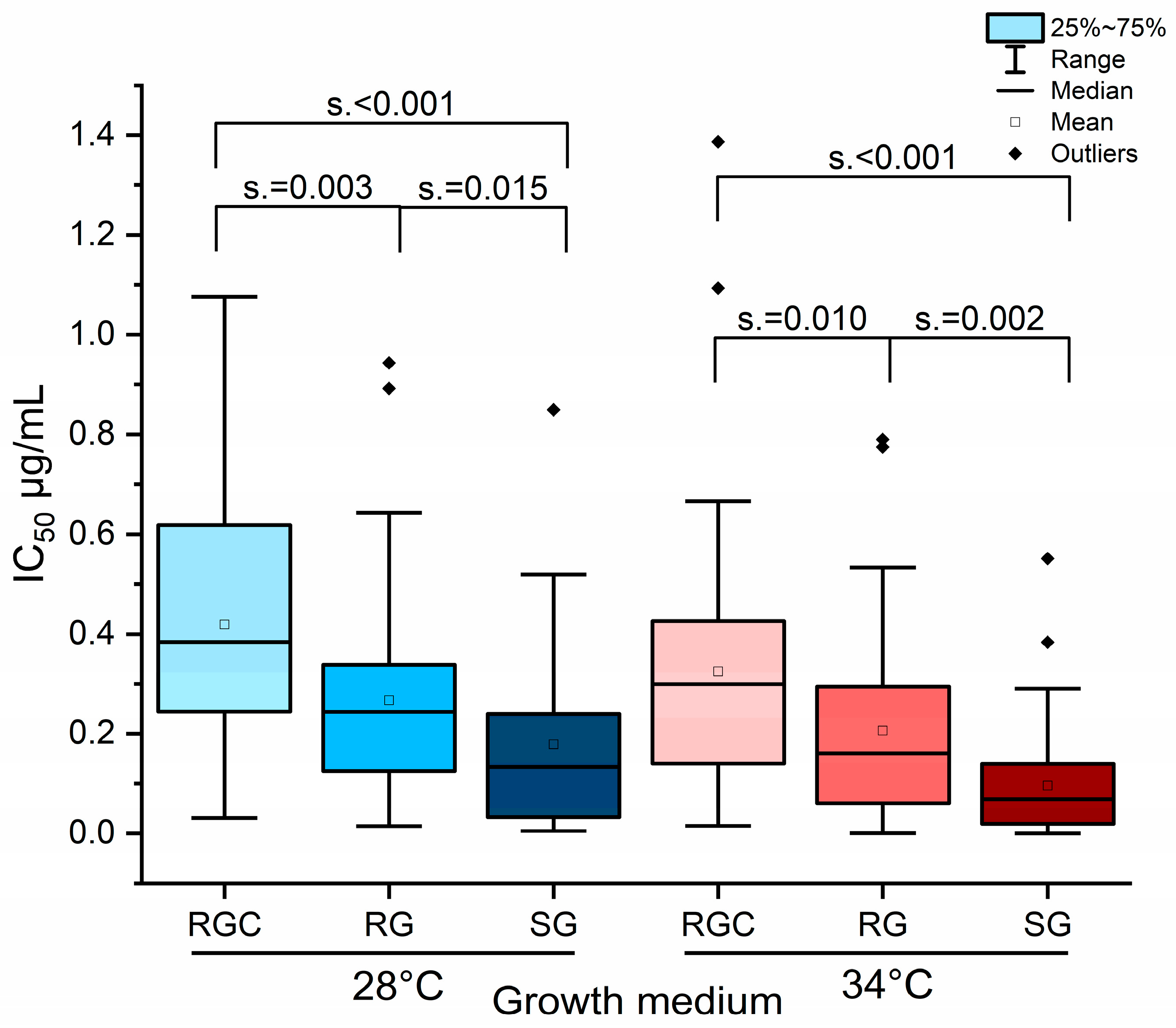
2.3. Influence of Growth Medium and Temperature on Phenotypic Discrimination Azole-Resistant and Sensitive Strains by Itraconazole Inhibitory Concentration (IC) Measurements
2.4. Temperature Shift to 34 °C Resulted in Reduced IC Values Across All Media, with the Strongest Effect Observed in SG Medium
2.5. RGC Medium Enhances Growth Compared to RG and Shows Intermediate Performance Between RG and SG Medium
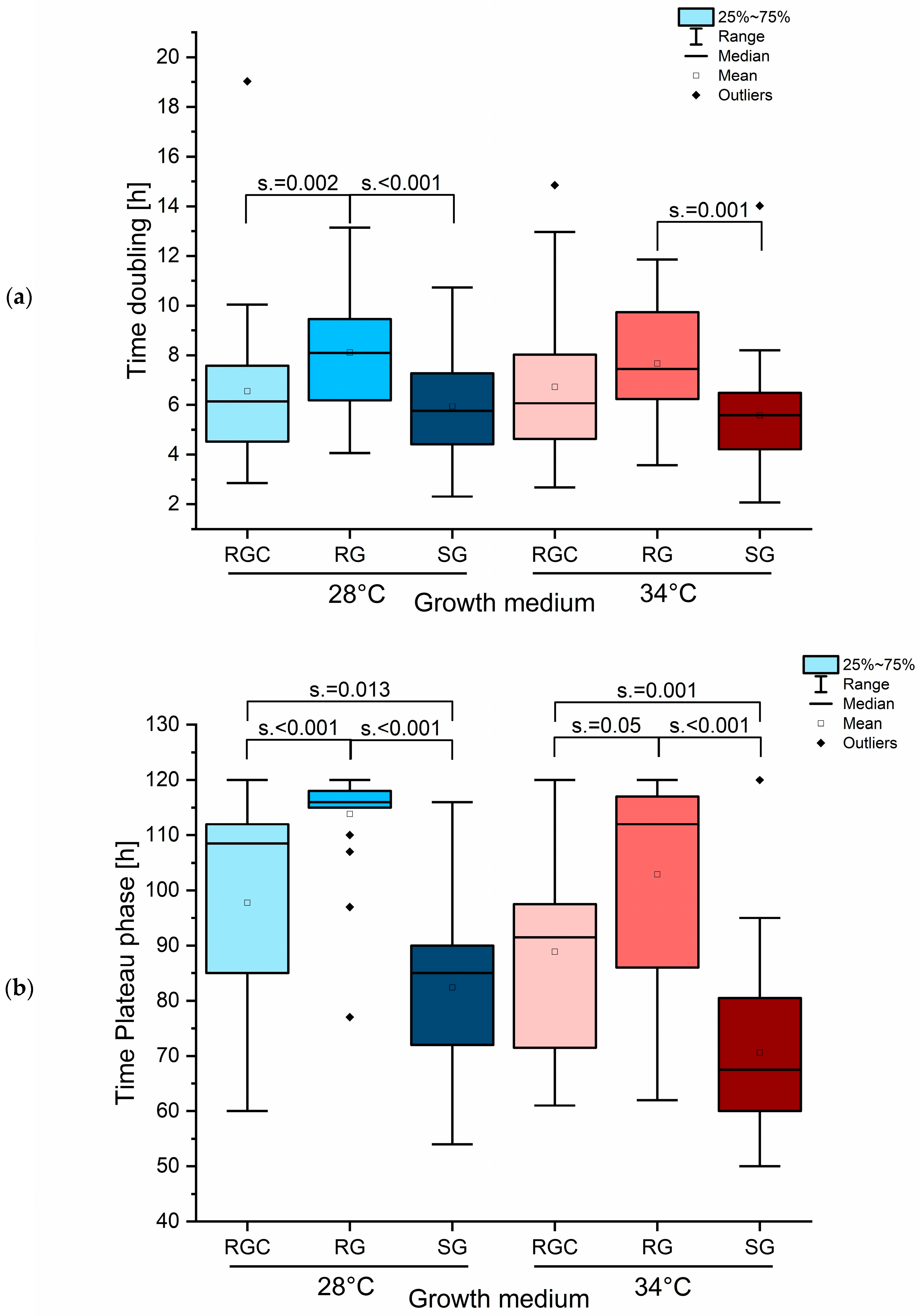
2.6. Species-Specific Growth Analysis Revealed Impaired Growth of T. benhamiae/erinacei and T. indotineae in RG Medium
2.7. Effect of Spore Titer Reduction on LAG Phase Duration Revealed Impaired Growth in RG Medium
3. Discussion
4. Materials and Methods
4.1. Strains and Growth Conditions
4.2. Type II and I Specific PCR, qPCR, and DNA Sequencing
4.3. Microplate Laser Nephelometry (MLN) Assays
4.4. Graphical Image Preparation, Data Performance, and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IC | Inhibitory concentration |
| RG | RPMI1640 derived medium, 2% glucose, MOPS pH 7 |
| RGC | RPMI1640 derived medium, 2% glucose, MOPS pH 7, casein |
| RP | RPMI1640 derived medium, MOPS pH 7 |
| SG | Sabouraud, 2% glucose medium |
References
- Yamada, T.; Maeda, M.; Alshahni, M.M.; Tanaka, R.; Yaguchi, T.; Bontems, O.; Salamin, K.; Fratti, M.; Monod, M. Terbinafine resistance of Trichophyton clinical isolates caused by specific point mutations of squalene epoxidase gene. Antimicrob. Agents Chemother. 2017, 61, e00115-17. [Google Scholar] [CrossRef]
- Jabet, A.; Normand, A.-C.; Brun, S.; Dannaoui, E.; Bachmeyer, C.; Piarroux, R.; Hennequin, C.; Moreno-Sabater, A. Trichophyton indotineae from epidemiology to therapeutic. J. Med. Mycol. 2023, 33, 101383. [Google Scholar] [CrossRef]
- Gupta, A.K.; Wang, T.; Mann, A.; Ravi, S.P.; Talukder, M.; Lincoln, S.A.; Foreman, H.-C.-; Kaplan, B.; Galili, E.; Piguet, V.; et al. Antifungal resistance in dermatophytes—Review of the epidemiology, diagnostic challenges, and treatment strategies for managing Trichophyton indotineae infections. Expert Rev. Anti-Infect. Ther. 2024, 22, 739–751. [Google Scholar] [CrossRef]
- Sonego, B.; Corio, A.; Mazzoletti, V.; Zerbato, V.; Benini, A.; di Meo, N.; Zalaudek, I.; Stinco, G.; Errichetti, E.; Zelin, E. Trichophyton indotineae, an emerging drug-resistant dermatophyte: A review of the treatment options. J. Clin. Med. 2024, 13, 3558. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Susmita; Nguyen, H.C.; Liddy, A.; Economopoulus, V.; Wang, T. Terbinafine resistance in Trichophyton rubrum and Trichophyton indotineae: A literature review. Antibiotics 2025, 14, 472. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Masih, A.; Khurana, A.; Singh, P.K.; Gupta, M.; Hagen, F.; Meis, J.F.; Chowdhary, A. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses 2018, 61, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Rudramurthy, S.M.; Shankarnarayan, S.A.; Dogra, S.; Shaw, D.; Mushtaq, K.; Paul, R.A.; Narang, T.; Chakrabarti, A. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with allylamine resistance. Antimicrob. Agents Chemother. 2018, 62, e02522-17. [Google Scholar] [CrossRef]
- Khurana, A.; Masih, A.; Chowdhary, A.; Sardana, K.; Borker, S.; Gupta, A.; Gautam, R.K.; Sharma, P.K.; Jain, D. Correlation of in vitro susceptibility based on MICs and squalene epoxidase mutations with clinical response to terbinafine in patients with tinea corporis/cruris. Antimicrob. Agents Chemother. 2018, 62, e01038-18. [Google Scholar] [CrossRef]
- Nenoff, P.; Verma, S.B.; Vasani, R.; Burmester, A.; Hipler, U.-C.; Wittig, F.; Krüger, M.; Nenoff, K.; Wiegand, C.; Saraswat, A.; et al. The current epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes—A molecular study. Mycoses 2019, 62, 336–356. [Google Scholar] [CrossRef]
- Ebert, A.; Monod, M.; Salamin, K.; Burmester, A.; Uhrlaß, S.; Wiegand, C.; Hipler, U.-C.; Krüger, C.; Koch, D.; Wittig, F.; et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicenter study. Mycoses 2020, 63, 717–728. [Google Scholar] [CrossRef]
- Singh, S.; Chandra, U.; Anchan, V.N.; Verma, P.; Tilak, R. Limited effectiveness of four oral antifungal drugs (fluconazole, griseofulvin, itraconazole and terbinafine) in the current epidemic of altered dermatophytosis in India: Results of a randomized pragmatic trial. Br. J. Dermatol. 2020, 183, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Pchelin, I.M.; Azarov, D.V.; Churina, M.A.; Scherbak, S.G.; Apalko, S.V.; Vasilyeva, N.V.; Taraskina, A.E. Species boundaries in the Trichophyton mentagrophytes/ T. interdigitale species complex. Med. Mycol. 2019, 57, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Uhrlaß, S.; Verma, S.B.; Gräser, Y.; Rezaei-Matehkolaei, A.; Hatami, M.; Schaller, M.; Nenoff, P. Trichophyton indotineae—An emerging pathogen causing recalcitrant dermatophytoses in India and worldwide—A multidimensional perspective. J. Fungi 2022, 8, 757. [Google Scholar] [CrossRef] [PubMed]
- Kano, R.; Kimura, U.; Kakurai, M.; Hiruma, J.; Kamata, H.; Suga, Y.; Harada, K. Trichophyton indotineae sp. nov.: A new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia 2020, 185, 947–958. [Google Scholar] [CrossRef]
- Tang, C.; Kong, X.; Ahmed, S.A.; Thakur, R.; Chowdhary, A.; Nenoff, P.; Uhrlaß, S.; Verma, S.B.; Meis, J.F.; Kandemir, H.; et al. Taxonomy of the Trichophyton mentagrophytes/T. interdigitale species complex, harboring the highly virulent, multiresistent genotype T. indotineae. Mycopathologia 2021, 186, 315–326. [Google Scholar] [CrossRef]
- Verma, S.B.; Khurana, A.; Bosshard, P.P.; Kargl, A.; Singal, A.; Saraswat, A.; Guenova, E.; Schaller, M.; Panda, S.; Rezaei-Matehkolaei, A.; et al. ‘Trichophyton indotineae’ is an inaccurate and pejorative term. Indian J. Dermatol. Venereol. Leprol. 2025, 91, 277–280. [Google Scholar] [CrossRef]
- Burmester, A.; Hipler, U.-C.; Elsner, P.; Wiegand, C. Point mutations in the squalene epoxidase erg1 and sterol 14-α demethylase erg11 gene of T. indotineae isolates indicate that the resistant mutant strains evolved independently. Mycoses 2022, 65, 97–102. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.I.; Verma, S.B.; Illigner, G.-M.; Uhrlaß, S.; Klonowski, E.; Burmester, A.; Noor, T.; Nenoff, P. Trichophyton mentagrophytes ITS genotype VIII/Trichophyton indotineae infection and antifungal resistance in Bangladesh. J. Fungi 2024, 10, 768. [Google Scholar] [CrossRef]
- Song, J.; Zhang, S.; Lu, L. Fungal cytochrome P450 protein Cyp51: What we can learn from its evolution, regulons and Cyp51-based azole resistance. Fungal Biol. Rev. 2018, 32, 131–142. [Google Scholar] [CrossRef]
- Mellado, E.; Diaz-Guerra, T.M.; Cuenca-Estrella, M.; Rodriguez-Tudela, J.L. Identification of two different 14-α sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J. Clin. Microbiol. 2001, 39, 2431–2438. [Google Scholar] [CrossRef]
- Yamada, T.; Yaguchi, T.; Maeda, M.; Alshahni, M.M.; Salamin, K.; Guenova, E.; Feuermann, M.; Monod, M. Gene amplification of CYP51B: A new mechanism of resistance to azole compounds in Trichophyton indotineae. Antimicrob. Agents Chemother. 2022, 66, e0005922. [Google Scholar] [CrossRef]
- Yamada, T.; Maeda, M.; Nagai, H.; Salamin, K.; Chang, Y.-T.; Guenova, E.; Feuermann, M.; Monod, M. Two different types of tandem sequences mediate the overexpression of TinCYP51B in azole-resistant Trichophyton indotineae. Antimicrob. Agents Chemother. 2023, 67, e0093323. [Google Scholar] [CrossRef] [PubMed]
- Berstecher, N.; Burmester, A.; Gregersen, D.M.; Tittelbach, J.; Wiegand, C. Trichophyton indotineae Erg1Ala448Thr strain expressed constitutively high levels of sterol 14-α demethylase Erg11B mRNA, while transporter MDR3 and Erg11A expression was induced after addition of short chain azoles. J. Fungi 2024, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Astvad, K.M.T.; Hare, R.K.; Jorgensen, K.M.; Saunte, D.M.L.; Thomsen, P.K.; Arendrup, M.C. Increasing terbinafine resistance in Danish Trichophyton isolates. J. Fungi 2022, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Kano, R.; Kimura, U.; Noguchi, H.; Hiruma, M. Clinical isolate of a multi-antifungal-resistant Trichophyton rubrum. Antimicrob. Agents Chemother. 2022, 66, e0239321. [Google Scholar] [CrossRef]
- Rosam, K.; Monk, B.C.; Lackner, M. Sterol 14-α demethylase ligand-binding pocket-mediated acquired and intrinsic azole resistance in fungal pathogens. J. Fungi 2021, 7, 1. [Google Scholar] [CrossRef]
- Nenoff, P.; Stahl, M.; Schaller, M.; Burmester, A.; Monod, M.; Ebert, A.; Uhrlaß, S. Recurrent tinea corporis generalisata due to terbinafine-resistant Trichophyton rubrum strain—Long-term treatment with super-availability itraconazole. Dermatologie 2023, 74, 864–873. [Google Scholar] [CrossRef]
- Gnat, S.; Lagowski, D.; Nowakiewicz, A.; Dylag, M.; Osinska, M. Complementary effect of mechanism of multidrug resistance in Trichophyton mentagrophytes isolated from human dermatophytosis of animal origin. Mycoses 2021, 64, 537–549. [Google Scholar] [CrossRef]
- Winter, P.; Burmester, A.; Tittelbach, J.; Wiegand, C. A new genotype of Trichophyton quinckeanum with point mutations in Erg11A encoding sterol 14-α demethylase exhibits increased itraconazole resistance. J. Fungi 2023, 9, 1006. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Guinea, J.; Meletiadis, J. Twenty years in EUCAST anti-fungal susceptibility testing: Progress & remaining challenges. Mycopathologia 2024, 189, 64. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Kahlmeter, G.; Guinea, J.; Meletiadis, J. How to: Perform antifungal susceptibility testing of microconidia-forming dermatophytes following the new reference EUCAST method E.Def 11.0 exemplified by Trichophyton. Clin. Microbiol. Inf. 2021, 27, 55–60. [Google Scholar] [CrossRef]
- CLSI Document M38: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017.
- Georg, L.K.; Camp, L.B. Routine nutritional tests for the identification of dermatophytes. J. Bacteriol. 1957, 74, 113–121. [Google Scholar] [CrossRef]
- Negroni, R.; Arechavala, A.I. Itraconazole: Pharmacokinetics and indications. Arch. Med. Res. 1993, 24, 387–393. [Google Scholar]
- Prentice, A.G.; Glasmacher, A. Making sense of itraconazole pharmacokinetics. J. Antimicrob. Chemother. 2005, 56, i17–i22. [Google Scholar] [CrossRef]
- Mikami, Y.; Sakamoto, T.; Yazawa, K.; Gonoi, T.; Ueno, S.; Hasegawa, S. Comparison of in vitro antifungal activity of itraconazole and hydroxyl-itraconazole by colorimetric MTT assay. Mycoses 1994, 37, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; Stewart, C.S. Keratin hydrolysis by dermatophytes. Med. Mycol. 2019, 57, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, G.; Amarov, B.; Fratti, M.; Salamin, K.; Bontems, O.; Chang, Y.-T.; Sabou, A.M.; Künzle, N.; Monod, M.; Guenova, E. Reliable and rapid identification of terbinafine resistance in dermatophytic nail and skin infections. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 2080–2089. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Liu, Z.; Luo, X. Impact of pH on the antifungal susceptibility of vaginal Candida albicans. Int. J. Gynecol. Obstet. 2011, 114, 278–280. [Google Scholar] [CrossRef]
- Yin, X.; Daintree, L.S.; Ding, S.; Ledger, D.M.; Wang, B.; Zhao, W.; Qi, J.; Wu, W. Itraconazole solid dispersion prepared by a supercritical fluid technique: Preparation, in vitro characterization, and bioavailability in beagle dogs. Drug Des. Dev. Ther. 2015, 9, 2801–2810. [Google Scholar]
- Lee, J.-H.; Park, C.; Weon, K.-Y.; Kang, C.-Y.; Lee, B.-J.; Park, J.-B. Improved bioavailability of poorly water-soluble drug by targeting increased absorption through solubility enhancement and precipitation inhibition. Pharmaceuticals 2021, 14, 1255. [Google Scholar] [CrossRef]
- Silveira, H.C.S.; Gras, D.E.; Cazzaniga, R.A.; Sanches, P.R.; Rossi, A.; Martinez-Rossi, N.M. Transcriptional profiling reveals genes in the human pathogen Trichophyton rubrum that are expressed in response to pH signaling. Microb. Pathog. 2010, 48, 91–96. [Google Scholar] [CrossRef]
- Brunner, P.C.; Stefanato, F.L.; McDonald, B.A. Evolution of the CYP51 gene in Mycosphaerella graminicola: Evidence for intragenic recombination and selective replacement. Mol. Plant Pathol. 2008, 9, 305–316. [Google Scholar] [CrossRef]
- Becher, R.; Wirsel, S.G.R. Fungal cytochrome P450 sterol 14-α demethylase (CYP51) and azole resistance in plant and human pathogens. Appl. Microbiol. Biotechnol. 2012, 95, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, N.; Li, D.; Zheng, H.; Zhang, L.; Ge, H.; Liu, W. cyp51A-based mechanism of azole resistance in Aspergillus fumigatus: Illustration by a new 3D structural model of Aspergillus fumigatus CYP51 protein. Med. Mycol. 2016, 54, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Monod, M.; Feuermann, M.; Salamin, K.; Fratti, M.; Makino, M.; Alshahni, M.M.; Makimura, K.; Yamada, T. Trichophyton rubrum azole resistance mediated by a new ABC transporter, TruMDR3. Antimicrob. Agents Chemother. 2019, 63, e00863-19. [Google Scholar] [CrossRef]
- Sammadar, S.; Redhwan, M.A.M.; Eraiah, M.M.; Koneri, R. Clinical isolates of the anthropophilic dermatophyte Trichophyton tonsurans exhibit transcriptional regulation of multidrug efflux transporters that induce antifungal resistance. Mol. Biol. Rep. 2025, 52, 612. [Google Scholar] [CrossRef]
- Weitzman, I.; Allerdice, P.W.; Silva-Hutner, M.; Miller, O.J. Meiosis in Arthroderma benhamiae (=Trichophyton mentagrophytes). Sabouraudia J. Med. Vet. Mycol. 1968, 6, 232–237. [Google Scholar] [CrossRef]
- Elsaman, H.; Golubtsov, E.; Brazil, S.; Ng, N.; Klugherz, I.; Martin, R.; Dichtl, K.; Müller, C.; Wagener, J. Toxic eburicol accumulation drives the antifungal activity of azoles against Aspergillus fumigatus. Nat. Commun. 2024, 15, 6312. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
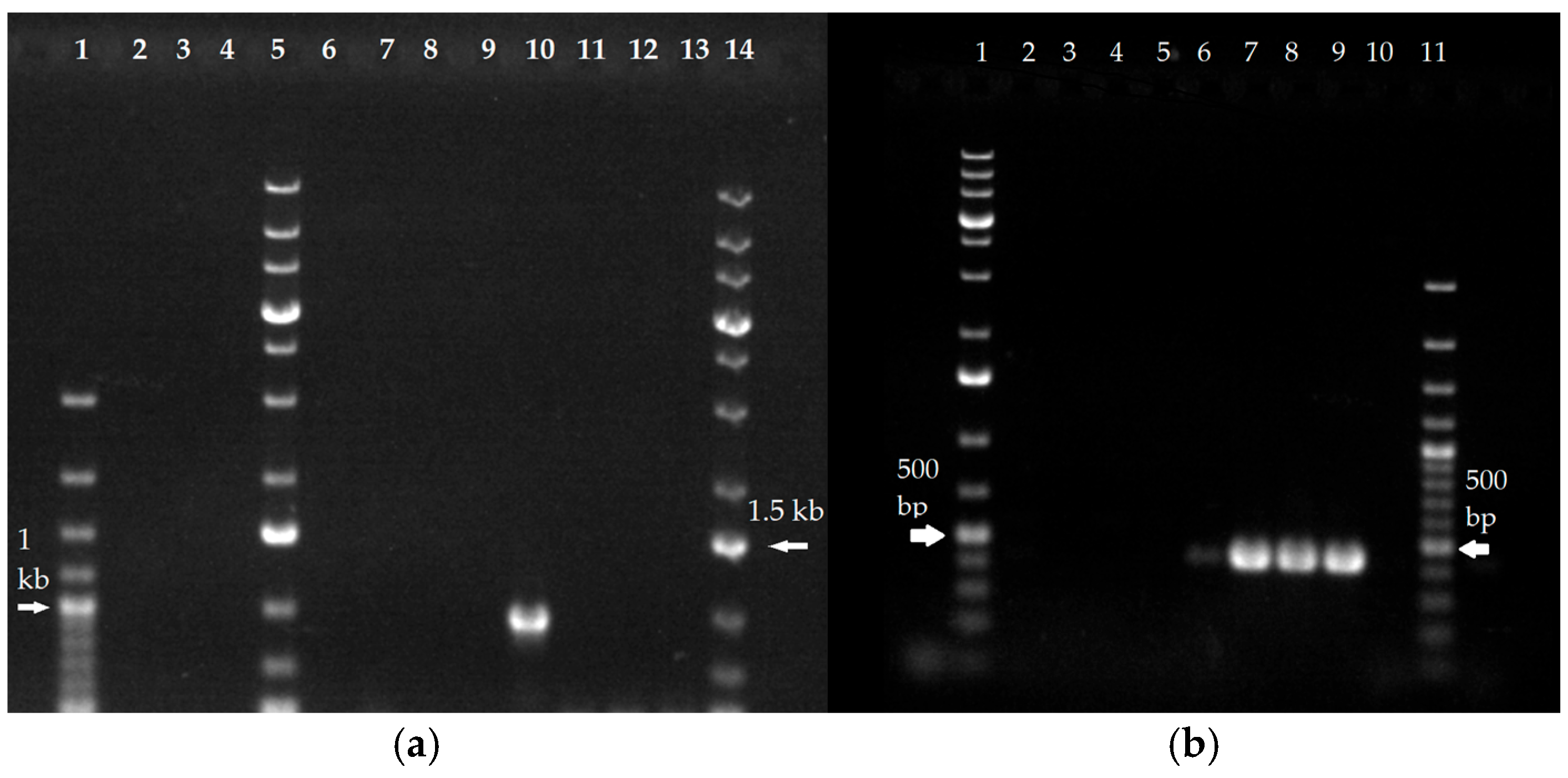



| Genus | Species | ITS Subtype, Variety | Collection No. | Collection Syn. | Erg1 Amino Acid Exchanges | Erg11B Amino Acid Exchanges | Erg11B Amplification Type | Source, Cited |
|---|---|---|---|---|---|---|---|---|
| Trichophyton | rubrum | UKJ705/21 | 202247/21 | Ile479Thr | Gly443Cys | No amplificates | [27] | |
| UKJ706/21 | 202252/21 | Ile479Val | Wild-type | No amplificates | [27] | |||
| DSM16111 | Wild-type | Wild-type | No amplificates | DSMZ | ||||
| tonsurans | UKJ186/23 | n.d. | n.d. | n.d. | Routine | |||
| UKJ1163/23 | n.d. | n.d. | n.d. | RV491/23 | ||||
| interdigitale | I | DSM16110 | n.d. | n.d. | n.d. | DSMZ | ||
| II | DSM4167 | n.d. | n.d. | n.d. | DSMZ | |||
| II | UKJ1780/22 | n.d. | n.d. | n.d. | Routine | |||
| indotineae | VIII | UKJ1676/17 | TIMM20114 | Ala448Thr | Wild-type | No amplificates | [10,21] | |
| VIII | UKJ1687/17 | TIMM20118 | Phe397Leu | Gly443Glu | Type I | [10,21] | ||
| VIII | UKJ1708/17 | Ala448Thr | Wild-type | Type II | [10,21] | |||
| VIII | UKJ392/18 | TIMM20117, 200087/18 | Ala448Thr | Wild-type | Type II | [10,21] | ||
| VIII | CBS146726 | UKJ594/19 | Phe397Leu | Tyr444His | No amplificates | [17,23] | ||
| VIII | CBS146727 | UKJ1145/19 | Phe397Leu | Ala230Thr, Asp441Gly | No amplificates | [17,23] | ||
| VIII | UKJ262/21 | Ala448Thr | Tyr444His | No amplificates | [17,23] | |||
| VIII | UKJ476/21 | Ala448Thr | Wild-type | Type II | [17,23] | |||
| mentagrophytes | VII | UKJ1722/22 | n.d. | n.d. | n.d. | Routine | ||
| XXV | UKJ173/19 | 218292/17 | n.d. | n.d. | n.d. | [13] | ||
| benhamiae | v. benhamiae | DSM6916 | RV26680 | n.d. | n.d. | n.d. | DSMZ | |
| v. luteum | UKJ117/23 | n.d. | n.d. | n.d. | Routine | |||
| UKJ488/23 | n.d. | n.d. | n.d. | Routine | ||||
| erinacei | UKJ314/23 | n.d. | n.d. | n.d. | RV491/23 | |||
| quinckeanum | I * | IHEM13697 | Wild-type | Wild-type | n.d. | BCCM | ||
| II * | UKJ1506/20 | Wild-type | Wild-type | n.d. | [29] | |||
| Microsporum | audouinii | UKJ317/23 | n.d. | n.d. | n.d. | RV491/23 | ||
| canis | UKJ299/23 | n.d. | n.d. | n.d. | Routine | |||
| UKJ300/23 | n.d. | n.d. | n.d. | Routine | ||||
| Epidermophyton | floccosum | UKJ317/23 | n.d. | n.d. | n.d. | RV491/23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krauße, L.; Burmester, A.; Uhrlaß, S.; Fabri, M.; Nenoff, P.; Tittelbach, J.; Wiegand, C. Significant Impact of Growth Medium on Itraconazole Susceptibility in Azole-Resistant Versus Wild-Type Trichophyton indotineae, rubrum, and quinckeanum Isolates. Int. J. Mol. Sci. 2025, 26, 7090. https://doi.org/10.3390/ijms26157090
Krauße L, Burmester A, Uhrlaß S, Fabri M, Nenoff P, Tittelbach J, Wiegand C. Significant Impact of Growth Medium on Itraconazole Susceptibility in Azole-Resistant Versus Wild-Type Trichophyton indotineae, rubrum, and quinckeanum Isolates. International Journal of Molecular Sciences. 2025; 26(15):7090. https://doi.org/10.3390/ijms26157090
Chicago/Turabian StyleKrauße, Luisa, Anke Burmester, Silke Uhrlaß, Mario Fabri, Pietro Nenoff, Jörg Tittelbach, and Cornelia Wiegand. 2025. "Significant Impact of Growth Medium on Itraconazole Susceptibility in Azole-Resistant Versus Wild-Type Trichophyton indotineae, rubrum, and quinckeanum Isolates" International Journal of Molecular Sciences 26, no. 15: 7090. https://doi.org/10.3390/ijms26157090
APA StyleKrauße, L., Burmester, A., Uhrlaß, S., Fabri, M., Nenoff, P., Tittelbach, J., & Wiegand, C. (2025). Significant Impact of Growth Medium on Itraconazole Susceptibility in Azole-Resistant Versus Wild-Type Trichophyton indotineae, rubrum, and quinckeanum Isolates. International Journal of Molecular Sciences, 26(15), 7090. https://doi.org/10.3390/ijms26157090






