A Novel Selenium-Based Nanozyme (GSH-Se) Ameliorates Colitis in Mice by Modulating the Nrf2/Keap1 and GPx4 Pathways
Abstract
1. Introduction
2. Results
2.1. Toxicity of GSH-Se
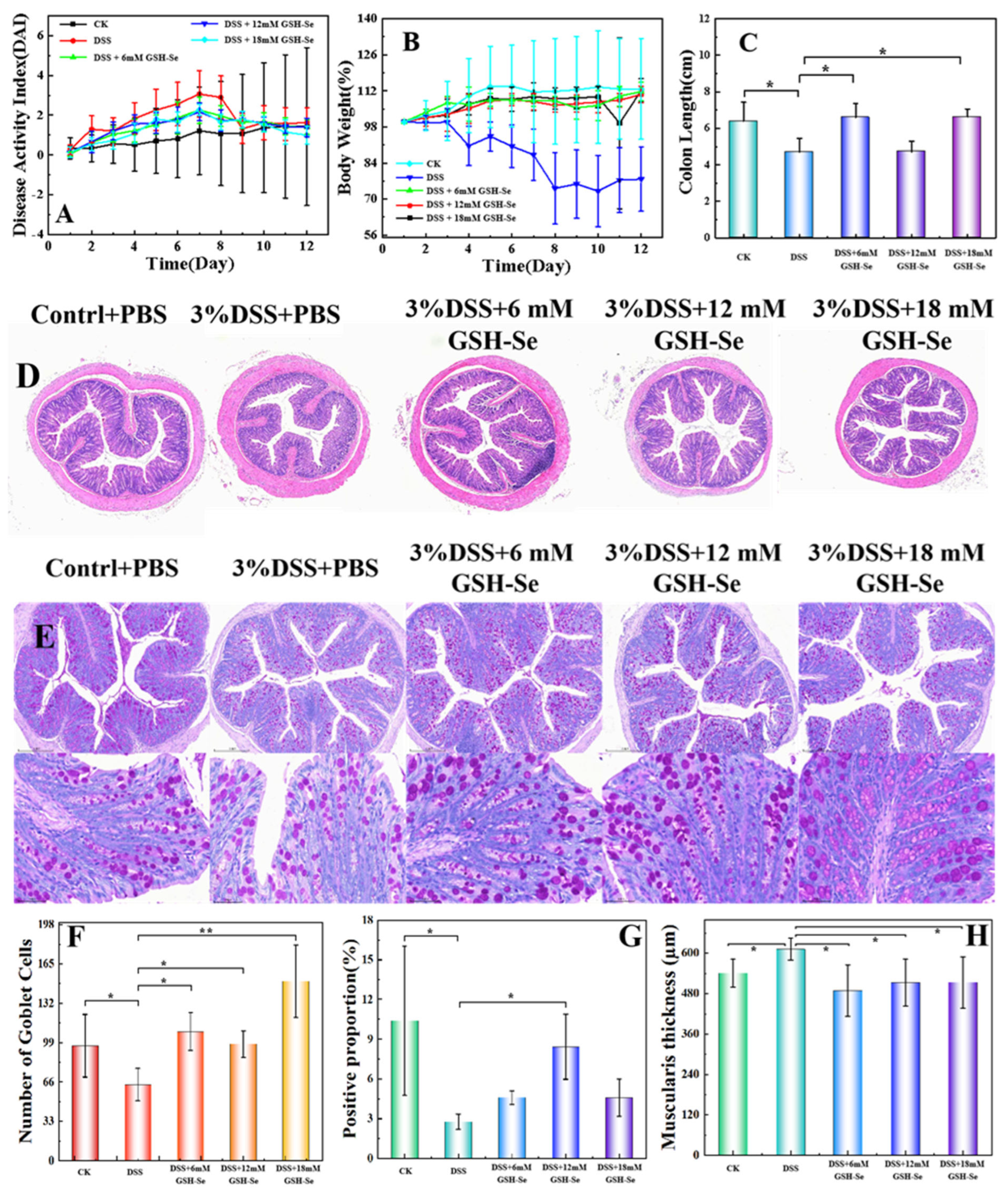
2.2. DSS-Induced Colitis and the Attenuating Effects of GSH-Se
2.3. Effects of DSS and GSH-Se on Oxidative Status
2.4. Effects of DSS, LPS, and GSH-Se on Inflammatory Responses
2.5. Effects of DSS and GSH-Se on the Colonic Microbiome
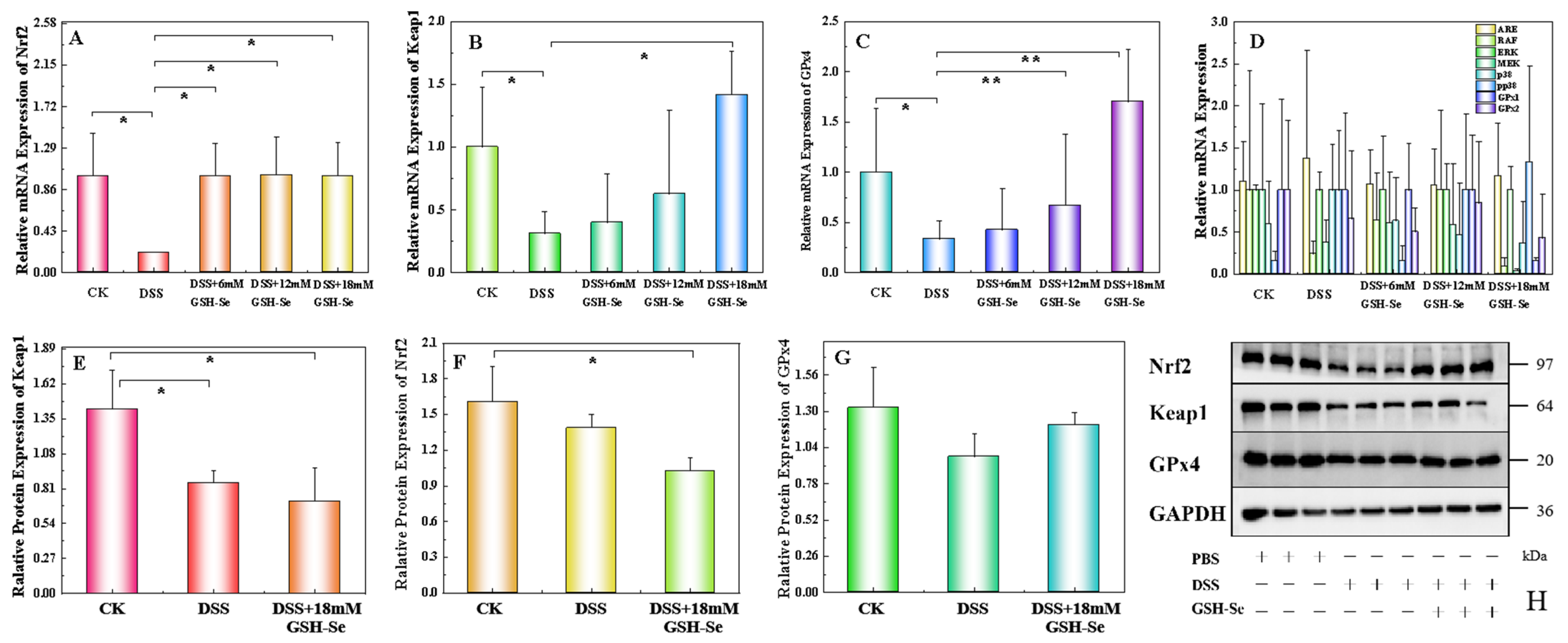
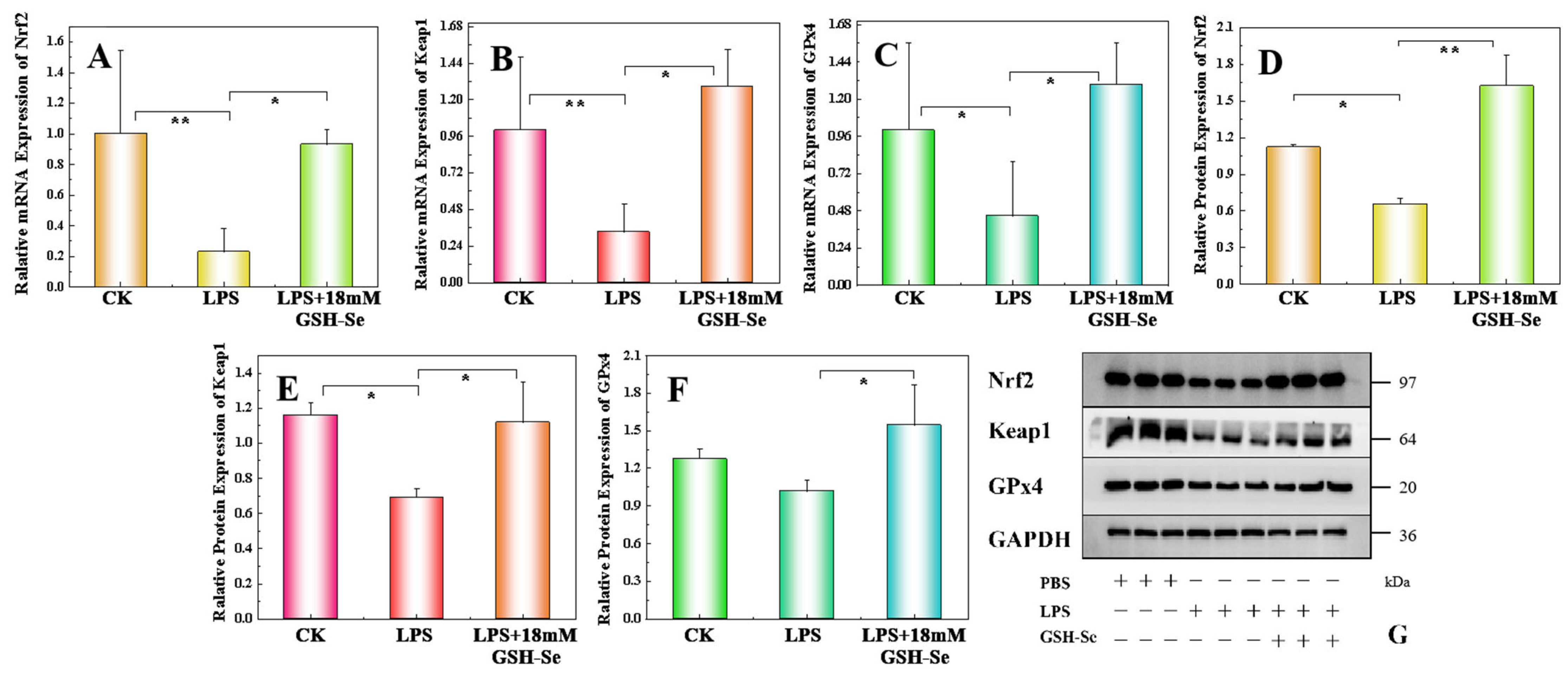
2.6. Effect of DSS, LPS, and GSH-Se on the Nrf 2/Keap 1/GPx 4 Pathway
3. Discussion
4. Materials and Methods
4.1. Synthesis of GSH-Se
4.2. Animal and Experimental Design
4.2.1. The Use of Animals and Cells
4.2.2. Evaluation of GSH-Se Biosafety
4.3. Experimental Design
4.4. Clinical Evaluation
4.5. Assessment of Oxidative Stress and Inflammatory Status
4.6. Microbiome Analysis
4.7. mRNA and Protein Expression
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Se | Selenium |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| DSS | Dextran sulphate sodium salt |
| Nrf2 | Nuclear factor E2-related factor 2 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| UC | Ulcerative colitis |
| IBD | Inflammatory bowel disease |
| ROS | Reactive oxygen species |
| DNTB | 5-thio-2-nitrobenzoic acid |
| PBS | Phosphate-buffered saline |
| MCEC | Colonocytes of mouse colon epithelial cells |
| LPS | Lipopolysaccharide |
| AST | Aspartate aminotransferase |
| ALT | Alanine aminotransferase |
| T-AOC | Total antioxidant capacity |
| T-SOD | Total superoxide dismutase |
| MDA | Malondialdehyde |
| TNF | Tumour necrosis factor |
| IL | Interleukin |
| qPCR | Quantitative real-time polymerase chain reaction |
| PCA | Principal component analysis |
| SCFAs | Short-chain fatty acids |
| DAI | Disease activity index |
References
- Honap, S.; Jairath, V.; Sands, B.E.; Dulai, P.S.; Danese, S.; Peyrin-Biroulet, L. Acute severe ulcerative colitis trials: The past, the present and the future. Gut 2024, 73, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Gros, B.; Kaplan, G.G. Ulcerative colitis in adults: A review. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Honap, S.; Buisson, A.; Danese, S.; Beaugerie, L.; Peyrin-Biroulet, L. Patient and public involvement in research: Lessons for inflammatory bowel disease. J. Crohn’s Colitis 2023, 17, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Feelisch, M.; Faber, K.N.; Pasch, A.; Dijkstra, G.; van Goor, H. Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol. Med. 2020, 26, 1034–1046. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, L.; Jiang, S.; Qian, D.; Duan, J. Excessive apoptosis in ulcerative colitis: Crosstalk between apoptosis, ROS, ER stress, and intestinal homeostasis. Inflamm. Bowel Dis. 2022, 28, 639–648. [Google Scholar] [CrossRef]
- Hwang, J.; Jing, J.; Jeon, S.; Moon, S.H.; Park, M.Y.; Yum, D.Y.; Kim, J.H.; Kang, J.E.; Park, M.H.; Kim, E.J.; et al. SOD1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis. Redox Biol. 2020, 37, 101760. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, H.; Yu, B.; He, J.; Mao, X.; Yu, J.; Zheng, P.; Huang, Z.; Luo, Y.; Luo, J.; et al. Protective effects of natural antioxidants on inflammatory bowel disease: Thymol and its pharmacological properties. Antioxidants 2022, 11, 1947. [Google Scholar] [CrossRef]
- Moura, F.A.; de Andrade, K.Q.; Dos Santos, J.C.F.; Araújo, O.R.P.; Goulart, M.O.F. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015, 6, 617–639. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, Y.; Lu, H.; Xie, T.; Hu, Z.; Chu, Z.; Luo, F. The Potential Role of Vitamin E and the Mechanism in the Prevention and Treatment of Inflammatory Bowel Disease. Foods 2024, 13, 898. [Google Scholar] [CrossRef]
- Shapiro, H.; Singer, P.; Halpern, Z.; Bruck, R. Polyphenols in the treatment of inflammatory bowel disease and acute pancreatitis. Gut 2007, 56, 426–436. [Google Scholar] [CrossRef]
- Faghfouri, A.H.; Zarezadeh, M.; Tavakoli-Rouzbehani, O.M.; Radkhah, N.; Faghfuri, E.; Kord-Varkaneh, H.; Tan, S.C.; Ostadrahimi, A. The effects of N-acetylcysteine on inflammatory and oxidative stress biomarkers: A systematic review and meta-analysis of controlled clinical trials. Eur. J. Pharmacol. 2020, 884, 173368. [Google Scholar] [CrossRef] [PubMed]
- Mertens, R.T.; Gukathasan, S.; Arojojoye, A.S.; Olelewe, C.; Awuah, S.G. Next generation gold drugs and probes: Chemistry and biomedical applications. Chem. Rev. 2023, 123, 6612–6667. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.; Hryckowian, A.J.; Tholen, M.; Bender, K.O.; Van Treuren, W.W.; Loscher, S.; Sonnenburg, J.L.; Bogyo, M. The clinical drug ebselen attenuates inflammation and promotes microbiome recovery in mice after antibiotic treatment for CDI. Cell Rep. Med. 2020, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- Kudva, A.K.; Shay, A.E.; Prabhu, K.S. Selenium and inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G71–G77. [Google Scholar] [CrossRef]
- Short, S.P.; Pilat, J.M.; Barrett, C.W.; Reddy, V.K.; Haberman, Y.; Hendren, J.R.; Marsh, B.J.; Keating, C.E.; Motley, A.K.; Hill, K.E.; et al. Colonic epithelial-derived selenoprotein P is the source for antioxidant-mediated protection in colitis-associated cancer. Gastroenterology 2020, 160, 1694–1708. [Google Scholar] [CrossRef]
- Speckmann, B.; Steinbrenner, H. Selenium and selenoproteins in inflammatory bowel diseases and experimental colitis. Inflamm. Bowel Dis. 2014, 20, 1110–1119. [Google Scholar] [CrossRef]
- Brenneisen, P.; Steinbrenner, H.; Sies, H. Selenium, oxidative stress, and health aspects. Mol. Aspects Med. 2005, 26, 256–267. [Google Scholar] [CrossRef]
- Flohé, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free Radical Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The role of glutathione peroxidase-1 in health and disease. Free Radical Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Ai, Y.; Hu, Z.N.; Liang, X.; Sun, H.B.; Xin, H.; Liang, Q. Recent advances in nanozymes: From matters to bioapplications. Adv. Funct. Mater. 2022, 32, 2110432. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, X.; Fan, K. Nanozymes inspired by natural enzymes. Acc. Mater. Res. 2021, 2, 534–547. [Google Scholar] [CrossRef]
- Dos Santos, M.; Penteado, J.O.; Baisch, P.R.M.; Soares, B.M.; Muccillo-Baisch, A.L.; da Silva Júnior, F.M.R. Selenium dietary intake, urinary excretion, and toxicity symptoms among children from a coal mining area in Brazil. Environ. Geochem. Health 2021, 43, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Breugelmans, T.; Oosterlinck, B.; Arras, W.; Ceuleers, H.; De Man, J.; Hold, G.L.; Hold, G.L.; De Winter, B.Y.; Smet, A. The role of mucins in gastrointestinal barrier function during health and disease. Lancet Gastroenterol. 2022, 7, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Kai, Y. Mechanical regulation of tissues that reproduces wrinkle patterns of gastrointestinal tracts. Phys. Biol. 2022, 19, 036006. [Google Scholar] [CrossRef]
- Muraleedharan, C.K.; Mierzwiak, J.; Feier, D.; Nusrat, A.; Quiros, M. Generation of murine primary colon epithelial monolayers from intestinal crypts. J. Vis. Exp. 2021, 168, e62156. [Google Scholar] [CrossRef]
- Yao, D.; Dai, W.; Dong, M.; Dai, C.; Wu, S. MUC2 and related bacterial factors: Therapeutic targets for ulcerative colitis. eBioMedicine 2021, 74, 103751. [Google Scholar] [CrossRef]
- Sanmarco, L.M.; Chao, C.C.; Wang, Y.C.; Kenison, J.E.; Li, Z.; Rone, J.M.; Polonio, C.M.; Gutierrez-Vazquez, C.; Piester, G.; Plasencia, A.; et al. Identification of environmental factors that promote intestinal inflammation. Nature 2022, 611, 801–809. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, Y.; Wang, H.; Kong, D.; Jin, W.; Hu, Y.; Qin, Y.; Zhang, B.; Li, X.; Hao, J.; et al. IL-1β pre-stimulation enhances the therapeutic effects of endometrial regenerative cells on experimental colitis. Stem Cell Res. Ther. 2021, 12, 324. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, L.; Dai, L.; Zuo, D.; Li, X.; Zhu, H.; Xu, F. TNF-α promotes the malignant transformation of intestinal stem cells through the NF-κB and Wnt/β-catenin signaling pathways. Oncol. Rep. 2020, 44, 577–588. [Google Scholar] [CrossRef]
- Jin, X.; Zimmers, T.A.; Zhang, Z.; Pierce, R.H.; Koniaris, L.G. Interleukin-6 is an important in vivo inhibitor of intestinal epithelial cell death in mice. Gut 2010, 59, 186–196. [Google Scholar] [CrossRef]
- Pott, J.; Kabat, A.M.; Maloy, K.J. Intestinal epithelial cell autophagy is required to protect against TNF-induced apoptosis during chronic colitis in mice. Cell Host Microbe 2018, 23, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 7282–7300. [Google Scholar] [CrossRef] [PubMed]
- Josh, F.; Soekamto, T.H.; Adriani, J.R.; Jonatan, B.; Mizuno, H.; Faruk, M. The combination of stromal vascular fraction cells and platelet-rich plasma reduces malondialdehyde and nitric oxide levels in deep dermal burn injury. J. Inflamm. Res. 2021, 14, 3049–3061. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.; Stanton, C.; Ross, R.P.; Zhao, J.; Chen, W.; Yang, B. Exopolysaccharides produced by bifidobacterium longum subsp. Longum ys108r ameliorates dss-induced ulcerative colitis in mice by improving the gut barrier and regulating the gut microbiota. J. Agric. Food Chem. 2024, 72, 7055–7073. [Google Scholar] [CrossRef]
- Willing, B.P.; Dicksved, J.; Halfvarson, J.; Andersson, A.F.; Lucio, M.; Zheng, Z.; Järnerot, G.; Tysk, C.; Jansson, J.K.; Engstrand, L. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology 2010, 139, 1844–1854. [Google Scholar] [CrossRef]
- Papamichael, K.; Konstantopoulos, P.; Mantzaris, G.J. Helicobacter pylori infection and inflammatory bowel disease: Is there a link? World J. Gastroenterol. 2014, 20, 6374. [Google Scholar] [CrossRef]
- Koelink, P.J.; Bloemendaal, F.M.; Li, B.; Westera, L.; Vogels, E.W.; van Roest, M.; Gloudemans, A.K.; van’t Wout, A.B.; Korf, H.; Vermeire, S.; et al. Anti-TNF therapy in IBD exerts its therapeutic effect through macrophage IL-10 signalling. Gut 2020, 69, 1053–1063. [Google Scholar] [CrossRef]
- Aghamohammad, S.; Sepehr, A.; Miri, S.T.; Najafi, S.; Pourshafie, M.R.; Rohani, M. Anti-inflammatory and immunomodulatory effects of Lactobacillus spp. as a preservative and therapeutic agent for IBD control. Immun. Inflamm. Dis. 2022, 10, e635. [Google Scholar] [CrossRef]
- Dang, J.T.; Mocanu, V.; Park, H.; Laffin, M.; Hotte, N.; Karmali, S.; Birch, D.W.; Madsen, K.L. Roux-en-Y gastric bypass and sleeve gastrectomy induce substantial and persistent changes in microbial communities and metabolic pathways. Gut Microbes 2022, 14, 2050636. [Google Scholar] [CrossRef]
- Wu, X.; Huang, X.; Ma, W.; Li, M.; Wen, J.; Chen, C.; Liu, L.; Nie, S. Bioactive polysaccharides promote gut immunity via different ways. Food Funct. 2023, 14, 1387–1400. [Google Scholar] [CrossRef]
- Khajah, M.A.; Hawai, S. Effect of minocycline, methyl prednisolone, or combination treatment on the colonic bacterial population in a state of colonic inflammation using the murine dextran sulfate sodium model. Microb. Cell Fact. 2023, 22, 232. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Chen, S.Y.; Wu, Y.H.; Liao, Y.L.; Yen, G.C. Ameliorative effect of buckwheat polysaccharides on colitis via regulation of the gut microbiota. Int. J. Biol. Macromol. 2023, 227, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Lemons, J.M.; Conrad, M.; Tanes, C.; Chen, J.; Friedman, E.S.; Roggiani, M.; Curry, D.; Chau, L.; Hecht, A.L.; Harling, L.; et al. Enterobacteriaceae Growth Promotion by Intestinal Acylcarnitines, a Biomarker of Dysbiosis in Inflammatory Bowel Disease. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, W.; Zhu, X.; Sun, X.; Xiao, J.; Li, D.; Cui, Y.; Wang, C.; Shi, Y. Response of gut microbiota to dietary fiber and metabolic interaction with SCFAs in piglets. Front. Microbiol. 2018, 9, 2344. [Google Scholar] [CrossRef]
- Suzuki, I.; Shimizu, T.; Senpuku, H. Role of SCFAs for fimbrillin-dependent biofilm formation of Actinomyces oris. Microorganisms 2018, 6, 114. [Google Scholar] [CrossRef]
- Guo, X.; Cao, X.; Fang, X.; Guo, A.; Li, E. Inhibitory effects of fermented Ougan (Citrus reticulata cv. Suavissima) juice on high-fat diet-induced obesity associated with white adipose tissue browning and gut microbiota modulation in mice. Food Funct. 2021, 12, 9300–9314. [Google Scholar] [CrossRef]
- Ruiz-Saavedra, S.; Del Rey, C.G.; Suárez, A.; Díaz, Y.; Zapico, A.; Arboleya, S.; Salazar, N.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; González, S. Associations of dietary factors and xenobiotic intake with faecal microbiota composition according to the presence of intestinal mucosa damage. Food Funct. 2023, 14, 9591–9605. [Google Scholar] [CrossRef]
- Bailey, J.R.; Vince, V.; Williams, N.A.; Cogan, T.A. Streptococcus thermophilus NCIMB 41856 ameliorates signs of colitis in an animal model of inflammatory bowel disease. Benef. Microbes 2017, 8, 605–614. [Google Scholar] [CrossRef]
- Yang, N.; Xia, Z.; Shao, N.; Li, B.; Xue, L.; Peng, Y.; Zhi, F.; Yang, Y. Carnosic acid prevents dextran sulfate sodium-induced acute colitis associated with the regulation of the Keap1/Nrf2 pathway. Sci. Rep. 2017, 7, 11036. [Google Scholar] [CrossRef]
- Antonuccio, P.; Pallio, G.; Marini, H.R.; Irrera, N.; Romeo, C.; Puzzolo, D.; Freni, J.; Santoro, G.; Pirrotta, I.; Squadrito, F.; et al. Involvement of Hypoxia-Inducible Factor 1-α in Experimental Testicular Ischemia and Reperfusion: Effects of Polydeoxyribonucleotide and Selenium. Int. J. Mol. Sci. 2022, 23, 13144. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Muramatsu, A.; Saito, R.; Iso, T.; Shibata, T.; Kuwata, K.; Kawaguchi, S.; Iwawaki, T.; Adachi, S.; Suda, H.; et al. Molecular mechanism of cellular oxidative stress sensing by Keap1. Cell Rep. 2019, 28, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Gene. Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef]
- Baird, L.; Dinkova-Kostova, A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011, 85, 241–272. [Google Scholar] [CrossRef]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radical Biol. Med. 2018, 129, 454–462. [Google Scholar] [CrossRef]
- Mayr, L.; Grabherr, F.; Schwärzler, J.; Reitmeier, I.; Sommer, F.; Gehmacher, T.; Niederreiter, L.; He, G.; Ruder, B.; Kunz, K.T.R.; et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn’s disease. Nature Commun. 2020, 11, 1775. [Google Scholar] [CrossRef]
- Li, D.; Xie, T.; Guo, T.; Hu, Z.; Li, M.; Tang, Y.; Wu, Q.; Luo, F.; Lin, Q.; Wang, H. Sialic acid exerts anti-inflammatory effect through inhibiting MAPK-NF-κB/AP-1 pathway and apoptosis in ulcerative colitis. J. Funct. Foods 2023, 101, 105416. [Google Scholar] [CrossRef]
- Yin, J.; Ren, Y.; Yang, K.; Wang, W.; Wang, T.; Xiao, W.; Yang, H. The role of hypoxia-inducible factor 1-alpha in inflammatory bowel disease. Cell Biol. Int. 2022, 46, 46–51. [Google Scholar] [CrossRef]
- Marini, H.R. Mediterranean Diet and Soy Isoflavones for Integrated Management of the Menopausal Metabolic Syndrome. Nutrients 2022, 14, 1550. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Han, M.; Zhang, R.; Li, H.; Wu, F.; Wu, A.; Wang, X. Selenium-based nanozyme as a fluorescence-enhanced probe and imaging for chlortetracycline in living cells and foods. Food Chem. 2024, 432, 137147. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, Q.; Zhu, H.; Fang, Z.; Che, L.; Lin, Y.; Xu, S.; Zhuo, Y.; Hua, L.; Wang, J.; et al. Hepatic leptin signaling improves hyperglycemia by stimulating MAPK phosphatase-3 protein degradation via STAT3. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 983–1001. [Google Scholar] [CrossRef] [PubMed]
- Mou, D.; Ding, D.; Yang, M.; Jiang, X.; Zhao, L.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; Zhuo, Y.; et al. Maternal organic selenium supplementation during gestation improves the antioxidant capacity and reduces the inflammation level in the intestine of offspring through the NF-κB and ERK/Beclin-1 pathways. Food Funct. 2021, 12, 315–327. [Google Scholar] [CrossRef] [PubMed]


| Items | CK | DSS | DSS + 6 mM GSH-Se | DSS + 12 mM GSH-Se | DSS + 18 mM GSH-Se |
|---|---|---|---|---|---|
| Colon | |||||
| GPx (U/mg protein) | 129.44 ± 4.57 a | 102.01 ± 0.97 b | 119.28 ± 3.13 ab | 106.83 ± 7.75 a | 129.33 ± 9.28 a |
| T-AOC (U/mg protein) | 1.98 ± 0.14 | 1.86 ± 0.22 | 1.27 ± 0.10 | 1.63 ± 0.18 | 1.45 ± 0.46 |
| T-SOD (U/mg protein) | 78.49 ± 1.12 a | 70.40 ± 1.27 b | 74.85 ± 1.56 b | 76.68 ± 1.94 a | 76.68 ± 1.94 a |
| GSH (mg/g protein) | 50.23 ± 5.10 a | 14.94 ± 1.52 c | 26.27 ± 2.99 b | 45.93 ± 3.01 a | 53.12 ± 3.77 a |
| MDA (nmol/mL homogenate) | 1.45 ± 0.13 b | 6.30 ± 0.26 a | 5.13 ± 0.54 a | 6.40 ± 0.68 a | 1.96 ± 0.22 b |
| Liver | |||||
| GPx (U/mg protein) | 210.41 ± 28.85 a | 77.01 ± 14.60 b | 83.55 ± 68.09 b | 120.99 ± 16.04 b | 275.72 ± 12.29 a |
| T-AOC (U/mg protein) | 2.50 ± 0.29 b | 4.01 ± 0.24 a | 3.01 ± 0.15 ab | 3.30 ± 0.35 a | 2.61 ± 0.16 b |
| T-SOD (U/mg protein) | 40.68 ± 5.33 | 42.63 ± 3.23 | 33.50 ± 2.22 | 44.02 ± 3.25 | 44.14 ± 6.52 |
| GSH (mg/g protein) | 45.30 ± 7.45 b | 46.08 ± 3.59 b | 40.54 ± 2.83 b | 52.77 ± 7.16 b | 97.09 ± 10.24 a |
| MDA (nmol/mL homogenate) | 2.54 ± 0.36 b | 5.87 ± 0.79 a | 4.13 ± 0.70 ab | 3.71 ± 0.41 b | 3.74 ± 0.68 b |
| Serum | |||||
| GPx (U/mg protein) | 455.03 ± 7.98 a | 175.94 ± 9.40 d | 275.81 ± 5.42 b | 243.77 ± 5.74 c | 458.71 ± 3.26 a |
| T-AOC (U/mg protein) | 1.33 ± 0.10 a | 0.64 ± 0.01 c | 0.62 ± 0.01 c | 0.71 ± 0.01 c | 0.97 ± 0.13 b |
| T-SOD (U/mg protein) | 84.56 ± 1.96 b | 68.81 ± 0.69 c | 77.68 ± 1.08 bc | 285.54 ± 13.94 a | 94.71 ± 1.56 b |
| GSH (mg/g protein) | 197.21 ± 5.28 a | 173.22 ± 8.88 b | 197.62 ± 3.08 a | 191.61 ± 4.39 a | 210.59 ± 6.13 a |
| MDA (nmol/mL supernatant) | 3.25 ± 0.92 bc | 7.54 ± 0.24 a | 4.15 ± 0.24 b | 2.25 ± 0.35 c | 2.48 ± 0.52 c |
| Colon | CK | DSS | DSS + 6 mM GSH-Se | DSS + 12 mM GSH-Se | DSS + 18 mM GSH-Se |
|---|---|---|---|---|---|
| IL-1β (ng/L) | 181.77 ± 5.40 c | 266.58 ± 7.8 a | 237.10 ± 8.01 b | 164.50 ± 10.04 a | 125.51 ± 10.46 d |
| IL-6 (ng/L) | 78.01 ± 9.07 b | 107.88 ± 10.56 a | 73.82 ± 6.08 b | 74.37 ± 1.74 b | 72.07 ± 7.10 b |
| IL-8 (ng/L) | 50.99 ± 1.86 | 54.71 ± 3.60 | 47.44 ± 2.77 | 50.97 ± 1.74 | 53.15 ± 4.56 |
| TNF-γ (pg/mL) | 45.75 ± 4.37 b | 75.96 ± 1.56 a | 67.23 ± 10.36 a | 45.36 ± 4.88 b | 67.67 ± 7.01 a |
| TNF-α (pg/mL) | 44.33 ± 4.00 b | 73.45 ± 1.64 a | 65.21 ± 9.91 a | 44.24 ± 4.64 b | 65.53 ± 6.79 a |
| Cell | CK | LPS | LPS + 18 mM GSH-Se | ||
| IL-1β (ng/L) | 136.82 ± 14.53 c | 343.12 ± 13.90 a | 208.54 ± 9.55 b | ||
| IL-6 (ng/L) | 84.47 ± 3.35 b | 106.10 ± 5.12 a | 88.57 ± 9.78 b | ||
| IL-8 (ng/L) | 34.33 ± 1.61 | 38.09 ± 3.61 | 46.11 ± 1.25 | ||
| TNF-γ (pg/mL) | 60.58 ± 3.81 | 68.15 ± 7.08 | 63.18 ± 6.81 | ||
| TNF-α (pg/mL) | 62.59 ± 4.11 b | 70.74 ± 7.28 a | 65.38 ± 7.06 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zhang, Y.; Zhou, Z.; Zhang, K.; Zhou, Y.; Tang, J.; Zhang, R.; Li, H.; Wu, F.; Bai, S.; et al. A Novel Selenium-Based Nanozyme (GSH-Se) Ameliorates Colitis in Mice by Modulating the Nrf2/Keap1 and GPx4 Pathways. Int. J. Mol. Sci. 2025, 26, 1866. https://doi.org/10.3390/ijms26051866
Wu C, Zhang Y, Zhou Z, Zhang K, Zhou Y, Tang J, Zhang R, Li H, Wu F, Bai S, et al. A Novel Selenium-Based Nanozyme (GSH-Se) Ameliorates Colitis in Mice by Modulating the Nrf2/Keap1 and GPx4 Pathways. International Journal of Molecular Sciences. 2025; 26(5):1866. https://doi.org/10.3390/ijms26051866
Chicago/Turabian StyleWu, Caimei, Yuwei Zhang, Ziyun Zhou, Kun Zhang, Yixuan Zhou, Jiayong Tang, Ruinan Zhang, Hua Li, Fali Wu, Shipping Bai, and et al. 2025. "A Novel Selenium-Based Nanozyme (GSH-Se) Ameliorates Colitis in Mice by Modulating the Nrf2/Keap1 and GPx4 Pathways" International Journal of Molecular Sciences 26, no. 5: 1866. https://doi.org/10.3390/ijms26051866
APA StyleWu, C., Zhang, Y., Zhou, Z., Zhang, K., Zhou, Y., Tang, J., Zhang, R., Li, H., Wu, F., Bai, S., Wang, X., & Lyu, Y. (2025). A Novel Selenium-Based Nanozyme (GSH-Se) Ameliorates Colitis in Mice by Modulating the Nrf2/Keap1 and GPx4 Pathways. International Journal of Molecular Sciences, 26(5), 1866. https://doi.org/10.3390/ijms26051866









