CSE/H2S Signaling Pathways in Enhancing Muscle Function and Insulin Sensitivity During Exercise
Abstract
1. Introduction
2. Methods
3. Biological Basis of CSE/H2S Signaling
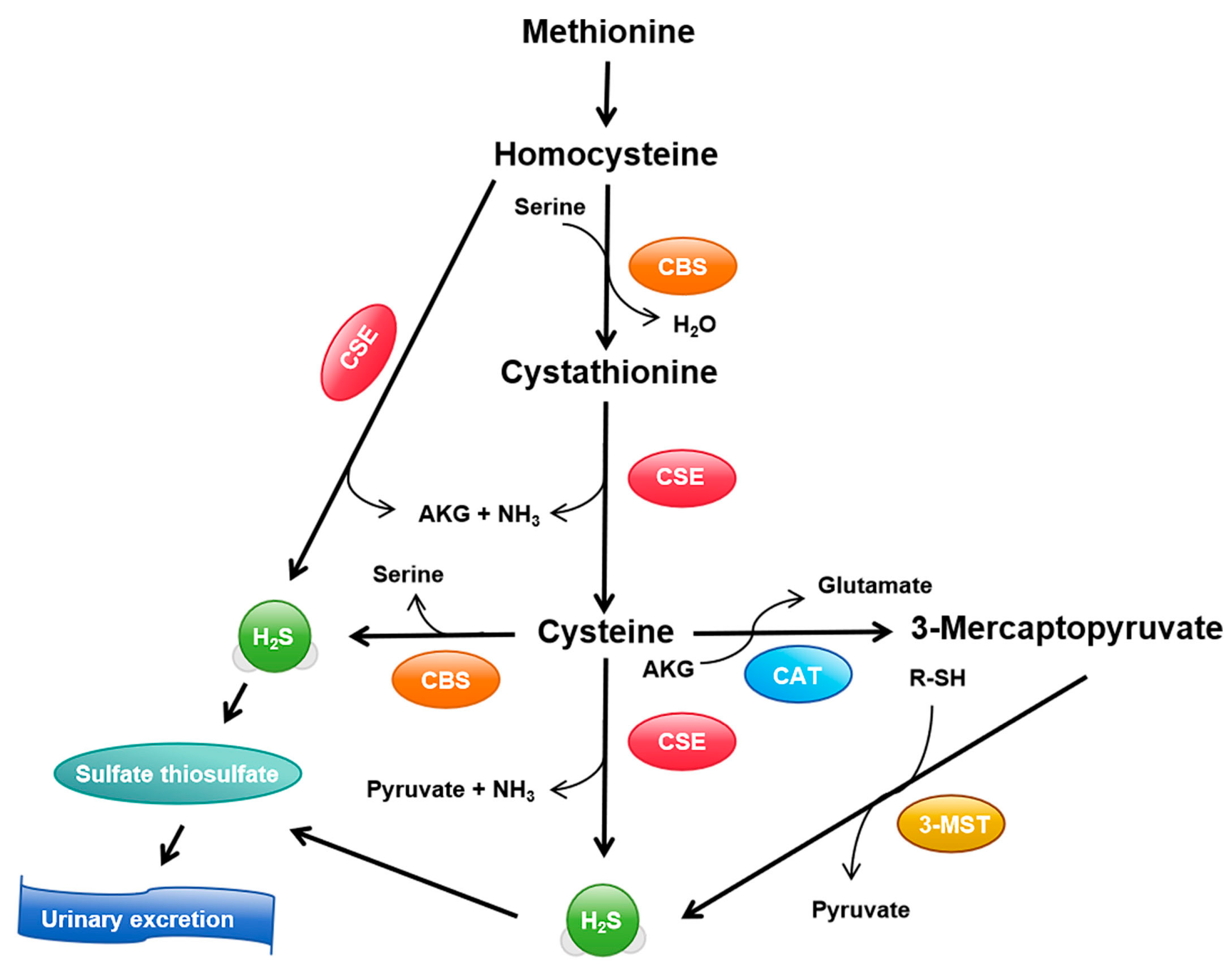
3.1. Synthesis and Metabolism of H2S
3.2. Distribution and Expression in Muscle Tissues
3.3. H2S as a Signaling Molecule
4. Exercise-Induced Molecular and Physiological Adaptations
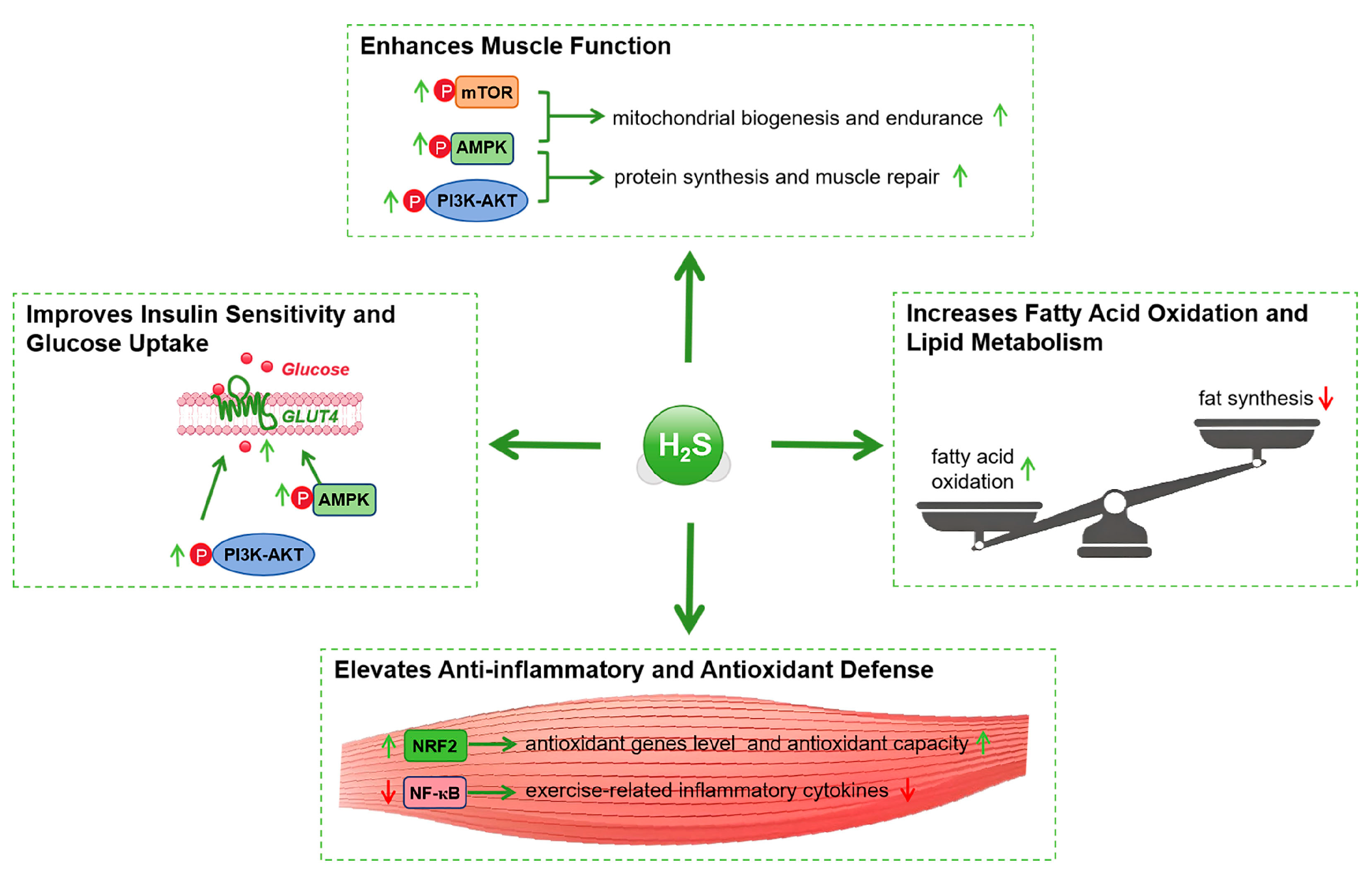
4.1. Enhancements in Muscle Function
4.2. Improvements in Insulin Sensitivity
4.3. Multi-Omics Approaches in Exercise Research
| Omics Technology | Application in Exercise Research | Key Findings and Insights | References |
|---|---|---|---|
| Genomics | Genome-wide association studies (GWAS) to identify genetic variations related to exercise capacity | Detected genetic variations that influence exercise performance and adaptive responses, helping predict individual exercise capacity | [76,77] |
| Transcriptomics | RNA sequencing to analyze gene expression changes before and after exercise | Revealed key genes regulating muscle adaptation, mitochondrial function, and metabolism | [66,67,78] |
| Proteomics | Mass spectrometry to identify protein expression and post-translational modifications | Identified proteins involved in muscle repair, protein synthesis, and mitochondrial biogenesis, highlighting adaptive responses to exercise | [68,69,78] |
| Metabolomics | High-throughput metabolite analysis to study exercise’s effects on metabolism | Uncovered metabolite changes related to energy metabolism, oxidative stress, and inflammation, highlighting metabolic adaptation | [70,71,72,75] |
| Integrated Multi-Omics | Combining genomics, transcriptomics, proteomics, and metabolomics to study exercise adaptations | Integrated data revealed key insights into mitochondrial function, fatty acid metabolism, and antioxidant pathways | [79,80] |
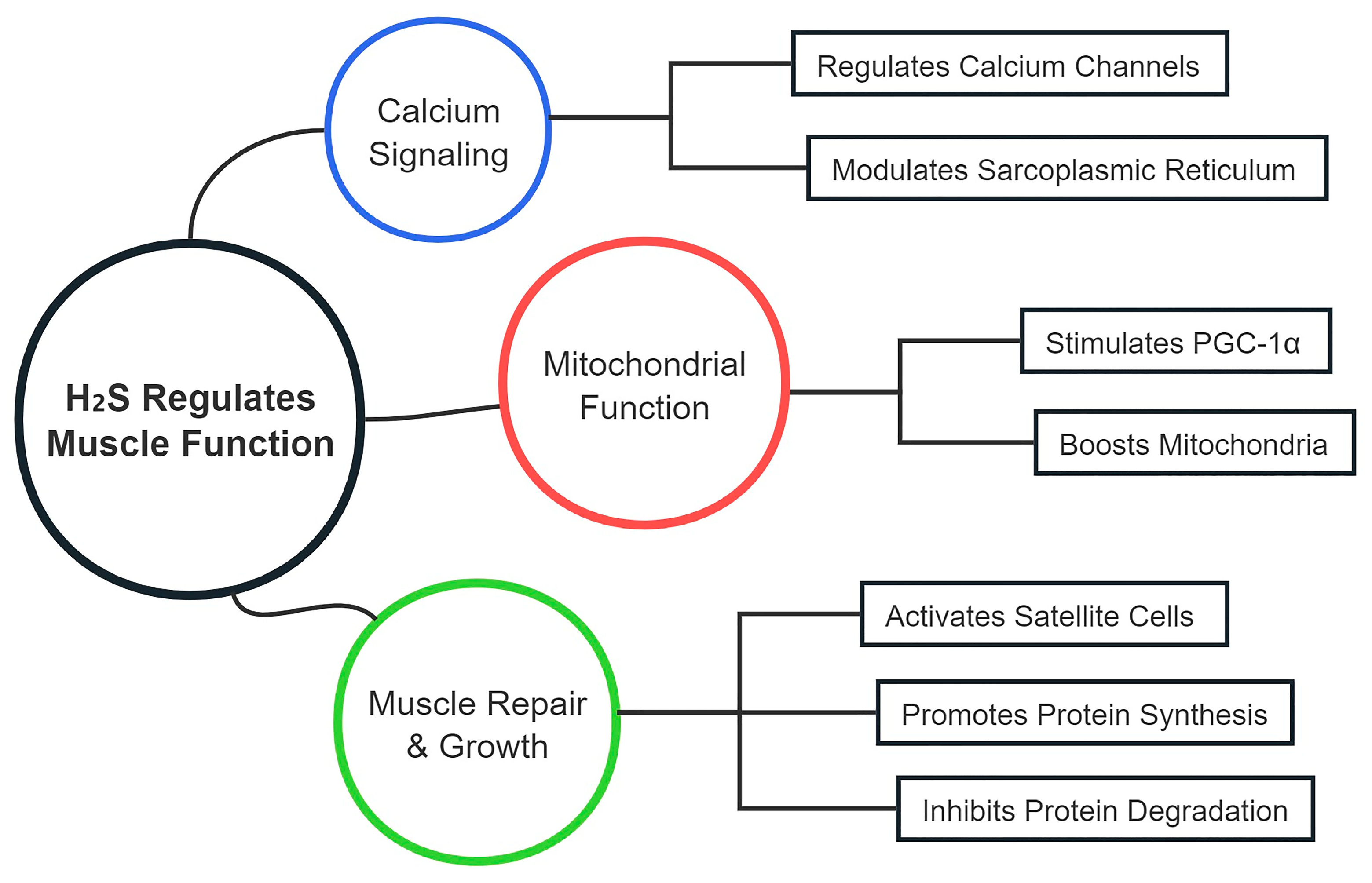
5. Mechanisms of CSE/H2S in Enhancing Muscle Function
5.1. Regulation of Muscle Contraction and Relaxation
5.2. Muscle Repair and Regeneration
5.3. Protein Synthesis and Degradation
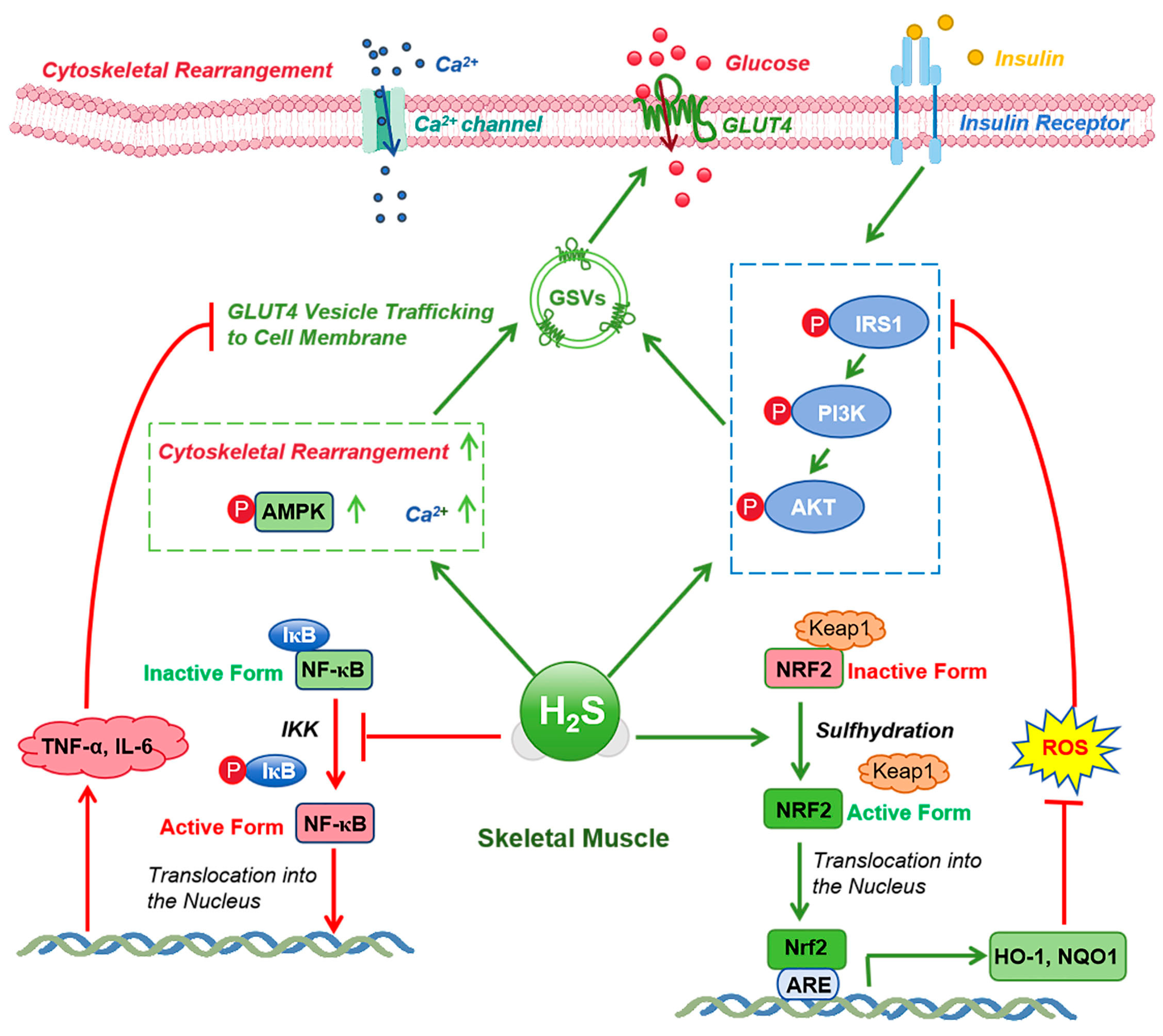
6. Mechanisms of CSE/H2S Signaling in Enhancing Insulin Sensitivity During Exercise
6.1. Regulation of Insulin Signaling
6.2. Regulation of Glucose and Lipid Metabolism
6.3. Anti-Inflammatory and Antioxidant Effects
7. H2S Supplementation in Exercise
7.1. Types of H2S Donors
7.2. Effects on Exercise Performance and Metabolic Health
7.3. Mechanistic Insights from Supplementation Studies
8. Current Research, Technological Advances, and Future Directions
8.1. Summary of Current Research
8.2. Technological and Methodological Advances
8.3. Future Research Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pesta, D.; Anadol-Schmitz, E.; Sarabhai, T.; den Kamp, Y.O.; Gancheva, S.; Trinks, N.; Zaharia, O.-P.; Mastrototaro, L.; Lyu, K.; Habets, I.; et al. Determinants of Increased Muscle Insulin Sensitivity of Exercise-Trained versus Sedentary Normal Weight and Overweight Individuals. Sci. Adv. 2025, 11, eadr8849. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Li, J.; Wang, W.; Yu, Y.; Pu, B.; Peng, Y.; Zhang, L.; Zhao, Z. The Associations of “Weekend Warrior” and Regularly Active Physical Activity with Abdominal and General Adiposity in US Adults. Obesity 2024, 32, 822–833. [Google Scholar] [CrossRef]
- Mambrini, S.P.; Grillo, A.; Colosimo, S.; Zarpellon, F.; Pozzi, G.; Furlan, D.; Amodeo, G.; Bertoli, S. Diet and Physical Exercise as Key Players to Tackle MASLD through Improvement of Insulin Resistance and Metabolic Flexibility. Front. Nutr. 2024, 11, 1426551. [Google Scholar] [CrossRef]
- Mcleod, J.C.; Currier, B.S.; Lowisz, C.V.; Phillips, S.M. The Influence of Resistance Exercise Training Prescription Variables on Skeletal Muscle Mass, Strength, and Physical Function in Healthy Adults: An Umbrella Review. J. Sport Health Sci. 2024, 13, 47–60. [Google Scholar] [CrossRef]
- Rosdiana, R.; Rasyid, D.; Djunaedi, D.; Noviar, R.A. Foot Exercises in Controlling Blood Sugar Levels in Elderly People with DM at the Baraka Community Health Center, Enrekang Regency, South Sulawesi. Int. J. Health Sci. 2024, 2, 167–178. [Google Scholar] [CrossRef]
- Thomas, A.C.Q.; Stead, C.A.; Burniston, J.G.; Phillips, S.M. Exercise-Specific Adaptations in Human Skeletal Muscle: Molecular Mechanisms of Making Muscles Fit and Mighty. Free Radic. Biol. Med. 2024, 223, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, S.; Li, C.; Zhang, X.; Shan, Y.; Zhang, Z.; Bo, H.; Zhang, Y. Endurance Exercise-Induced Histone Methylation Modification Involved in Skeletal Muscle Fiber Type Transition and Mitochondrial Biogenesis. Sci. Rep. 2024, 14, 21154. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Huang, C. Role of the Gut–Muscle Axis in Mitochondrial Function of Ageing Muscle under Different Exercise Modes. Ageing Res. Rev. 2024, 98, 102316. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Yoo, G.D.; Heo, W.; Oh, H.T.; Park, J.; Shin, S.; Do, Y.; Jeong, M.G.; Hwang, E.S.; Hong, J.-H. TAZ Stimulates Exercise-Induced Muscle Satellite Cell Activation via Pard3–P38 MAPK–TAZ Signalling Axis. J. Cachexia Sarcopenia Muscle 2023, 14, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Guo, D.; Luo, M.; Zhang, Q.; Zhang, L.; Zhang, D. Exercise Improves Heart Function after Myocardial Infarction: The Merits of AMPK. Cardiovasc. Drugs Ther. 2024. [Google Scholar] [CrossRef]
- Lu, J.; Tang, Z.; Xu, M.; Lu, J.; Wang, F.; Ni, X.; Wang, C.; Yu, B. Skeletal Muscle Cystathionine γ-Lyase Deficiency Promotes Obesity and Insulin Resistance and Results in Hyperglycemia and Skeletal Muscle Injury upon HFD in Mice. Redox Rep. 2024, 29, 2347139. [Google Scholar] [CrossRef]
- Kaleta, K.; Janik, K.; Rydz, L.; Wróbel, M.; Jurkowska, H. Bridging the Gap in Cancer Research: Sulfur Metabolism of Leukemic Cells with a Focus on L-Cysteine Metabolism and Hydrogen Sulfide-Producing Enzymes. Biomolecules 2024, 14, 746. [Google Scholar] [CrossRef]
- Flori, L.; Benedetti, G.; Calderone, V.; Testai, L. Hydrogen Sulfide and Irisin, Potential Allies in Ensuring Cardiovascular Health. Antioxidants 2024, 13, 543. [Google Scholar] [CrossRef] [PubMed]
- Ligi, S.; Ali, A.; Yang, G. Cystathionine Gamma-Lyase Deficiency Exaggerates Diethylnitrosamine-Induced Liver Damage in Mice. Nitric Oxide 2024, 151, 1–9. [Google Scholar] [CrossRef]
- Yang, J.-H.; Gao, J.; E, Y.-Q.; Jiao, L.-J.; Wu, R.; Yan, Q.-Y.; Wei, Z.-Y.; Yan, G.-L.; Liang, J.-L.; Li, H.-Z. Hydrogen Sulfide Inhibits Skeletal Muscle Ageing by Up-Regulating Autophagy through Promoting Deubiquitination of Adenosine 5′-Monophosphate (AMP)-Activated Protein Kinase A1 via Ubiquitin Specific Peptidase 5. J. Cachexia Sarcopenia Muscle 2024, 15, 2118–2133. [Google Scholar] [CrossRef]
- Machado-Neto, J.A.; Cerqueira, A.R.A.; Veríssimo-Filho, S.; Muscará, M.N.; Costa, S.K.P.; Lopes, L.R. Hydrogen Sulfide Signaling in the Tumor Microenvironment: Implications in Cancer Progression and Therapy. Antioxid. Redox Signal. 2024, 40, 250–271. [Google Scholar] [CrossRef]
- Shi, X.; Li, H.; Guo, F.; Li, D.; Xu, F. Novel Ray of Hope for Diabetic Wound Healing: Hydrogen Sulfide and Its Releasing Agents. J. Adv. Res. 2024, 58, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Rotimi, D.E.; Acho, M.A.; Falana, B.M.; Olaolu, T.D.; Mgbojikwe, I.; Ojo, O.A.; Adeyemi, O.S. Oxidative Stress-Induced Hormonal Disruption in Male Reproduction. Reprod. Sci. 2024, 31, 2943–2956. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, R.; Matson, J.B. Hydrogels for Gasotransmitter Delivery: Nitric Oxide, Carbon Monoxide, and Hydrogen Sulfide. Macromol. Biosci. 2024, 24, 2300138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-J.; Wang, Y.; Jin, Y.-Q.; Zhu, Y.-W.; Zhu, S.-G.; Wang, Q.-M.; Jing, M.-R.; Zhang, Y.-X.; Cai, C.-B.; Feng, Z.-F.; et al. Recent Advances in the Role of Hydrogen Sulfide in Age-Related Diseases. Exp. Cell Res. 2024, 441, 114172. [Google Scholar] [CrossRef]
- Munteanu, C.; Popescu, C.; Vlădulescu-Trandafir, A.-I.; Onose, G. Signaling Paradigms of H2S-Induced Vasodilation: A Comprehensive Review. Antioxidants 2024, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Slade, L.; Deane, C.S.; Szewczyk, N.J.; Etheridge, T.; Whiteman, M. Hydrogen Sulfide Supplementation as a Potential Treatment for Primary Mitochondrial Diseases. Pharmacol. Res. 2024, 203, 107180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Zhang, Y.-X.; Zhu, Y.-W.; Tang, A.-Q.; Liang, H.-B.; Yang, Y.-L.; Zhai, Y.-K.; Ji, X.-Y.; Wu, D.-D. Hydrogen Sulfide in Musculoskeletal Diseases: Molecular Mechanisms and Therapeutic Opportunities. Antioxid. Redox Signal. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Xu, M.; Liu, X.; Bao, P.; Wang, Y.; Zhu, X.; Liu, Y.; Ni, X.; Lu, J. Skeletal Muscle CSE Deficiency Leads to Insulin Resistance in Mice. Antioxidants 2022, 11, 2216. [Google Scholar] [CrossRef]
- Dawoud, A.; Youness, R.A.; Nafea, H.; Manie, T.; Bourquin, C.; Szabo, C.; Abdel-Kader, R.M.; Gad, M.Z. Pan-Inhibition of the Three H2S Synthesizing Enzymes Restrains Tumor Progression and Immunosuppression in Breast Cancer. Cancer Cell Int. 2024, 24, 136. [Google Scholar] [CrossRef] [PubMed]
- Al-Taee, K.; Al-Helaly, L.; Al-Helaly, L.; Al-Helaly, L. Hydrogen Sulfide and Cystathionine γ–Lyase with Oxidants and Antioxidants Levels for Patients with Epilepsy Diseases. Pharmacogn. J. 2024, 16, 319–322. [Google Scholar] [CrossRef]
- Si, Y.; Song, N.; Ji, Y. Bioinformatics Analysis of the Clinical Value and Potential Mechanisms of CBS/H2S in Glioma Through 1027 Samples; Research Square Platform: Durham, NC, USA, 2024. [Google Scholar]
- Manna, S.; Agrawal, R.; Yadav, T.; Kumar, T.A.; Kumari, P.; Dalai, A.; Kanade, S.; Balasubramanian, N.; Singh, A.; Chakrapani, H. Orthogonal Persulfide Generation through Precision Tools Provides Insights into Mitochondrial Sulfane Sulfur. Angew. Chem. 2024, 136, e202411133. [Google Scholar] [CrossRef]
- He, K.; Tan, B.; Lu, A.; Bai, L.; Song, C.; Miao, Y.; Liu, B.; Chen, Q.; Teng, X.; Dai, J.; et al. Asynchronous Changes of Hydrogen Sulfide and Its Generating Enzymes in Most Tissues with the Aging Process. Biosci. Rep. 2024, 44, BSR20240320. [Google Scholar] [CrossRef]
- Derry, P.J.; Liopo, A.V.; Mouli, K.; McHugh, E.A.; Vo, A.T.T.; McKelvey, A.; Suva, L.J.; Wu, G.; Gao, Y.; Olson, K.R.; et al. Oxidation of Hydrogen Sulfide to Polysulfide and Thiosulfate by a Carbon Nanozyme: Therapeutic Implications with an Emphasis on down Syndrome. Adv. Mater. 2024, 36, 2211241. [Google Scholar] [CrossRef]
- Wu, D.; Lu, Z.; Li, M.; Feng, Z.; Li, Y. Treadmill Exercise Increases Cystathionine γ Lyase Expression and Decreases Inflammation in Skeletal Muscles of High Fat Diet Induced Obese Rats. Int. J. Clin. Exp. Med. 2016, 9, 23119–23126. [Google Scholar]
- Zhang, H.; Huang, Y.; Chen, S.; Tang, C.; Wang, G.; Du, J.; Jin, H. Hydrogen Sulfide Regulates Insulin Secretion and Insulin Resistance in Diabetes Mellitus, a New Promising Target for Diabetes Mellitus Treatment? A Review. J. Adv. Res. 2021, 27, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Parsanathan, R.; Jain, S.K. Hydrogen Sulfide Regulates Irisin and Glucose Metabolism in Myotubes and Muscle of HFD-Fed Diabetic Mice. Antioxidants 2022, 11, 1369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Zheng, H.; Xu, Q.; Zhou, K.; Liu, H.; Xia, Y.; Wei, D.-H.; Jiang, M.; Tang, Z.-H.; et al. Hydrogen Sulfide Upregulates SIRT1 to Inhibit Ox-HDL-Induced Endothelial Cell Damage and Mitochondrial Dysfunction. Nitric Oxide 2024, 152, 78–89. [Google Scholar] [CrossRef]
- Pandey, T.; Pandey, V. Hydrogen Sulfide (H2S) Metabolism: Unraveling Cellular Regulation, Disease Implications, and Therapeutic Prospects for Precision Medicine. Nitric Oxide 2024, 144, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Quinzii, C.M.; Lopez, L.C. Abnormalities of Hydrogen Sulfide and Glutathione Pathways in Mitochondrial Dysfunction. J. Adv. Res. 2021, 27, 79–84. [Google Scholar] [CrossRef]
- Ng, P.C.; Hendry-Hofer, T.B.; Witeof, A.E.; Brenner, M.; Mahon, S.B.; Boss, G.R.; Haouzi, P.; Bebarta, V.S. Hydrogen Sulfide Toxicity: Mechanism of Action, Clinical Presentation, and Countermeasure Development. J. Med. Toxicol. 2019, 15, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Stoltzfus, A.T.; Ballot, J.G.; Vignane, T.; Li, H.; Worth, M.M.; Muller, L.; Siegler, M.A.; Kane, M.A.; Filipovic, M.R.; Goldberg, D.P.; et al. Chemoselective Proteomics, Zinc Fingers, and a Zinc(II) Model for H2S Mediated Persulfidation. Angew. Chem. Int. Ed. 2024, 63, e202401003. [Google Scholar] [CrossRef]
- Wang, R.; Li, K.; Wang, H.; Jiao, H.; Wang, X.; Zhao, J.; Lin, H. Endogenous CSE/Hydrogen Sulfide System Regulates the Effects of Glucocorticoids and Insulin on Muscle Protein Synthesis. Oxid. Med. Cell. Longev. 2019, 2019, 9752698. [Google Scholar] [CrossRef] [PubMed]
- Omorou, M.; Liu, N.; Huang, Y.; Al-Ward, H.; Gao, M.; Mu, C.; Zhang, L.; Hui, X. Cystathionine Beta-Synthase in Hypoxia and Ischemia/Reperfusion: A Current Overview. Arch. Biochem. Biophys. 2022, 718, 109149. [Google Scholar] [CrossRef]
- Rao, S.P.; Dobariya, P.; Bellamkonda, H.; More, S.S. Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease. Antioxidants 2023, 12, 603. [Google Scholar] [CrossRef]
- Petrovic, D.; Slade, L.; Paikopoulos, Y.; D’Andrea, D.; Savic, N.; Stancic, A.; Miljkovic, J.L.; Vignane, T.; Drekolia, M.K.; Mladenovic, D.; et al. Ergothioneine Improves Healthspan of Aged Animals by Enhancing cGPDH Activity through CSE-Dependent Persulfidation. Cell Metab. 2025, 37, 542–556.e14. [Google Scholar] [CrossRef]
- Bekheit, M.; Kamera, B.; Colacino, L.; Dropmann, A.; Delibegovic, M.; Almadhoob, F.; Hanafy, N.; Bermano, G.; Hammad, S. Mechanisms Underpinning the Effect of Exercise on the Non-Alcoholic Fatty Liver Disease: Review. EXCLI J. 2025, 24, 238–266. [Google Scholar] [CrossRef]
- Veeranki, S.; Tyagi, S.C. Role of Hydrogen Sulfide in Skeletal Muscle Biology and Metabolism. Nitric Oxide 2015, 46, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Uyanga, V.A.; Wang, X.; Jiao, H.; Zhao, J.; Zhou, Y.; Li, H.; Lin, H. Allicin Promotes Glucose Uptake by Activating AMPK through CSE/H2S-Induced S-Sulfhydration in a Muscle-Fiber Dependent Way in Broiler Chickens. Mol. Nutr. Food Res. 2024, 68, 2300622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, J.; Huang, S.; Chen, X.; Chang, A.C.Y.; Wang, C.; Zhang, J.; Zhang, H. Hydrogen Sulfide Protects Cardiomyocytes from Doxorubicin-Induced Ferroptosis through the SLC7A11/GSH/GPx4 Pathway by Keap1 S-Sulfhydration and Nrf2 Activation. Redox Biol. 2024, 70, 103066. [Google Scholar] [CrossRef]
- Pang, P.-P.; Zhang, H.-Y.; Zhang, D.-C.; Tang, J.-X.; Gong, Y.; Guo, Y.-C.; Zheng, C.-B. Investigating the Impact of Protein S-Sulfhydration Modification on Vascular Diseases: A Comprehensive Review. Eur. J. Pharmacol. 2024, 966, 176345. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Bai, D.; Gong, C.; Cao, Y.; Yan, X.; Peng, R. Hydrogen Sulfide Mitigates Mitochondrial Dysfunction and Cellular Senescence in Diabetic Patients: Potential Therapeutic Applications. Biochem. Pharmacol. 2024, 230, 116556. [Google Scholar] [CrossRef]
- Li, R.-Y.; Guo, L. Exercise in Diabetic Nephropathy: Protective Effects and Molecular Mechanism. Int. J. Mol. Sci. 2024, 25, 3605. [Google Scholar] [CrossRef]
- Birulina, J.G.; Ivanov, V.V.; Buyko, E.E.; Voronkova, O.V.; Chernyshov, N.A. The role of endogenous H2S in experimental metabolic syndrome. RUDN J. Med. 2024, 28, 331–339. [Google Scholar] [CrossRef]
- Silva-Velasco, D.L.; Hong, E.; Beltran-Ornelas, J.H.; Sánchez-López, A.; Huerta de la Cruz, S.; Tapia-Martínez, J.A.; Gomez, C.B.; Centurión, D. Hydrogen Sulfide Ameliorates Hypertension and Vascular Dysfunction Induced by Insulin Resistance in Rats by Reducing Oxidative Stress and Activating eNOS. Eur. J. Pharmacol. 2024, 963, 176266. [Google Scholar] [CrossRef]
- Cirino, G.; Szabo, C.; Papapetropoulos, A. Physiological Roles of Hydrogen Sulfide in Mammalian Cells, Tissues, and Organs. Physiol. Rev. 2023, 103, 31–276. [Google Scholar] [CrossRef]
- Guo, Q.; Luo, Q.; Song, G. Control of Muscle Satellite Cell Function by Specific Exercise-Induced Cytokines and Their Applications in Muscle Maintenance. J. Cachexia Sarcopenia Muscle 2024, 15, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-C.; Gao, B. Integrative Effects of Resistance Training and Endurance Training on Mitochondrial Remodeling in Skeletal Muscle. Eur. J. Appl. Physiol. 2024, 124, 2851–2865. [Google Scholar] [CrossRef]
- Kong, S.; Cai, B.; Nie, Q. PGC-1α Affects Skeletal Muscle and Adipose Tissue Development by Regulating Mitochondrial Biogenesis. Mol. Genet. Genomics 2022, 297, 621–633. [Google Scholar] [CrossRef]
- Yendrizal, Y.; Okilanda, A.; Masrun, M.; Ridwan, M.; Ahmed, M.; Crisari, S.; Tulyakul, S. Unlocking the Science of Physical Endurance: Training Techniques and Biological Factors. Retos Nuevas Tend. En Educ. Física Deporte Recreación 2024, 55, 504–512. [Google Scholar]
- Luo, X.; Zhang, H.; Cao, X.; Yang, D.; Yan, Y.; Lu, J.; Wang, X.; Wang, H. Endurance Exercise-Induced Fgf21 Promotes Skeletal Muscle Fiber Conversion through TGF-Β1 and P38 MAPK Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 11401. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, N.; Ye, H.; Miao, J.; Xia, B.; Yang, Y.; Peng, H.; Xu, S.; Wu, T.; Tao, C.; et al. Glucose Restriction Enhances Oxidative Fiber Formation: A Multi-Omic Signal Network Involving AMPK and CaMK2. iScience 2024, 27, 108590. [Google Scholar] [CrossRef]
- Richter, E.A. Is GLUT4 Translocation the Answer to Exercise-Stimulated Muscle Glucose Uptake? Am. J. Physiol.-Endocrinol. Metab. 2021, 320, E240–E243. [Google Scholar] [CrossRef]
- Richter, E.A.; Sylow, L.; Hargreaves, M. Interactions between Insulin and Exercise. Biochem. J. 2021, 478, 3827–3846. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. Exercise Performance and Health: Role of GLUT4. Free Radic. Biol. Med. 2024, 224, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Zhu, Y.; Yan, H.; Lu, Y. Effects of Different Intensity Exercise on Glucose Metabolism and Hepatic IRS/PI3K/AKT Pathway in SD Rats Exposed with TCDD. Int. J. Environ. Res. Public Health 2021, 18, 13141. [Google Scholar] [CrossRef]
- Yang, B.; Yu, Q.; Chang, B.; Guo, Q.; Xu, S.; Yi, X.; Cao, S. MOTS-c Interacts Synergistically with Exercise Intervention to Regulate PGC-1α Expression, Attenuate Insulin Resistance and Enhance Glucose Metabolism in Mice via AMPK Signaling Pathway. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2021, 1867, 166126. [Google Scholar] [CrossRef]
- Atakan, M.M.; Koşar, Ş.N.; Güzel, Y.; Tin, H.T.; Yan, X. The Role of Exercise, Diet, and Cytokines in Preventing Obesity and Improving Adipose Tissue. Nutrients 2021, 13, 1459. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Almeida, J.A.; Franco, O.L.; Petriz, B. Chapter Three—Omics and the Molecular Exercise Physiology. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 96, pp. 55–84. [Google Scholar]
- Kusano, T.; Sotani, Y.; Takeda, R.; Hatano, A.; Kawata, K.; Kano, R.; Matsumoto, M.; Kano, Y.; Hoshino, D. Time-Series Transcriptomics Reveals Distinctive mRNA Expression Dynamics Associated with Gene Ontology Specificity and Protein Expression in Skeletal Muscle after Electrical Stimulation-Induced Resistance Exercise. FASEB J. 2024, 38, e70153. [Google Scholar] [CrossRef]
- Beiter, T.; Zügel, M.; Hudemann, J.; Schild, M.; Fragasso, A.; Burgstahler, C.; Krüger, K.; Mooren, F.C.; Steinacker, J.M.; Nieß, A.M. The Acute, Short-, and Long-Term Effects of Endurance Exercise on Skeletal Muscle Transcriptome Profiles. Int. J. Mol. Sci. 2024, 25, 2881. [Google Scholar] [CrossRef] [PubMed]
- Cervone, D.T.; Moreno-Justicia, R.; Quesada, J.P.; Deshmukh, A.S. Mass Spectrometry-Based Proteomics Approaches to Interrogate Skeletal Muscle Adaptations to Exercise. Scand. J. Med. Sci. Sports 2024, 34, e14334. [Google Scholar] [CrossRef]
- Bhat, A.; Abu, R.; Jagadesan, S.; Vellichirammal, N.N.; Pendyala, V.V.; Yu, L.; Rudebush, T.L.; Guda, C.; Zucker, I.H.; Kumar, V.; et al. Quantitative Proteomics Identifies Novel Nrf2-Mediated Adaptative Signaling Pathways in Skeletal Muscle Following Exercise Training. Antioxidants 2023, 12, 151. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; Cao, X.; Tian, Y.; Li, J. Effect of Acute High-Intensity Exercise on Myocardium Metabolic Profiles in Rat and Human Study via Metabolomics Approach. Sci. Rep. 2022, 12, 6791. [Google Scholar] [CrossRef]
- Ohmura, H.; Mukai, K.; Takahashi, Y.; Takahashi, T. Metabolomic Analysis of Skeletal Muscle before and after Strenuous Exercise to Fatigue. Sci. Rep. 2021, 11, 11261. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Luo, W.; Kong, C.; Xie, W.; Chen, X.; Qiu, J.; Wang, K.; Wei, H.; Zhou, Y. Impact of Aerobic Exercise on Brain Metabolism: Insights from Spatial Metabolomic Analysis. Behav. Brain Res. 2025, 478, 115339. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wu, W.; He, X.; Qian, P.; Chang, J.; Lu, Z.; Guo, J.; Bao, Y.; Guan, H.; Zhang, T. Effects of Moderate Intensity Exercise on Liver Metabolism in Mice Based on Multi-Omics Analysis. Sci. Rep. 2024, 14, 31072. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhao, R.; Chen, J.; Yan, Z.; Sui, X.; Li, H.; Li, Q.; Du, X.; Liu, Y.; Yao, S.; et al. Integrative Transcriptomic, Proteomic and Metabolomic Analyses Yields Insights into Muscle Fiber Type in Cattle. Food Chem. 2025, 468, 142479. [Google Scholar] [CrossRef]
- Khoramipour, K.; Rajizadeh, M.A.; Akbari, Z.; Arjmand, M. The Effect of High-Intensity Interval Training on Type 2 Diabetic Muscle: A Metabolomics-Based Study. Heliyon 2024, 10, e34917. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.H.; Lindholm, M.E.; Ashley, E.A. Charting the Molecular Terrain of Exercise: Energetics, Exerkines, and the Future of Multiomic Mapping. Physiology 2025, 40. [Google Scholar] [CrossRef] [PubMed]
- Vetr, N.G.; Gay, N.R.; Montgomery, S.B. The Impact of Exercise on Gene Regulation in Association with Complex Trait Genetics. Nat. Commun. 2024, 15, 3346. [Google Scholar] [CrossRef]
- Amar, D.; Gay, N.R.; Jean-Beltran, P.M.; Bae, D.; Dasari, S.; Dennis, C.; Evans, C.R.; Gaul, D.A.; Ilkayeva, O.; Ivanova, A.A.; et al. Temporal Dynamics of the Multi-Omic Response to Endurance Exercise Training. Nature 2024, 629, 174–183. [Google Scholar] [CrossRef]
- Jacques, M.; Landen, S.; Sharples, A.P.; Garnham, A.; Schittenhelm, R.; Stele, J.; Heikkinen, A.; Sillanpää, E.; Ollikainen, M.; Broatch, J.; et al. Molecular Landscape of Modality-Specific Exercise Adaptation in Human Skeletal Muscle through Large-Scale Multi-OMICs Integration. BioRxiv 2024, 2024-07. [Google Scholar]
- Owens, D.J.; Bennett, S. An Exercise Physiologist’s Guide to Metabolomics. Exp. Physiol. 2024, 109, 1066–1079. [Google Scholar] [CrossRef]
- Castro, A.A.; Perez, M.B.L.; Gonzalez, G.A.; Bonilla, N.C.; Santiago, G.G.; Singh, I. Significance of calcium: Its correlation with red and white muscle contraction, fatigue and potential. Int. J. Res. Med. Sci. 2024, 12, 1311–1316. [Google Scholar] [CrossRef]
- Quan, X.; Chen, W.; Qin, B.; Wang, J.; Luo, H.; Dai, F. The Excitatory Effect of Hydrogen Sulfide on Rat Colonic Muscle Contraction and the Underlying Mechanism. J. Pharmacol. Sci. 2022, 149, 100–107. [Google Scholar] [CrossRef]
- Dai, L.; Qian, Y.; Zhou, J.; Zhu, C.; Jin, L.; Li, S. Hydrogen Sulfide Inhibited L-Type Calcium Channels (CaV1.2) via up-Regulation of the Channel Sulfhydration in Vascular Smooth Muscle Cells. Eur. J. Pharmacol. 2019, 858, 172455. [Google Scholar] [CrossRef]
- Majumder, A.; Singh, M.; George, A.K.; Tyagi, S.C. Restoration of Skeletal Muscle Homeostasis by Hydrogen Sulfide during Hyperhomocysteinemia-Mediated Oxidative/ER Stress Condition. Can. J. Physiol. Pharmacol. 2019, 97, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Liu, W.; Yu, C.; Ren, J.; Li, Y.; Shi, X.; Li, Q.; Zhang, J. Protective Effects of Allicin on Acute Myocardial Infarction in Rats via Hydrogen Sulfide-Mediated Regulation of Coronary Arterial Vasomotor Function and Myocardial Calcium Transport. Front. Pharmacol. 2022, 12, 752244. [Google Scholar] [CrossRef]
- Asunción-Alvarez, D.; Palacios, J.; Ybañez-Julca, R.O.; Rodriguez-Silva, C.N.; Nwokocha, C.; Cifuentes, F.; Greensmith, D.J. Calcium Signaling in Endothelial and Vascular Smooth Muscle Cells: Sex Differences and the Influence of Estrogens and Androgens. Am. J. Physiol.-Heart Circ. Physiol. 2024, 326, H950–H970. [Google Scholar] [CrossRef]
- Donato, L.; Mordà, D.; Scimone, C.; Alibrandi, S.; D’Angelo, R.; Sidoti, A. From Powerhouse to Regulator: The Role of Mitoepigenetics in Mitochondrion-Related Cellular Functions and Human Diseases. Free Radic. Biol. Med. 2024, 218, 105–119. [Google Scholar] [CrossRef]
- Huang, S.; Chen, X.; Pan, J.; Zhang, H.; Ke, J.; Gao, L.; Yu Chang, A.C.; Zhang, J.; Zhang, H. Hydrogen Sulfide Alleviates Heart Failure with Preserved Ejection Fraction in Mice by Targeting Mitochondrial Abnormalities via PGC-1α. Nitric Oxide 2023, 136–137, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Long, J.; Zhao, L.; Liu, J. Hydrogen: A Rising Star in Gas Medicine as a Mitochondria-Targeting Nutrient via Activating Keap1-Nrf2 Antioxidant System. Antioxidants 2023, 12, 2062. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Jing, G.; Zhu, S. Regulation of Mitochondrial Respiration by Hydrogen Sulfide. Antioxidants 2023, 12, 1644. [Google Scholar] [CrossRef]
- Lu, F.; Zhang, S.; Dong, S.; Wang, M.; Pang, K.; Zhao, Y.; Huang, J.; Kang, J.; Liu, N.; Zhang, X.; et al. Exogenous Hydrogen Sulfide Enhances Myogenic Differentiation of C2C12 Myoblasts under High Palmitate Stress. Heliyon 2024, 10, e38661. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Q.; Liu, X.; Lu, L.; Li, Z. Impact of Alpha-Ketoglutarate on Skeletal Muscle Health and Exercise Performance: A Narrative Review. Nutrients 2024, 16, 3968. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Kaczmarek, M.; Ciałowicz, M.; Clemente, F.M.; Wolański, P.; Badicu, G.; Murawska-Ciałowicz, E. The Role of Satellite Cells in Skeletal Muscle Regeneration—The Effect of Exercise and Age. Biology 2021, 10, 1056. [Google Scholar] [CrossRef]
- Shuang, T. The Interaction Among Hydrogen Sulfide, Estrogen and Insulin-like Growth Factor-1 in Vascular Smooth Muscle Cells. Ph.D. Thesis, Laurentian University, Sudbury, ON, Canada, 2019. [Google Scholar]
- Kaziród, K.; Myszka, M.; Dulak, J.; Łoboda, A. Hydrogen Sulfide as a Therapeutic Option for the Treatment of Duchenne Muscular Dystrophy and Other Muscle-Related Diseases. Cell. Mol. Life Sci. 2022, 79, 608. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, G. H2S Signaling and Extracellular Matrix Remodeling in Cardiovascular Diseases: A Tale of Tense Relationship. Nitric Oxide 2021, 116, 14–26. [Google Scholar] [CrossRef]
- Wang, R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration That Blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of Hydrogen Sulfide on Mitochondrial Function and Cellular Bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef]
- Qiu, Y.; Fernández-García, B.; Lehmann, H.I.; Li, G.; Kroemer, G.; López-Otín, C.; Xiao, J. Exercise Sustains the Hallmarks of Health. J. Sport Health Sci. 2023, 12, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Soori, R.; Ghram, A.; Zare Shahneh, M.; Choobineh, S.; Costa, P.B.; Voltarelli, F.A. Effects of High Intensity Interval Training and Aging on Cardiac Muscle Apoptosis Markers in C57BL/6 Mice. Sport Sci. Health 2021, 17, 173–179. [Google Scholar] [CrossRef]
- Zhang, Y.; Masters, L.; Wang, Y.; Wu, L.; Pei, Y.; Guo, B.; Parissenti, A.; Lees, S.J.; Wang, R.; Yang, G. Cystathionine Gamma-Lyase/H2S Signaling Facilitates Myogenesis under Aging and Injury Condition. FASEB J. 2021, 35, e21511. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, Y.-F.; Zhang, Y.-X.; Wang, Y.; Jin, Y.-Q.; Yuan, H.; Liang, X.-Y.; Ji, X.-Y.; Jiang, Q.-Y.; Wu, D.-D. The Potential Role of Hydrogen Sulfide in Cancer Cell Apoptosis. Cell Death Discov. 2024, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Luo, N.; Wang, L.; Zhao, Z.; Bu, H.; Xu, G.; Yan, Y.; Che, X.; Jiao, Z.; Zhao, T.; et al. Hydrogen Sulfide Ameliorates Chronic Renal Failure in Rats by Inhibiting Apoptosis and Inflammation through ROS/MAPK and NF-κB Signaling Pathways. Sci. Rep. 2017, 7, 455. [Google Scholar] [CrossRef]
- Tao, B.-B.; Zhu, Q.; Zhu, Y.-C. Mechanisms Underlying the Hydrogen Sulfide Actions: Target Molecules and Downstream Signaling Pathways. Antioxid. Redox Signal. 2024, 40, 86–109. [Google Scholar] [CrossRef]
- Majumder, A. Effects of Hydrogen Sulfide in Hyperhomocysteinemia-Mediated Skeletal Muscle Myopathy. Ph.D. Thesis, University of Louisville, Louisville, KS, USA, 2018. [Google Scholar] [CrossRef]
- Łoboda, A.; Dulak, J. Cardioprotective Effects of Hydrogen Sulfide and Its Potential Therapeutic Implications in the Amelioration of Duchenne Muscular Dystrophy Cardiomyopathy. Cells 2024, 13, 158. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Ho, T.-J.; Tsai, B.C.-K.; Chiang, C.-Y.; Kao, H.-C.; Kuo, W.-W.; Chen, R.-J.; Viswanadha, V.P.; Huang, C.-W.; Huang, C.-Y. Exercise Renovates H2S and Nrf2-Related Antioxidant Pathways to Suppress Apoptosis in the Natural Ageing Process of Male Rat Cortex. Biogerontology 2021, 22, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, X.; Bao, P.; Wang, Y.J.; Lu, J.; Liu, Y.J. H2S Protects against Immobilization-Induced Muscle Atrophy via Reducing Oxidative Stress and Inflammation. Front. Physiol. 2022, 13, 844539. [Google Scholar] [CrossRef]
- Blackwood, E.A.; Glembotski, C.C. Hydrogen Sulfide: The Gas That Fuels Longevity. J. Cardiovasc. Aging 2022, 2, 26. [Google Scholar] [CrossRef]
- Zheng, X.-B.; Wang, C.; Zhang, M.; Yao, B.-Q.; Wu, H.-Y.; Hou, S.-X. Exogenous H2S Targeting PI3K/AKT/mTOR Pathway Alleviates Chronic Intermittent Hypoxia-Induced Myocardial Damage through Inhibiting Oxidative Stress and Enhancing Autophagy. Sleep Breath. 2024, 29, 43. [Google Scholar] [CrossRef]
- Soria, M.; González-Haro, C.; Esteva, S.; Escanero, J.F.; Pina, J.R. Effect of Sulphurous Mineral Water in Haematological and Biochemical Markers of Muscle Damage after an Endurance Exercise in Well-Trained Athletes. J. Sports Sci. 2014, 32, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.S.; Nader, J.; Al-Ali, W.; Al Madhoun, A.; Arefanian, H.; Al-Mulla, F. Hydrogen Sulfide Donor NaHS Improves Metabolism and Reduces Muscle Atrophy in Type 2 Diabetes: Implication for Understanding Sarcopenic Pathophysiology. Oxid. Med. Cell. Longev. 2018, 2018, 6825452. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Elsayed, H.R.H.; El-Nablaway, M.; Hamed, S.; Eladl, A.; Fouad, S.; El Nashar, E.M.; Al-Otaibi, M.L.; Rabei, M.R. Targeting Hydrogen Sulfide Modulates Dexamethasone-Induced Muscle Atrophy and Microvascular Rarefaction, through Inhibition of NOX4 and Induction of MGF, M2 Macrophages and Endothelial Progenitors. Cells 2022, 11, 2500. [Google Scholar] [CrossRef]
- Tian, X.; Zhou, D.; Zhang, Y.; Song, Y.; Zhang, Q.; Bu, D.; Sun, Y.; Wu, L.; Long, Y.; Tang, C.; et al. Persulfidation of Transcription Factor FOXO1 at Cysteine 457: A Novel Mechanism by Which H2S Inhibits Vascular Smooth Muscle Cell Proliferation. J. Adv. Res. 2021, 27, 155–164. [Google Scholar] [CrossRef]
- Haberecht-Müller, S.; Krüger, E.; Fielitz, J. Out of Control: The Role of the Ubiquitin Proteasome System in Skeletal Muscle during Inflammation. Biomolecules 2021, 11, 1327. [Google Scholar] [CrossRef]
- Majumder, A.; Singh, M.; Behera, J.; Theilen, N.T.; George, A.K.; Tyagi, N.; Metreveli, N.; Tyagi, S.C. Hydrogen Sulfide Alleviates Hyperhomocysteinemia-Mediated Skeletal Muscle Atrophy via Mitigation of Oxidative and Endoplasmic Reticulum Stress Injury. Am. J. Physiol.-Cell Physiol. 2018, 315, C609–C622. [Google Scholar] [CrossRef]
- Chen, R.; Zheng, Y.; Zhou, C.; Dai, H.; Wang, Y.; Chu, Y.; Luo, J. N-Acetylcysteine Attenuates Sepsis-Induced Muscle Atrophy by Downregulating Endoplasmic Reticulum Stress. Biomedicines 2024, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Liu, H.; Wang, H. Exogenous Hydrogen Sulfide Plays an Important Role by Regulating Autophagy in Diabetic-Related Diseases. Int. J. Mol. Sci. 2021, 22, 6715. [Google Scholar] [CrossRef] [PubMed]
- Strutynska, N.; Strutynskyi, R.; Mys, L.; Luchkova, A.; Korkach, Y.; Goshovska, Y.; Chorna, S.; Sagach, V. Exercise Restores Endogenous H2S Synthesis and Mitochondrial Function in the Heart of Old Rats. Eur. J. Clin. Investig. 2022, 52, e13829. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, W.; Zhu, X.; Xu, N.; Meng, Q.; Jiang, W.; Zhang, L.; Yang, M.; Xu, F.; Li, Y. VEGFB Ameliorates Insulin Resistance in NAFLD via the PI3K/AKT Signal Pathway. J. Transl. Med. 2024, 22, 976. [Google Scholar] [CrossRef]
- Munteanu, C.; Rotariu, M.; Turnea, M.; Dogaru, G.; Popescu, C.; Spînu, A.; Andone, I.; Postoiu, R.; Ionescu, E.V.; Oprea, C.; et al. Recent Advances in Molecular Research on Hydrogen Sulfide (H2S) Role in Diabetes Mellitus (DM)—A Systematic Review. Int. J. Mol. Sci. 2022, 23, 6720. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Zeng, W.-T.; Lee, D.-Y. H2S- and Redox-State-Mediated PTP1B S-Sulfhydration in Insulin Signaling. Int. J. Mol. Sci. 2023, 24, 2898. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, X.; Lan, F.; Zhou, T.; Cai, H.; Sun, H.; Kong, W.; Kong, W. Hydrogen Sulphide Treatment Increases Insulin Sensitivity and Improves Oxidant Metabolism through the CaMKKbeta-AMPK Pathway in PA-Induced IR C2C12 Cells. Sci. Rep. 2017, 7, 13248. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mesfin, F.M.; Hunter, C.E.; Olson, K.R.; Shelley, W.C.; Brokaw, J.P.; Manohar, K.; Markel, T.A. Recent Development of the Molecular and Cellular Mechanisms of Hydrogen Sulfide Gasotransmitter. Antioxidants 2022, 11, 1788. [Google Scholar] [CrossRef]
- Youssef, N.; Noureldein, M.H.; Riachi, M.E.; Haddad, A.; Eid, A.A. Macrophage Polarization and Signaling in Diabetic Kidney Disease: A Catalyst for Disease Progression. Am. J. Physiol.-Ren. Physiol. 2024, 326, F301–F312. [Google Scholar] [CrossRef]
- Deng, N.-H.; Luo, W.; Gui, D.-D.; Yan, B.-J.; Zhou, K.; Tian, K.-J.; Ren, Z.; Xiong, W.-H.; Jiang, Z.-S. Hydrogen Sulfide Plays a Potential Alternative for the Treatment of Metabolic Disorders of Diabetic Cardiomyopathy. Mol. Cell. Biochem. 2022, 477, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Mateus, I.; Prip-Buus, C. Hydrogen Sulphide in Liver Glucose/Lipid Metabolism and Non-Alcoholic Fatty Liver Disease. Eur. J. Clin. Investig. 2022, 52, e13680. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Untereiner, A.; Wu, L.; Yang, G. H2S-Induced S-Sulfhydration of Pyruvate Carboxylase Contributes to Gluconeogenesis in Liver Cells. Biochim. Biophys. Acta BBA Gen. Subj. 2015, 1850, 2293–2303. [Google Scholar] [CrossRef] [PubMed]
- Piragine, E.; Calderone, V. Pharmacological Modulation of the Hydrogen Sulfide (HS) System by Dietary HS-Donors: A Novel Promising Strategy in the Prevention and Treatment of Type 2 Diabetes Mellitus. Phytother. Res. 2021, 35, 1817–1846. [Google Scholar] [CrossRef] [PubMed]
- Mihaylov, S.R.; Castelli, L.M.; Lin, Y.-H.; Gül, A.; Soni, N.; Hastings, C.; Flynn, H.R.; Păun, O.; Dickman, M.J.; Snijders, A.P.; et al. The Master Energy Homeostasis Regulator PGC-1α Exhibits an mRNA Nuclear Export Function. Nat. Commun. 2023, 14, 5496. [Google Scholar] [CrossRef]
- Shahgaldi, S.; Kahmini, F.R. A Comprehensive Review of Sirtuins: With a Major Focus on Redox Homeostasis and Metabolism. Life Sci. 2021, 282, 119803. [Google Scholar] [CrossRef]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef]
- Bourque, C.; Zhang, Y.; Fu, M.; Racine, M.; Greasley, A.; Pei, Y.; Wu, L.; Wang, R.; Yang, G. H2S Protects Lipopolysaccharide-Induced Inflammation by Blocking NFκB Transactivation in Endothelial Cells. Toxicol. Appl. Pharmacol. 2018, 338, 20–29. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Li, M.; Mao, J.; Zhu, Y. New Therapeutic Approaches Using Hydrogen Sulfide Donors in Inflammation and Immune Response. Antioxid. Redox Signal. 2021, 35, 341–356. [Google Scholar] [CrossRef]
- He, Z.; Zhu, Y.; Ma, H.; Shen, Q.; Chen, X.; Wang, X.; Shao, H.; Wang, Y.; Yang, S. Hydrogen Sulfide Regulates Macrophage Polarization and Necroptosis to Accelerate Diabetic Skin Wound Healing. Int. Immunopharmacol. 2024, 132, 111990. [Google Scholar] [CrossRef]
- Merz, T.; McCook, O.; Brucker, C.; Waller, C.; Calzia, E.; Radermacher, P.; Datzmann, T. H2S in Critical Illness—A New Horizon for Sodium Thiosulfate? Biomolecules 2022, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.-H.; Moore, P.K. Characterization of a Novel, Water-Soluble Hydrogen Sulfide–Releasing Molecule (GYY4137). Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Lu, H.; Chen, Y.; Hu, P. Current Status and Future Prospects of Hydrogen Sulfide Donor-Based Delivery Systems. Adv. Ther. 2023, 6, 2200349. [Google Scholar] [CrossRef]
- Comas, F.; Latorre, J.; Ortega, F.; Arnoriaga Rodríguez, M.; Kern, M.; Lluch, A.; Ricart, W.; Blüher, M.; Gotor, C.; Romero, L.C.; et al. Activation of Endogenous H2S Biosynthesis or Supplementation with Exogenous H2S Enhances Adipose Tissue Adipogenesis and Preserves Adipocyte Physiology in Humans. Antioxid. Redox Signal. 2021, 35, 319–340. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Onose, G.; Poștaru, M.; Turnea, M.; Rotariu, M.; Galaction, A.I. Hydrogen Sulfide and Gut Microbiota: Their Synergistic Role in Modulating Sirtuin Activity and Potential Therapeutic Implications for Neurodegenerative Diseases. Pharmaceuticals 2024, 17, 1480. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.T.; Huynh, T.D.; Wang, C.-S.; Lai, K.-H.; Lin, Z.-C.; Lin, W.-N.; Chen, Y.-L.; Peng, T.-Y.; Wu, H.-C.; Lee, I.-T. The Potential Implications of Hydrogen Sulfide in Aging and Age-Related Diseases through the Lens of Mitohormesis. Antioxidants 2022, 11, 1619. [Google Scholar] [CrossRef]
- Olson, K.R.; Derry, P.J.; Kent, T.A.; Straub, K.D. The Effects of Antioxidant Nutraceuticals on Cellular Sulfur Metabolism and Signaling. Antioxid. Redox Signal. 2023, 38, 68–94. [Google Scholar] [CrossRef]
- Batallé, G.; Bai, X.; Pol, O. The Interaction between Carbon Monoxide and Hydrogen Sulfide during Chronic Joint Pain in Young Female Mice. Antioxidants 2022, 11, 1271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, X.; Zhang, J.; Dong, G.; Xiao, W.; Xu, X. Hydrogen Sulfide Alleviates Skeletal Muscle Fibrosis via Attenuating Inflammation and Oxidative Stress. Front. Physiol. 2020, 11, 533690. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. H2S and Polysulfide Metabolism: Conventional and Unconventional Pathways. Biochem. Pharmacol. 2018, 149, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, H.; Yang, Y.; Lan, T.; Wang, H.; Wu, D. Hydrogen Sulfide Plays an Important Role by Regulating Endoplasmic Reticulum Stress in Diabetes-Related Diseases. Int. J. Mol. Sci. 2022, 23, 7170. [Google Scholar] [CrossRef] [PubMed]
- Luchkova, A.; Mys, L.; Strutynska, N.; Sagach, V. Regular Exercise Increases H2S Levels in Mitochondria and Cardiac Tissue, Enhances Genes Expression of H2S-Synthesizing Enzymes and Prevents Mitochondrial Swelling. Cardiovasc. Res. 2022, 118, cvac066.041. [Google Scholar] [CrossRef]
- Magli, E.; Perissutti, E.; Santagada, V.; Caliendo, G.; Corvino, A.; Esposito, G.; Esposito, G.; Fiorino, F.; Migliaccio, M.; Scognamiglio, A.; et al. H2S Donors and Their Use in Medicinal Chemistry. Biomolecules 2021, 11, 1899. [Google Scholar] [CrossRef]
- Wallace, J.L.; Wang, R. Hydrogen Sulfide-Based Therapeutics: Exploiting a Unique but Ubiquitous Gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zeng, J.; Gu, Q. Exercise Restores Bioavailability of Hydrogen Sulfide and Promotes Autophagy Influx in Livers of Mice Fed with High-Fat Diet. Can. J. Physiol. Pharmacol. 2017, 95, 667–674. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Read, E.; Fu, M.; Pei, Y.; Wu, L.; Wang, R.; Yang, G. Golgi Stress Response, Hydrogen Sulfide Metabolism, and Intracellular Calcium Homeostasis. Antioxid. Redox Signal. 2020, 32, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, M.; Habashi, A.N. The Effect of Eight Weeks of High-Intensity Interval Training with L-Cysteine Consumption on Interleukin-13 and Oxidative Stress of Heart Tissue in Young Rats with Type 2 Diabetes. J. Shahrekord Univ. Med. Sci. 2023, 25, 172–177. [Google Scholar] [CrossRef]
- Munteanu, C.; Munteanu, D.; Onose, G. Hydrogen Sulfide (H2S)—Therapeutic Relevance in Rehabilitation and Balneotherapy Systematic Literature Review and Meta-Analysis Based on the PRISMA Paradigm. Balneo PRM Res. J. 2021, 12, 176–195. [Google Scholar] [CrossRef]
- Ibrahim, H.; Serag, A.; Farag, M.A. Emerging Analytical Tools for the Detection of the Third Gasotransmitter H2S, a Comprehensive Review. J. Adv. Res. 2021, 27, 137–153. [Google Scholar] [CrossRef]
- Tovar-Lopez, F.J. Recent Progress in Micro- and Nanotechnology-Enabled Sensors for Biomedical and Environmental Challenges. Sensors 2023, 23, 5406. [Google Scholar] [CrossRef] [PubMed]
- Chun, Y.L.; Eom, W.-J.; Lee, J.H.; Nguyen, T.N.C.; Park, K.-H.; Chung, H.-J.; Seo, H.; Huh, Y.; Kim, S.H.; Yeo, S.G.; et al. Investigation of the Hydrogen Sulfide Signaling Pathway in Schwann Cells during Peripheral Nerve Degeneration: Multi-Omics Approaches. Antioxidants 2022, 11, 1606. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; He, P.; Jia, R. Effects of Μ-Conotoxin GIIIB on the Cellular Activity of Mouse Skeletal Musculoblast: Combined Transcriptome and Proteome Analysis. Proteome Sci. 2023, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Pandey, T.; Kaundal, R.S.; Pandey, V. Biophysical Characterization of Hydrogen Sulfide: A Fundamental Exploration in Understanding Significance in Cell Signaling. Biophys. Chem. 2024, 314, 107317. [Google Scholar] [CrossRef] [PubMed]
| Research Area | Role of H2S | Associated Signaling Pathways and Mechanisms | Potential Impact and Clinical Relevance | References |
|---|---|---|---|---|
| Regulation of muscle contraction and relaxation | Modulates calcium signaling to influence muscle contraction and relaxation | Activates L-type calcium channels, regulates sarcoplasmic reticulum (RyR, SERCA pumps) | Enhances calcium influx during contraction, improving muscle contraction efficiency and endurance | [81,82,83,84,85,86] |
| Mitochondrial function and energy metabolism | Stimulates mitochondrial biogenesis and enhances ATP production, reducing ROS generation | Upregulates PGC-1α, NRF1, TFAM; modulates electron transport chain (ETC) | Improves muscle energy production, reduces oxidative damage, and enhances endurance | [48,87,88,89,90] |
| Muscle repair and regeneration | Promotes satellite cell activation and proliferation for muscle repair and regeneration | Modulates Notch, Wnt, IGF-1/AKT pathways, promoting cell proliferation and differentiation | Accelerates muscle recovery post-exercise, reducing muscle damage | [91,92,93,94,95,96,97] |
| Anti-apoptotic effects | Inhibits apoptotic pathways to protect muscle cells from damage | Upregulates Bcl-2, inhibits Bax, caspases, and activates PI3K/AKT, ERK1/2 pathways | Maintains muscle cell integrity, reduces exercise-induced cell death | [98,99,100,101,102,103,104,105,106] |
| Protein synthesis and degradation regulation | Promotes protein synthesis and reduces protein degradation to support muscle growth and strength | Activates mTOR signaling, modulates MyoD and myogenin expression, inhibits UPS and ALP pathways | Enhances muscle hypertrophy, strength, and reduces protein degradation | [107,108,109,110,111,112,113] |
| Metabolism and protein homeostasis | Regulates FoxO transcription factors to inhibit muscle atrophy | Inhibits FoxO activity, reducing the expression of genes involved in protein degradation | Maintains muscle mass, prevents atrophy, especially after intense physical activity | [114,115,116,117,118] |
| H2S Donor | Effects on Exercise Performance | Effects on Metabolic Health | References |
|---|---|---|---|
| NaHS | Increases in vivo H2S levels rapidly, suitable for acute studies | Enhances aerobic capacity, increases muscle strength and power, improves recovery | [137] |
| GYY4137 | Improves endurance performance, increases running time and oxygen consumption | Enhances insulin sensitivity, promotes fatty acid oxidation, reduces fat synthesis | [138,140] |
| AP39 | Enhances mitochondrial function and ATP production, improving endurance | Improves glucose metabolism, reduces oxidative stress | [139] |
| JK1 | Increases exercise performance by modulating oxidative stress and muscle function | Promotes muscle fiber hypertrophy, improves metabolic health | [139] |
| Dietary sources | Supports aerobic performance and muscle strength through increased H2S production | Enhances insulin sensitivity, improves lipid profile and glucose metabolism | [141,142,143] |
| Endogenous H2S production | Supports exercise performance by maintaining H2S levels through endogenous pathways | Improves metabolic health by supporting glucose uptake and reducing adiposity | [142,143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Liu, X.; Hu, D.; Li, Z.; Lu, L. CSE/H2S Signaling Pathways in Enhancing Muscle Function and Insulin Sensitivity During Exercise. Int. J. Mol. Sci. 2025, 26, 1741. https://doi.org/10.3390/ijms26041741
Xu M, Liu X, Hu D, Li Z, Lu L. CSE/H2S Signaling Pathways in Enhancing Muscle Function and Insulin Sensitivity During Exercise. International Journal of Molecular Sciences. 2025; 26(4):1741. https://doi.org/10.3390/ijms26041741
Chicago/Turabian StyleXu, Miaomiao, Xiaoguang Liu, Danting Hu, Zhaowei Li, and Liming Lu. 2025. "CSE/H2S Signaling Pathways in Enhancing Muscle Function and Insulin Sensitivity During Exercise" International Journal of Molecular Sciences 26, no. 4: 1741. https://doi.org/10.3390/ijms26041741
APA StyleXu, M., Liu, X., Hu, D., Li, Z., & Lu, L. (2025). CSE/H2S Signaling Pathways in Enhancing Muscle Function and Insulin Sensitivity During Exercise. International Journal of Molecular Sciences, 26(4), 1741. https://doi.org/10.3390/ijms26041741







