Carboxylated Osteocalcin as an Independent Predictor of Mean Arterial Pressure and the Atherogenic Index in Adults
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects and Ethical Considerations
4.2. Clinical and Anthropometric Measurements
4.3. Biochemical Measurements
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G. Update on the biology of osteocalcin. Endocr. Pract. 2017, 23, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Franco, M.C.; Franco-Diaz de Leon, R.; Villafan-Bernal, J.R. Osteocalcin-GPRC6A: An update of its clinical and biological multi-organic interactions. Mol. Med. Rep. 2018, 19, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Polonskaya, Y.V.; Kashtanova, E.V.; Murashov, I.S.; Volkov, A.M.; Kurguzov, A.V.; Chernyavsky, A.M.; Ragino, Y.I. Associations of Osteocalcin, Osteoprotegerin, and Calcitonin with Inflammation Biomarkers in Atherosclerotic Plaques of Coronary Arteries. Bull. Exp. Biol. Med. 2017, 162, 726–729. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.J.; Si, D.L.; Wang, C.; Yang, J.C.; Jiang, P.; Du, C.; Wang, J.J. Correlation between osteocalcin-positive endothelial progenitor cells and spotty calcification in patients with coronary artery disease. Clin. Exp. Pharmacol. Physiol. 2015, 42, 734–739. [Google Scholar] [CrossRef]
- Chi, P.-J.; Lin, Y.-L.; Tasi, J.-P.; Wang, C.-H.; Hou, J.-S.; Lee, C.-J.; Hsu, B.-G. Osteocalcin and carotid–femoral pulse wave velocity in patients on peritoneal dialysis. Tzu Chi Med. J. 2018, 31, 23–28. [Google Scholar]
- Tacey, A.; Hayes, A.; Zulli, A.; Levinger, I. Osteocalcin and vascular function: Is there across-talk? Mol. Metab. 2021, 49, 101205. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Lian, J.B.; Cole, D.E.; Gundberg, C.M. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol. Rev. 1989, 69, 990–1047. [Google Scholar] [CrossRef]
- Krzanowski, M.; Janda, K.; Dumnicka, P.; Dubiel, M.; Stompór, M.; Kusnierz-Cabala, B.; Grodzicki, T.; Sulowicz, W. Relationship between aortic pulse wave velocity, selected proinflammatory cytokines, and vascular calcification parameters in peritoneal dialysis patients. J. Hypertens. 2014, 32, 142–148. [Google Scholar] [CrossRef]
- Sánchez-Enriquez, S.; Ballesteros-Gonzalez, I.T.; Villafán-Bernal, J.R.; Pascoe-Gonzalez, S.; Rivera-Leon, E.A.; Bastidas-Ramirez, B.E.; Rivas-Carrillo, J.D.; Alcala-Zermeno, J.L.; Armendariz-Borunda, J.; Llamas-Covarrubias, I.M. Serum levels of undercarboxylated osteocalcin are related to cardiovascular risk factors in patients with type 2 diabetes mellitus and healthy subjects. World J. Diabetes 2017, 8, 11. [Google Scholar] [CrossRef]
- Idelevich, A.; Rais, Y.; Monsonego-Ornan, E. Bone Gla protein increases HIF-1alpha dependent glucose metabolism and induces cartilage and vascular calcification. Arter. Thromb. Vasc. Biol. 2011, 31, e55–e71. [Google Scholar] [CrossRef] [PubMed]
- Marinova, E.; Dimova-Mileva, M.; Gancheva, S.; Boyadzhieva, M.; Kanazirev, B. Carboxylated osteocalcin levels correlate with early change of arterial stiffness parameters in patients with type 2 diabetes mellitus. J. Hypertens. 2021, 39, e401. [Google Scholar] [CrossRef]
- Shioi, A.; Ikari, Y. Plaque calcification during atherosclerosis progression and regression. J. Atheroscler. Thromb. 2017, 25, RV17020. [Google Scholar] [CrossRef]
- Mackey, R.H.; Venkitachalam, L.; Sutton-Tyrrell, K. Calcifications, arterial stiffness and atherosclerosis. Atheroscler. Large Arter. Cardiovasc. Risk 2007, 44, 234–244. [Google Scholar]
- Lemogoum, D.; Flores, G.; Van den Abeele, W.; Ciarka, A.; Leeman, M.; Degaute, J.P.; Van De Borne, P.; Van Bortel, L. Validity of pulse pressure and augmentation index as surrogate measures of arterial stiffness during beta-adrenergic stimulation. J. Hypertens. 2004, 22, 511–517. [Google Scholar] [CrossRef]
- Polgreen, L.E.; Jacobs, D.R., Jr.; Nathan, B.M.; Steinberger, J.; Moran, A.; Sinaiko, A.R. Association of osteocalcin with obesity, insulin resistance, and cardiovascular risk factors in young adults. Obesity 2012, 20, 2194–2201. [Google Scholar] [CrossRef]
- Joffres, M.R.; Hamet, P.; MacLean, D.R.; L’italien, G.J.; Fodor, G. Distribution of blood pressure and hypertension in Canada and the United States. Am. J. Hypertens. 2001, 14, 1099–1105. [Google Scholar] [CrossRef]
- Vassalle, C.; Sabatino, L.; Cecco, P.D.; Maltinti, M.; Ndreu, R.; Maffei, S.; Pingitore, A. Relationship between Bone Health Biomarkers and Cardiovascular Risk in a General Adult Population. Diseases 2017, 5, 24. [Google Scholar] [CrossRef]
- Zwakenberg, S.R.; Schouw, Y.T.; Schalkwijk, C.G.; Spijkerman, A.M.; Beulens, J.W. Bone markers and cardiovascular risk in type 2 diabetes patients. Cardiovasc. Diabetol. 2018, 17, 45. [Google Scholar] [CrossRef]
- Montagnana, M.; Lippi, G.; Danese, E.; Guidi, G.C. The role of osteoprotegerin in cardiovascular disease. Ann. Med. 2013, 45, 254–264. [Google Scholar] [CrossRef]
- Berezin, A.E.; Kremzer, A.A. Predictive value of circulating osteonectin in patients with ischemic symptomatic chronic heart failure. Biomed. J. 2015, 38, 523–530. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Genre, F.; Rueda-Gotor, J.; Remuzgo-Martínez, S.; Irure-Ventura, J.; Corrales, A.; Mijares, V.; Portilla, V.; Blanco, R.; Rodríguez-Rodríguez, L.; Hernández, J. AB0143 Implication of osteonectin on cardiovascular risk in axial spondyloarthritis: A serological and genetic study. Ann. Rheum. Dis. 2018, 77, 1263. [Google Scholar] [CrossRef]
- Barrett, H.; O’Keeffe, M.; Kavanagh, E.; Walsh, M.; O’Connor, E. Is Matrix Gla Protein Associated with Vascular Calcification? A Systematic Review. Nutrients 2018, 10, 415. [Google Scholar] [CrossRef]
- Moon, J.-Y.; Park, S.; Ahn, C.M.; Cho, J.R.; Park, C.M.; Ko, Y.-G.; Choi, D.; Jeong, M.H.; Jang, Y.; Chung, N. Increase of Metabolic Syndrome Score is an Independent Determinant of Increasing Pulse Pressure. Yonsei Med. J. 2008, 49, 63–70. [Google Scholar] [CrossRef]
- De Pergola, G.; Nardecchia, A.; Ammirati, A.; Caccavo, D.; Bavaro, S.; Silvestris, F. Abdominal obesity is characterized by higher pulse pressure: Possible role of free triiodothyronine. J. Obes. 2012, 1, 656303. [Google Scholar] [CrossRef]
- Hadi, H.A.; Suwaidi, J.A. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk Manag. 2007, 3, 853–876. [Google Scholar]
- Levy, R.J.; Gundberg, C.; Scheinman, R. The identification of the vitamin K-dependent bone protein osteocalcin as one of the γ-carboxyglutamic acid containing proteins present in calcified atherosclerotic plaque and mineralized heart valves. Atherosclerosis 1983, 46, 49–56. [Google Scholar] [CrossRef]
- Foresta, C.; Strapazzon, G.; De Toni, L.; Fabris, F.; Grego, F.; Gerosa, G.; Vettore, S.; Garolla, A. Platelets express and release osteocalcin and co-localize in human calcified atherosclerotic plaques. J. Thromb. Haemost. 2013, 11, 357–365. [Google Scholar] [CrossRef]
- Millar, S.A.; Patel, H.; Anderson, S.I.; England, T.J.; O’Sullivan, S.E. Osteocalcin, Vascular Calcification, and Atherosclerosis: A Systematic Review and Meta-analysis. Front. Endocrinol. 2017, 8, 183. (In English) [Google Scholar] [CrossRef]
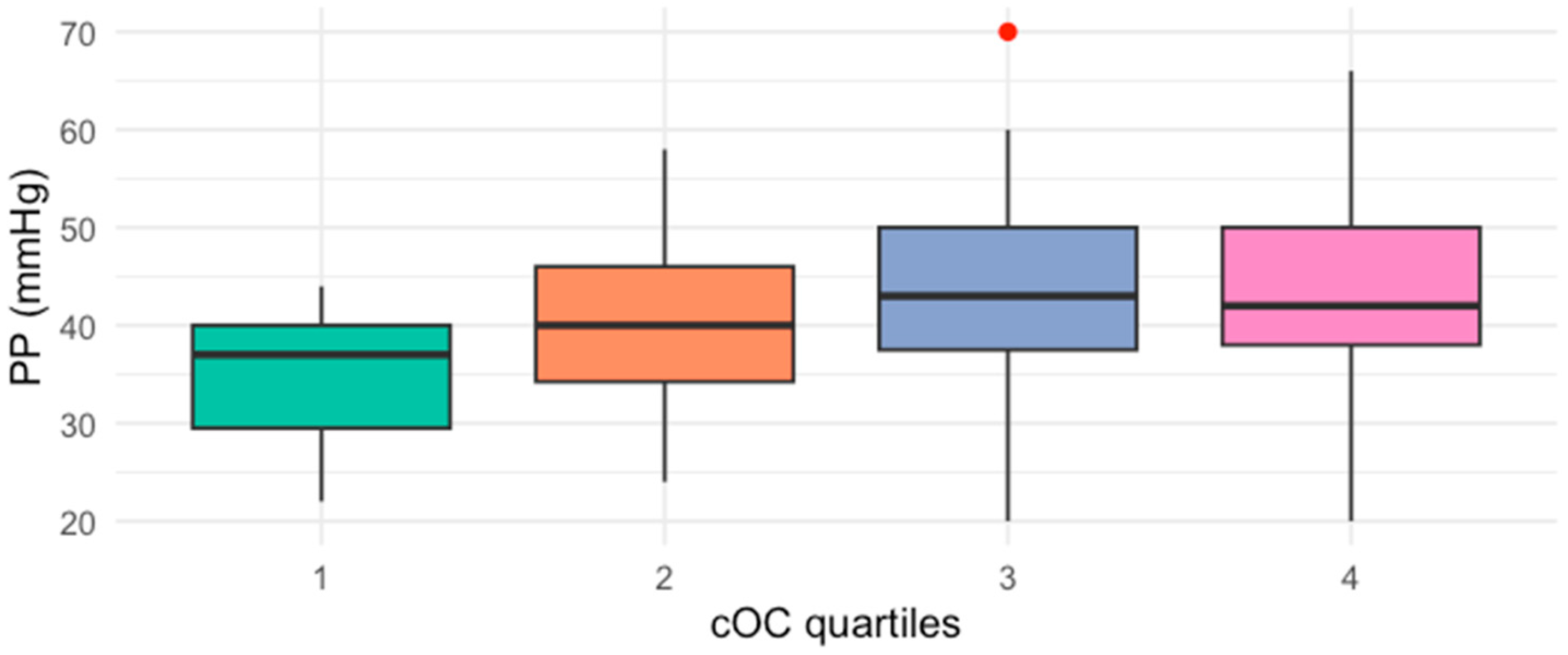
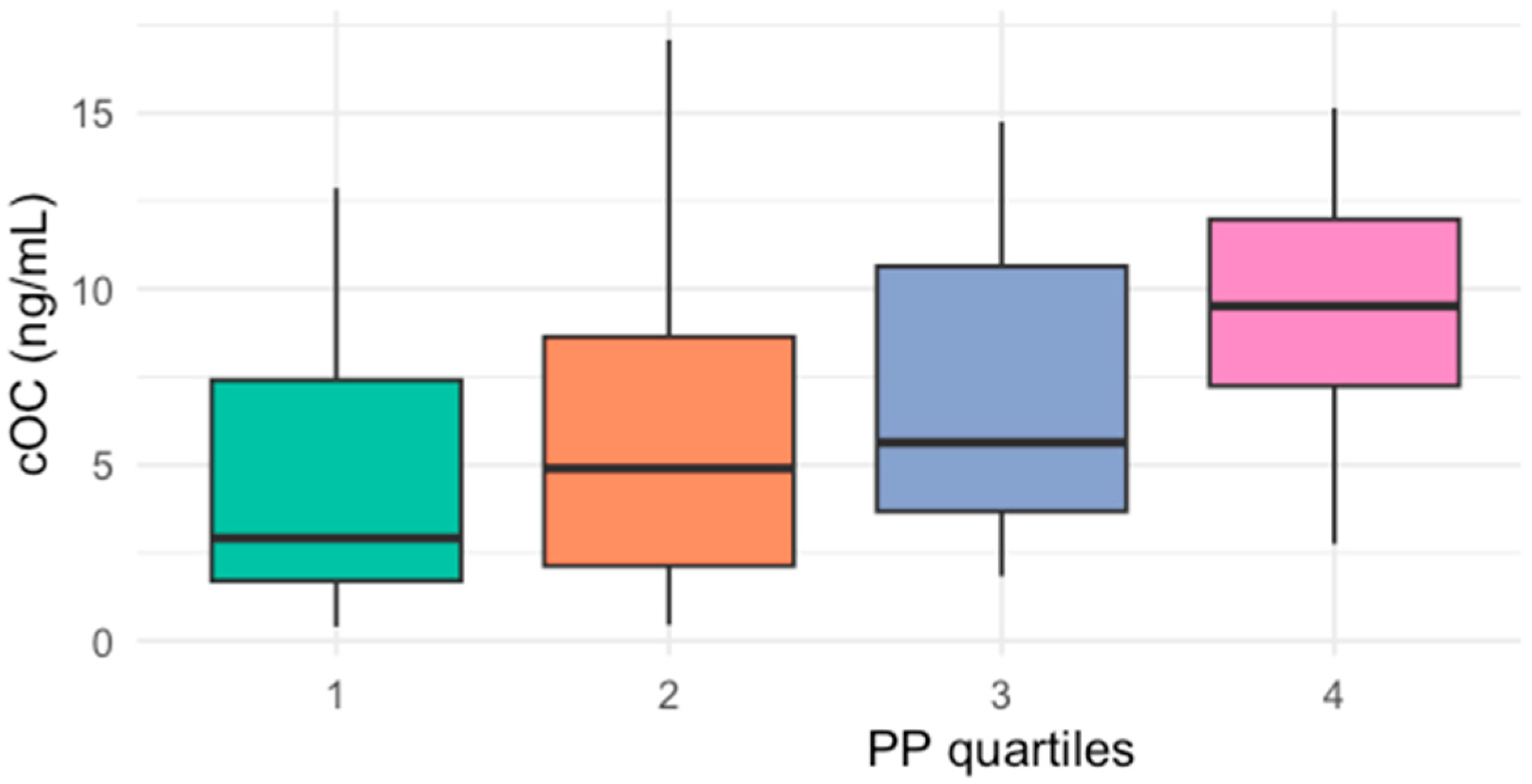
| Characteristic | Median (IQR) * |
|---|---|
| Age (years) | 47.0 (42–53) |
| Gender (male/female), %[n] | 30.9 (25)/69.1 (56) |
| Diabetes, %[n] | 33.3 (17) |
| Hypertension, %[n] | 17.3 (14) |
| Weight (kg) | 70.2 (63.3–79.3) |
| BMI (kg/m2) | 26.7 (24.6–30.3) |
| Waist circumference (cm) | 92.5 (85.18–101.13) |
| Body fat % | 32.7 (24.48–40.50) |
| SBP (mmHg) | 116 (110–124) |
| DBP (mmHg) | 78 (72–82) |
| PP (mmHg) | 40 (34–46) |
| cOC (ng/mL) | 5.32 (2.64–10.80) |
| Total cholesterol (mg/dL) | 182 (154.7–226) |
| HDL-c (mg/dL) | 40.25 (34–48.71) |
| LDL-c (mg/dL) | 105.5 (88.75–127) |
| Triglycerides (mg/dL) | 120 (86.10–156.50) |
| Insulin (μU/mL) | 9.96 (6.63–18.15) |
| HOMA-IR | 3.12 (1.39–5.90) |
| HbA1c (%) | 5.17 (4.71–7.17) |
| Creatinine (mg/dL) | 1 (0.80–1.23) |
| Parameter | cOC | PP |
|---|---|---|
| cOC (ng/mL) | ----- | 0.342 ** |
| PP (mmHg) | 0.342 ** | ----- |
| Total cholesterol (mg/dL) | −0.122 | 0.019 |
| HDL-c (mg/dL) | −0.239 * | 0.005 |
| LDL-c (mg/dL) | 0.097 | −0.044 |
| Triglycerides (mg/dL) | 0.083 | 0.079 |
| Insulin (μU/mL) | 0.007 | 0.208 |
| HOMA-IR | 0.079 | 0.285 * |
| HbA1c (%) | 0.346 * | 0.230 |
| Atherogenic index | 0.224 * | −0.027 |
| Characteristic | cOC <5.32 ng/mL (Q1–Q2) | cOC >5.32 ng/mL (Q3–Q4) | p-Value * |
|---|---|---|---|
| Age (years) | 45 (40.75–49.25) | 49 (45–54.5) | 0.139 |
| Gender (%male/female) | 34/66 | 25/75 | |
| Weight (kg) | 73 (62.48–86.15) | 68.9 (63.65–75.4) | 0.366 |
| BMI (kg/m2) | 27 (25.2–30.6) | 26.05 (23.73–29.7) | 0.314 |
| Waist circumference (cm) | 96 (85.5–105.75) | 88.88 (84.53–95.38) | 0.402 |
| Body fat % | 33.95 (27.33–40.80) | 31.35 (22.78–38.05) | 0.435 |
| SBP (mmHg) | 110 (108–120) | 120 (110–135) | <0.001 |
| DBP (mmHg) | 78 (70–80.5) | 80 (72–87) | 0.557 |
| PP (mmHg) | 39 (30–42) | 42 (37–87) | <0.001 |
| Total cholesterol (mg/dL) | 177.5 (161.25–216.63) | 190.0 (149.05–238.35) | 0.711 |
| HDL-c (mg/dL) | 41 (36.25–52.8) | 38.6 (31.58–46.52) | 0.311 |
| LDL-c (mg/dL) | 102.5 (87.5–126.5) | 108.5 (89.5–128.25) | 0.965 |
| Triglycerides (mg/dL) | 108.5 (84–148.5) | 126.6 (88.13–182.95) | 0.601 |
| Insulin (μU/mL) | 10.12 (6.03–23.3) | 9.96 (7.27–14.49) | 0.333 |
| HOMA-IR | 2.84 (1.28–5.61) | 3.35 (1.92–6.07) | 0.323 |
| HbA1c (%) | 4.89 (4.61–6.87) | 5.79 (4.98–7.17) | 0.976 |
| Characteristic | B Coefficient | IC95% | p-Value |
|---|---|---|---|
| Age (years) | 0.033 | −0.140 to 0.207 | 0.700 |
| MAP (mmHg) | 0.138 | 0.028 to 0.247 | 0.015 |
| HbA1c (%) | −0.099 | −1.128 to 0.930 | 0.847 |
| HOMA-IR | 0.142 | −0.313 to 0.598 | 0.532 |
| BMI (kg/m2) | −0.224 | −0.501 to 0.052 | 0.109 |
| Atherogenic index | 0.599 | −0.039 to 1.161 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villafán-Bernal, J.R.; Rivas-Carrillo, J.D.; Guzmán-Guzmán, I.P.; Frias-Cabrera, J.L.; Rivera-León, E.A.; Martinez-Portilla, R.J.; Sánchez-Enríquez, S. Carboxylated Osteocalcin as an Independent Predictor of Mean Arterial Pressure and the Atherogenic Index in Adults. Int. J. Mol. Sci. 2025, 26, 1733. https://doi.org/10.3390/ijms26041733
Villafán-Bernal JR, Rivas-Carrillo JD, Guzmán-Guzmán IP, Frias-Cabrera JL, Rivera-León EA, Martinez-Portilla RJ, Sánchez-Enríquez S. Carboxylated Osteocalcin as an Independent Predictor of Mean Arterial Pressure and the Atherogenic Index in Adults. International Journal of Molecular Sciences. 2025; 26(4):1733. https://doi.org/10.3390/ijms26041733
Chicago/Turabian StyleVillafán-Bernal, José Rafael, Jorge David Rivas-Carrillo, Iris Paola Guzmán-Guzmán, Jose Luis Frias-Cabrera, Edgar Alfonso Rivera-León, Raigam Jafet Martinez-Portilla, and Sergio Sánchez-Enríquez. 2025. "Carboxylated Osteocalcin as an Independent Predictor of Mean Arterial Pressure and the Atherogenic Index in Adults" International Journal of Molecular Sciences 26, no. 4: 1733. https://doi.org/10.3390/ijms26041733
APA StyleVillafán-Bernal, J. R., Rivas-Carrillo, J. D., Guzmán-Guzmán, I. P., Frias-Cabrera, J. L., Rivera-León, E. A., Martinez-Portilla, R. J., & Sánchez-Enríquez, S. (2025). Carboxylated Osteocalcin as an Independent Predictor of Mean Arterial Pressure and the Atherogenic Index in Adults. International Journal of Molecular Sciences, 26(4), 1733. https://doi.org/10.3390/ijms26041733






