Characterization of Anti-Insulin Antibodies in Type 1 and Type 2 Diabetes Mellitus: Clinical Relevance
Abstract
1. Introduction
2. Results
2.1. Clinical and Demographic Characteristics of the Study Population
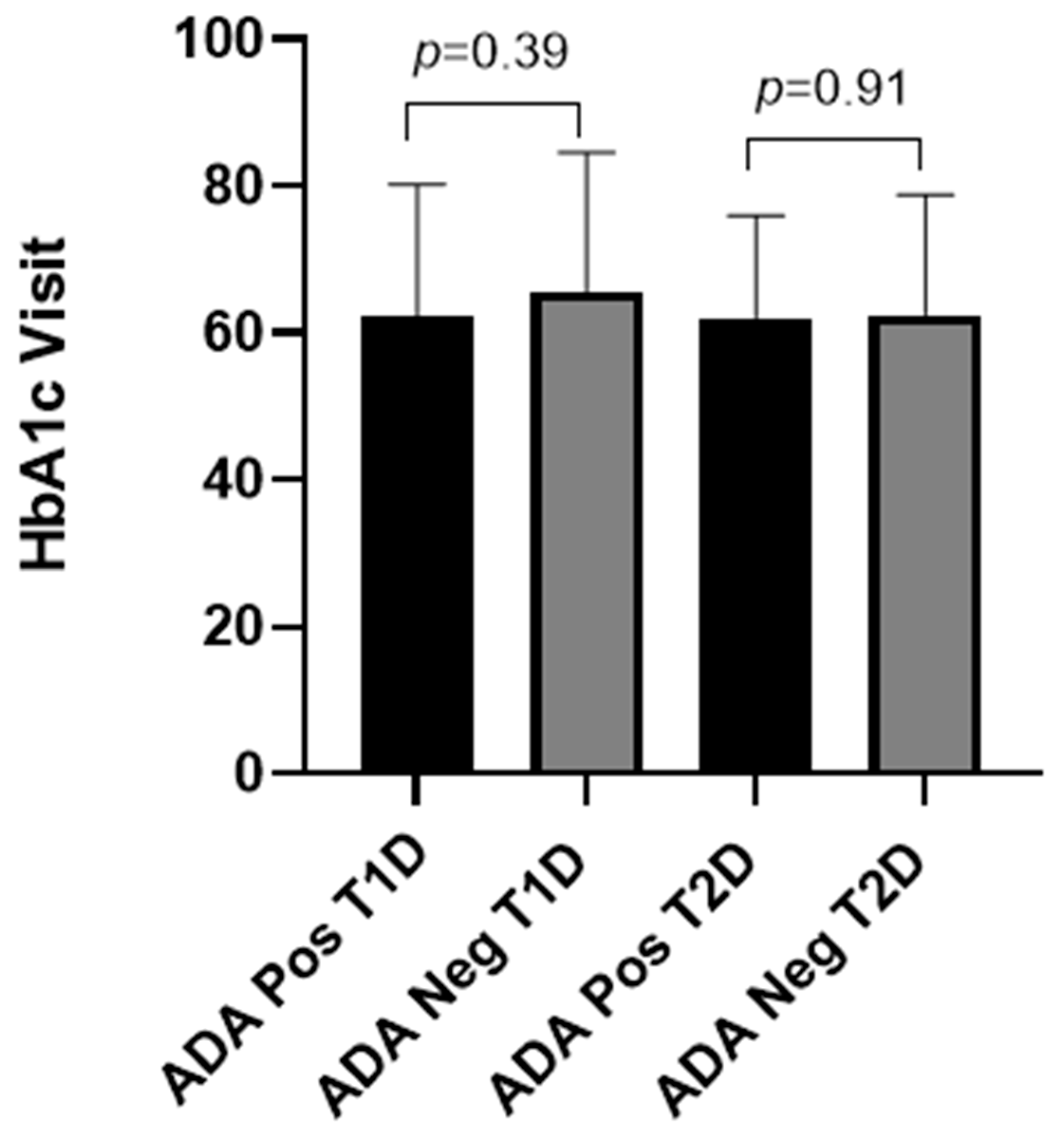
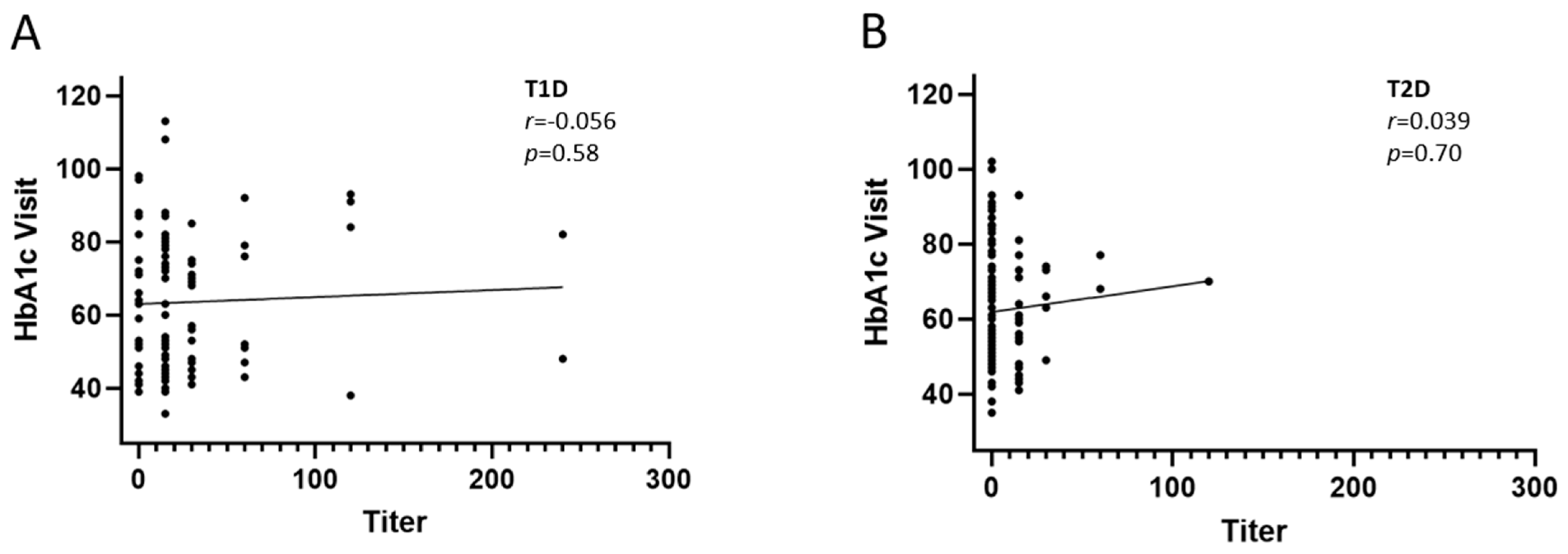
2.2. Prevalence and Titers of Insulin Antibodies and Glycemic Control
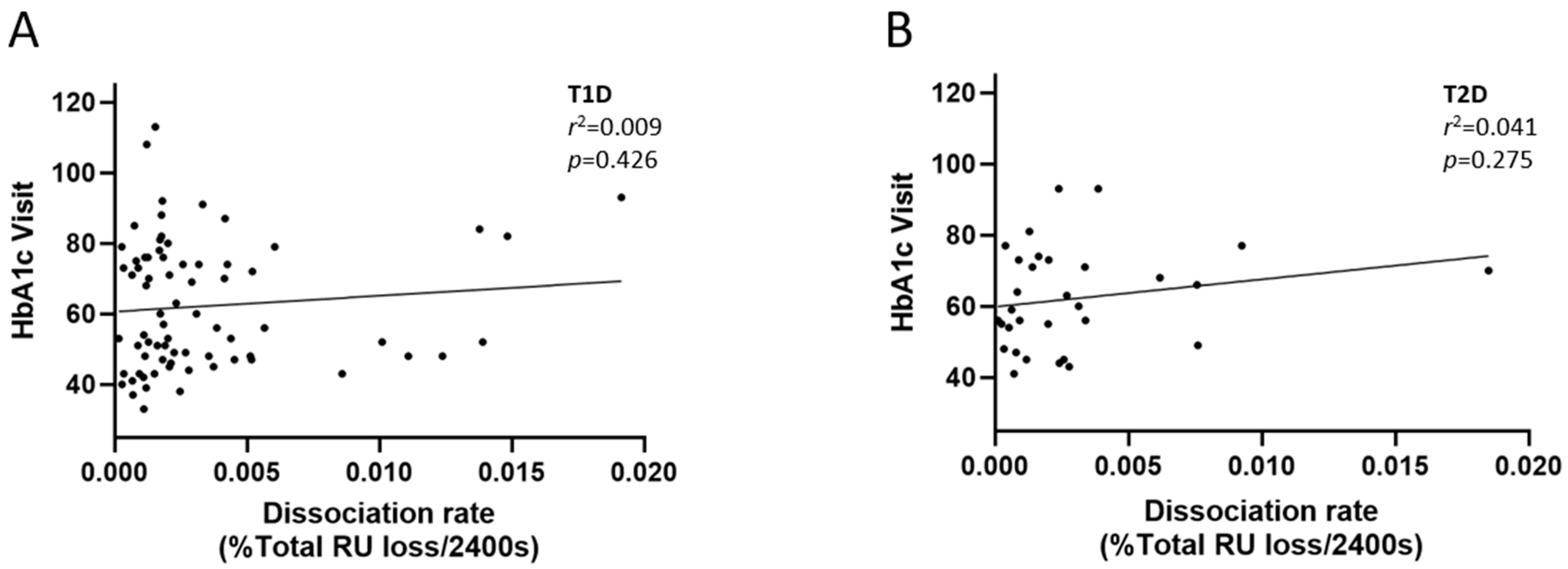
2.3. Avidity of Insulin Antibodies and Glycemic Control
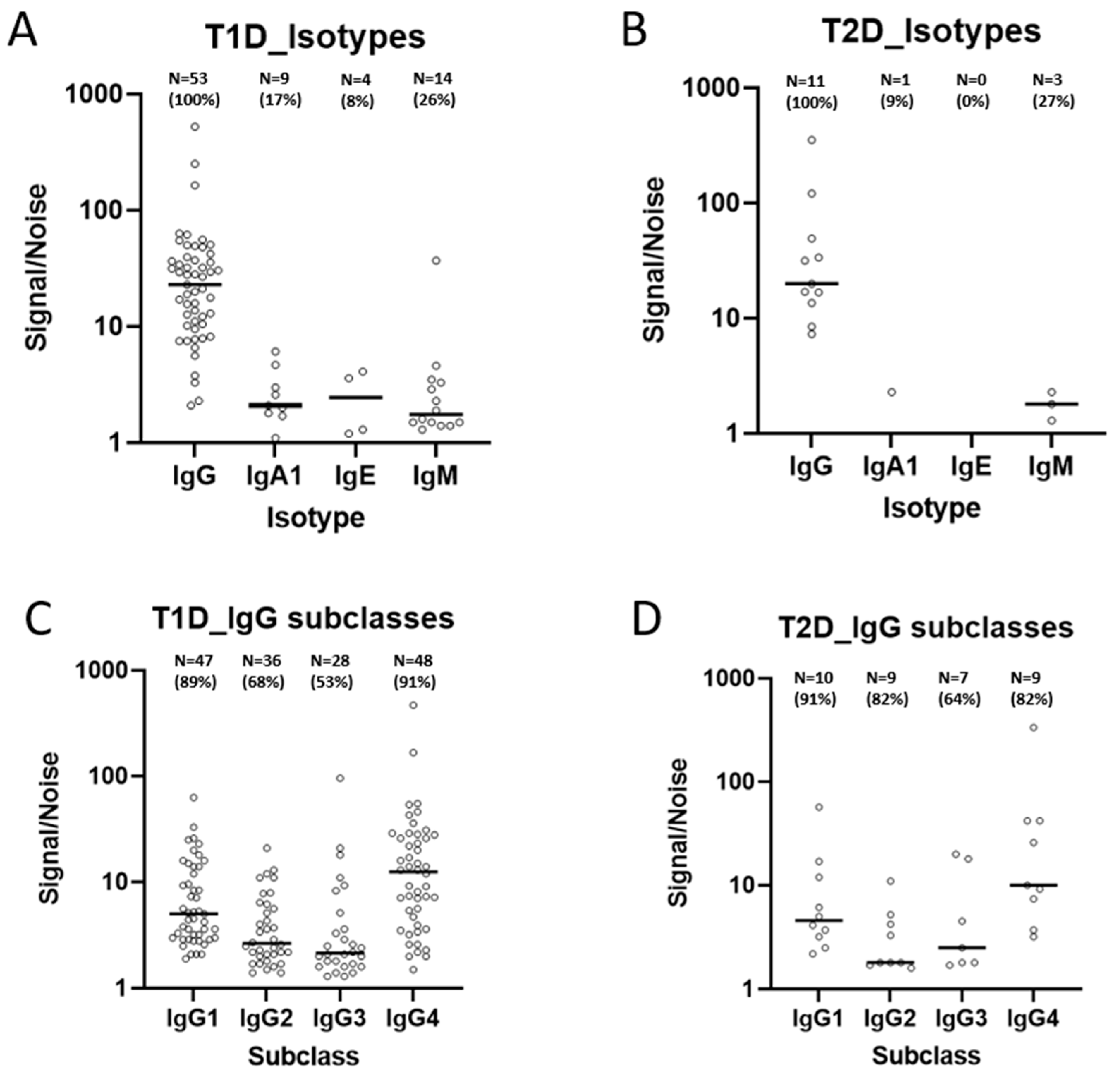
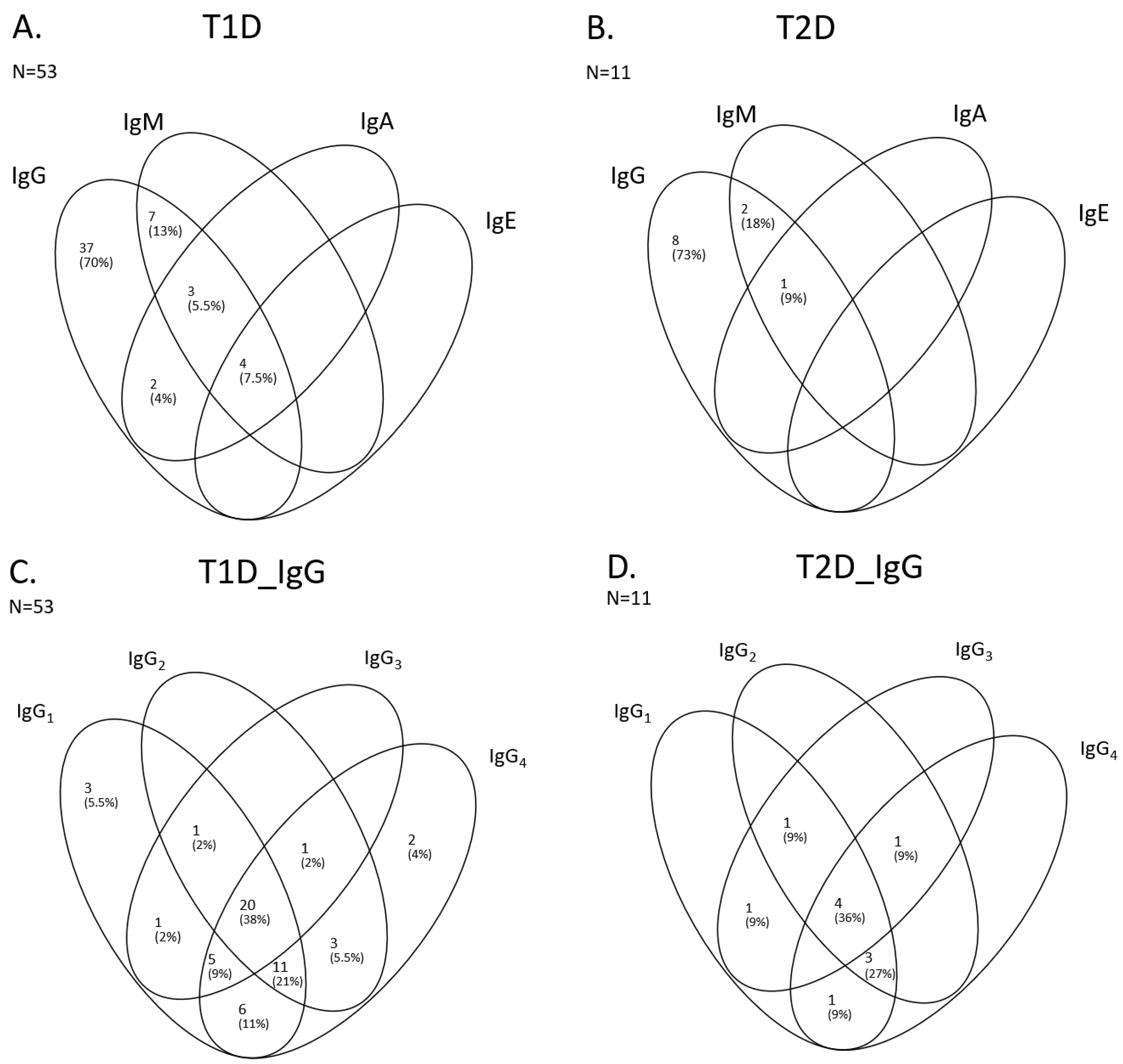
2.4. Isotypes and Subclasses of Insulin Antibodies
2.5. Evaluation of the Neutralizing Activity of IAs
2.6. Evaluation of IA Titer and Insulin Dose
2.7. Effect of Concomitant Medication
3. Discussion
4. Materials and Methods
4.1. Analyses of Insulin Antibodies and Titer
4.2. Dissociation Kinetics (Avidity Score)
4.3. IA Isotype and Subclass Determination
4.4. Neutralizing Capacity
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hameed, I.; Masoodi, S.R.; A Mir, S.; Nabi, M.; Ghazanfar, K.; A Ganai, B. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes 2015, 6, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, S.E.; Kawabata, T.T.; Finco-Kent, D.; Fountaine, R.J.; Finch, G.L.; Krasner, A.S. Immunological responses to exogenous insulin. Endocr. Rev. 2007, 28, 625–652. [Google Scholar] [CrossRef]
- Boulton, A.J.; Malik, R.A.; Arezzo, J.C.; Sosenko, J.M. Diabetic somatic neuropathies. Diabetes Care 2004, 27, 1458–1486. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Invest. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Cryer, P.E. Severe hypoglycemia predicts mortality in diabetes. Diabetes Care 2012, 35, 1814–1816. [Google Scholar] [CrossRef] [PubMed]
- The International Hypoglycaemia Study Group. Hypoglycaemia, cardiovascular disease, and mortality in diabetes: Epidemiology, pathogenesis, and management. Lancet Diabetes Endocrinol. 2019, 7, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Badik, J.; Chen, J.; Letvak, K.; So, T.-Y. Hypersensitivity Reaction to Insulin Glargine and Insulin Detemir in a Pediatric Patient: A Case Report. J. Pediatr. Pharmacol. Ther. 2016, 21, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.C.; Wang, J.; Hon, Y.Y.; Zhou, L.; Fang, L.; Ahn, H.Y. Evaluating and Reporting the Immunogenicity Impacts for Biological Products--a Clinical Pharmacology Perspective. AAPS J. 2016, 18, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Fineberg, S.E.; Kawabata, T.; Finco-Kent, D.; Liu, C.; Krasner, A. Antibody response to inhaled insulin in patients with type 1 or type 2 diabetes. An analysis of initial phase II and III inhaled insulin (Exubera) trials and a two-year extension trial. J. Clin. Endocrinol. Metab. 2005, 90, 3287–3294. [Google Scholar] [CrossRef]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. MAbs 2010, 2, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Uchida, H.A.; Wada, Y. Poor glycemic control attributable to insulin antibody in a fulminant type 1 diabetes patient under hemodialysis: Successful treatment with double filtration plasmapheresis and prednisolone. Ren. Replace. Ther. 2017, 3, 14. [Google Scholar] [CrossRef][Green Version]
- Cappellani, D.; Macchia, E.; Falorni, A.; Marchetti, P. Insulin Autoimmune Syndrome (Hirata Disease): A Comprehensive Review Fifty Years After Its First Description. Diabetes Metab. Syndr. Obes. 2020, 13, 963–978. [Google Scholar] [CrossRef]
- Church, D.S.; Barker, P.; Burling, K.A.; Shinwari, S.K.; Kennedy, C.; Smith, D.; Semple, R.K. Diagnosis and treatment of anti-insulin antibody-mediated labile glycaemia in insulin-treated diabetes. Diabet. Med. 2023, 40, e15194. [Google Scholar] [CrossRef] [PubMed]
- Han, C.-Y.; Ye, X.-M.; Lu, J.-P.; Jin, H.-Y.; Xu, W.-W.; Wang, P.; Zhang, M. Exogenous Insulin Antibody Syndrome in Patients with Type 2 Diabetes. Diabetes Metab. Syndr. Obes. 2023, 16, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, C.; Tashima-Horie, K.; Fukushima, S.; Saito, S.; Tanaka, S.; Haruki, T.; Ogino, J.; Suzuki, Y.; Hashimoto, N. Association of morning fasting blood glucose variability with insulin antibodies and clinical factors in type 1 diabetes. Endocr. J. 2016, 63, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, R.; Di Pietro, S.; Giaccari, A.; Petrone, A.; Locatelli, M.; Suraci, C. High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care 2007, 30, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Achenbach, P.; Warncke, K.; Reiter, J.; Naserke, H.E.; Williams, A.J.; Bingley, P.J.; Bonifacio, E.; Ziegler, A.-G. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 2004, 53, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Bingley, P.J.; Bonifacio, E.; Mueller, P.W. Diabetes Antibody Standardization Program: First assay proficiency evaluation. Diabetes 2003, 52, 1128–1136. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Jiang, Y.; Zheng, X.; Zhang, M.; Yang, T.; Gu, Y. Association of subclass distribution of insulin antibody with glucose control in insulin-treated type 2 diabetes mellitus: A retrospective observational study. Front. Endocrinol. 2023, 14, 1141414. [Google Scholar] [CrossRef]
- Kucuksezer, U.C.; Ozdemir, C.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy and allergen tolerance. Allergol. Int. 2020, 69, 549–560. [Google Scholar] [CrossRef]
- Wildner, P.; Selmaj, K.W. Multiple sclerosis: Skin-induced antigen-specific immune tolerance. J. Neuroimmunol. 2017, 311, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, C.; Powers, R.; Satyabhama, L.; Cui, A.; Tipton, C.; Michaeli, M.; Skountzou, I.; Mittler, R.S.; Kleinstein, S.H.; Mehr, R.; et al. Long-lived antigen-induced IgM plasma cells demonstrate somatic mutations and contribute to long-term protection. Nat. Commun. 2016, 7, 11826. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Bolton, M.J.; Santos, J.J.; Arevalo, C.P.; Griesman, T.; Watson, M.; Li, S.H.; Bates, P.; Ramage, H.; Wilson, P.C.; Hensley, S.E. IgG3 subclass antibodies recognize antigenically drifted influenza viruses and SARS-CoV-2 variants through efficient bivalent binding. bioRxiv 2022, 120, e2216521120. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, L.; Li, J.; Bai, S.; Wang, Y.; Zhang, B.; Zheng, Q.; Chen, M.; Zhao, W.; Wu, J. The kinetics of IgG subclasses and contributions to neutralizing activity against SARS-CoV-2 wild-type strain and variants in healthy adults immunized with inactivated vaccine. Immunology 2022, 167, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Rispens, T.; Huijbers, M.G. The unique properties of IgG4 and its roles in health and disease. Nat. Rev. Immunol. 2023, 23, 763–778. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Shankar, G.; Devanarayan, V.; Amaravadi, L.; Barrett, Y.C.; Bowsher, R.; Finco-Kent, D.; Fiscella, M.; Gorovits, B.; Kirschner, S.; Moxness, M.; et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J. Pharm. Biomed. Anal. 2008, 48, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Finco-Kent, D.; Morrone, A.; Moxness, M.; Bedian, V.; Krasner, A.; Foley, J.; Stene, M.; Kawabata, T. Development and validation of a radioligand binding assay to measure insulin specific IgG subclass antibodies in human serum. Ann. N. Y. Acad. Sci. 2003, 1005, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Moxness, M.; Foley, J.; Stene, M.; Finco-Kent, D.; Bedian, V.; Krasner, A.; Kawabata, T. Development and validation of radioligand binding assays to measure total, IgA, IgE, IgG, and IgM insulin antibodies in human serum. Ann. N. Y. Acad. Sci. 2003, 1005, 265–268. [Google Scholar] [CrossRef] [PubMed]


| Diabetes Mellitus Type | ||
|---|---|---|
| Type 1 | Type 2 | |
| Demography | ||
| Age (years) (mean, median, range) | (N = 101) 48, 48, 18–79 | (N = 101) 67, 66, 34–95 |
| Sex (F, M) | 50, 51 | 34, 67 |
| Clinical parameters | ||
| BMI (mean, median, range) | (N = 100) 26.7, 26.0, 19.7–38.6 | (N = 101) 30.9, 30.3, 18.5–46.9 |
| GAD antibodies (U/mL) (mean, median, range) | (N = 69) 111, 69, 0–250 | (N = 75) 13, 5, 0–250 |
| C-pep (pmol/L) (mean, median, range) | (N = 87) 162, 30, 1.5–906 | (N = 101) 1020, 821, 11–3496 |
| Well-controlled glycemia at screening (HbA1c ≤ 53 mmol/mol) (mean, median HbA1c, range) | (N = 51) 47, 48, 35–52 | (N = 47) 48, 48, 35–53 |
| Poorly controlled glycemia at screening (HbA1c ≥ 69 mmol/mol) (mean, median HbA1c, range) | (N = 50) 82, 78.5, 70–112 | (N = 54) 80, 78, 70–109 |
| Age (years) at onset (mean, median, range) | (N = 101) 26, 24, 5–65 | (N = 100) 49, 50, 27–83 |
| Duration (years) of insulin treatment (mean, median, range) | (N = 100) 22, 19, <1–68 | (N = 96) 9, 8, <1–30 |
| Insulin dose (IU/kg) (mean, median, range) | (N = 82) 0.49, 0.48, 0.04–1.14 | (N = 92) 0.76, 0.47, 0.05–13.37 |
| DM Type 1 (N = 101) | DM Type 2 (N = 101) | |||
|---|---|---|---|---|
| ADA Positive | ADA Negative | ADA Positive | ADA Negative | |
| N (proportion) | 73 (72.3%) | 28 (27.7%) | 32 (31.7%) | 69 (68.3%) |
| Clinical parameters | ||||
| GAD antibodies (U/mL) (mean, median, range) | (N = 47) 116, 76, 0–250 | (N = 22) 99, 29, 0–250 | (25) 22, 5, 0–250 | (N = 50) 9, 5, 0–250 |
| C-pep (pmol/L) (median, range) | (N = 62) 28, 1.5–616 | (N = 25) 41, 1.5–906 | (N = 32) 592, 11–3410 | (N = 69) 944, 42–3496 |
| Well-controlled glycemia (HbA1c ≤ 53 mmol/mol) (mean, median HbA1c, range) | (N = 38) 46, 48, 35–52 | (N = 13) 48, 49, 42–52 | (N = 15) 48, 49, 43–53 | (N = 32) 47, 48, 35–53 |
| Proportion of ADA pos/neg at Visit among well-controlled at Screening (N, %) | 38/51 (74.5%) | 13/51 (25.5%) | 15/47 (31.9%) | 32/47 (68.1%) |
| Poorly controlled glycemia (HbA1c ≥ 69 mmol/mol) (mean, median HbA1c, range) | (N = 35) 81, 76, 70–112 | (N = 15) 86, 89, 71–100 | (N = 17) 79, 76, 70–100 | (N = 37) 81, 80, 70–109 |
| Proportion of ADA pos/neg at Visit among poorly controlled at Screening (N, %) | 35/50 (70%) | 15/50 (30%) | 17/54 (31.5%) | 37/54 (68.5%) |
| Age at onset (median years, range) | (N = 73) 27, 5–65 | (N = 28) 20.5, 6–46 | (N = 32) 46.5, 30–79 | (N = 68) 51, 27–83 |
| Duration of insulin treatment (mean, median years, range) | (N = 72) 20, 18, 0–57 | (N = 18) 26, 26, 0–68 | (N = 30) 10, 10, 0–27 | (n = 66) 8, 8, 0–30 |
| Insulin dose (IU/kg) (mean, median, range) | (N = 58) 0.48, 0.46, 0.04–1.09 | (N = 24) 0.52, 0.48, 0.10–1.14 | (N = 30) 1.00, 0.49, 0.12–13.37 | (N = 62) 0.64, 0.46, 0.05–4.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toft-Hansen, H.; Aniol-Nielsen, C.; Elias, D.; Dahlbäck, M.; Rossing, P.; Sivalingam, S.; Hagopian, W.A.; Schneider, D.A.; Nielsen, C.H.; Solberg, H. Characterization of Anti-Insulin Antibodies in Type 1 and Type 2 Diabetes Mellitus: Clinical Relevance. Int. J. Mol. Sci. 2025, 26, 1730. https://doi.org/10.3390/ijms26041730
Toft-Hansen H, Aniol-Nielsen C, Elias D, Dahlbäck M, Rossing P, Sivalingam S, Hagopian WA, Schneider DA, Nielsen CH, Solberg H. Characterization of Anti-Insulin Antibodies in Type 1 and Type 2 Diabetes Mellitus: Clinical Relevance. International Journal of Molecular Sciences. 2025; 26(4):1730. https://doi.org/10.3390/ijms26041730
Chicago/Turabian StyleToft-Hansen, Henrik, Christina Aniol-Nielsen, Daniel Elias, Madeleine Dahlbäck, Peter Rossing, Suvanjaa Sivalingam, William A. Hagopian, Darius A. Schneider, Claus H. Nielsen, and Helene Solberg. 2025. "Characterization of Anti-Insulin Antibodies in Type 1 and Type 2 Diabetes Mellitus: Clinical Relevance" International Journal of Molecular Sciences 26, no. 4: 1730. https://doi.org/10.3390/ijms26041730
APA StyleToft-Hansen, H., Aniol-Nielsen, C., Elias, D., Dahlbäck, M., Rossing, P., Sivalingam, S., Hagopian, W. A., Schneider, D. A., Nielsen, C. H., & Solberg, H. (2025). Characterization of Anti-Insulin Antibodies in Type 1 and Type 2 Diabetes Mellitus: Clinical Relevance. International Journal of Molecular Sciences, 26(4), 1730. https://doi.org/10.3390/ijms26041730






