Abstract
Antibody therapies are a crucial component of modern lymphoid malignancy treatment and an exciting area of active research. We performed a review of modern antibody therapies used in the treatment of lymphoid malignancies, with an emphasis on landmark studies and current directions. We describe the indications for rituximab, obinutuzumab, ADCs, and bispecific antibody therapies. Finally, we summarize early data from ongoing trials on emerging novel therapy combination regimens and discuss the role of machine learning in future therapy development.
Keywords:
antibody; malignancy; CLL; FL; DLBCL; rituximab; obinutuzumab; polatuzumab; mosunetuzumab; epcoritamab 1. Introduction
Antibody therapies have revolutionized the landscape of lymphoid malignancy therapies since their inception. In 1975, Kohler and Milstein developed the first method for fusing murine B lymphocytes with immortal myeloma cells as a bioreactor to produce monoclonal antibodies with in vitro immortality. These fusion cell lines, or “hybridomas”, had promising clinical applications [1]. However, pure murine antibodies were limited in terms of their efficacy due to the short half-life, poor antibody-dependent cellular cytotoxicity (ADCC), and development of human anti-mouse antibodies (HAMA) in almost all patients with repeated infusion [2]. However, the core concept of developing targeted therapies that activate the patient’s own immune system was sound, particularly in the context of lymphoid malignancies, where a monoclonal species with unique targetable surface proteins is the source of disease. These therapies have been continually refined, from chimerized, to glycoengineered, to modern novel therapies, including antibody–drug conjugates (ADCs) and bispecific antibodies (BsAbs), as noted in Figure 1. Novel therapies are constantly being developed, and new combination regimens are now under investigation, with promising results. A summary of the reviewed studies can be found in Table 1.
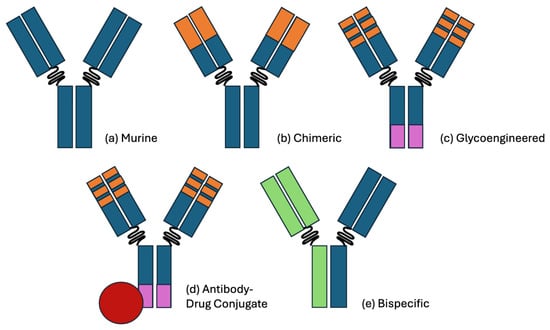
Figure 1.
Visual representation of the antibody therapy classes. (a) A pure murine antibody, as derived from hybridoma cell lines. (b) A chimeric antibody with humanized (orange) fAb regions, improving the immunogenicity and half-life. A notable example is rituximab, which targets CD20. (c) A glycoengineered antibody with a reduced fucose Fc region (purple), improving the antibody-dependent cellular cytotoxicity (ADCC). A notable example is obinutuzumab, which also targets CD20. (d) An antibody–drug conjugate with cytotoxic payload (red). A notable example is polatuzumab vedotin, which targets CD79b and delivers a payload of monomethyl auristatin E. (e) A bispecific antibody with distinct FAb complementarity. A notable example is glofitamab, which targets CD20 and CD3. Binding CD3 on a CD8+ T-cell activates its cytotoxic activity toward the approximated CD20+ cell.

Table 1.
Summary of antibody therapies in lymphoid malignancies, organized by study, population, therapy regimen with target, and primary outcomes listed, with p-values included where available.
2. Chimeric Monoclonal Therapies—Rituximab
2.1. Introduction
The first step beyond pure murine monoclonal therapies was the production of chimeric murine–human monoclonal antibodies. First described in 1984 by Morrison et al., these chimeric antibodies consisted of murine variable regions affixed to a human Fc region [43]. Utilizing DNA recombination techniques, murine hybridoma cells carrying genes for preselected variable regions are transfected with a viral vector carrying human Fc regions. The final product is a large (~150 dKa) IgG glycoprotein composed of a human Fc region with murine variable regions that have been selected for specific targets. The presence of a human Fc region improves the therapeutic potential of purely murine antibody therapies (-omabs) by improving the immunogenicity, half-life, and cell-mediated cytotoxic response [44,45]. Additionally, only ~10% of patients generate an anti-idiotype response of modest intensity to early chimeric antibodies [2].
Within the context of lymphoid malignancies, the most therapeutically notable chimeric antibody is rituximab, an anti-CD20 human–murine chimeric IgG monoclonal antibody [46]. CD20 is a transmembrane phosphoprotein that is initially expressed during early B-cell development and acts as a regulatory molecule in B-cell maturation. CD20 is expressed shortly before B-cells gain the ability to generate cytoplasmic heavy chains, with continued expression in mature B-cells until plasma cell differentiation. Thus, its high sensitivity and specificity to antibody-secreting B-cells makes CD20 an excellent target in B-cell malignancies.
Once rituximab binds to CD20, the bound cells undergo cell death through a variety of mechanisms, which include complement-mediated cell death (CDC), ADCC, phagocytic activation, and multiple other cell death cascades, including upregulated calcium influx, as noted in Figure 2 [47,48]. This leads to the cytoreduction of CD20+ cell populations. Rituximab is then cleared by hepatic non-specific catabolism [49].
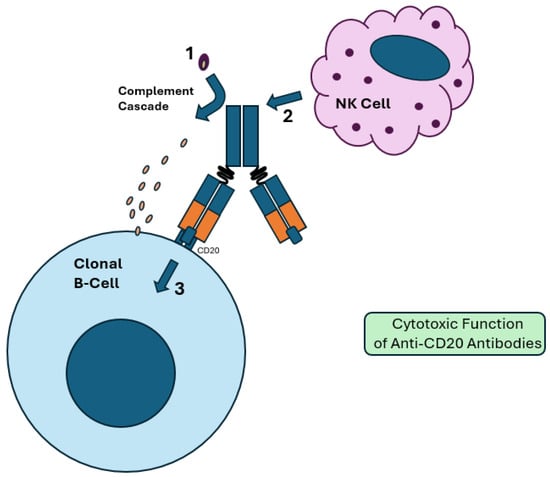
Figure 2.
Cytotoxic function of anti-CD20 antibodies. (1) Proteolytic cleavage via the Fc region results in activation of the complement cascade. A membrane attack complex (MAC) is formed, resulting in complement-dependent cytotoxicity (CDC). (2) The Fc region of the bound antibody activates NK and other effector cells, resulting in antibody-dependent cellular cytotoxicity (ADCC). This is more effective in glycoengineered antibody therapies, which have a reduced fucose Fc region. (3) Binding of CD20 in clonal B-cells upregulates intracellular pathways that promote apoptosis. These include decreased expression of antiapoptotic proteins like BCL-2 and upregulated calcium influx, which results in cell death.
While rituximab has theoretical benefits in all CD20+ malignancies, it is best studied in chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma, including follicular lymphoma (FL), and diffuse large B-cell lymphoma (DLBCL) [50].
2.2. Clinical Applications
2.2.1. Chronic Lymphocytic Leukemia
CLL is the most prevalent form of leukemia in the United States, accounting for 25% of diagnoses [51]. CLL is defined by the presence of a monoclonal B-cell population greater than 5 × 109/L in the peripheral blood, with classic phenotypic characteristics of CLL, which include CD5+, CD19+, and CD43+ [3,4]. Rituximab monotherapy was a promising concept in CLL but has been largely inferior to combination therapies, with an overall response rate (ORR) of 51% and progression-free survival (PFS) of 18.6 months in previously untreated patients who were symptomatic or Rai stage 2–4 [5]. In patients who presented with Rai stage 0–2 disease, rituximab performed better, with an ORR of 81%, a median overall time to progression (TTP) of 23 months, and a time to retreatment (TTR) of 43 months (PFS was not assessed due to the high survival rates in this population) [6].
Rituximab was used throughout the 2000s in combination therapies, most notably the fludarabine and cyclophosphamide + rituximab regimen (FCR), which provided survival benefit as both new and salvage therapy [7].
While these combination regimens outperformed pure chemotherapy, they have been largely supplanted by the advent of targeted therapies. The MURANO trial was a phase III randomized study for patients with relapsed or refractory CLL that compared the efficacy of bendamustine + rituximab (BR) and venetoclax + rituximab (venR). Here, 62% of patients treated with venR achieved undetectable minimal residual disease (uMRD), while only 13% of patients in the BR arm reached uMRD, leading to higher rates of OS in the venR arm [8]. At the 5-year follow-up, the median PFS was 53.6 vs. 17.0 months, respectively, with 5-year OS of 82.1% vs. 62.2%, respectively [9].
Rituximab has also been shown to be highly efficacious in combination with targeted therapy. In the ECOG 1912 trial, patients with previously untreated CLL were treated with either FCR or ibrutinib + rituximab (IR). At three years, the IR arm outperformed FCR in PFS (89.4% vs. 72.9%, respectively), OS (98.8% vs. 91.5%, respectively) and tolerability, with decreased grade 3 or higher infectious complications in the IR arm [52,53].
2.2.2. Follicular Lymphoma
FL is the third most common non-Hodgkin lymphoma, with an incidence of 17.1% in the United States [54]. It is classically characterized by a t(14;18), which results in overexpression of BCL-2, an antiapoptotic protein with resultant clonal expansion [3,10,11]. These clonal populations are nearly always positive for monoclonal immunoglobulin light chain, BCL-6, CD10, CD19, and CD20, making FL a prime target for anti-CD20 therapy [12].
Rituximab has been used for induction monotherapy in grades I–III follicular lymphoma with good effect. In a clinical trial by Colombat et al., 41% of the study population reached CR/CRu and 39% reached PR, with an ORR of 80% within the first year of therapy [13]. A study performed by the North Central Cancer Treatment Group also demonstrated an ORR of 72%, with only 56% of responders developing disease progression within 2.6 years [55]. The RESORT trial (E4402) tried to elucidate whether rituximab should be continued after induction. Patients were randomized to either maintenance rituximab (MR) or rituximab retreatment (RR), who only received treatment following disease progression. There was no statistical significance between the groups in terms of the primary endpoint of time to treatment failure (TTF). The MR group had an overall decreased dependency on cytotoxic therapy at three years and an improved duration of the first remission; however, the OS did not change at 10 years between the groups [14,15].
Rituximab’s first approval for FL was in 1997, following a phase II non-inferiority trial in relapsed/refractory FL as salvage therapy. Patients underwent 4 weekly infusions of rituximab. With an ORR of 48% and CR of 6%, rituximab performed similarly to single-agent cytotoxic regimens in this population [16]. Extending the therapy to eight weeks improved the outcomes further, with an ORR of 57%, of which 14% achieved CR [17].
This improved performance with longer therapy windows led to a landmark study evaluating the benefit of maintenance rituximab therapy. In the PRIMA trial, patients were separated into an observation group or given 2 years of maintenance rituximab monotherapy after induction with either CVP, CHOP, or FCM. At two years, 71.5% of patients in the rituximab group were in CR or uCR compared to 52.2% in the observation group [18]. Additionally, the PFS was 74.9% vs. 57.6%, respectively. Of note, the overall survival did not change between the groups after long-term follow-up analysis [19].
Rituximab in combination with chemotherapy has also been evaluated. The BRIGHT study evaluated bendamustine + rituximab (BR) against chemotherapy + rituximab regimens (R-CHOP or R-CVP) in untreated patients with indolent NHL or MCL. The differences in the complete response rate and OS were not statistically significant between the groups. However, the 5-year PFS was improved in the BR group at 65.5% compared to 55.8% in the R-CHOP / R-CVP group, and the adverse effect profile of the BR group was considered more favorable, with higher incidences of vomiting and infusion reactions but lower incidences of peripheral neuropathy and alopecia [56,57].
More recently, rituximab has been studied in combination with lenalidomide in the phase III multicenter AUGMENT trial for patients with relapsed or refractory indolent FL and MZL. Lenalidomide has shown increased NK-cell-mediated cytotoxicity and ADCC and so enhances the efficacy of rituximab. Patients were randomized to either a rituximab–placebo arm or a rituximab + lenalidomide. The lenalidomide group had a significantly long median PFS at 29.4% compared with the placebo at 14.1%. However, more adverse effects were noted in the lenalidomide group, most notable neutropenia (58% vs. 23%, respectively) [58].
2.2.3. Diffuse Large B-Cell Lymphoma
DLBCL is the most common form of NHL, comprising nearly 30–40% of NHL diagnoses in the United States [59]. DLBCL is often aggressive and can be life-threatening without prompt initiation of treatment. These NHLs tend to express pan-B-cell markers, including CD19, CD20, CD22, CD45, and CD79a, making them a target for anti-CD20 therapies [60]. At a basic level, these malignancies can be separated into three distinct treatment groups: germinal-center B-cell type (GC), non-germinal center (non-GC), also known as activated B-cell type (ABC), and high-grade DLBCL, not otherwise specified [3]. Germinal-center DLBCL is characterized by CD10+ and is considered the lowest risk. High-grade DLBCL has an MYC gene rearrangement in combination with a BCL-2 or BCL-6 rearrangement, which confers the highest risk of a poor outcome. Non-GC DLBCL is considered an intermediate risk and is CD10- without rearrangement in the MYC plus BCL-2 or BCL-6 genes.
Rituximab as a monotherapy has not been shown to outperform the standard of care in DLBCL, including cutaneous forms [61,62]. In combination with cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP), however, rituximab has demonstrated significant improvements in PFS and OS in low-risk young patients and high-risk elderly patients [20,21,63]. This regimen is widely used for initiation therapy in both GC and non-GC DLBCL. In high-grade DLBCL, rituximab in combination with DA-EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) has conferred improved PFS rates at two years [64].
The combination with other regimens has also been studied. In young patients with high-risk disease, rituximab in combination with doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (R-ACVBP) outperforms R-CHOP, with improved 3-year EFS (81% to 67%) and OS (92% to 84%, respectively) [65]. The more recent POLARIX trial subgroup analyses demonstrated improved 2-year PFS in non-GC DLBCL patients who received Pola-R-CHP (polatuzumab vedotin, rituximab, cyclophosphamide, doxorubicin, prednisone) against a standard of care R-CHOP arm [66].
2.3. Adverse Effects of Rituximab
2.3.1. Infusion-Related Reactions
In a 5-year review of infusion reactions to rituximab at a large academic infusion center, there was ~77% incidence of mild to severe infusion reactions, with 63% occurring during the first transfusion. Most of these reactions were classified as mild to moderate, with only 12% progressing to severe or life-threatening. Moreover, 84% of patients with grade 2 moderate reactions tolerated a same-day rechallenge [67].
2.3.2. B-Cell Depletion and Immunosuppression
In a retrospective review, patients treated with rituximab for rheumatologic and lymphoid malignancies had a 73.3% chance of having an infection event (IE) within 3 months of infusion. In addition, 58% of the infections in the malignancy group were severe, requiring hospitalization [68]. These IEs often coincide with leukopenia, with a median onset time of 13 days following infusion, or late-onset neutropenia, which typically occurs at least four weeks following infusion [69]. Patients with chronic or latent hepatitis B have a 50–64% risk of reactivation following rituximab initiation [70]. Additionally, patients with NHL started on rituximab are at a 12.6% risk of reactivation of herpes zoster, with an odds ratio of 1.38 compared to the general population [71]. These adverse effects have been mitigated by recombinant VZV vaccination prior to treatment, hepatitis B screening prior to initiation, and close follow-up during initiation to screen for early signs of infection [72].
2.4. Resistance Mechanisms
Tumor cell lines have exhibited multiple resistance mechanisms to rituximab, including complement inhibition via upregulation of CD55 and CD59, internalization of CD20 complexes, and alteration of pro-apoptotic and anti-apoptotic pathways [73,74,75,76]. The most common resistance mechanism seen is decreased total CD20 surface expression, which is mediated by the MS4A1 gene. This effectively hides the tumor cell from rituximab and immune surveillance and is commonly found in relapsed/refractory DLBCL (R/R DLBCL) [76,77].
3. Glycoengineered Antibody Therapies—Obinutuzumab
3.1. Introduction
Obinutuzumab is a type II, glycoengineered monoclonal antibody that binds to CD-20, similarly to rituximab. However, obinutuzumab binds to a distinct epitope on the CD20 protein with a different orientation, allowing for a unique mechanism of action [78]. An IgG1 subclass, this glycoengineered antibody has a modified Fc region of reduced fucose content. This allows for enhanced ability to engage with the FcγRIII receptors of immune effector cells, like natural killer (NK) cells [22].
This antibody demonstrates several mechanisms that enhance its effectiveness in targeting CD20-expressing B-cells. First, it enhances ADCC through FcγRIII-mediated CD20 internalization, allowing for greater recruitment of immune effector cells, which increases the lysis of CD20-expressing B-cells. Additionally, it induces homotypic aggregation, where the clustering of B-cells results in a lysosome-mediated, non-apoptotic form of cell death, which is caspase-independent [78]. Furthermore, it promotes antibody-dependent cellular phagocytosis by enhancing the interactions with FcγRIII receptors, leading to greater recruitment and activation of macrophages, which then engulf the B-cells [79].
3.2. Clinical Applications
In the setting of lymphoid malignancies, obinutuzumab is FDA-approved for the treatment of CLL, FL, and MZL. In CLL, the drug is combined with chlorambucil in treatment-naive patients. A phase III trial showed that this combination (Clb-Obi) improved the median progression-free survival (26.7 months) compared to patients treated with rituximab plus chlorambucil (15.2 months) and chlorambucil alone (11.1 months). It also improved the overall survival, with a hazard ratio for death of 0.41 (p = 0.002) [80]. More recently, obinutuzumab in combination with venetoclax (Ven-Obi) was shown to significantly outperform Clb-Obi in terms of the median PFS (76.2 vs. 36.4 months), six-year rate of time to next treatment (65.2% vs. 37.1%), and rates of uMRD in bone marrow at three months (57% vs. 17%) [81].
In FL, the drug is combined with chemotherapy, followed as a monotherapy, in patients with treatment-naive stage II bulky, III, or IV FL. It can also be used in combination with bendamustine, followed by monotherapy, for patients with relapsed disease or disease refractory to a regimen containing rituximab [82].
Due to their similarities, obinutuzumab and rituximab have had their efficacy compared in several head-to-head trials. In the GALLIUM trial, patients with treatment-naive FL were randomized to the obinutuzumab + chemotherapy or rituximab + chemotherapy arm for first-line treatment. At 7 years, obinutuzumab outperformed rituximab, with a PFS of 63.4% and 55.7%, respectively [24,25]. In the GAUSS trial, 175 patients with relapsed indolent CD20+ DLBCL who responded to rituximab but developed progression were randomized to either a rituximab maintenance or obinutuzumab arm. The obinutuzumab arm had an independently confirmed improvement in the ORR (44.6% vs. 26.7%, respectively) without statistical improvement in the PFS [23]. However, the GOYA trial demonstrated that there was no statistical difference in PFS when compared to rituximab in combination with CHOP for first-line treatment of DLBCL, with an increased adverse event rate [27,83]. Thus, in summary, it does appear the obinutuzumab has improved efficacy for indolent lymphoid neoplasms; however, in aggressive lymphomas, there has not been demonstrated a significant clinical benefit over rituximab.
3.3. Adverse Effects of Obinutuzumab
However, the drug has some weaknesses. Infusion-related reactions (IRRs) are common, with the GALLIUM study reporting that 59.3% of patients receiving obinutuzumab experienced IRRs, compared to 48.9% of patients receiving rituximab (p < 0.001). These reactions included nausea, chills, fever and emesis, with more severe IRRs occurring in 12% of patients compared to 8% with rituximab. Careful monitoring and vigilance may be necessary with obinutuzumab, as well as premedication with Tylenol, antihistamines and corticosteroids to mitigate the severity of the IRRs [25]. Additionally, hematologic toxicities have been reported, with the GREEN trial indicating that 49.9% of patients developed neutropenia and 16.4% of patients developed thrombocytopenia when taking obinutuzumab [84].
4. Antibody–Drug Conjugate (ADC) Therapies
4.1. Introduction
Antibody–drug conjugate (ADC) therapies are an emerging class of targeted cancer treatments. Structurally, ADCs involve a tumor-specific monoclonal antibody (mAb), a cytotoxic molecule called the payload, and a covalent chemical bond that links the mAb and the payload. This design allows the targeted delivery of cytotoxins to cancerous cells while minimizing the off-target effects on normal tissue.
Once ADCs bind to the corresponding antigen of tumor cells, the ADC–antigen complex is internalized. The cytotoxic payload is then released by the linker once the ADC undergoes lysosomal degradation, allowing the linker to bind to its intracellular target, resulting in cell death, as depicted in Figure 3. ADCs also exhibit “bystander killing” by diffusing into adjacent cells and inducing cell death. This design allows ADCs to specifically target tumor cells while reducing the damage to healthy tissue [28,29,85].
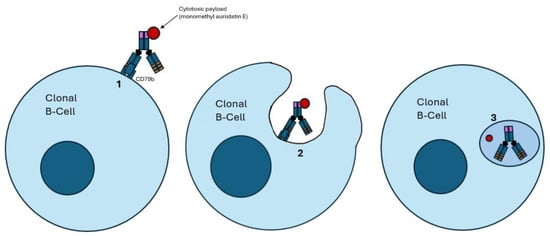
Figure 3.
Mechanism of action of antibody–drug conjugates (ADCs). (1) The ADC binds to its target antigen; in this example, CD79b. (2) CD79b binding leads to endocytosis of the ADC. (3) The ADC undergoes proteolytic cleavage once it enters a lysosome, resulting in the freedom of the bound cytotoxic payload, which results in cell death. A notable example is polatuzumab vedotin, an anti-CD79b ADC, which carries a payload of monomethyl auristatin E.
4.2. Clinical Applications
Clinically, ADCs have been particularly effective in treating lymphoid malignancies, where cell surface markers like CD30, CD22 and CD79b are commonly expressed on malignant lymphocytes. The FDA-approved ADCs for lymphoid malignancies include loncastuximab teserine, brentuximab vedotin, inotuzumab ozogamicin, moxetumomab pasudotox, and polatuzumab vedotin.
Loncastuximab teserine (LT) is an ADC that targets the B-cell marker CD19. Its payload, SG3199, is an irreversible DNA cross-linker that leads to cell death. The LOTIS-2 trial demonstrated efficacy in R/R DLBCL, with a CR rate of 24.8%. While the median PFS was 4.9 months and the median OS was 9.5 months, patients who achieved CR surpassed these values, with a 2-year PFS rate of 72.5% and 2-year OS rate of 68.2% [86,87]. The LOTIS-3 trial exploring the use of LT in combination with ibrutinib for R/R DLBCL had promising preliminary data, with an ORR of 57.1% and overall CRR of 34.3%, but was terminated early. The LOTIS-5 trial investigating a regimen of LT with rituximab is ongoing, with recruitment scheduled until 2028 and with preliminary data showing an ORR of 80% and a CRR of 50% [26].
Brentuximab vedotin (BV) targets CD30, a TNF receptor found on Reed–Sternberg cells in Hodgkin lymphoma, anaplastic large-cell lymphoma cells, and some cutaneous T-cell lymphoma cells. The cytotoxic component of BV is monomethyl auristatin E, a microtubule-inhibiting agent [88]. In the recently updated ESCHELON-1 trial, BV in combination with doxorubicin, vinblastine, and dacarbazine (A+AVD) was placed head to head against the gold standard therapy of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) for first-line treatment in patients with untreated stage III or IV classical Hodgkin lymphoma. A+AVD outperformed ABVD in seven-year OS (94.6% vs. 89.4%, respectively), with maintained PFS rates of 82.3% vs. 75.3%. A+AVD also had a favorable AE profile, with the removal of bleomycin decreasing the pulmonary adverse outcomes [89,90,91]. A phase II study in patients with relapsed or refractory Hodgkin lymphoma following autologous stem-cell transplantation has also shown a 75% overall response rate, with complete remission in 34% of patients [92].
Polatuzumab vedotin targets CD79b, a component of the B-cell receptor complex. This ADC has shown significant clinical efficacy in treating DLBCL. Similarly to BV, its cytotoxic payload is monomethyl auristatin E. In the phase II GO29365 study, the combination of polatuzumab vedotin with bendamustine and rituximab significantly improved the complete response rate (40% vs. 17.5%, p = 0.026) and progression-free survival (median of 9.5 months vs. 3.7 months, p < 0.001, HR 0.36) compared to bendamustine and rituximab alone [93]. The POLARIX trial demonstrated the efficacy of polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin, and prednisone (Pola-R-CHP) against the standard-of-care R-CHOP. The two-year PFS in Pola-R-CHP was 76.7% compared to 70.2% for R-CHOP [30]. A subgroup network meta-analysis revealed improved hazard ratios in patients with untreated activated B-cell-like (ABC) DLBCL, an aggressive and generally treatment-resistant subtype, who were treated with Pola-R-CHP when compared to other regimens, including R-CHOP plus bortezomib (HR: 0.52, p = 0.02), R-CHOP plus ibrutinib (HR: 0.43, p = 0.001), R-CHOP plus lenalidomide (HR: 0.51, p = 0.009), obinutuzumab-CHOP (HR: 0.46, p = 0.008), and R-CHOP (HR: 0.40, p < 0.001) [31].
4.3. Adverse Effects of ADCs
While effective, ADCs have a notable AE profile and several described resistance mechanisms. Since tumor antigens are not specific to tumor cells, toxicity can theoretically occur to all mitotically active cells when a potent cytotoxic is delivered by an ADC. For example, toxicity, including grade 3–4 neutropenia, anemia, thrombocytopenia and peripheral neuropathy are clear with polatuzumab vedotin [85]. The resistance mechanisms also limit the clinical efficacy of ADCs through tumor-induced downregulation or mutation of the target antigen, resulting in suboptimal binding by the ADC. Furthermore, there have been cases of multidrug resistance 1 (MDR1) gene overexpression, with consequent P-glycoprotein-mediated drug efflux of the cytotoxic payload [33,94]. However, ongoing research aims to overcome these challenges. Efforts include developing novel linkers to enhance the bystander effect and designing unique antibody structures to stabilize the therapeutic potential of ADCs.
5. Bispecific Antibodies—Immunotherapy
5.1. Introduction
Bispecific antibodies (BsAbs) offer another innovative therapeutic approach. Structurally, they are designed to bind two unique antigens simultaneously, often redirecting immune effector T-cells to the tumor cell, to induce cytotoxicity [95]. BsAbs can be divided into two main categories, IgG-like and non-IgG-like BsAbs. IgG-like BsAbs resemble natural antibodies and include an Fc region, which stabilizes and extends the half-life of the drug and enables immune effector processes, including antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. However, IgG-like antibodies can be challenging to engineer due to potential mispairing of the heavy and light chains. In contrast, non-IgG-like BsAbs lack an Fc region, making them smaller and with a shorter half-life. However, without the need for Fc receptor interactions, they have a more flexible and straightforward design. Bispecific T-cell engagers (BiTEs) are a type of non-IgG-like BsAb [34]. Functionally, BsAbs facilitate the recruitment and activation of T-cells, resulting in tumor cell lysis, as seen in Figure 4. One arm targets a tumor cell antigen, such as CD19, CD20 or BCMA, while the other arm binds to the CD3 antigen on T-cells. This CD3 engagement on cytotoxic CD8+ T-cells upregulates activation markers and releases inflammatory cytokines, driving tumor cell lysis. The dual binding function of BiTEs forms an immunologic synapse between the T-cell and the tumor cell, triggering T-cell activation, proliferation and tumor cell destruction. [34,95]. Given their mechanism of action, BsAbs have a unique side effect profile, including CRS and neurological toxicity related to their ability to stimulate the immune system [32].
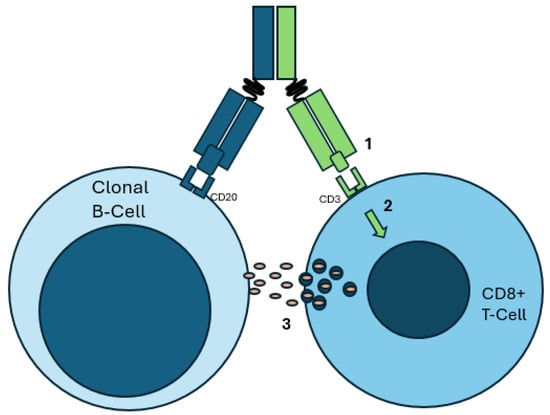
Figure 4.
Example of a malignant B-cell as targeted by a CD20xCD3 bispecific antibody therapy. Note the approximation of a CD3+ T-cell with the clonal B-cell via the bispecific antibody. (1) The bispecific antibody binds to CD20 on the clonal B-cell and CD3 on the CD8+ T-cell. (2) CD3 binding leads to TCR-independent signaling, resulting in activation. (3) Cytotoxic granules and cytokines are released, resulting in immune activation and clonal B-cell demise.
5.2. Clinical Applications
Clinically, BsAbs have shown great promise. Blinatumomab, a BiTE that targets CD19 and CD3, is particularly effective in treating B-cell acute lymphoblastic leukemia, especially in patients with minimal residual disease (MRD). The phase II BLAST study demonstrated that 78% of patients with the B-cell precursor ALL in complete remission but with MRD achieved a great MRD response after one cycle of blinatumomab [35]. Similarly, mosunetuzumab, a BsAb that targets CD20 and CD3, has shown efficacy in treating relapsed or refractory B-cell non-Hodgkin lymphoma. A phase I/II clinical trial demonstrated that mosunetuzumab achieved an ORR of 66% and a CRR of 48.5% in patients with indolent relapsed or refractory B-cell non-Hodgkin lymphoma [96].
More recently, there have been multiple CD3xCD20 BsAbs with approval as third-line therapies in lymphoid malignancies. Epcoritamab is a subcutaneous CD3xCD20 BsAb, which recently obtained FDA approval for third-line treatment of R/R DLBCL. In the EPCORE NHL-1 trial, patients with R/R DLBCL treated with epcoritamab had a 24-month follow-up ORR of 63.1%, with 40.1% achieving CR. The estimated 24-month PFS was 65.1%, with an OSR of 78.2%. Patients with R/R FL were also evaluated, with an ORR of 82% and a CR of 62.5%, although FDA approval for third-line therapy has not been granted [36,37,97]. Glofitamab, another promising CD3xCD20 BsAb, has also received accelerated approval for third-line treatment of R/R DLBCL and LBCL arising from FL. These patients are pretreated with obinutuzumab to mitigate the risk of cytokine release syndrome (obinutuzumab depletes the number of B-cells available as a target). Patients achieved a CRR of 39% at 12.6 months. Of note, the response was independent of prior treatment, including CAR-T and histological, or molecular subtype apart from the high-grade DLBCL cohort, which only experienced PR [38]. Finally, mosunetuzumab has also received approval for the treatment of R/R FL. In the clinical trial GO29781, patients with R/R FL who had failed two prior therapies achieved an ORR of 80%, and a CRR of 60%, with median PFS of 17.9 months. The current regimen uses step-up dosing to mitigate the main adverse effect of CRS [39,96].
5.3. Adverse Effects of Bispecific Antibody Therapies
Given their mechanism of action, the AE profile of BsAbs is generally characterized by cytokine release syndrome (up to 72% in LBCL), neutropenia with hypogammaglobulinemia, CNS toxicity, and infections, which are primarily driven by T-cell activation [32].
6. Future Directions
6.1. Combination Regimens
There is active investigation into the use of antibody therapy for patients with lymphoid malignancies. Fortunately, antibodies are readily able to be administered with one another to allow for combination therapy, thus increasing the potential of synergy. To date, the most common tumor-associated antigen targeted has been CD20; however, ongoing investigation into other targets is actively being pursued. However, we do not expect CD20-directed therapies to be abandoned in the near future. Unlike cellular therapies, there is a lot more potential for novel treatment approaches with antibodies. From a clinical perspective, ongoing studies are investigating the use of BsAbs and ADCs in combination with each other and with chemotherapy regimens like R-CHOP as early-line therapies in both DLBCL and FL.
The EPCORE NHL-2 trial is underway to evaluate the combination of epcoritamab with other regimens. Preliminary data from several arms are promising. Arm 1 is actively investigating epcoritamab in combination with R-CHOP for previously untreated DLBCL. Phase 1 and 2 early data report an ORR of 100% and a complete metabolic response (CMR) of 96% at 9 months. Notably, the response rate was similar across all the DLBCL subtypes [98]. They have recently begun a phase III trial (NCT04663347).
Arm 6 of the study is investigating the use of epcoritamab in combination with lenalidomide for previously untreated FL. Early data presented in abstract show an ORR of 95% and a CRR of 85% at the 21-month follow-up. Maintenance therapy with epcoritamab in patients with FL who have undergone standard-of-care treatment is being investigated in arm 7, where all eight patients enrolled with PR have had a 100% conversion rate to CR, with an acceptable AE profile thus far [99]. These findings are still preliminary but are hopeful.
Arm 9 of EPCORE NHL-2 is still in the recruitment phase and is set to investigate epcoritamab + lenalidomide for second-line treatment in patients with R/R FL who progressed within 24 months of initiation of first-line anti-CD20-containing immunochemotherapy (NCT04663347).
BsAbs are also being investigated in combination with ADCs and immunotherapies. An ongoing phase 1b/2 trial of mosunetuzumab in combination with polatuzumab for R/R LBCL has reported an ORR and a CRR of 59.2% and 45.9%, respectively, at a median of 23.9 months. Notably, the rate of CRS was only 16.7% in the early data [100]. A recent subgroup update of the phase 1b/2 trial reported no statistical difference in the durable response regardless of prior treatment history, with a CR of 35.9% (95% CI 21.9–51.2) in the refractory group and a CRR of 59.3% (95% CI 38.8–77.6) in the relapsed group [101]. Combination therapy of glofitamab and polatuzumab is also under investigation in R/R DLBCL [102]. Epcoritamab and Pola-R-CHP are being investigated as first-line therapy in patients with DLBCL in the phase 1b/2 EPCORE NHL-5 study, with an early reported ORR of 100% and CRR of 89% at a median time to follow-up of 5.8 months [103]. Finally, preliminary results from a phase 1b/2 trial for mosunetuzumab in combination with lenalidomide in patients with previously untreated FL reported an ORR of 88.9% and a CR of 81.5% at the time of reporting. No median follow-up was reported (NCT04246086) [104]. These results promoted a phase III trial, which is ongoing (NCT04712097).
CD47 is another novel target that has been identified in recent years, with several exciting developments. CD47, the so-called “Don’t Eat Me” signal, is a surface protein that is expressed in normal cells as an inhibitor of phagocytosis by macrophages and dendritic cells. CD47 binds the SIRPα receptor on these phagocytic immune surveillance cells and prevents phagocytosis. CD47 is commonly highly expressed in tumor cells. Several therapeutic antibody therapies are being developed to block this pathway with the goal of increasing immune surveillance and the death of tumor cells [105].
The first described anti-CD47 therapy is magrolimab, a humanized anti-CD47 antibody. Magrolimab has been primarily studied in combination therapy with CD20 (NCT02953509]. In R/R NHL, patients treated with magrolimab + rituximab achieved an ORR of 52.2% and a CR of 30.4%. The median PFS at 3 years was 7.4 months, with 21% having developed grade III/IV anemia [40,41]. In R/R DLBCL, magrolimab + rituximab (MR) was compared to a regimen of MR, gemcitabine, and oxaliplatin (MR+GemOx). The regimens achieved ORRs of 24.2% and 51.5%, with CRRs of 12.1% and 39.4%, respectively. Notably, there was one treatment-related death (grade V colitis) in the MR+GemOx arm [42]. Ligufalimab, another anti-CD47 humanized antibody therapy, is currently under investigation for multiple malignancies (NCT0472830) [106].
There are many other therapies targeting the CD47 pathway in the pipeline, with 15 current clinical trials [105]. A notable example is IMM0306, a BsAb targeting CD20 and the CD47-binding domain of SIRPα [107]. IMM0306 is being studied both as a monotherapy for R/R CD20+ B-cell NHL and in combination with lenalidomide (NCT05805943 and NCT05805943, respectively). There are not yet preliminary data from these studies.
6.2. Role of Machine Learning
From a therapy development perspective, antibody therapies should continue to address the common shared weaknesses while improving clinical efficacy by improving target selection, immunogenicity, poor tumor penetration/resistance mechanisms, and difficulties with production or delivery (e.g., high production cost, low half-life).
The problems of immunogenicity and poor tumor penetration are both related to the core structures that form the therapeutic antibody. Researchers are addressing these problems with predictive modeling via machine learning algorithms. Identifying and avoiding peptide motifs associated with high anti-therapy immunogenicity will theoretically decrease adverse effects and improve efficacy. Machine learning models offer the ability to predict the possible anti-therapy immunogenicity of future antibody therapies. Currently, there are many different models that aim to improve the drug development pipeline at multiple stages, including analyzing tumor genomics, identifying targetable motifs, and predicting immunogenicity [108]. In 2020, Liang and Zhang generated a machine learning model that predicted immunogenicity with 83% accuracy when given crystalized structures of therapeutic monoclonal antibodies, and 65% accuracy with modeled structures during leave-one-out cross-validation [109]. Another example is TAP 1.0, an ML system designed to analyze protein sequences to identify potential tumor antigens with high immunogenicity for future targeted therapies. The use of such processes has the potential to greatly decrease the development cost of targeted therapies by decreasing broad in vitro assays, which are both time- and labor-intensive [110]. These advancements show great promise in improving the feasibility and development of targeted therapies for lymphoid malignancies.
Author Contributions
Conceptualization, B.M.H. and J.N.; investigation, J.N. and N.S.; resources, J.N. and N.S.; writing—original draft preparation, J.N. and N.S.; writing—review and editing, J.N., N.S. and B.M.H.; visualization, J.N.; supervision, B.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- LoBuglio, A.F.; Wheeler, R.H.; Trang, J.; Haynes, A.; Rogers, K.; Harvey, E.B.; Sun, L.; Ghrayeb, J.; Khazaeli, M.B. Mouse/human chimeric monoclonal antibody in man: Kinetics and immune response. Proc. Natl. Acad. Sci. USA 1989, 86, 4220–4224. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S.; et al. The International Consensus Classification of Mature Lymphoid Neoplasms: A report from the Clinical Advisory Committee. Blood 2022, 140, 1229–1253. [Google Scholar] [CrossRef] [PubMed]
- Hainsworth, J.D.; Litchy, S.; Barton, J.H.; Houston, G.A.; Hermann, R.C.; Bradof, J.E.; Greco, F.A. Single-Agent Rituximab as First-Line and Maintenance Treatment for Patients With Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma: A Phase II Trial of the Minnie Pearl Cancer Research Network. J. Clin. Oncol. 2003, 21, 1746–1751. [Google Scholar] [CrossRef]
- Ferrajoli, A.; Keating, M.J.; O’Brien, S.; Cortes, J.; Thomas, D.A. Experience with rituximab immunotherapy as an early intervention in patients with Rai stage 0 to II chronic lymphocytic leukemia. Cancer 2011, 117, 3182–3186. [Google Scholar] [CrossRef][Green Version]
- Hallek, M.; Fischer, K.; Fingerle-Rowson, G.; Fink, A.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; von Grünhagen, U.; et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet 2010, 376, 1164–1174. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.; Hillmen, P.; D’rozario, J.; Assouline, S.; Owen, C.; Gerecitano, J.; Robak, T.; De la Serna, J.; et al. Venetoclax–Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2018, 378, 1107–1120. [Google Scholar] [CrossRef]
- Seymour, J.F.; Kipps, T.J.; Eichhorst, B.F.; D’rozario, J.; Owen, C.J.; Assouline, S.; Lamanna, N.; Robak, T.; de la Serna, J.; Jaeger, U.; et al. Enduring undetectable MRD and updated outcomes in relapsed/refractory CLL after fixed-duration venetoclax-rituximab. Blood 2022, 140, 839–850. [Google Scholar] [CrossRef]
- Zelenetz, A.; Chu, G.; Galili, N.; Bangs, C.; Horning, S.; Donlon, T.; Cleary, M.; Levy, R. Enhanced detection of the t(14;18) translocation in malignant lymphoma using pulsed-field gel electrophoresis. Blood 1991, 78, 1552–1560. [Google Scholar] [CrossRef]
- Fisher, R.I.; LeBlanc, M.; Press, O.W.; Maloney, D.G.; Unger, J.M.; Miller, T.P. New Treatment Options Have Changed the Survival of Patients With Follicular Lymphoma. J. Clin. Oncol. 2005, 23, 8447–8452. [Google Scholar] [CrossRef] [PubMed]
- Roulland, S.; Kelly, R.S.; Morgado, E.; Sungalee, S.; Solal-Celigny, P.; Colombat, P.; Jouve, N.; Palli, D.; Pala, V.; Tumino, R.; et al. t(14;18) Translocation: A Predictive Blood Biomarker for Follicular Lymphoma. J. Clin. Oncol. 2014, 32, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Colombat, P.; Salles, G.; Brousse, N.; Eftekhari, P.; Soubeyran, P.; Delwail, V.; Deconinck, E.; Haïoun, C.; Foussard, C.; Sebban, C.; et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: Clinical and molecular evaluation. Blood 2001, 97, 101–106. [Google Scholar] [CrossRef]
- Kahl, B.S.; Hong, F.; Williams, M.E.; Gascoyne, R.D.; Wagner, L.I.; Krauss, J.C.; Habermann, T.M.; Swinnen, L.J.; Schuster, S.J.; Peterson, C.G.; et al. Rituximab Extended Schedule or Re-Treatment Trial for Low–Tumor Burden Follicular Lymphoma: Eastern Cooperative Oncology Group Protocol E4402. J. Clin. Oncol. 2014, 32, 3096–3102. [Google Scholar] [CrossRef]
- Kahl, B.S.; Jegede, O.A.; Peterson, C.; Swinnen, L.J.; Habermann, T.M.; Schuster, S.J.; Weiss, M.; Fishkin, P.A.; Fenske, T.S.; Williams, M.E. Long-Term Follow-Up of the RESORT Study (E4402): A Randomized Phase III Comparison of Two Different Rituximab Dosing Strategies for Low–Tumor Burden Follicular Lymphoma. J. Clin. Oncol. 2024, 42, 774–778. [Google Scholar] [CrossRef]
- McLaughlin, P.; Grillo-López, A.J.; Link, B.K.; Levy, R.; Czuczman, M.S.; Williams, M.E.; Heyman, M.R.; Bence-Bruckler, I.; White, C.A.; Cabanillas, F.; et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998, 16, 2825–2833. [Google Scholar] [CrossRef]
- Piro, L.D.; White, C.A.; Grillo-López, A.J.; Janakiraman, N.; Saven, A.; Beck, T.M.; Varns, C.; Shuey, S.; Czuczman, M.; Lynch, J.W.; et al. Extended Rituximab (anti-CD20 monoclonal antibody) therapy for relapsed or refractory low-grade or follicular non-Hodgkin’s lymphoma. Ann. Oncol. 1999, 10, 655–661. [Google Scholar] [CrossRef]
- Salles, G.; Seymour, J.F.; Offner, F.; López-Guillermo, A.; Belada, D.; Xerri, L.; Feugier, P.; Bouabdallah, R.; Catalano, J.V.; Brice, P.; et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet 2010, 377, 42–51. [Google Scholar] [CrossRef]
- Bachy, E.; Seymour, J.F.; Feugier, P.; Offner, F.; López-Guillermo, A.; Belada, D.; Xerri, L.; Catalano, J.V.; Brice, P.; Lemonnier, F.; et al. Sustained Progression-Free Survival Benefit of Rituximab Maintenance in Patients With Follicular Lymphoma: Long-Term Results of the PRIMA Study. J. Clin. Oncol. 2019, 37, 2815–2824. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Kuhnt, E.; Trümper, L.; Österborg, A.; Trneny, M.; Shepherd, L.; Gill, D.S.; Walewski, J.; Pettengell, R.; Jaeger, U.; et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011, 12, 1013–1022. [Google Scholar] [CrossRef]
- Coiffier, B.; Lepage, E.; Brière, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Gagez, A.-L.; Cartron, G. Obinutuzumab. Curr. Opin. Oncol. 2014, 26, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Townsend, W.; Hiddemann, W.; Buske, C.; Cartron, G.; Cunningham, D.; Dyer, M.J.; Gribben, J.G.; Phillips, E.H.; Dreyling, M.; Seymour, J.F.; et al. Obinutuzumab Versus Rituximab Immunochemotherapy in Previously Untreated iNHL: Final Results From the GALLIUM Study. HemaSphere 2023, 7, e919. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.; Davies, A.; Ando, K.; Klapper, W.; Opat, S.; Owen, C.; Phillips, E.; Sangha, R.; Schlag, R.; Seymour, J.F.; et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N. Engl. J. Med. 2017, 377, 1331–1344. [Google Scholar] [CrossRef]
- Hiddemann, W.; Barbui, A.M.; Canales, M.A.; Cannell, P.K.; Collins, G.P.; Dürig, J.; Forstpointner, R.; Herold, M.; Hertzberg, M.; Klanova, M.; et al. Immunochemotherapy With Obinutuzumab or Rituximab for Previously Untreated Follicular Lymphoma in the GALLIUM Study: Influence of Chemotherapy on Efficacy and Safety. J. Clin. Oncol. 2018, 36, 2395–2404. [Google Scholar] [CrossRef]
- Caimi, P.F.; Ai, W.Z.; Alderuccio, J.P.; Ardeshna, K.M.; Hamadani, M.; Hess, B.; Kahl, B.S.; Radford, J.; Solh, M.; Stathis, A.; et al. Loncastuximab tesirine in relapsed/refractory diffuse large B-cell lymphoma: Long-term efficacy and safety from the phase 2 LOTIS-2 study. Haematologica 2023, 109, 1184–1193. [Google Scholar] [CrossRef]
- Sehn, L.H.; Goy, A.; Offner, F.C.; Martinelli, G.; Caballero, M.D.; Gadeberg, O.; Baetz, T.; Zelenetz, A.D.; Gaidano, G.; Fayad, L.E.; et al. Randomized Phase II Trial Comparing Obinutuzumab (GA101) With Rituximab in Patients With Relapsed CD20+ Indolent B-Cell Non-Hodgkin Lymphoma: Final Analysis of the GAUSS Study. J. Clin. Oncol. 2015, 33, 3467–3474. [Google Scholar] [CrossRef]
- Leblond, V.; Aktan, M.; Coll, C.M.F.; Dartigeas, C.; Kisro, J.; Montillo, M.; Raposo, J.; Merot, J.-L.; Robson, S.; Gresko, E.; et al. Safety of obinutuzumab alone or combined with chemotherapy for previously untreated or relapsed/refractory chronic lymphocytic leukemia in the phase IIIb GREEN study. Haematologica 2018, 103, 1889–1898. [Google Scholar] [CrossRef]
- Tolcher, A.W. Antibody drug conjugates: Lessons from 20 years of clinical experience. Ann. Oncol. 2016, 27, 2168–2172. [Google Scholar] [CrossRef]
- Sehn, L.H.; Herrera, A.F.; Flowers, C.R.; Kamdar, M.K.; McMillan, A.; Hertzberg, M.; Assouline, S.; Kim, T.M.; Kim, W.S.; Ozcan, M.; et al. Polatuzumab Vedotin in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2020, 38, 155–165. [Google Scholar] [CrossRef]
- Tilly, H.; Morschhauser, F.; Sehn, L.H.; Friedberg, J.W.; Trněný, M.; Sharman, J.P.; Herbaux, C.; Burke, J.M.; Matasar, M.; Rai, S.; et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Pretelli, G.; Desai, J.; Garralda, E.; Siu, L.L.; Steiner, T.M.; Au, L. Bispecific antibodies: Advancing precision oncology. Trends Cancer 2024, 10, 893–919. [Google Scholar] [CrossRef] [PubMed]
- Abelman, R.O.; Wu, B.; Spring, L.M.; Ellisen, L.W.; Bardia, A. Mechanisms of Resistance to Antibody–Drug Conjugates. Cancers 2023, 15, 1278. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.J.; Zweegman, S. T-cell-engaging bispecific antibodies in cancer. Lancet 2023, 402, 142–158. [Google Scholar] [CrossRef]
- Bayly-McCredie, E.; Treisman, M.; Fiorenza, S. Safety and Efficacy of Bispecific Antibodies in Adults with Large B-Cell Lymphomas: A Systematic Review of Clinical Trial Data. Int. J. Mol. Sci. 2024, 25, 9736. [Google Scholar] [CrossRef]
- Frampton, J.E. Epcoritamab: First Approval. Drugs 2023, 83, 1331–1340. [Google Scholar] [CrossRef]
- Linton, K.M.; Vitolo, U.; Jurczak, W.; Lugtenburg, P.J.; Gyan, E.; Sureda, A.; Christensen, J.H.; Hess, B.; Tilly, H.; Cordoba, R.; et al. Epcoritamab monotherapy in patients with relapsed or refractory follicular lymphoma (EPCORE NHL-1): A phase 2 cohort of a single-arm, multicentre study. Lancet Haematol. 2024, 11, e593–e605. [Google Scholar] [CrossRef]
- Thieblemont, C.; Karimi, Y.H.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Jurczak, W.; Do, Y.R.; Gasiorowski, R.; Lewis, D.J.; et al. Epcoritamab in relapsed/refractory large B-cell lymphoma: 2-year follow-up from the pivotal EPCORE NHL-1 trial. Leukemia 2024, 38, 2653–2662. [Google Scholar] [CrossRef]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Wróbel, T.; Offner, F.; Trněný, M.; et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 387, 2220–2231. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, H.; Jiang, Z.; Tian, W.; Cang, S.; Yu, J. Targeting the CD47/SIRPα pathway in malignancies: Recent progress, difficulties and future perspectives. Front. Oncol. 2024, 14, 1378647. [Google Scholar] [CrossRef]
- Sikic, B.I.; Lakhani, N.; Patnaik, A.; Shah, S.A.; Chandana, S.R.; Rasco, D.; Colevas, A.D.; O’rourke, T.; Narayanan, S.; Papadopoulos, K.; et al. First-in-Human, First-in-Class Phase I Trial of the Anti-CD47 Antibody Hu5F9-G4 in Patients With Advanced Cancers. J. Clin. Oncol. 2019, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Popplewell, L.; Collins, G.P.; Smith, S.M.; Flinn, I.W.; Bartlett, N.L.; Ghosh, N.; Hacohen-Kleiman, G.; Huo, Y.; Su-Feher, L.; et al. Magrolimab plus rituximab in relapsed/refractory indolent non-Hodgkin lymphoma: 3-year follow-up of a phase 1/2 trial. Blood Adv. 2024, 8, 5855–5863. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.L.; Johnson, M.J.; Herzenberg, L.A.; Oi, V.T. Chimeric human antibody molecules: Mouse antigen-binding domains with human constant region domains. Proc. Natl. Acad. Sci. USA 1984, 81, 6851–6855. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Y.; Robinson, R.R.; Murray, E.D.; Ledbetter, J.A.; Hellström, I.; Hellström, K.E. Production of a mouse-human chimeric monoclonal antibody to CD20 with potent Fc-dependent biologic activity. J. Immunol. 1987, 139, 3521–3526. [Google Scholar] [CrossRef]
- Nishimura, Y.; Yokoyama, M.; Araki, K.; Ueda, R.; Kudo, A.; Watanabe, T. Recombinant Human-Mouse Chimeric Monoclonal-Antibody Specific for Common Acute Lymphocytic-Leukemia Antigen. Cancer Res. 1987, 47, 999–1005. [Google Scholar]
- Reff, M.E.; Carner, K.; Chambers, K.S.; Chinn, P.C.; Leonard, J.E.; Raab, R.; Newman, R.A.; Hanna, N.; Anderson, D. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994, 83, 435–445. [Google Scholar] [CrossRef]
- Weiner, G.J. Rituximab: Mechanism of Action. Semin. Hematol. 2010, 47, 115–123. [Google Scholar] [CrossRef]
- Abulayha, A.; Bredan, A.; El Enshasy, H.; Daniels, I. Rituximab: Modes of Action, Remaining Dispute and Future Perspective. Futur. Oncol. 2014, 10, 2481–2492. [Google Scholar] [CrossRef]
- Cartron, G.; Blasco, H.; Paintaud, G.; Watier, H.; Le Guellec, C. Pharmacokinetics of rituximab and its clinical use: Thought for the best use? Crit. Rev. Oncol. 2007, 62, 43–52. [Google Scholar] [CrossRef]
- Keating, G.M. Spotlight on Rituximab in Chronic Lymphocytic Leukemia, Low-Grade or Follicular Lymphoma, and Diffuse Large B-Cell Lymphoma†. BioDrugs 2011, 25, 55–61. [Google Scholar] [CrossRef]
- ZERO Prostate Cancer. Cancer Facts & Figures 2024; American Cancer Society: Atlanta, GA, USA, 2024. [Google Scholar]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib–Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Wang, X.V.; Hanson, C.A.; Paietta, E.M.; O’brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Long-term outcomes for ibrutinib–rituximab and chemoimmunotherapy in CLL: Updated results of the E1912 trial. Blood 2022, 140, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamadani, M.; Habermann, T.M.; Cerhan, J.R.; Macon, W.R.; Maurer, M.J.; Go, R.S. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am. J. Hematol. 2015, 90, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Witzig, T.E.; Vukov, A.M.; Habermann, T.M.; Geyer, S.; Kurtin, P.J.; Friedenberg, W.R.; White, W.L.; Chalchal, H.I.; Flynn, P.J.; Fitch, T.R.; et al. Rituximab Therapy for Patients With Newly Diagnosed, Advanced-Stage, Follicular Grade I Non-Hodgkin’s Lymphoma: A Phase II Trial in the North Central Cancer Treatment Group. J. Clin. Oncol. 2005, 23, 1103–1108. [Google Scholar] [CrossRef]
- Flinn, I.W.; van der Jagt, R.; Kahl, B.S.; Wood, P.; Hawkins, T.E.; MacDonald, D.; Hertzberg, M.; Kwan, Y.-L.; Simpson, D.; Craig, M.; et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: The BRIGHT study. Blood 2014, 123, 2944–2952. [Google Scholar] [CrossRef]
- Flinn, I.W.; van der Jagt, R.; Kahl, B.; Wood, P.; Hawkins, T.; MacDonald, D.; Simpson, D.; Kolibaba, K.; Issa, S.; Chang, J.; et al. First-Line Treatment of Patients with Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma with Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study. J. Clin. Oncol. 2019, 37, 984–991. [Google Scholar] [CrossRef]
- Leonard, J.P.; Trneny, M.; Izutsu, K.; Fowler, N.H.; Hong, X.; Zhu, J.; Zhang, H.; Offner, F.; Scheliga, A.; Nowakowski, G.S.; et al. AUGMENT: A Phase III Study of Lenalidomide Plus Rituximab Versus Placebo Plus Rituximab in Relapsed or Refractory Indolent Lymphoma. J. Clin. Oncol. 2019, 37, 1188–1199. [Google Scholar] [CrossRef]
- Wang, S.S. Epidemiology and etiology of diffuse large B-cell lymphoma. Semin. Hematol. 2023, 60, 255–266. [Google Scholar] [CrossRef]
- Liu, Y.; Barta, S.K. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019, 94, 604–616. [Google Scholar] [CrossRef]
- Coiffier, B.; Haioun, C.; Ketterer, N.; Engert, A.; Tilly, H.; Ma, D.; Johnson, P.; Lister, A.; Feuring-Buske, M.; A Radford, J.; et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood 1998, 92, 1927–1932. [Google Scholar]
- Fenot, M.; Quereux, G.; Brocard, A.; Renaut, J.-J.; Dreno, B. Rituximab for primary cutaneous diffuse large B-cell lymphoma-leg type. Eur. J. Dermatol. 2010, 20, 753–757. [Google Scholar] [PubMed]
- Coiffier, B.; Thieblemont, C.; Van Den Neste, E.; Lepeu, G.; Plantier, I.; Castaigne, S.; Lefort, S.; Marit, G.; Macro, M.; Sebban, C.; et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010, 116, 2040–2045. [Google Scholar] [CrossRef]
- Oki, Y.; Noorani, M.; Lin, P.; Davis, R.E.; Neelapu, S.S.; Ma, L.; Ahmed, M.; Rodriguez, M.A.; Hagemeister, F.B.; Fowler, N.; et al. Double hit lymphoma: The MD Anderson Cancer Center clinical experience. Br. J. Haematol. 2014, 166, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Récher, C.; Coiffier, B.; Haioun, C.; Molina, T.J.; Fermé, C.; Casasnovas, O.; Thiéblemont, C.; Bosly, A.; Laurent, G.; Morschhauser, F.; et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): An open-label randomised phase 3 trial. Lancet 2011, 378, 1858–1867. [Google Scholar] [CrossRef]
- Song, Y.; Tilly, H.; Rai, S.; Zhang, H.; Jin, J.; Goto, H.; Terui, Y.; Shin, H.-J.; Kim, W.S.S.; Cao, J.; et al. Polatuzumab vedotin in previously untreated DLBCL: An Asia subpopulation analysis from the phase 3 POLARIX trial. Blood 2023, 141, 1971–1981. [Google Scholar] [CrossRef]
- Levin, A.S.; Otani, I.M.; Lax, T.; Hochberg, E.; Banerji, A. Reactions to Rituximab in an Outpatient Infusion Center: A 5-Year Review. J. Allergy Clin. Immunol. Pract. 2017, 5, 107–113.e1. [Google Scholar] [CrossRef]
- Tudesq, J.-J.; Cartron, G.; Rivière, S.; Morquin, D.; Iordache, L.; Mahr, A.; Pourcher, V.; Klouche, K.; Cerutti, D.; Le Quellec, A.; et al. Clinical and microbiological characteristics of the infections in patients treated with rituximab for autoimmune and/or malignant hematological disorders. Autoimmun. Rev. 2018, 17, 115–124. [Google Scholar] [CrossRef]
- Wolach, O.; Bairey, O.; Lahav, M. Late-Onset Neutropenia After Rituximab Treatment. Medicine 2010, 89, 308–318. [Google Scholar] [CrossRef]
- Hou, K.; Su, T.; Kao, C.; Cheng, H.; Tseng, T.; Liu, C.; Hsieh, S.; Kao, J. Rituximab carries high risks of hepatitis B virus reactivation in hematologic and rheumatic patients with chronic or resolved hepatitis B. J. Gastroenterol. Hepatol. 2024, 39, 2447–2455. [Google Scholar] [CrossRef]
- Cho, S.-F.; Wu, W.-H.; Yang, Y.-H.; Liu, Y.-C.; Hsiao, H.-H.; Chang, C.-S. Longitudinal risk of herpes zoster in patients with non-Hodgkin lymphoma receiving chemotherapy: A nationwide population-based study. Sci. Rep. 2015, 5, 14008. [Google Scholar] [CrossRef]
- Kamboj, M.; Bohlke, K.; Baptiste, D.M.; Dunleavy, K.; Fueger, A.; Jones, L.; Kelkar, A.H.; Law, L.Y.; LeFebvre, K.B.; Ljungman, P.; et al. Vaccination of Adults With Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 1699–1721. [Google Scholar] [CrossRef] [PubMed]
- Bordron, A.; Bagacean, C.; Mohr, A.; Tempescul, A.; Bendaoud, B.; Deshayes, S.; Dalbies, F.; Buors, C.; Saad, H.; Berthou, C.; et al. Resistance to complement activation, cell membrane hypersialylation and relapses in chronic lymphocytic leukemia patients treated with rituximab and chemotherapy. Oncotarget 2018, 9, 31590–31605. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Vaughan, A.T.; Ashton-Key, M.; Williams, E.L.; Dixon, S.V.; Chan, H.T.C.; Beers, S.A.; French, R.R.; Cox, K.L.; Davies, A.J.; et al. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011, 118, 2530–2540. [Google Scholar] [CrossRef] [PubMed]
- Jazirehi, A.R.; Vega, M.I.; Bonavida, B. Development of Rituximab-Resistant Lymphoma Clones with Altered Cell Signaling and Cross-Resistance to Chemotherapy. Cancer Res. 2007, 67, 1270–1281. [Google Scholar] [CrossRef]
- Olejniczak, S.H.; Hernandez-Ilizaliturri, F.J.; Clements, J.L.; Czuczman, M.S. Acquired Resistance to Rituximab Is Associated with Chemotherapy Resistance Resulting from Decreased Bax and Bak Expression. Clin. Cancer Res. 2008, 14, 1550–1560. [Google Scholar] [CrossRef]
- Rushton, C.K.; Arthur, S.E.; Alcaide, M.; Cheung, M.; Jiang, A.; Coyle, K.M.; Cleary, K.L.S.; Thomas, N.; Hilton, L.K.; Michaud, N.; et al. Genetic and evolutionary patterns of treatment resistance in relapsed B-cell lymphoma. Blood Adv. 2020, 4, 2886–2898. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Fischer, K.; Engelke, A.; Pflug, N.; Hallek, M.; Goede, V. Obinutuzumab in chronic lymphocytic leukemia: Design, development and place in therapy. Drug Des. Dev. Ther. 2017, 11, 295–304. [Google Scholar] [CrossRef]
- Golay, J.; Da Roit, F.; Bologna, L.; Ferrara, C.; Leusen, J.H.; Rambaldi, A.; Klein, C.; Introna, M. Glycoengineered CD20 antibody obinutuzumab activates neutrophils and mediates phagocytosis through CD16B more efficiently than rituximab. Blood 2013, 122, 3482–3491. [Google Scholar] [CrossRef]
- Goede, V.; Fischer, K.; Busch, R.; Engelke, A.; Eichhorst, B.; Wendtner, C.M.; Chagorova, T.; de la Serna, J.; Dilhuydy, M.-S.; Illmer, T.; et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2014, 370, 1101–1110. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Robrecht, S.; Zhang, C.; Olivieri, S.; Chang, Y.M.; Fink, A.M.; Tausch, E.; Schneider, C.; Ritgen, M.; Kreuzer, K.-A.; et al. Venetoclax-obinutuzumab for previously untreated chronic lymphocytic leukemia: 6-year results of the randomized phase 3 CLL14 study. Blood 2024, 144, 1924–1935. [Google Scholar] [CrossRef]
- Gibiansky, E.; Gibiansky, L.; Buchheit, V.; Frey, N.; Brewster, M.; Fingerle-Rowson, G.; Jamois, C. Pharmacokinetics, exposure, efficacy and safety of obinutuzumab in rituximab-refractory follicular lymphoma patients in the GADOLIN phase III study. Br. J. Clin. Pharmacol. 2019, 85, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, U.; Trněný, M.; Belada, D.; Burke, J.M.; Carella, A.M.; Chua, N.; Abrisqueta, P.; Demeter, J.; Flinn, I.; Hong, X.; et al. Obinutuzumab or Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone in Previously Untreated Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2017, 35, 3529–3537. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Martelli, M.; Trněný, M.; Liu, W.; Bolen, C.R.; Knapp, A.; Sahin, D.; Sellam, G.; Vitolo, U. A randomized, open-label, Phase III study of obinutuzumab or rituximab plus CHOP in patients with previously untreated diffuse large B-Cell lymphoma: Final analysis of GOYA. J. Hematol. Oncol. 2020, 13, 71. [Google Scholar] [CrossRef]
- Chu, Y.; Zhou, X.; Wang, X. Antibody-drug conjugates for the treatment of lymphoma: Clinical advances and latest progress. J. Hematol. Oncol. 2021, 14, 88. [Google Scholar] [CrossRef]
- Fu, Y.; Ho, M. DNA damaging agent-based antibody-drug conjugates for cancer therapy. Antib. Ther. 2018, 1, 43–53. [Google Scholar] [CrossRef]
- Caimi, P.F.; Ai, W.; Alderuccio, J.P.; Ardeshna, K.M.; Hamadani, M.; Hess, B.; Kahl, B.S.; Radford, J.; Solh, M.; Stathis, A.; et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 790–800. [Google Scholar] [CrossRef]
- Kwiatek, M.; Grosicki, S.; Jiménez, J.L.; Mariño, S.F.P.; Snauwaert, S.; Kingsley, E.; Zacchetti, G.; Wang, Y.; Wang, L.; Depaus, J. ABCL-515 Updated Results of the Safety Run-In of the Phase 3 LOTIS-5 Trial: Novel Combination of Loncastuximab Tesirine With Rituximab (Lonca-R) Versus Immunochemotherapy in Patients With R/R DLBCL. Clin. Lymphoma Myeloma Leuk. 2023, 23, S439–S440. [Google Scholar] [CrossRef]
- Deutsch, Y.E.; Tadmor, T.; Podack, E.R.; Rosenblatt, J.D. CD30: An important new target in hematologic malignancies. Leuk. Lymphoma 2011, 52, 1641–1654. [Google Scholar] [CrossRef]
- Connors, J.M.; Jurczak, W.; Straus, D.J.; Ansell, S.M.; Kim, W.S.; Gallamini, A.; Younes, A.; Alekseev, S.; Illés, Á.; Picardi, M.; et al. Brentuximab Vedotin with Chemotherapy for Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 378, 331–344. [Google Scholar] [CrossRef]
- Straus, D.J.; Długosz-Danecka, M.; Alekseev, S.; Illés, Á.; Picardi, M.; Lech-Maranda, E.; Feldman, T.; Smolewski, P.; Savage, K.J.; Bartlett, N.L.; et al. Brentuximab vedotin with chemotherapy for stage III/IV classical Hodgkin lymphoma: 3-year update of the ECHELON-1 study. Blood 2020, 135, 735–742. [Google Scholar] [CrossRef]
- Ansell, S.M.; Radford, J.; Connors, J.M.; Długosz-Danecka, M.; Kim, W.-S.; Gallamini, A.; Ramchandren, R.; Friedberg, J.W.; Advani, R.; Hutchings, M.; et al. Overall Survival with Brentuximab Vedotin in Stage III or IV Hodgkin’s Lymphoma. N. Engl. J. Med. 2022, 387, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Ramchandren, R.; Bartlett, N.L.; Cheson, B.D.; De Vos, S.; et al. Results of a Pivotal Phase II Study of Brentuximab Vedotin for Patients With Relapsed or Refractory Hodgkin’s Lymphoma. J. Clin. Oncol. Off. 2012, 30, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Li, D.; Chen, B.; Zhao, C.; Zhang, W.; Ding, B.; Wang, L. Superiority of polatuzumab vedotin over other novel agents in previously untreated ABC-type diffuse large B-cell lymphoma: A network meta-analysis of 20 RCTs. Ann. Hematol. 2023, 102, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Loganzo, F.; Sung, M.; Gerber, H.-P. Mechanisms of Resistance to Antibody–Drug Conjugates. Mol. Cancer Ther. 2016, 15, 2825–2834. [Google Scholar] [CrossRef]
- Goekbuget, N.; Dombret, H.; Bonifacio, M.; Reichle, A.; Graux, C.; Havelange, V.; Buss, E.C.; Faul, C.; Bruggemann, M.; Ganser, A.; et al. BLAST: A Confirmatory, Single-Arm, Phase 2 Study of Blinatumomab, a Bispecific T-Cell Engager (BiTE®) Antibody Construct, in Patients with Minimal Residual Disease B-Precursor Acute Lymphoblastic Leukemia (ALL). Blood 2014, 124, 379. [Google Scholar] [CrossRef]
- Budde, L.E.; Assouline, S.; Sehn, L.H.; Schuster, S.J.; Yoon, S.-S.; Yoon, D.H.; Matasar, M.J.; Bosch, F.; Kim, W.S.; Nastoupil, L.J.; et al. Single-Agent Mosunetuzumab Shows Durable Complete Responses in Patients With Relapsed or Refractory B-Cell Lymphomas: Phase I Dose-Escalation Study. J. Clin. Oncol. 2022, 40, 481–491. [Google Scholar] [CrossRef]
- E Budde, L.; Sehn, L.H.; Matasar, M.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: A single-arm, multicentre, phase 2 study. Lancet Oncol. 2022, 23, 1055–1065. [Google Scholar] [CrossRef]
- Clausen, M.R.; Belada, D.; Offner, F.; de Vos, S.; Brody, J.; Linton, K.; Snauwaert, S.; Cordoba, R.; Wu, J.; Bykhovski, I.; et al. P1116: HIGH COMPLETE METABOLIC RESPONSE RATES WITH EPCORITAMAB + R-CHOP IN PREVIOUSLY UNTREATED (1L) PATIENTS WITH HIGH-RISK DIFFUSE LARGE B-CELL LYMPHOMA, INCLUDING DOUBLE/TRIPLE-HIT: EPCORE NHL-2 UPDATE. HemaSphere 2023, 7, e55140cd. [Google Scholar] [CrossRef]
- Lori, L.A.; Falchi, L.; Vermaat, J.S.; Musuraca, G.; Belada, D.; Nijland, M.; Christensen, J.H.; Offner, F.; Hoehn, D.; Marek, J.; et al. Epcoritamab with rituximab + lenalidomide (R2) in previously untreated (1L) follicular lymphoma (FL) and epcoritamab maintenance in FL: EPCORE NHL-2 arms 6 and 7. J. Clin. Oncol. 2024, 42, 7014. [Google Scholar] [CrossRef]
- Budde, L.E.; Olszewski, A.J.; Assouline, S.; Lossos, I.S.; Diefenbach, C.; Kamdar, M.; Ghosh, N.; Modi, D.; Sabry, W.; Naik, S.; et al. Mosunetuzumab with polatuzumab vedotin in relapsed or refractory aggressive large B cell lymphoma: A phase 1b/2 trial. Nat. Med. 2023, 30, 229–239. [Google Scholar] [CrossRef]
- Assouline, S.; Budde, L.E.; Chavez, J.C.; Diefenbach, C.S.; Dorritie, K.A.; Ghosh, N.; Olszewski, A.J.; Lossos, I.S.; Mehta, A.; Modi, D.; et al. Mosunetuzumab With Polatuzumab Vedotin: Subgroup Analyses in Patients (pts) With Primary Refractory or Early Relapsed Large B-Cell Lymphoma (LBCL). Clin. Lymphoma Myeloma Leuk. 2024, 24, S212. [Google Scholar] [CrossRef]
- Hutchings, M.; Avigdor, A.; Balari, A.M.; Terol, M.J.; Bosch, F.; Corradini, P.; Larsen, T.S.; Dominguez, A.R.; Skarbnik, A.; Joergensen, J.M.; et al. Glofitamab Plus Polatuzumab Vedotin Continues to Demonstrate Frequent and Durable Responses and Has a Manageable Safety Profile in Patients with ≥2L Relapsed/Refractory DLBCL, Including HGBCL, and in Patients with Prior CAR T-Cell Therapy: Updated Results from a Phase Ib/II Study. Blood 2023, 142, 4460. [Google Scholar]
- Kerr, D.A.; Lavie, D.; Avigdor, A.; Avivi, I.; Belada, D.; Patel, K.; Thieblemont, C.; Abrisqueta, P.; Izutsu, K.; Seliem, M.; et al. First Data From Subcutaneous Epcoritamab + Polatuzumab Vedotin, Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone (Pola-R-CHP) for First-Line Diffuse Large B-Cell Lymphoma (DLBCL): EPCORE NHL-5. Clin. Lymphoma Myeloma Leuk. 2024, 24, S217. [Google Scholar] [CrossRef]
- Morschhauser, F.; Patel, K.; Bobillo, S.; Cordoba, R.; Eyre, T.A.; Bishton, M.; Houot, R.; Zhang, H.-L.; Zou, L.; Osborne, W.; et al. Preliminary Findings of a Phase Ib/II Trial Indicate Manageable Safety and Promising Efficacy for Mosunetuzumab in Combination with Lenalidomide (M+Len) in Previously Untreated (1L) Follicular Lymphoma (FL). Blood 2023, 142, 605. [Google Scholar] [CrossRef]
- Maakaron, J.E.; Asch, A.; Popplewell, L.; Collins, G.P.; Flinn, I.W.; Ghosh, N.; Keane, C.; Ku, M.; Mehta, A.; Roschewski, M.; et al. Magrolimab plus rituximab with or without chemotherapy in patients with relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2024, 8, 5864–5874. [Google Scholar] [CrossRef]
- Qu, T.; Zhong, T.; Pang, X.; Huang, Z.; Jin, C.; Wang, Z.M.; Li, B.; Xia, Y. Ligufalimab, a novel anti-CD47 antibody with no hemagglutination demonstrates both monotherapy and combo antitumor activity. J. Immunother. Cancer 2022, 10, e005517. [Google Scholar] [CrossRef]
- Piccione, E.C.; Juarez, S.; Liu, J.; Tseng, S.; Ryan, C.E.; Narayanan, C.; Wang, L.; Weiskopf, K.; Majeti, R. A bispecific antibody targeting CD47 and CD20 selectively binds and eliminates dual antigen expressing lymphoma cells. mAbs 2015, 7, 946–956. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, R.; Gao, S.; Li, W.; Liu, Y.; Su, G.; Song, M.; Jiang, M.; Jiang, C.; Zhang, X. Artificial intelligence applied in neoantigen identification facilitates personalized cancer immunotherapy. Front. Oncol. 2023, 12, 1054231. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, C. Prediction of immunogenicity for humanized and full human therapeutic antibodies. PLoS ONE 2020, 15, e0238150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).