Arabidopsis thaliana Plants’ Overexpression of the MYB Transcription Factor VhMYB60 in the Face of Stress Hazards Enhances Salt and Cold Tolerance
Abstract
:1. Introduction
2. Research Results
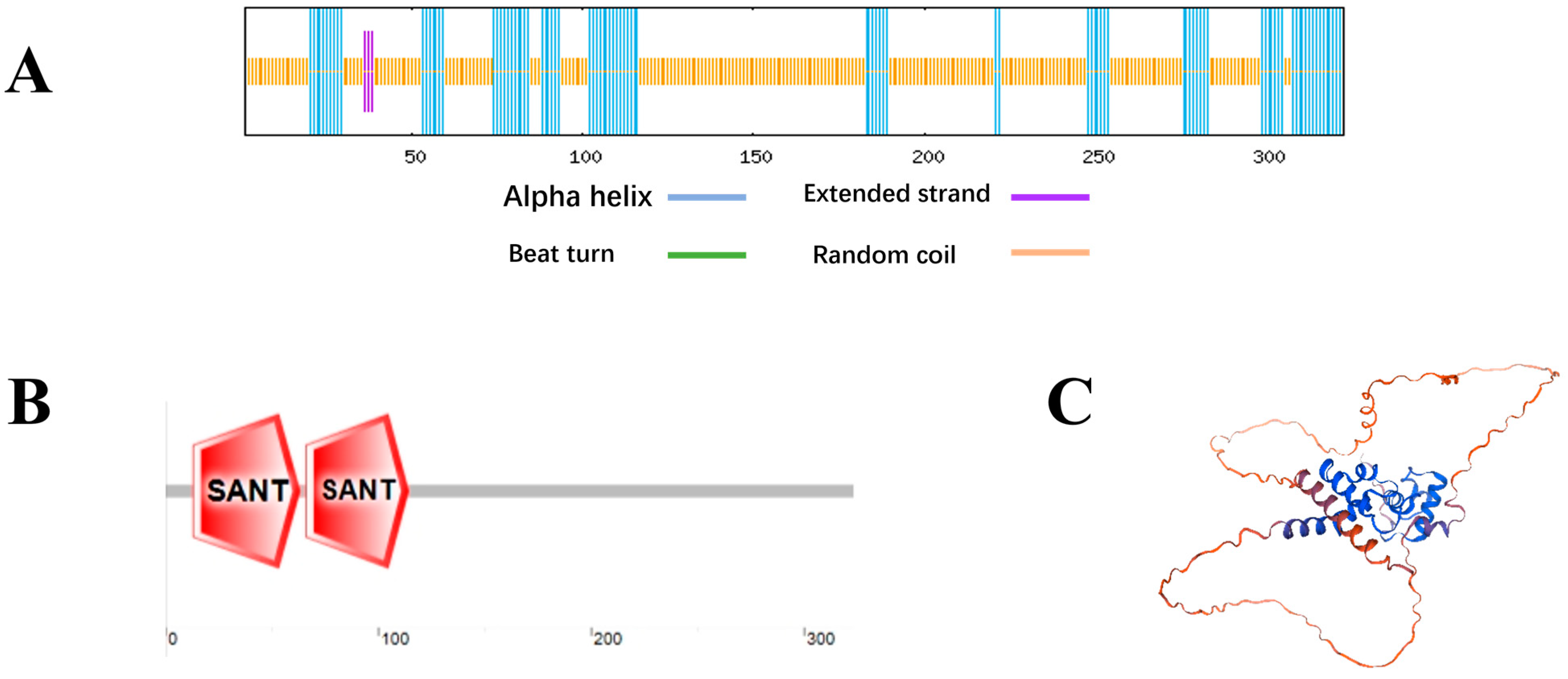
2.1. VhMYB60 Gene Cloning and Bioinformatic Analysis
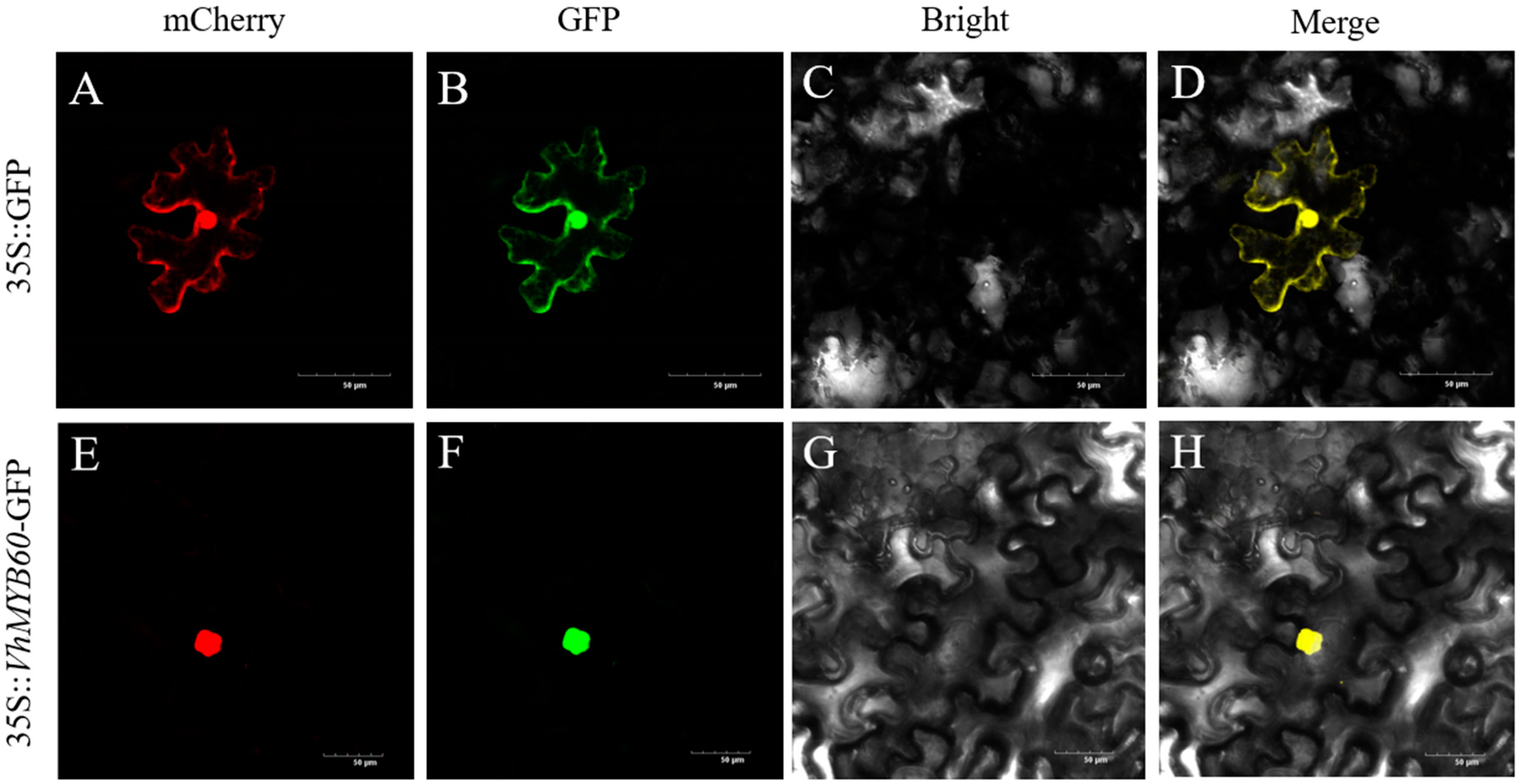
2.2. Subcellular Localisation of the VhMYB60 Gene in Tobacco Benjamina
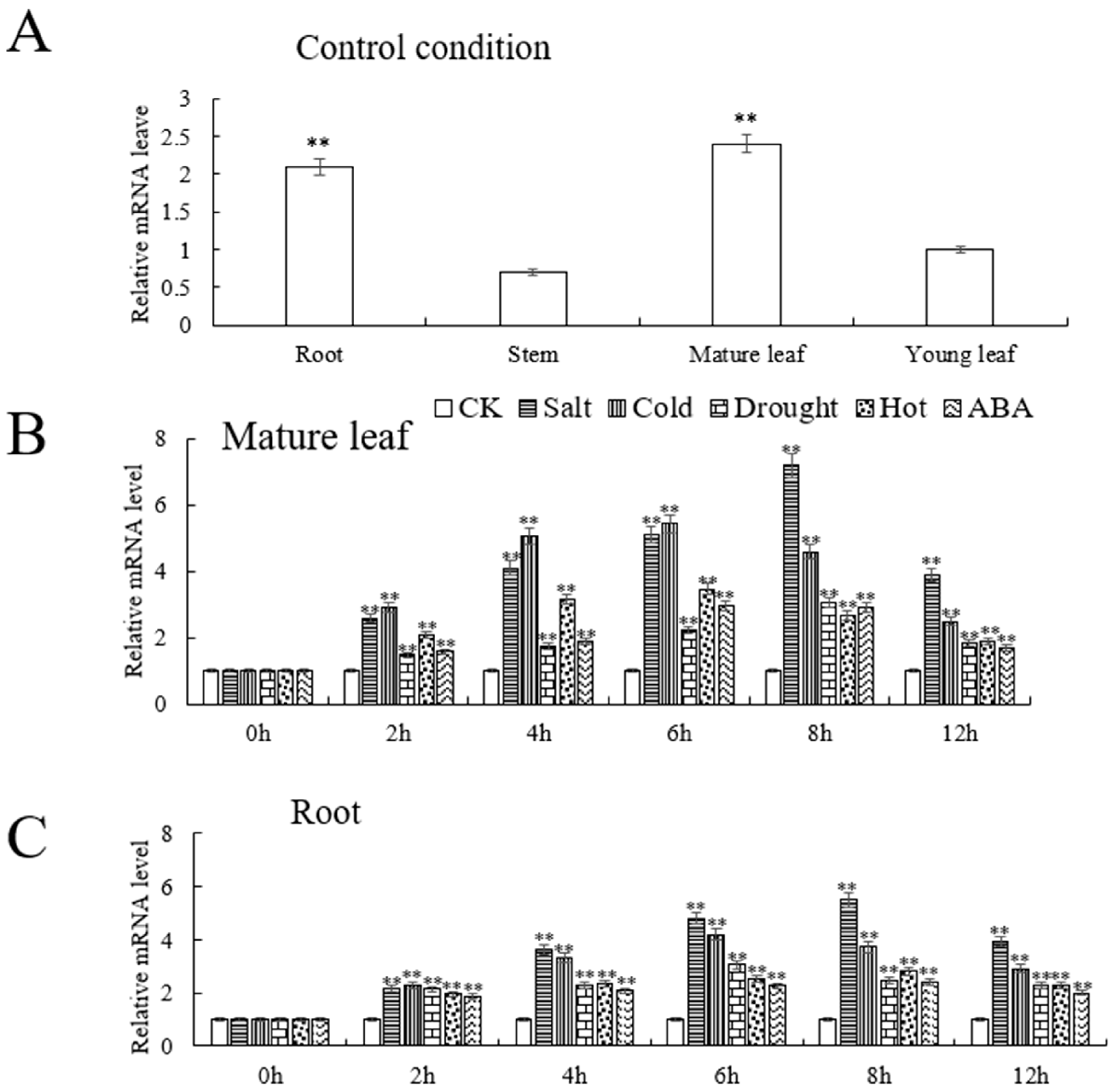
2.3. Analysis of the Expression Level of VhMYB60 in ‘Beta’
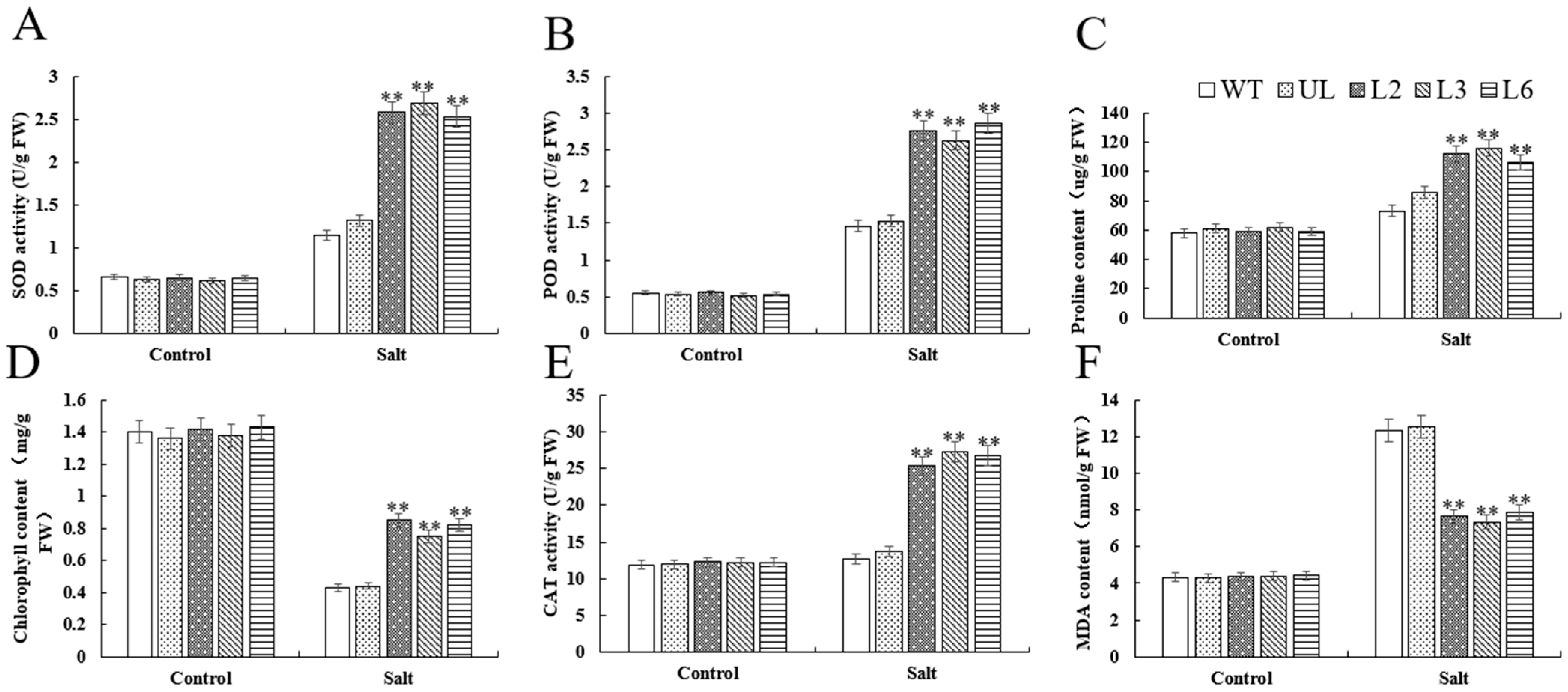
2.4. Overexpression of VhMYB60 Significantly Enhances Salt Tolerance in Arabidopsis
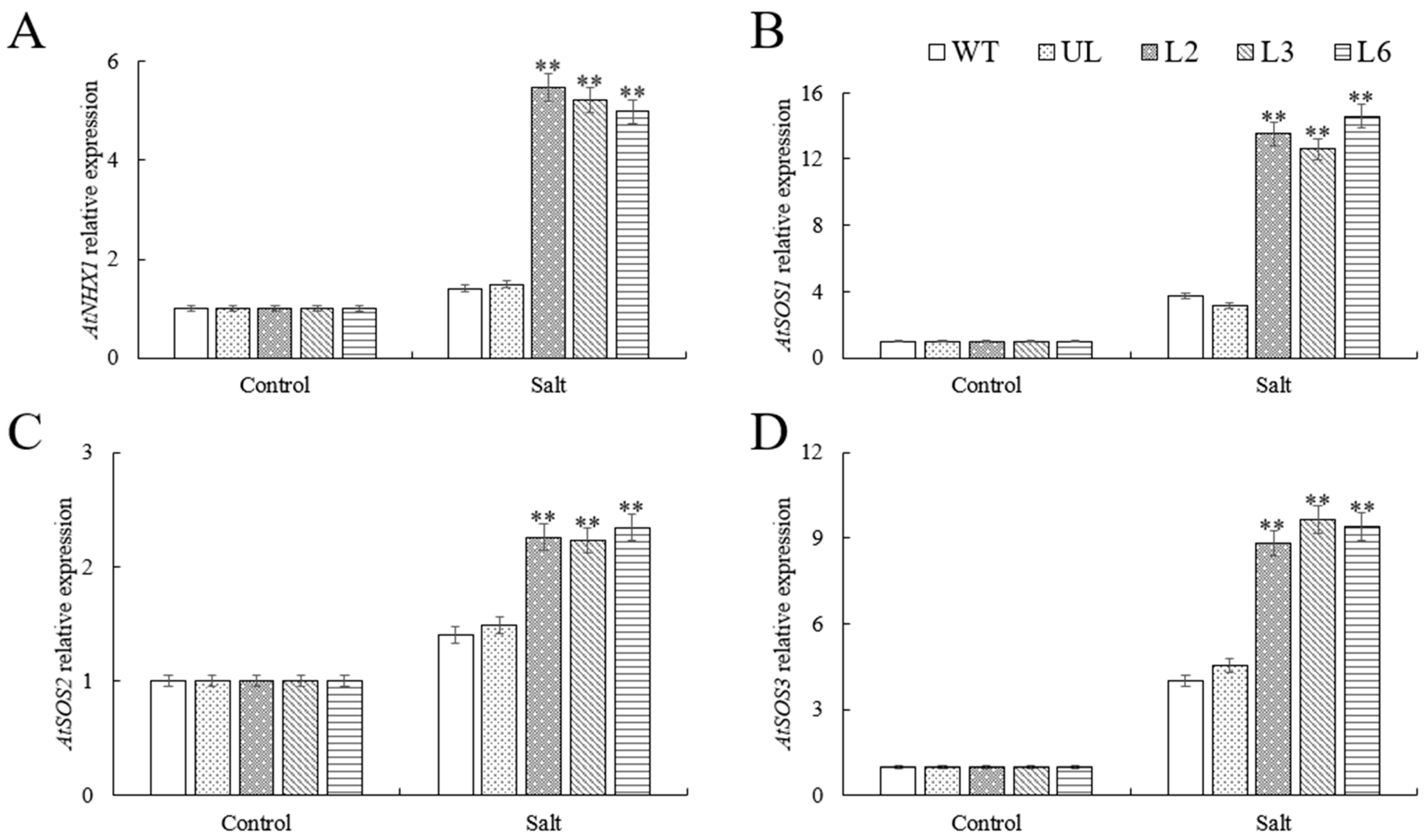
2.5. VhMYB60 Activates Salt Stress-Related Genes
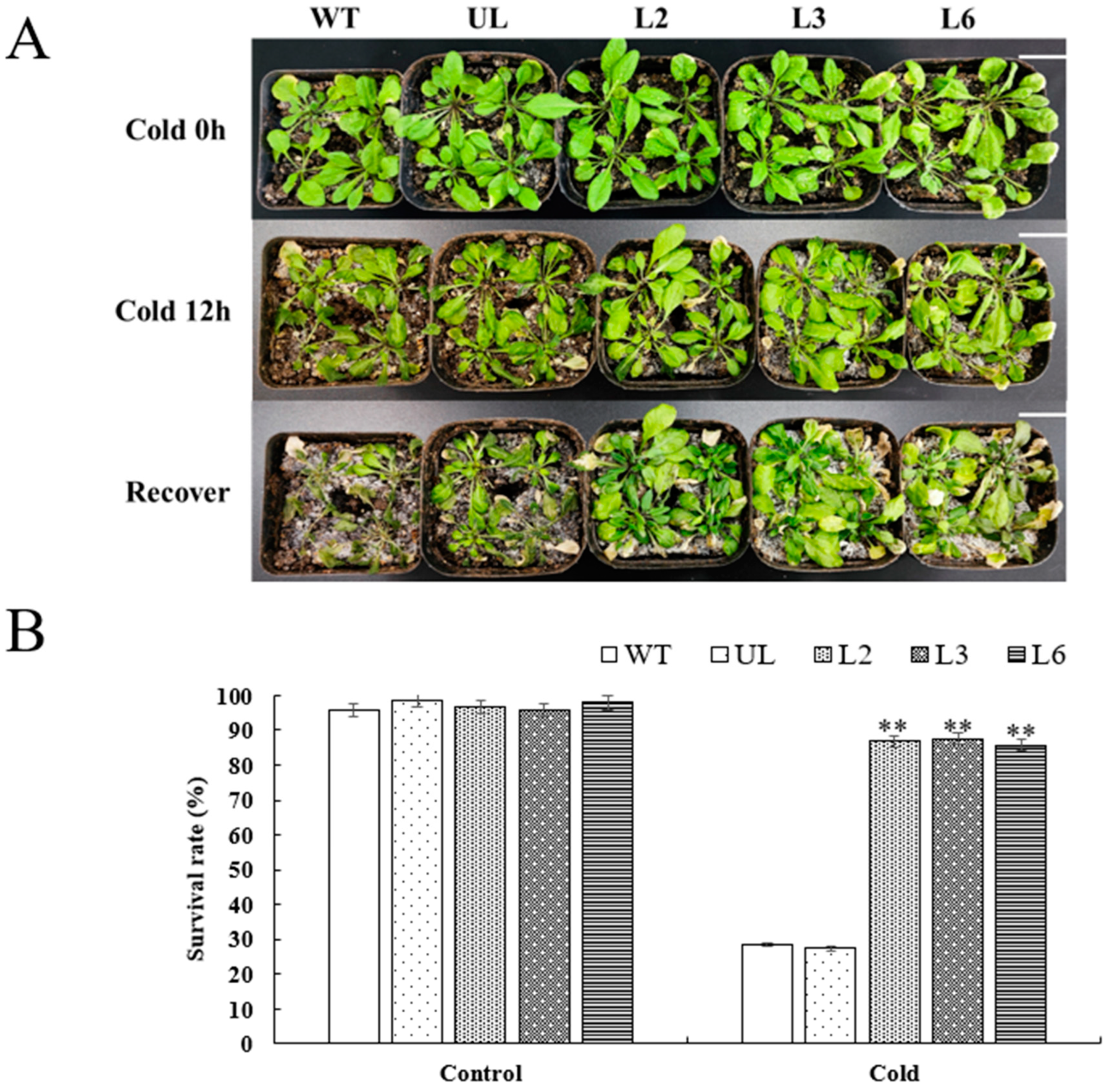
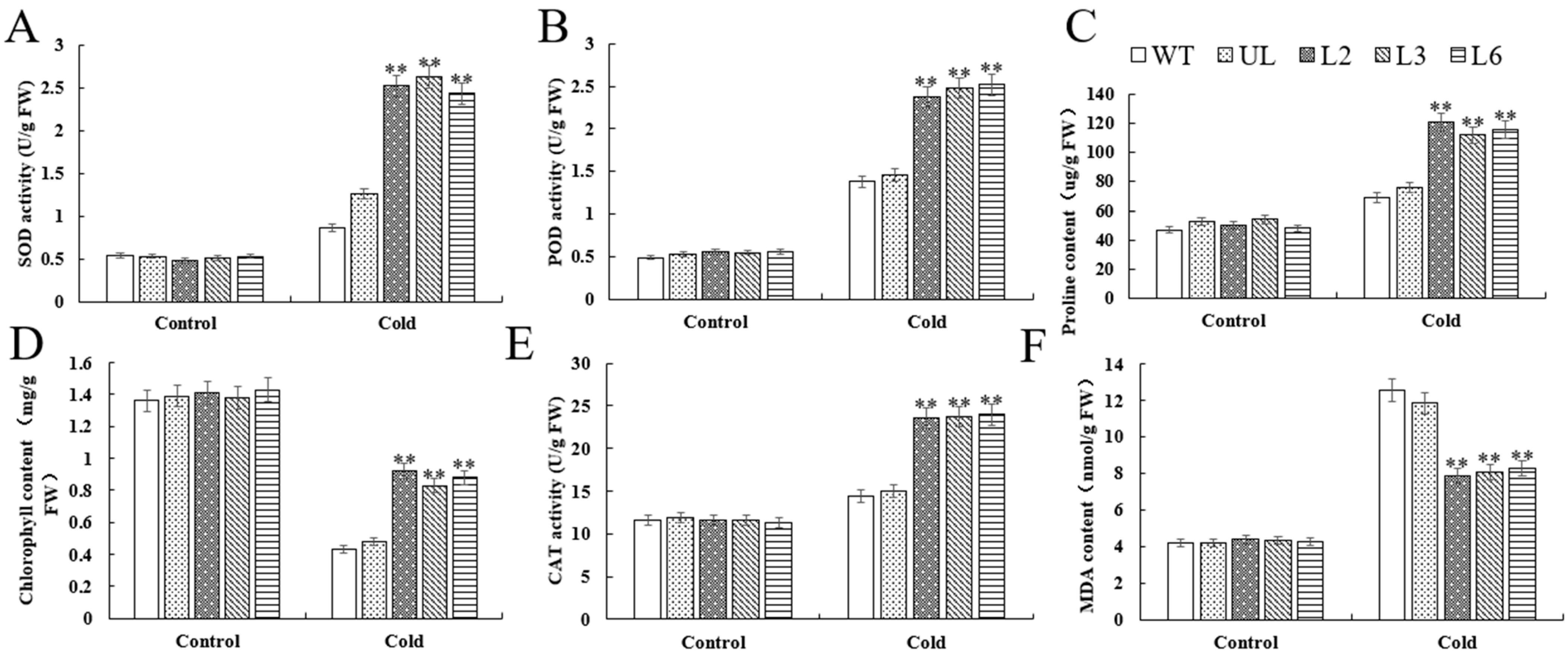
2.6. Overexpression of VhMYB60 Significantly Enhances Cold Resistance in Arabidopsis
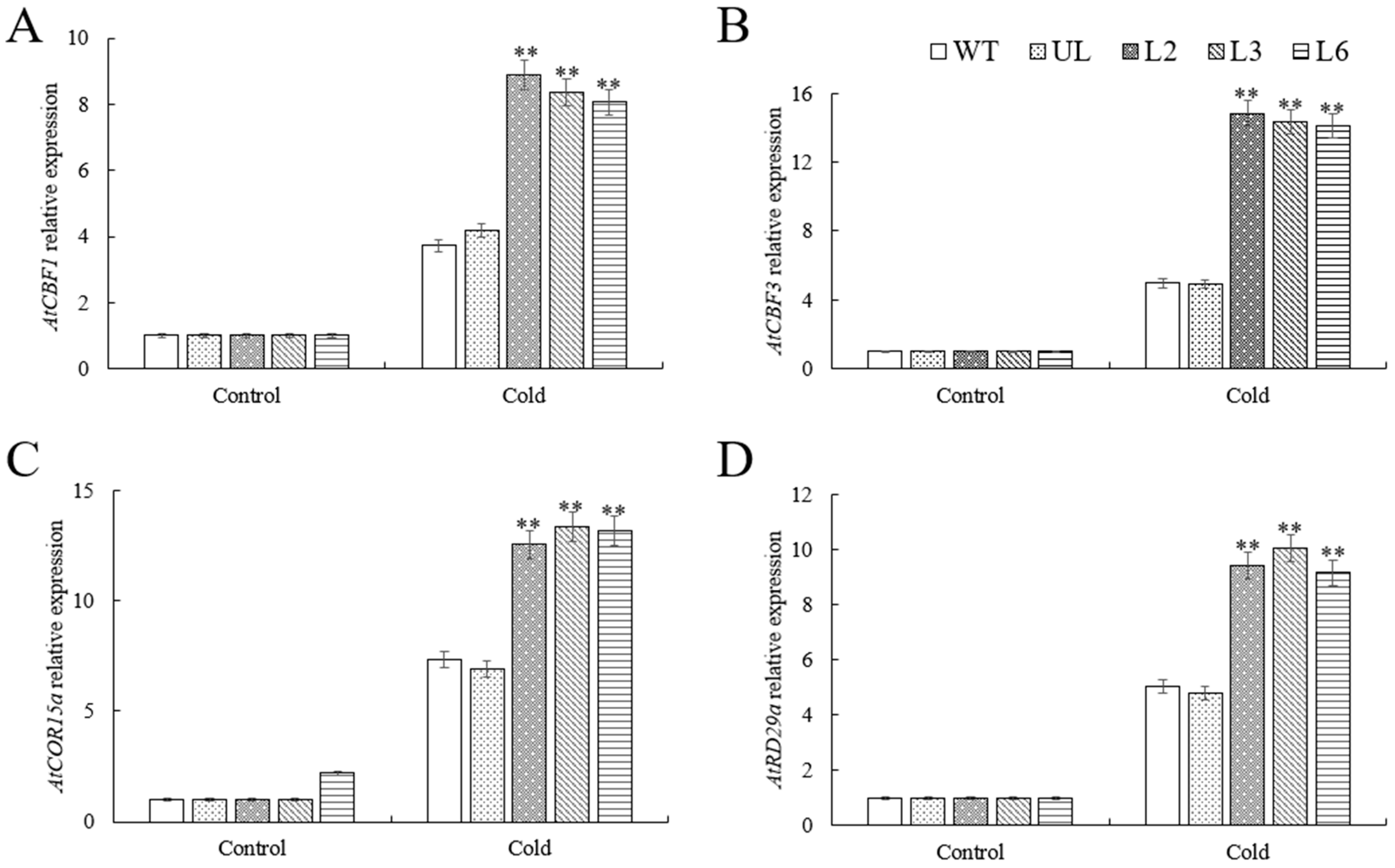
2.7. VhMYB60 Activates Cold Stress-Related Genes
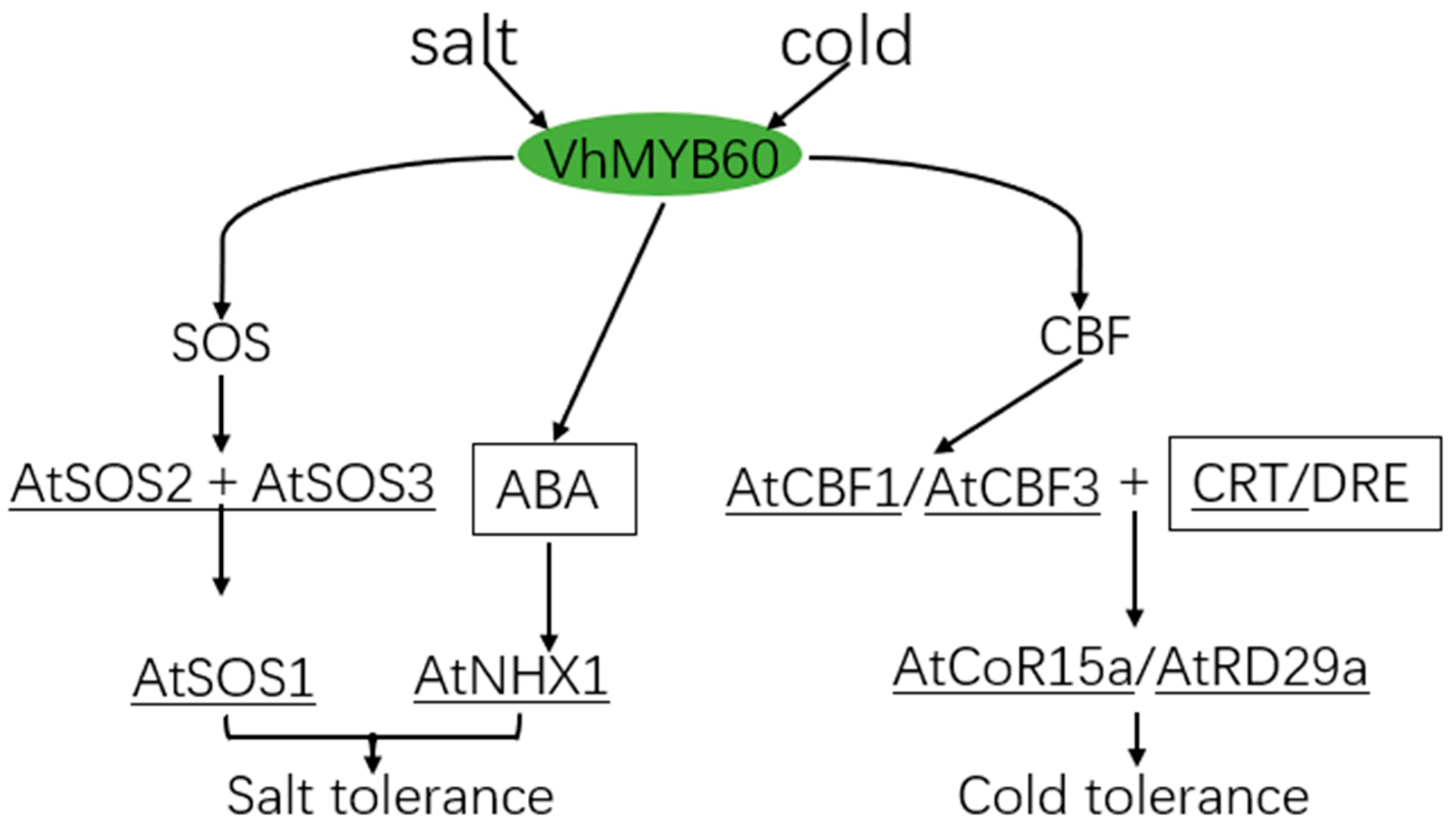
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Isolation and Cloning of VhMYB60
4.3. Subcellular Localisation of VhMYB60
4.4. Bioinformatics Analysis of VhMYB60
4.5. Expression Analysis of VhMYB60 Gene
4.6. Vector Preparation and Transformation of Arabidopsis Plants
4.7. Analysis of Physiological Indicators After Stress Overexpression of VhMYB60 in Arabidopsis thaliana
4.8. Analysis of Stress-Related Genes in Arabidopsis thaliana-Overexpressing VhMYB60
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Hou, Y.; Ding, H.; Zhou, Z.; Li, H.; Yang, G. Isolation and preliminary functional analysis of MbWRKY4 gene involved in salt tolerance in transgenic tobacco. Int. J. Agric. Biol. 2018, 20, 2045–2052. [Google Scholar]
- Hao, S.; Wang, Y.; Yan, Y. A review on plant responses to salt stress and their mechanisms of salt resistance. Sci. Hortic. 2021, 7, 132. [Google Scholar] [CrossRef]
- Han, D.; Du, M.; Zhou, Z.; Wang, S.; Li, T.; Han, J.; Xu, T.; Yang, G. An NAC transcription factor gene from Malus baccata, MbNAC29, increases cold and high salinity tolerance in Arabidopsis. Vitr. Cell. Dev. Biol.-Plant. 2020, 56, 588–599. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Tan, L.; Ijaz, U.; Salih, H.; Cheng, Z.; Ni Win Htet, N.; Ge, Y.; Azeem, F. Genome-Wide Identification and Comparative Analysis of MYB Transcription Factor Family in Musa acuminata and Musa balbisiana. Plants 2020, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Brendolise, C.; Espley, R.V.; Lin-Wang, K.; Laing, W.; Peng, Y.; McGhie, T.; Allan, A.C. Multiple Copies of a Simple MYB-Binding Site Confers Trans-regulation by Specific Flavonoid-Related R2R3 MYBs in Diverse Species. Front. Plant Sci. 2017, 8, 1864. [Google Scholar] [CrossRef] [PubMed]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB Transcription Factor Family in Plants. Trends Plant Sci. 2001, 6, 1–8. [Google Scholar]
- Gang, H.; Zhang, Q.; Chen, J.; Qin, D.; Huo, J. Identification of R2R3-MYB gene family reveal candidate genes for anthocyanin biosynthesis in Lonicera caerulea fruit based on RNA-seq data. J. Berry Res. 2021, 11, 669–687. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Guo, T.; Guo, B.; Chen, Z.; An, X. Comprehensive analysis of the R2R3-MYB transcription factor gene family in Populus trichocarpa. Ind. Crops Prod. 2021, 168, 113614. [Google Scholar] [CrossRef]
- Rahim, M.A.; Resentini, F.; Dalla Vecchia, F.; Trainotti, L. Effects on plant growth and reproduction of a peach R2R3-MYB transcription factor overexpressed in tobacco. Front. Plant Sci. 2019, 10, 1143. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Teng, Y.; Cen, Q.; Fang, Y.; Tian, Q.; Zhang, X.; Xue, D. Genome-wide identification of R2R3-MYB transcription factor and expression analysis under abiotic stress in rice. Plants 2022, 11, 1928. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, X.; Yang, Q.; Wu, X.; Cao, J. Function analysis of a cotton R2R3 MYB transcription factor GhMYB3 in regulating plant trichome development. Plant Biol. 2021, 23, 1118–1127. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.Y.; Huang, Y.; Xu, Z.S.; Wang, F.; Xiong, A.S. An R2R3-MYB transcription factor, SlMYB28, involved in the regulation of TYLCV infection in tomato. Sci. Hortic. 2018, 237, 192–200. [Google Scholar] [CrossRef]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Feller, A.; Machemer, K.; Braun, E.L.; Grotewold, E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011, 66, 94–116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, G.; Sun, C.; Sui, N. Research advances of MYB transcription factors in plant stress resistance and breeding. Plant Signal. Behav. 2019, 14, 1613131. [Google Scholar] [CrossRef]
- Zhou, Z.; Wei, X.; Lan, H. CgMYB1, an R2R3-MYB transcription factor, can alleviate abiotic stress in an annual halophyte Chenopodium glaucum. Plant Physiol. Biochem. 2023, 196, 484–496. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, T.; Wang, J.; Ji, G.; Cui, C.; Song, J.; Sun, N.; Qi, S.; Xu, N.; Zhang, H. Genome-wide identification of R2R3-MYB transcription factors in Betula platyphylla and functional analysis of BpMYB95 in salt tolerance. Int. J. Biol. Macromol. 2024, 279, 135193. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, S.; Zhang, H.; Dong, S.; Chen, J.; Sun, Y.; Liu, Q. Genome-wide identification, expression analysis of the R2R3-MYB gene family and their potential roles under cold stress in Prunus sibirica. BMC Genom. 2024, 25, 953. [Google Scholar]
- Zhou, X.; Lei, D.; Yao, W.; Li, S.; Wang, H.; Lu, J.; Zhang, Y. A novel R2R3-MYB transcription factor PbMYB1L of Pyrus bretschneideri regulates cold tolerance and anthocyanin accumulation. Plant Cell Rep. 2024, 43, 34. [Google Scholar] [CrossRef]
- Oh, J.E.; Kwon, Y.; Kim, J.H.; Noh, H.; Hong, S.W.; Lee, H. A dual role for MYB60 in stomatal regulation and root growth of Arabidopsis thaliana under drought stress. Plant Mol. Biol. 2011, 77, 91–103. [Google Scholar] [CrossRef]
- Zhou, S.; He, L.; Iqbal, Z.; Su, Y.; Huang, J.; He, L.; Xiao, L. The ZOS7-MYB60 module confers drought-stress tolerance in rice. Crop J. 2024, 12, 1369–1378. [Google Scholar] [CrossRef]
- Wang, X.; Niu, Y.; Zheng, Y. Multiple functions of MYB transcription factors in abiotic stress responses. Int. J. Mol. Sci. 2021, 22, 6125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peng, Z.; Khan, S.; Qayyum, A.; Rehman, A.; Du, X. Unveiling the power of MYB transcription factors: Master regulators of multi-stress responses and development in cotton. Int. J. Biol. Macromol. 2024, 276, 133885. [Google Scholar] [CrossRef] [PubMed]
- Batista, V.G.L.; Fernandes, P.D.; dos Santos, R.C.; Melo Filho, P.A.; de Lima, L.M. Expression profile of MYB60 and GUSP1 genes during early growth of cotton genotypes submitted to water stress. Genet. Mol. Res. 2019, 18, GMR18319. [Google Scholar] [CrossRef]
- Li, M.; Yan, X.; Guo, Z.; Jia, N.; Yuan, J.; Han, B.; Zhao, S. Rootstock influence on vegetative growth, yield, and fruit quality of ‘Petit Verdot’. Eur. J. Hortic. Sci. 2019, 84, 343–349. [Google Scholar] [CrossRef]
- Chaudhry, U.K.; Junaid, M.D.; Gökçe, A.F. Influence of environmental adversities on physiological changes in plants. In Developing Climate-Resilient Crops; CRC Press: Boca Raton, FL, USA, 2021; pp. 85–110. [Google Scholar]
- Saxena, R.; Kumar, M.; Tomar, R.S. Plant responses and resilience towards drought and salinity stress. Plant Arch. 2019, 19 (Suppl. 2), 50–58. [Google Scholar]
- Ferguson, J.N. Climate change and abiotic stress mechanisms in plants. Emerg. Top. Life Sci. 2019, 3, 165–181. [Google Scholar] [PubMed]
- Bhanuprakash, K.; Yogeesha, H.S. Seed priming for abiotic stress tolerance: An overview. In Abiotic Stress Physiology of Horticultural Crops; Springer: New Delhi, India, 2016; pp. 103–117. [Google Scholar]
- Arora, A.; Sairam, R.K.; Srivastava, G.C. Oxidative stress and antioxidative system in plants. Curr. Sci. 2002, 82, 1227–1238. [Google Scholar]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Tran, L.S.P. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Jia, Y.; Sha, H.; Zhao, H. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef] [PubMed]
- Meitha, K.; Pramesti, Y.; Suhandono, S. Reactive oxygen species and antioxidants in postharvest vegetables and fruits. Int. J. Food Sci. 2020, 8817778. [Google Scholar] [CrossRef] [PubMed]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive oxygen species (ROS) generation and detoxifying in plants. J. Plant Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Tavanti, T.R.; de Melo, A.A.R.; Moreira, L.D.K.; Sanchez, D.E.J.; dos Santos Silva, R.; da Silva, R.M.; Dos Reis, A.R. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Sengupta, A.; Chakraborty, M.; Gupta, B. Hydrogen peroxide and polyamines act as double edged swords in plant abiotic stress responses. Front. Plant Sci. 2016, 7, 1343. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.M. Hydrogen peroxide and plant stress: A challenging relationship. Plant Stress 2007, 1, 4–15. [Google Scholar]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol. Plant 2009, 31, 261–269. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Singh Sidhu, G.P.; Bali, A.S.; Zheng, B. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms regulating the dynamics of photosynthesis under abiotic stresses. Front. Plant Sci. 2021, 11, 615942. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Stals, E.; Panis, B.; Keulemans, J.; Swennen, R.L. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Yan, B.; Huang, S.; Liu, X.; Peng, S.; Miranda, M.L.L.; Olszyk, D.M. Response of oxidative stress defense systems in rice (Oryza sativa) leaves with supplemental UV-B radiation. Physiol. Plant. 1997, 101, 301–308. [Google Scholar] [CrossRef]
- Ma, R.; Liu, B.; Geng, X.; Ding, X.; Yan, N.; Sun, X.; Zheng, C. Biological function and stress response mechanism of MYB transcription factor family genes. J. Plant Growth Regul. 2023, 42, 83–95. [Google Scholar] [CrossRef]
- Roy, S. Function of MYB domain transcription factors in abiotic stress and epigenetic control of stress response in plant genome. Plant Signal. Behav. 2016, 11, e1117723. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xue, Z.; Zhao, X.; Wu, C.; Sun, Y.; Kou, X. Transcription factors, potential regulatory targets in fruit defense responses to pathogens. Postharvest Biol. Technol. 2023, 206, 112589. [Google Scholar] [CrossRef]
- Han, D.; Wang, L.; Wang, Y.; Yang, G.; Gao, C.; Yu, Z.; Li, T.; Zhang, X.; Ma, L.; Xu, X.; et al. Overexpression of Malus xiaojinensis CS1 gene in tobacco affects plant development and increases iron stress tolerance. Sci. Hortic. 2013, 150, 65–72. [Google Scholar] [CrossRef]
- Han, D.G.; Wang, Y.; Zhang, L.; Ma, L.; Zhang, X.Z.; Xu, X.F.; Han, Z.H. Isolation and functional characterization of MxCS1: A gene encoding a citrate synthase in Malus xiaojinensis. Biol. Plant. 2012, 56, 50–56. [Google Scholar] [CrossRef]
- Han, D.; Shi, Y.; Yu, Z.; Liu, W.; Lv, B.; Wang, B.; Yang, G. Isolation and functional analysis of MdCS1: A gene encoding a citrate synthase in Malus domestica (L.) Borkh. Plant Growth Regul. 2015, 75, 209–218. [Google Scholar] [CrossRef]
- Han, D.G.; Yang, G.H.; Xu, K.D.; Shao, Q.; Yu, Z.Y.; Wang, B.; Ge, Q.L.; Yu, Y. Overexpression of a Malus xiaojinensis Nas1 gene influences flower development and tolerance to iron stress in transgenic tobacco. Plant Mol. Biol. Rep. 2013, 31, 802–809. [Google Scholar] [CrossRef]
- Han, D.; Wang, Y.; Zhang, Z.; Pu, Q.; Ding, H.; Han, J.; Fan, T.; Bai, X.; Yang, G. Isolation and functional analysis of MxCS3: A gene encoding a citrate synthase in Malus xiaojinensis, with functions in tolerance to iron stress and abnormal flower in transgenic Arabidopsis thaliana. Plant Growth Regul. 2017, 82, 479–489. [Google Scholar] [CrossRef]
- Han, D.G.; Shi, Y.; Wang, B.; Liu, W.; Yu, Z.Y.; Lv, B.Y.; Yang, G.H. Isolation and preliminary functional analysis of MxCS2: A gene encoding a citrate synthase in Malus xiaojinensis. Plant Mol. Biol. Rep. 2015, 33, 133–142. [Google Scholar] [CrossRef]
- Ren, C.; Luo, G.; Li, X.; Yao, A.; Liu, W.; Zhang, L.; Han, D. MxFRO4 confers iron and salt tolerance through up-regulating antioxidant capacity associated with the ROS scavenging. J. Plant Physiol. 2023, 285, 154001. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Zhang, Z.; Ni, B.; Ding, H.; Liu, W.; Li, W.; Chai, L.; Yang, G. Isolation and functional analysis of MxNAS3 involved in enhanced iron stress tolerance and abnormal flower in transgenic Arabidopsis. J. Plant Interact. 2018, 13, 433–441. [Google Scholar] [CrossRef]
- Han, D.; Xu, T.; Han, J.; Liu, W.; Wang, Y.; Li, X.; Sun, X.; Wang, X.; Li, T.; Yang, G. Overexpression of MxWRKY53 increased iron and high salinity stress tolerance in Arabidopsis thaliana. Vitr. Cell. Dev. Biol.-Plant. 2022, 58, 266–278. [Google Scholar] [CrossRef]
- Ma, L.; Hou, C.W.; Zhang, X.Z.; Li, H.L.; Han, D.G.; Wang, Y.; Han, Z.H. Seasonal growth and spatial distribution of apple tree roots on different rootstocks or interstems. J. Am. Soc. Hort. Sci. 2013, 138, 79–87. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Wang, J.; Li, W.; Chen, X.; Zhao, C.; Guo, Y.; Li, Y.; Chen, Z.; Li, X.; Han, D. Arabidopsis thaliana Plants’ Overexpression of the MYB Transcription Factor VhMYB60 in the Face of Stress Hazards Enhances Salt and Cold Tolerance. Int. J. Mol. Sci. 2025, 26, 1695. https://doi.org/10.3390/ijms26041695
Chen Z, Wang J, Li W, Chen X, Zhao C, Guo Y, Li Y, Chen Z, Li X, Han D. Arabidopsis thaliana Plants’ Overexpression of the MYB Transcription Factor VhMYB60 in the Face of Stress Hazards Enhances Salt and Cold Tolerance. International Journal of Molecular Sciences. 2025; 26(4):1695. https://doi.org/10.3390/ijms26041695
Chicago/Turabian StyleChen, Zhe, Jinghan Wang, Wenhui Li, Xiang Chen, Changjia Zhao, Yanbo Guo, Yingnan Li, Zhuo Chen, Xingguo Li, and Deguo Han. 2025. "Arabidopsis thaliana Plants’ Overexpression of the MYB Transcription Factor VhMYB60 in the Face of Stress Hazards Enhances Salt and Cold Tolerance" International Journal of Molecular Sciences 26, no. 4: 1695. https://doi.org/10.3390/ijms26041695
APA StyleChen, Z., Wang, J., Li, W., Chen, X., Zhao, C., Guo, Y., Li, Y., Chen, Z., Li, X., & Han, D. (2025). Arabidopsis thaliana Plants’ Overexpression of the MYB Transcription Factor VhMYB60 in the Face of Stress Hazards Enhances Salt and Cold Tolerance. International Journal of Molecular Sciences, 26(4), 1695. https://doi.org/10.3390/ijms26041695






