High-Density Lipoprotein in Patients with Diabetic Kidney Disease: Friend or Foe?
Abstract
1. Introduction
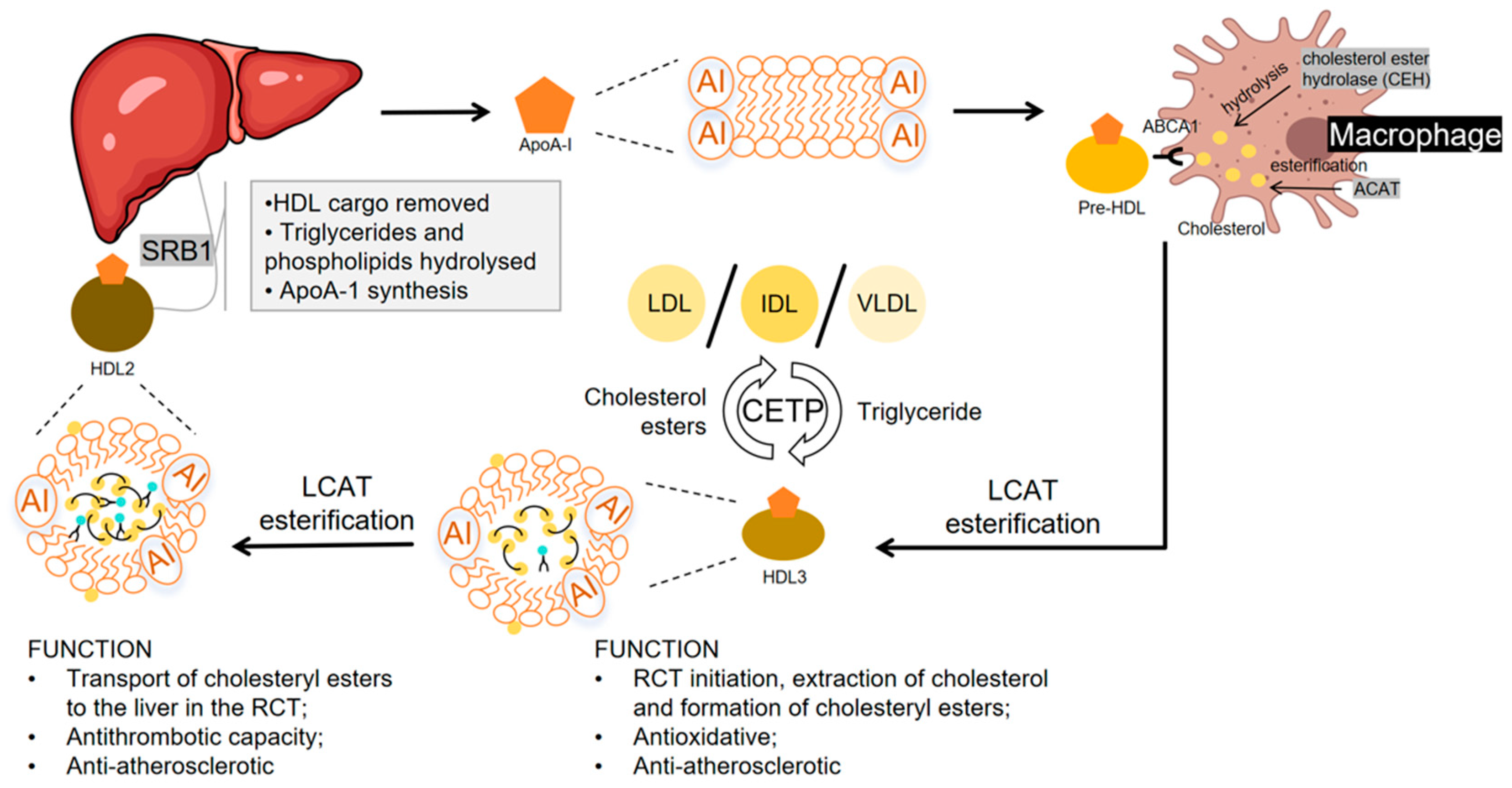
2. Structure and Function of High-Density Lipoproteins
2.1. HDL Production and Reverse Cholesterol Transport
2.2. Antioxidant Capability
2.3. Anti-Inflammatory Activity
2.4. Endothelial Protection
2.5. Anti-Thrombotic Effects
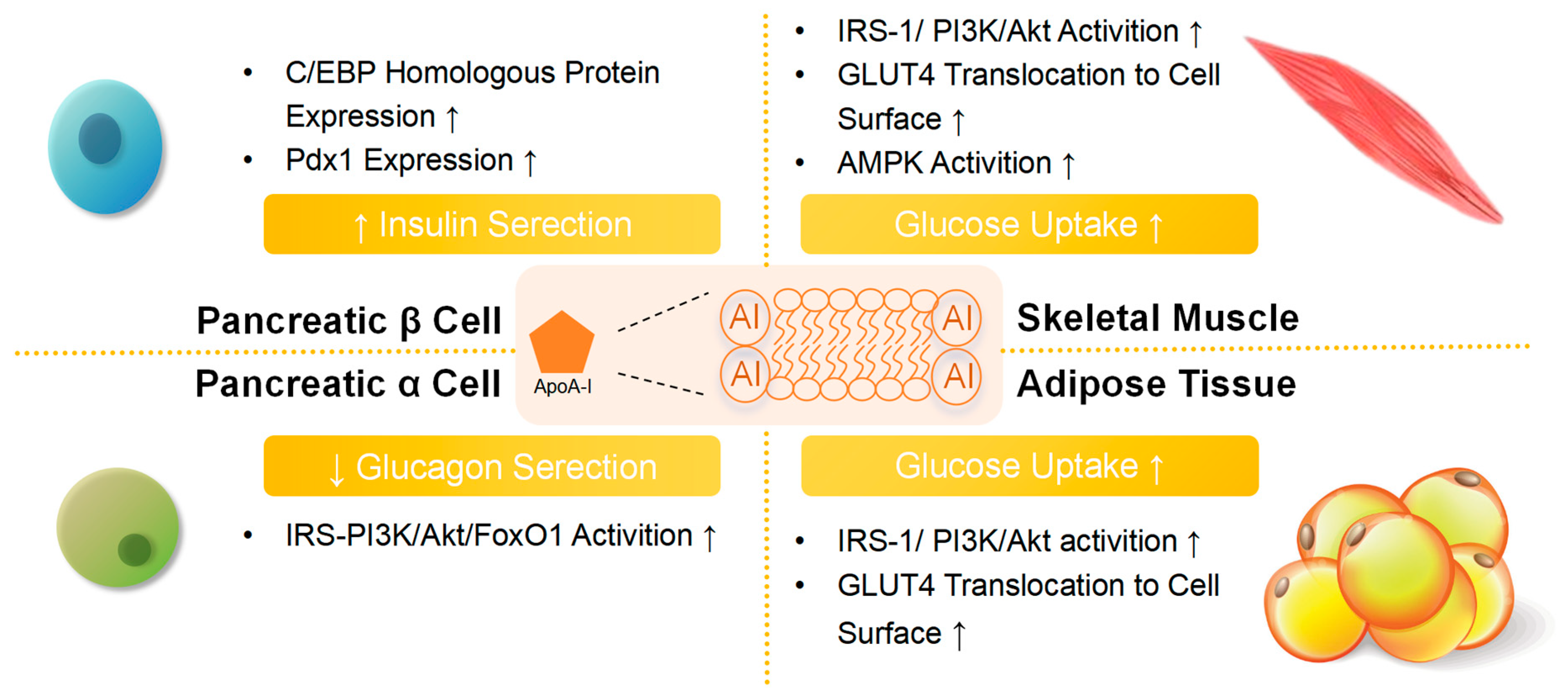
2.6. Antidiabetic Activities
2.7. Modulating Monocytes and Macrophages and Detoxification
2.8. Kidney Protection
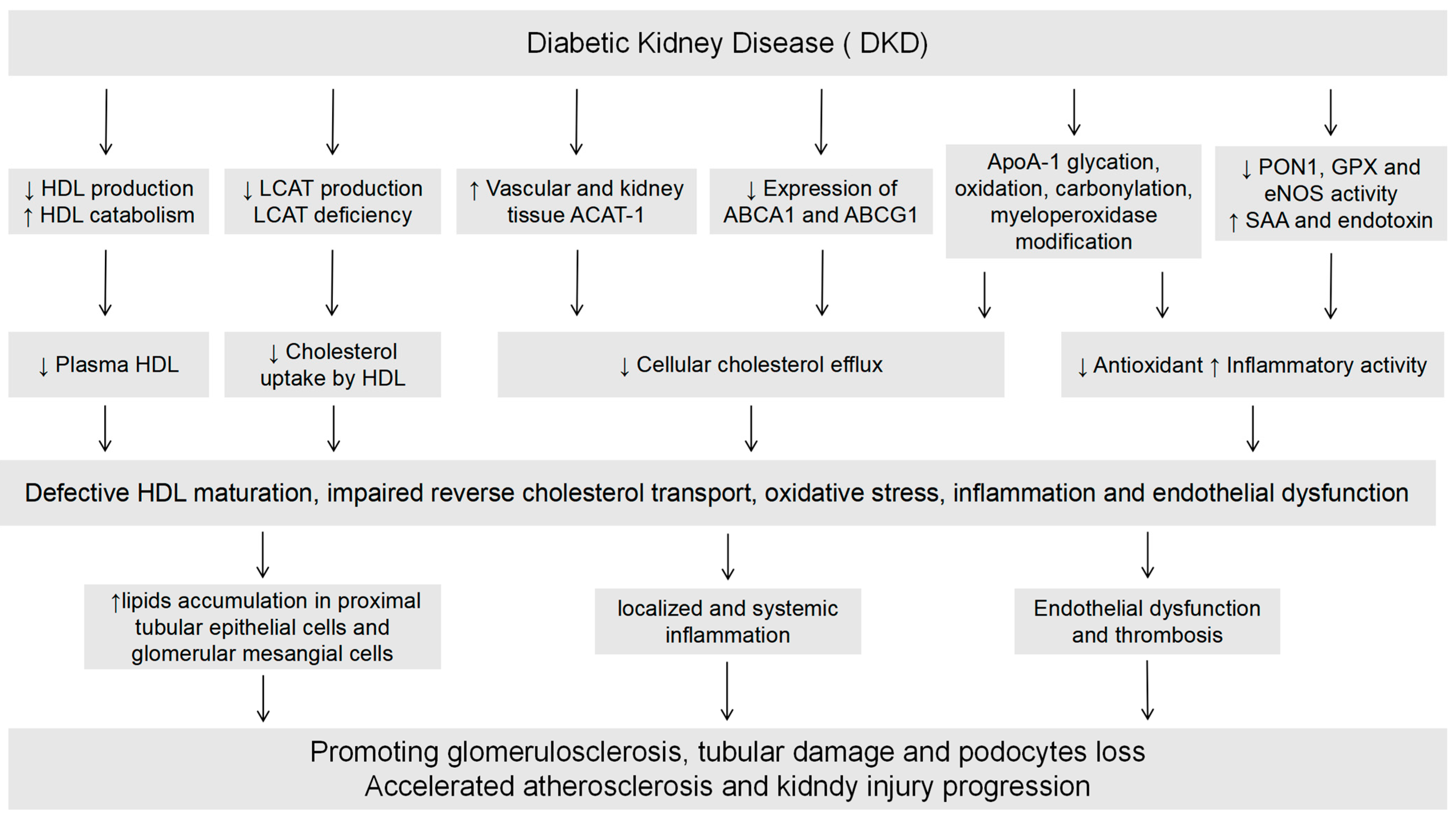
3. The Impact of Diabetic Kidney Disease on HDL
3.1. DKD Affects HDL Levels
3.2. DKD Affects HDL Function
3.3. DKD Affects HDL Components
4. U-Shaped Relationship Between DKD and HDL-C Level
5. The Potential of HDL as a Clinical Diagnostic and Therapeutic Target for Diabetic Kidney Disease
5.1. HDL as a Risk Biomarker for Development of DKD
5.2. Therapeutic Strategies to Enhance Levels and Function of HDL in DKD
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; Sheffer, K.E.; Carrillo-Larco, R.M.; Bennett, J.E.; Shaw, J.E.; Paciorek, C.J.; Singleton, R.K.; Pires, A.B.; et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Tuomilehto, J. International Diabetes Federation Position Statement on the 1-hour post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes. Diabetes Res. Clin. Pract. 2024, 209, 111589. [Google Scholar] [CrossRef]
- Rossing, P. Prediction, progression and prevention of diabetic nephropathy. The Minkowski Lecture 2005. Diabetologia 2006, 49, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, C.E.; Cooper, M.E. Diabetic renal disease: From recent studies to improved clinical practice. Diabet. Med. 2004, 21, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Bruseghin, M.; Berto, I.; Gallina, P.; Manzato, E.; Mussap, M. Renal protection in diabetes: Role of glycemic control. J. Am. Soc. Nephrol. 2006, 17, S86–S89. [Google Scholar] [CrossRef]
- Valmadrid, C.T.; Klein, R.; Moss, S.E.; Klein, B.E. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch. Intern. Med. 2000, 160, 1093–1100. [Google Scholar] [CrossRef]
- Wahba, I.M.; Mak, R.H. Obesity and obesity-initiated metabolic syndrome: Mechanistic links to chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, L.S.; Kaysen, G.A. The effect of lipoproteins on the development and progression of renal disease. Am. J. Nephrol. 2008, 28, 723–731. [Google Scholar] [CrossRef]
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R.; UKPDS Study Group. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Du, Q. HDL and ASCVD. Adv. Exp. Med. Biol. 2022, 1377, 109–118. [Google Scholar] [PubMed]
- King, T.W.; Cochran, B.J.; Rye, K.A. ApoA-I and Diabetes. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Fryirs, M.A.; Barter, P.J.; Appavoo, M.; Tuch, B.E.; Tabet, F.; Heather, A.K.; Rye, K.A. Effects of high-density lipoproteins on pancreatic beta-cell insulin secretion. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1642–1648. [Google Scholar] [CrossRef]
- Cochran, B.J.; Bisoendial, R.J.; Hou, L.; Glaros, E.N.; Rossy, J.; Thomas, S.R.; Barter, P.J.; Rye, K.A. Apolipoprotein A-I increases insulin secretion and production from pancreatic beta-cells via a G-protein-cAMP-PKA-FoxO1-dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2261–2267. [Google Scholar] [CrossRef]
- Morton, J.; Zoungas, S.; Li, Q.; Patel, A.A.; Chalmers, J.; Woodward, M.; Celermajer, D.S.; Beulens, J.W.; Stolk, R.P.; Glasziou, P.; et al. Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: Results of the ADVANCE study. Diabetes Care 2012, 35, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Xian, H.; Balasubramanian, S.; Al-Aly, Z. Low levels of high-density lipoprotein cholesterol increase the risk of incident kidney disease and its progression. Kidney Int. 2016, 89, 886–896. [Google Scholar] [CrossRef]
- Bermudez-Lopez, M.; Arroyo, D.; Betriu, À.; Masana, L.; Fernández, E.; Valdivielso, J.M. New perspectives on CKD-induced dyslipidemia. Expert. Opin. Ther. Targets 2017, 21, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C.; Feingold, K.F.; Anawalt, B.; et al. The Role of Lipids and Lipoproteins in Atherosclerosis. In Endotext; MDText.com, Inc: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Karathanasis, S.K.; Freeman, L.A.; Gordon, S.M.; Remaley, A.T. The Changing Face of HDL and the Best Way to Measure It. Clin. Chem. 2017, 63, 196–210. [Google Scholar] [CrossRef]
- Rohatgi, A.; Westerterp, M.; von Eckardstein, A.; Remaley, A.; Rye, K.A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation 2021, 143, 2293–2309. [Google Scholar] [CrossRef]
- Mineo, C.; Deguchi, H.; Griffin, J.H.; Shaul, P.W. Endothelial and antithrombotic actions of HDL. Circ. Res. 2006, 98, 1352–1364. [Google Scholar] [CrossRef]
- Shih, D.M.; Xia, Y.R.; Wang, X.P.; Miller, E.; Castellani, L.W.; Subbanagounder, G.; Cheroutre, H.; Faull, K.F.; Berliner, J.A.; Witztum, J.L.; et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J. Biol. Chem. 2000, 275, 17527–17535. [Google Scholar] [CrossRef]
- Riwanto, M.; Rohrer, L.; von Eckardstein, A.; Landmesser, U. Dysfunctional HDL: From structure-function-relationships to biomarkers. Handb. Exp. Pharmacol. 2015, 224, 337–366. [Google Scholar] [PubMed]
- Ajala, O.N.; Demler, O.V.; Liu, Y.; Farukhi, Z.; Adelman, S.J.; Collins, H.L.; Ridker, P.M.; Rader, D.J.; Glynn, R.J.; Mora, S. Anti-Inflammatory HDL Function, Incident Cardiovascular Events, and Mortality: A Secondary Analysis of the JUPITER Randomized Clinical Trial. J. Am. Heart Assoc. 2020, 9, e016507. [Google Scholar] [CrossRef]
- Didichenko, S.A.; Navdaev, A.V.; Cukier, A.M.; Gille, A.; Schuetz, P.; Spycher, M.O.; Thérond, P.; Chapman, M.J.; Kontush, A.; Wright, S.D. Enhanced HDL Functionality in Small HDL Species Produced Upon Remodeling of HDL by Reconstituted HDL, CSL112: Effects on Cholesterol Efflux, Anti-Inflammatory and Antioxidative Activity. Circ. Res. 2016, 119, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Garner, B.; Waldeck, A.R.; Witting, P.K.; Rye, K.A.; Stocker, R. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J. Biol. Chem. 1998, 273, 6088–6095. [Google Scholar] [CrossRef]
- Vaziri, N.D. HDL abnormalities in nephrotic syndrome and chronic kidney disease. Nat. Rev. Nephrol. 2016, 12, 37–47. [Google Scholar] [CrossRef]
- Terasaka, N.; Yu, S.; Yvan-Charvet, L.; Wang, N.; Mzhavia, N.; Langlois, R.; Pagler, T.; Li, R.; Welch, C.L.; Goldberg, I.J.; et al. ABCG1 and HDL protect against endothelial dysfunction in mice fed a high-cholesterol diet. J. Clin. Investig. 2008, 118, 3701–3713. [Google Scholar] [CrossRef] [PubMed]
- Linton, M.F.; Tao, H.; Linton, E.F.; Yancey, P.G. SR-BI: A Multifunctional Receptor in Cholesterol Homeostasis and Atherosclerosis. Trends Endocrinol. Metab. 2017, 28, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, E.; Jomard, A.; Osto, E. Crosstalk between high-density lipoproteins and endothelial cells in health and disease: Insights into sex-dependent modulation. Front. Cardiovasc. Med. 2022, 9, 989428. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.J.; Irimpen, A.M.; Siebenlist, U.; Chandrasekar, B. OxLDL induces endothelial dysfunction and death via TRAF3IP2: Inhibition by HDL3 and AMPK activators. Free Radic. Biol. Med. 2014, 70, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Partoush, A.; Volkova, N.; Aviram, M. Ox-LDL induces monocyte-to-macrophage differentiation in vivo: Possible role for the macrophage colony stimulating factor receptor (M-CSF-R). Atherosclerosis 2008, 196, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Birjmohun, R.S.; van Leuven, S.I.; Levels, J.H.; van ’t Veer, C.; Kuivenhoven, J.A.; Meijers, J.C.; Levi, M.; Kastelein, J.J.; van der Poll, T.; Stroes, E.S. High-density lipoprotein attenuates inflammation and coagulation response on endotoxin challenge in humans. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1153–1158. [Google Scholar] [CrossRef]
- Weichhart, T.; Kopecky, C.; Kubicek, M.; Haidinger, M.; Döller, D.; Katholnig, K.; Suarna, C.; Eller, P.; Tölle, M.; Gerner, C.; et al. Serum amyloid A in uremic HDL promotes inflammation. J. Am. Soc. Nephrol. 2012, 23, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Woollard, K.J.; Hoang, A.; Mukhamedova, N.; Stirzaker, R.A.; McCormick, S.P.; Remaley, A.T.; Sviridov, D.; Chin-Dusting, J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Di Bartolo, B.A.; Nakhla, S.; Heather, A.K.; Mitchell, T.W.; Jessup, W.; Celermajer, D.S.; Barter, P.J.; Rye, K.A. Anti-inflammatory effects of apolipoprotein A-I in the rabbit. Atherosclerosis 2010, 212, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, A.; Giral, H.; Landmesser, U. High-density lipoproteins as modulators of endothelial cell functions: Alterations in patients with coronary artery disease. Cardiovasc. Res. 2014, 103, 350–361. [Google Scholar] [CrossRef]
- Masson, W.; obo, M.; Siniawski, D.; Huerín, M.; Molinero, G.; Valéro, R.; Nogueira, J.P. Therapy with cholesteryl ester transfer protein (CETP) inhibitors and diabetes risk. Diabetes Metab. 2018, 44, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Keul, P.; Polzin, A.; Kaiser, K.; Gräler, M.; Dannenberg, L.; Daum, G.; Heusch, G.; Levkau, B. Potent anti-inflammatory properties of HDL in vascular smooth muscle cells mediated by HDL-S1P and their impairment in coronary artery disease due to lower HDL-S1P: A new aspect of HDL dysfunction and its therapy. FASEB J. 2019, 33, 1482–1495. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zeng, Y.; Zhu, X.; Tan, Y.; Li, Y.; Li, Q.; Yi, G. ApoM-S1P Modulates Ox-LDL-Induced Inflammation Through the PI3K/Akt Signaling Pathway in HUVECs. Inflammation 2019, 42, 606–617. [Google Scholar] [CrossRef]
- Lee, M.K.; Moore, X.L.; Fu, Y.; Al-Sharea, A.; Dragoljevic, D.; Fernandez-Rojo, M.A.; Parton, R.; Sviridov, D.; Murphy, A.J.; Chin-Dusting, J.P. High-density lipoprotein inhibits human M1 macrophage polarization through redistribution of caveolin-1. Br. J. Pharmacol. 2016, 173, 741–751. [Google Scholar] [CrossRef]
- De Nardo, D.; Labzin, L.I.; Kono, H.; Seki, R.; Schmidt, S.V.; Beyer, M.; Xu, D.; Zimmer, S.; Lahrmann, C.; Schildberg, F.; et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014, 15, 152–160. [Google Scholar] [CrossRef]
- Robert, J.; Osto, E.; von Eckardstein, A. The Endothelium Is Both a Target and a Barrier of HDL’s Protective Functions. Cells 2021, 10, 1041. [Google Scholar] [CrossRef]
- Rader, D.J. Molecular regulation of HDL metabolism and function: Implications for novel therapies. J. Clin. Investig. 2006, 116, 3090–3100. [Google Scholar] [CrossRef]
- Rye, K.A.; Barter, P.J. Antiinflammatory actions of HDL: A new insight. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1890–1891. [Google Scholar] [CrossRef]
- Recalde, D.; Ostos, M.A.; Badell, E.; Garcia-Otin, A.L.; Pidoux, J.; Castro, G.; Zakin, M.M.; Scott-Algara, D. Human apolipoprotein A-IV reduces secretion of proinflammatory cytokines and atherosclerotic effects of a chronic infection mimicked by lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 756–761. [Google Scholar] [CrossRef]
- Zhang, Q.; Yin, H.; Liu, P.; Zhang, H.; She, M. Essential role of HDL on endothelial progenitor cell proliferation with PI3K/Akt/cyclin D1 as the signal pathway. Exp. Biol. Med. 2010, 235, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Jozefczuk, E.; Guzik, T.J.; Siedlinski, M. Significance of sphingosine-1-phosphate in cardiovascular physiology and pathology. Pharmacol. Res. 2020, 156, 104793. [Google Scholar] [CrossRef]
- Zabczyk, M.; Hońdo, Ł.; Krzek, M.; Undas, A. High-density cholesterol and apolipoprotein AI as modifiers of plasma fibrin clot properties in apparently healthy individuals. Blood Coagul. Fibrinolysis 2013, 24, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Weidtmann, A.; Scheithe, R.; Hrboticky, N.; Pietsch, A.; Lorenz, R.; Siess, W. Mildly oxidized LDL induces platelet aggregation through activation of phospholipase A2. Arterioscler. Thromb. Vasc. Biol. 1995, 15, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Nofer, J.R.; Brodde, M.F.; Kehrel, B.E. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin. Exp. Pharmacol. Physiol. 2010, 37, 726–735. [Google Scholar] [CrossRef]
- Kaba, N.K.; Francis, C.W.; Moss, A.J.; Zareba, W.; Oakes, D.; Knox, K.L.; Fernández, I.D.; Rainwater, D.L.; THROMBO Investigators. Effects of lipids and lipid-lowering therapy on hemostatic factors in patients with myocardial infarction. J. Thromb. Haemost. 2004, 2, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, B.; Cochran, B.J.; Rye, K.A. Role of High-Density Lipoproteins in Cholesterol Homeostasis and Glycemic Control. J. Am. Heart Assoc. 2020, 9, e013531. [Google Scholar] [CrossRef]
- Petremand, J.; Puyal, J.; Chatton, J.Y.; Duprez, J.; Allagnat, F.; Frias, M.; James, R.W.; Waeber, G.; Jonas, J.C.; Widmann, C. HDLs protect pancreatic beta-cells against ER stress by restoring protein folding and trafficking. Diabetes 2012, 61, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.G.; Duffy, S.J.; Formosa, M.F.; Natoli, A.K.; Henstridge, D.C.; Penfold, S.A.; Thomas, W.G.; Mukhamedova, N.; de Courten, B.; Forbes, J.M.; et al. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation 2009, 119, 2103–2111. [Google Scholar] [CrossRef]
- Drew, B.G.; Rye, K.A.; Duffy, S.J.; Barter, P.; Kingwell, B.A. The emerging role of HDL in glucose metabolism. Nat. Rev. Endocrinol. 2012, 8, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Cochran, B.J.; Ryder, W.J.; Parmar, A.; Tang, S.; Reilhac, A.; Arthur, A.; Charil, A.; Hamze, H.; Barter, P.J.; Kritharides, L.; et al. In vivo PET imaging with [(18)F]FDG to explain improved glucose uptake in an apolipoprotein A-I treated mouse model of diabetes. Diabetologia 2016, 59, 1977–1984. [Google Scholar] [CrossRef]
- Tang, S.; Tabet, F.; Cochran, B.J.; Cuesta Torres, L.F.; Wu, B.J.; Barter, P.J.; Rye, K.A. Apolipoprotein A-I enhances insulin-dependent and insulin-independent glucose uptake by skeletal muscle. Sci. Rep. 2019, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- Karavia, E.A.; Hatziri, A.; Kalogeropoulou, C.; Papachristou, N.I.; Xepapadaki, E.; Constantinou, C.; Natsos, A.; Petropoulou, P.I.; Sasson, S.; Papachristou, D.J.; et al. Deficiency in apolipoprotein A-I ablates the pharmacological effects of metformin on plasma glucose homeostasis and hepatic lipid deposition. Eur. J. Pharmacol. 2015, 766, 76–85. [Google Scholar]
- Xepapadaki, E.; Maulucci, G.; Constantinou, C.; Karavia, E.A.; Zvintzou, E.; Daniel, B.; Sasson, S.; Kypreos, K.E. Impact of apolipoprotein A1- or lecithin:cholesterol acyltransferase-deficiency on white adipose tissue metabolic activity and glucose homeostasis in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; McKenna, B.; Li, C.; Reichert, M.; Nguyen, J.; Singh, T.; Yang, C.; Pannikar, A.; Doliba, N.; Zhang, T.; et al. Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab. 2014, 19, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, M.; Kerksiek, A.; Gebert, K.; Annema, W.; Sibler, R.; Radosavljevic, S.; Lütjohann, D.; Rohrer, L.; von Eckardstein, A. HDL inhibits endoplasmic reticulum stress-induced apoptosis of pancreatic beta-cells in vitro by activation of Smoothened. J. Lipid Res. 2020, 61, 492–504. [Google Scholar] [CrossRef]
- Mancuso, E.; Mannino, G.C.; Fuoco, A.; Leo, A.; Citraro, R.; Averta, C.; Spiga, R.; Russo, E.; De Sarro, G.; Andreozzi, F.; et al. HDL (High-Density Lipoprotein) and ApoA-1 (Apolipoprotein A-1) Potentially Modulate Pancreatic alpha-Cell Glucagon Secretion. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Dubuis, G.; Georgieva, M.; Mendes Ferreira, C.S.; Serulla, M.; Del Carmen Conde Rubio, M.; Trofimenko, E.; Mercier, T.; Decosterd, L.; Widmann, C. HDLs extract lipophilic drugs from cells. J. Cell Sci. 2022, 135, jcs258644. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fogo, A.B.; Kon, V. Kidneys: Key modulators of high-density lipoprotein levels and function. Curr. Opin. Nephrol. Hypertens. 2016, 25, 174–179. [Google Scholar] [CrossRef]
- Calabresi, L.; Simonelli, S.; Conca, P.; Busnach, G.; Cabibbe, M.; Gesualdo, L.; Gigante, M.; Penco, S.; Veglia, F.; Franceschini, G. Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J. Intern. Med. 2015, 277, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Ao, L.; Xie, Y. Research advance in the mechanism for oxidative stress-induced podocyte injury in diabetic kidney disease. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2021, 46, 1403–1408. [Google Scholar] [PubMed]
- Izquierdo-Lahuerta, A.; Martínez-García, C.; Medina-Gómez, G. Lipotoxicity as a trigger factor of renal disease. J. Nephrol. 2016, 29, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Sato, K.; Malchinkhuu, E.; Tomura, H.; Tamama, K.; Kuwabara, A.; Murakami, M.; Okajima, F. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Sharif-Kashani, B.; Hamraghani, A.; Salamzadeh, J.; Abbasi Nazari, M.; Malekmohammad, M.; Behzadnia, N.; Fahimi, F. The Effect of Amlodipine and Sildenafil on the NT-ProBNP Level of Patients with COPD-Induced Pulmonary Hypertension. Iran. J. Pharm. Res. 2014, 13, 161–168. [Google Scholar] [PubMed]
- Kronenberg, F. HDL in CKD-The Devil Is in the Detail. J. Am. Soc. Nephrol. 2018, 29, 1356–1371. [Google Scholar] [CrossRef] [PubMed]
- Attman, P.O.; Samuelsson, O.; Johansson, A.C.; Moberly, J.B.; Alaupovic, P. Dialysis modalities and dyslipidemia. Kidney Int. Suppl. 2003, 63, S110–S112. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Navab, M.; Fogelman, A.M. HDL metabolism and activity in chronic kidney disease. Nat. Rev. Nephrol. 2010, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Verges, B. Pathophysiology of diabetic dyslipidaemia: Where are we? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tan, K.C.; Shiu, S.W.; Wong, Y. Cellular cholesterol efflux to serum is impaired in diabetic nephropathy. Diabetes Metab. Res. Rev. 2008, 24, 617–623. [Google Scholar] [CrossRef]
- Tward, A.; Xia, Y.R.; Wang, X.P.; Shi, Y.S.; Park, C.; Castellani, L.W.; Lusis, A.J.; Shih, D.M. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation 2002, 106, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. Lipotoxicity and impaired high density lipoprotein-mediated reverse cholesterol transport in chronic kidney disease. J. Ren. Nutr. 2010, 20, S35–S43. [Google Scholar] [CrossRef] [PubMed]
- Cwiklinska, A.; Cackowska, M.; Wieczorek, E.; Król, E.; Kowalski, R.; Kuchta, A.; Kortas-Stempak, B.; Gliwińska, A.; Dąbkowski, K.; Zielińska, J.; et al. Progression of Chronic Kidney Disease Affects HDL Impact on Lipoprotein Lipase (LPL)-Mediated VLDL Lipolysis Efficiency. Kidney Blood Press. Res. 2018, 43, 970–978. [Google Scholar] [CrossRef]
- Honda, H.; Hirano, T.; Ueda, M.; Kojima, S.; Mashiba, S.; Hayase, Y.; Michihata, T.; Shibata, T. High-Density Lipoprotein Subfractions and Their Oxidized Subfraction Particles in Patients with Chronic Kidney Disease. J. Atheroscler. Thromb. 2016, 23, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zelnick, L.R.; Hoofnagle, A.N.; Vaisar, T.; Henderson, C.M.; Imrey, P.B.; Robinson-Cohen, C.; de Boer, I.H.; Shiu, Y.T.; Himmelfarb, J.; et al. Alteration of HDL Protein Composition with Hemodialysis Initiation. Clin. J. Am. Soc. Nephrol. 2018, 13, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Rye, K.A.; PBarter, J.; Cochran, B.J. Apolipoprotein A-I interactions with insulin secretion and production. Curr. Opin. Lipidol. 2016, 27, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Watanabe, T.; Sakaue, T.; Lewis, G.F. Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: The combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity. Clin. Biochem. 2003, 36, 421–429. [Google Scholar] [CrossRef]
- Sparks, D.L.; Davidson, W.S.; Lund-Katz, S.; Phillips, M.C. Effects of the neutral lipid content of high density lipoprotein on apolipoprotein A-I structure and particle stability. J. Biol. Chem. 1995, 270, 26910–26917. [Google Scholar] [CrossRef] [PubMed]
- Garg, A. Insulin resistance in the pathogenesis of dyslipidemia. Diabetes Care 1996, 19, 387–389. [Google Scholar] [CrossRef]
- Garg, A.; Haffner, S.M. Insulin resistance and atherosclerosis. Diabetes Care 1996, 19, 274. [Google Scholar] [CrossRef] [PubMed]
- Baynes, C.; enderson, A.D.; Anyaoku, V.; Richmond, W.; Hughes, C.L.; Johnston, D.G.; Elkeles, R.S. The role of insulin insensitivity and hepatic lipase in the dyslipidaemia of type 2 diabetes. Diabet. Med. 1991, 8, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Abnormal lipoprotein metabolism in diabetic nephropathy. Clin. Exp. Nephrol. 2014, 18, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Brott, D.; Gould, S.; Jones, H.; Schofield, J.; Prior, H.; Valentin, J.P.; Bjurstrom, S.; Kenne, K.; Schuppe-Koistinen, I.; Katein, A.; et al. Biomarkers of drug-induced vascular injury. Toxicol. Appl. Pharmacol. 2005, 207, 441–445. [Google Scholar] [CrossRef]
- Hirano, T.; Ookubo, K.; Kashiwazaki, K.; Tajima, H.; Yoshino, G.; Adachi, M. Vascular endothelial markers, von Willebrand factor and thrombomodulin index, are specifically elevated in type 2 diabetic patients with nephropathy: Comparison of primary renal disease. Clin. Chim. Acta 2000, 299, 65–75. [Google Scholar] [CrossRef]
- Kashiwazaki, K.; Hirano, T.; Yoshino, G.; Kurokawa, M.; Tajima, H.; Adachi, M. Decreased release of lipoprotein lipase is associated with vascular endothelial damage in NIDDM patients with microalbuminuria. Diabetes Care 1998, 21, 2016–2020. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, L.; Yamada-Fowler, N.; Smith, J.; Thornalley, P.J.; Rabbani, N. Arginine-directed glycation and decreased HDL plasma concentration and functionality. Nutr. Diabetes 2014, 4, e134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Marcel, Y.L. Serum albumin is a significant intermediate in cholesterol transfer between cells and lipoproteins. Biochemistry 1996, 35, 7174–7180. [Google Scholar] [CrossRef] [PubMed]
- Shearer, G.C.; Newman, J.W.; Hammock, B.D.; Kaysen, G.A. Graded effects of proteinuria on HDL structure in nephrotic rats. J. Am. Soc. Nephrol. 2005, 16, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Bulum, T.; Kolaric, B.; Duvnjak, L. Lower levels of total HDL and HDL3 cholesterol are associated with albuminuria in normoalbuminuric Type 1 diabetic patients. J. Endocrinol. Investig. 2013, 36, 574–578. [Google Scholar]
- Zhong, J.; Yang, H.; Kon, V. Kidney as modulator and target of “good/bad” HDL. Pediatr. Nephrol. 2019, 34, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tan, K.C.; Shiu, S.W.; Wong, Y. Increased serum advanced glycation end products are associated with impairment in HDL antioxidative capacity in diabetic nephropathy. Nephrol. Dial. Transplant. 2008, 23, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Nobecourt, E.; Tabet, F.; Lambert, G.; Puranik, R.; Bao, S.; Yan, L.; Davies, M.J.; Brown, B.E.; Jenkins, A.J.; Dusting, G.J.; et al. Nonenzymatic glycation impairs the antiinflammatory properties of apolipoprotein A-I. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 766–772. [Google Scholar] [CrossRef]
- Passarelli, M.; Tang, C.; McDonald, T.O.; O’Brien, K.D.; Gerrity, R.G.; Heinecke, J.W.; Oram, J.F. Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes 2005, 54, 2198–2205. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Marchionni, C.; Caldarelli, L.; Curatola, G. Effect of glycation of high density lipoproteins on their physicochemical properties and on paraoxonase activity. Acta Diabetol. 2001, 38, 163–169. [Google Scholar] [CrossRef]
- Murakami, H.; Tanabe, J.; Tamasawa, N.; Matsumura, K.; Yamashita, M.; Matsuki, K.; Murakami, H.; Matsui, J.; Suda, T. Reduction of paraoxonase-1 activity may contribute the qualitative impairment of HDL particles in patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 99, 30–38. [Google Scholar] [CrossRef]
- Shao, B.; Cavigiolio, G.; Brot, N.; Oda, M.N.; Heinecke, J.W. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc. Natl. Acad. Sci. USA 2008, 105, 12224–12229. [Google Scholar] [CrossRef]
- Lokeshwaran, K.; Hemadou, A.; Jayaprakash, N.S.; Prasanna, R.R.; Jacobin-Valat, M.J.; Dieryck, W.; Joucla, G.; Vijayalakshmi, M.A.; Clofent-Sanchez, G.; Santarelli, X.; et al. Development of anti-chloro (192) tyrosine HDL apoA-I antibodies for the immunodiagnosis of cardiovascular diseases. J. Immunol. Methods 2019, 474, 112637. [Google Scholar] [CrossRef]
- Shiu, S.W.; Xiao, S.M.; Wong, Y.; Chow, W.S.; Lam, K.S.; Tan, K.C. Carbamylation of LDL and its relationship with myeloperoxidase in type 2 diabetes mellitus. Clin. Sci. 2014, 126, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Femlak, M.; Gluba-Brzózka, A.; Ciałkowska-Rysz, A.; Rysz, J. The role and function of HDL in patients with diabetes mellitus and the related cardiovascular risk. Lipids Health Dis. 2017, 16, 207. [Google Scholar] [CrossRef]
- Shroff, R.; Speer, T.; Colin, S.; Charakida, M.; Zewinger, S.; Staels, B.; Chinetti-Gbaguidi, G.; Hettrich, I.; Rohrer, L.; O’Neill, F.; et al. HDL in children with CKD promotes endothelial dysfunction and an abnormal vascular phenotype. J. Am. Soc. Nephrol. 2014, 25, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Saleheen, D.; Scott, R.; Javad, S.; Zhao, W.; Rodrigues, A.; Picataggi, A.; Lukmanova, D.; Mucksavage, M.L.; Luben, R.; Billheimer, J.; et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes Endocrinol. 2015, 3, 507–513. [Google Scholar] [CrossRef]
- Klin, M.; Smogorzewski, M.; Ni, Z.; Zhang, G.; Massry, S.G. Abnormalities in hepatic lipase in chronic renal failure: Role of excess parathyroid hormone. J. Clin. Investig. 1996, 97, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Liang, K.; Vaziri, N.D. Protein restriction and AST-120 improve lipoprotein lipase and VLDL receptor in focal glomerulosclerosis. Kidney Int. 2003, 64, 1780–1786. [Google Scholar] [CrossRef]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Tsun, J.G.; Yung, S.; Chau, M.K.; Shiu, S.W.; Chan, T.M.; Tan, K.C. Cellular cholesterol transport proteins in diabetic nephropathy. PLoS ONE 2014, 9, e105787. [Google Scholar] [CrossRef]
- Annema, W.; von Eckardstein, A. High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ. J. 2013, 77, 2432–2448. [Google Scholar] [CrossRef] [PubMed]
- Ebtehaj, S.; Gruppen, E.G.; Parvizi, M.; Tietge, U.J.F.; Dullaart, R.P.F. The anti-inflammatory function of HDL is impaired in type 2 diabetes: Role of hyperglycemia, paraoxonase-1 and low grade inflammation. Cardiovasc. Diabetol. 2017, 16, 132. [Google Scholar] [CrossRef]
- Morgantini, C.; Natali, A.; Boldrini, B.; Imaizumi, S.; Navab, M.; Fogelman, A.M.; Ferrannini, E.; Reddy, S.T. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes 2011, 60, 2617–2623. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Pahl, M.V.; Elahimehr, R.; Vaziri, N.D. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl. Res. 2009, 153, 77–85. [Google Scholar] [CrossRef]
- Besler, C.; Heinrich, K.; Rohrer, L.; Doerries, C.; Riwanto, M.; Shih, D.M.; Chroni, A.; Yonekawa, K.; Stein, S.; Schaefer, N.; et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Investig. 2011, 121, 2693–2708. [Google Scholar] [CrossRef]
- Speer, T.; Rohrer, L.; Blyszczuk, P.; Shroff, R.; Kuschnerus, K.; Kränkel, N.; Kania, G.; Zewinger, S.; Akhmedov, A.; Shi, Y.; et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 2013, 38, 754–768. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.Y.; Sun, J.T.; Yang, K.; Shen, W.F.; Lu, L.; Zhang, R.Y.; Tong, X.; Liu, Y. Serum amyloid A enrichment impairs the anti-inflammatory ability of HDL from diabetic nephropathy patients. J. Diabetes Complicat. 2017, 31, 1538–1543. [Google Scholar] [CrossRef] [PubMed]
- Tolle, M.; Huang, T.; Schuchardt, M.; Jankowski, V.; Prüfer, N.; Jankowski, J.; Tietge, U.J.; Zidek, W.; van der Giet, M. High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc. Res. 2012, 94, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Drechsler, C.; Kleber, M.E.; Dressel, A.; Riffel, J.; Triem, S.; Lehmann, M.; Kopecky, C.; Säemann, M.D.; Lepper, P.M.; et al. Serum amyloid A: High-density lipoproteins interaction and cardiovascular risk. Eur. Heart J. 2015, 36, 3007–3016. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lundman, P.; Harmer, J.A.; Cutri, B.; Griffiths, K.A.; Rye, K.A.; Barter, P.J.; Celermajer, D.S. Consumption of saturated fat impairs the anti-inflammatory properties of high-density lipoproteins and endothelial function. J. Am. Coll. Cardiol. 2006, 48, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, M.; He, D.; Zhao, X.; Zhang, W.; Wei, L.; Huang, E.; Ji, L.; Zhang, M.; Willard, B.; et al. HDL in diabetic nephropathy has less effect in endothelial repairing than diabetes without complications. Lipids Health Dis. 2016, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.; Ducasa, G.M.; Fornoni, A. Systemic and renal lipids in kidney disease development and progression. Am. J. Physiol. Ren. Physiol. 2016, 310, F433–F445. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Wang, X.; Choudhury, D. Nuclear hormone receptors as therapeutic targets. Contrib. Nephrol. 2011, 170, 209–216. [Google Scholar] [PubMed]
- Muroya, Y.; Ito, O.; Rong, R.; Takashima, K.; Ito, D.; Cao, P.; Nakamura, Y.; Joh, K.; Kohzuki, M. Disorder of fatty acid metabolism in the kidney of PAN-induced nephrotic rats. Am. J. Physiol. Ren. Physiol. 2012, 303, F1070–F1079. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, T.; Li, J.; Proctor, G.; McManaman, J.L.; Lucia, S.; Chua, S.; Levi, M. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes 2005, 54, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Merscher-Gomez, S.; Guzman, J.; Pedigo, C.E.; Lehto, M.; Aguillon-Prada, R.; Mendez, A.; Lassenius, M.I.; Forsblom, C.; Yoo, T.; Villarreal, R.; et al. Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes 2013, 62, 3817–3827. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Vaziri, N.D. Upregulation of acyl-CoA: Cholesterol acyltransferase in chronic renal failure. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E676–E681. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Yuan, J.; Ni, Z.; Norris, K.; Vaziri, N.D. Reverse cholesterol transport pathway in experimental chronic renal failure. Am. J. Nephrol. 2009, 30, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Descamps-Latscha, B.; Witko-Sarsat, V.; Nguyen-Khoa, T.; Nguyen, A.T.; Gausson, V.; Mothu, N.; London, G.M.; Jungers, P. Advanced oxidation protein products as risk factors for atherosclerotic cardiovascular events in nondiabetic predialysis patients. Am. J. Kidney Dis. 2005, 45, 39–47. [Google Scholar] [CrossRef]
- Vaziri, N.D. Causes of dysregulation of lipid metabolism in chronic renal failure. Semin. Dial. 2009, 22, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Mori, K.; Mukoyama, M.; Kasahara, M.; Yokoi, H.; Saito, Y.; Ogawa, Y.; Imamaki, H.; Kawanishi, T.; Ishii, A.; et al. Exacerbation of diabetic nephropathy by hyperlipidaemia is mediated by Toll-like receptor 4 in mice. Diabetologia 2012, 55, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Mori, K.; Mukoyama, M.; Kasahara, M.; Yokoi, H.; Nakao, K. Macrophage-mediated glucolipotoxicity via myeloid-related protein 8/toll-like receptor 4 signaling in diabetic nephropathy. Clin. Exp. Nephrol. 2014, 18, 584–592. [Google Scholar] [CrossRef]
- Perego, C.; Da Dalt, L.; Pirillo, A.; Galli, A.; Catapano, A.L.; Norata, G.D. Cholesterol metabolism, pancreatic beta-cell function and diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Kruit, J.K.; Brunham, L.R.; Verchere, C.B.; Hayden, M.R. HDL and LDL cholesterol significantly influence beta-cell function in type 2 diabetes mellitus. Curr. Opin. Lipidol. 2010, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- De Cosmo, S.; Menzaghi, C.; Prudente, S.; Trischitta, V. Role of insulin resistance in kidney dysfunction: Insights into the mechanism and epidemiological evidence. Nephrol. Dial. Transplant. 2013, 28, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Jauregui, A.; Mintz, D.H.; Mundel, P.; Fornoni, A. Role of altered insulin signaling pathways in the pathogenesis of podocyte malfunction and microalbuminuria. Curr. Opin. Nephrol. Hypertens. 2009, 18, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Ni, Z.; Wang, X.Q.; Oveisi, F.; Zhou, X.J. Downregulation of nitric oxide synthase in chronic renal insufficiency: Role of excess PTH. Am. J. Physiol. 1998, 274, F642–F649. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Ni, Z.; Oveisi, F.; Liang, K.; Pandian, R. Enhanced nitric oxide inactivation and protein nitration by reactive oxygen species in renal insufficiency. Hypertension 2002, 39, 135–141. [Google Scholar]
- Deighan, C.J.; Caslake, M.J.; McConnell, M.; Boulton-Jones, J.M.; Packard, C.J. Atherogenic lipoprotein phenotype in end-stage renal failure: Origin and extent of small dense low-density lipoprotein formation. Am. J. Kidney Dis. 2000, 35, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Wang, H.L.; Cheng, X.L.; Wei, F.; Bai, X.; Lin, R.C.; Vaziri, N.D. Metabolomics analysis reveals the association between lipid abnormalities and oxidative stress, inflammation, fibrosis, and Nrf2 dysfunction in aristolochic acid-induced nephropathy. Sci. Rep. 2015, 5, 12936. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Sato, T.; Liang, K. Molecular mechanisms of altered cholesterol metabolism in rats with spontaneous focal glomerulosclerosis. Kidney Int. 2003, 63, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, S.; Pecoits-Filho, R.; Perreto, S.; Barberato, S.H.; Stinghen, A.E.; Lima, E.G.; Fuerbringer, R.; Sauthier, S.M.; Riella, M.C. Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol. Dial. Transplant. 2006, 21, 2788–2794. [Google Scholar] [CrossRef] [PubMed]
- Rye, K.A.; Barter, P.J. Cardioprotective functions of HDLs. J. Lipid Res. 2014, 55, 168–179. [Google Scholar] [CrossRef]
- Newman, J.W.; Kaysen, G.A.; Hammock, B.D.; Shearer, G.C. Proteinuria increases oxylipid concentrations in VLDL and HDL but not LDL particles in the rat. J. Lipid Res. 2007, 48, 1792–1800. [Google Scholar] [CrossRef] [PubMed]
- Kheniser, K.G.; Osme, A.; Kim, C.; Ilchenko, S.; Kasumov, T.; Kashyap, S.R. Temporal Dynamics of High-Density Lipoprotein Proteome in Diet-Controlled Subjects with Type 2 Diabetes. Biomolecules 2020, 10, 520. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.Q.; Brubaker, G.; Wu, Z.; Zheng, L.; Willard, B.; Kinter, M.; Hazen, S.L.; Smith, J.D. Apolipoprotein A-I tryptophan substitution leads to resistance to myeloperoxidase-mediated loss of function. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2063–2070. [Google Scholar] [CrossRef]
- Hedrick, C.C.; Thorpe, S.R.; Fu, M.X.; Harper, C.M.; Yoo, J.; Kim, S.M.; Wong, H.; Peters, A.L. Glycation impairs high-density lipoprotein function. Diabetologia 2000, 43, 312–320. [Google Scholar] [CrossRef]
- Aroner, S.A.; Furtado, J.D.; Sacks, F.M.; Tsai, M.Y.; Mukamal, K.J.; McClelland, R.L.; Jensen, M.K. Apolipoprotein C-III and its defined lipoprotein subspecies in relation to incident diabetes: The Multi-Ethnic Study of Atherosclerosis. Diabetologia 2019, 62, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Sakaue, T.; Misaki, A.; Murayama, S.; Takahashi, T.; Okada, K.; Takeuchi, H.; Yoshino, G.; Adachi, M. Very low-density lipoprotein-apoprotein CI is increased in diabetic nephropathy: Comparison with apoprotein CIII. Kidney Int. 2003, 63, 2171–2177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirano, T.; Hayashi, T.; Adachi, M.; Taira, T.; Hattori, H. Marked decrease of apolipoprotein A-V in both diabetic and nondiabetic patients with end-stage renal disease. Metabolism 2007, 56, 462–463. [Google Scholar] [CrossRef]
- Pennacchio, L.A.; Rubin, E.M. Apolipoprotein A5, a newly identified gene that affects plasma triglyceride levels in humans and mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Hermans, M.P.; Fioretto, P.; Valensi, P.; Davis, T.; Horton, E.; Wanner, C.; Al-Rubeaan, K.; Aronson, R.; Barzon, I.; et al. Association between plasma triglycerides and high-density lipoprotein cholesterol and microvascular kidney disease and retinopathy in type 2 diabetes mellitus: A global case-control study in 13 countries. Circulation 2014, 129, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Stancu, C.S.; Toma, L.; Sima, A.V. Dual role of lipoproteins in endothelial cell dysfunction in atherosclerosis. Cell Tissue Res. 2012, 349, 433–446. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Haase, C.L.; Tybjærg-Hansen, A.; Nordestgaard, B.G.; Frikke-Schmidt, R. HDL Cholesterol and Risk of Type 2 Diabetes: A Mendelian Randomization Study. Diabetes 2015, 64, 3328–3333. [Google Scholar] [CrossRef] [PubMed]
- Vaisar, T.; Couzens, E.; Hwang, A.; Russell, M.; Barlow, C.E.; DeFina, L.F.; Hoofnagle, A.N.; Kim, F. Type 2 diabetes is associated with loss of HDL endothelium protective functions. PLoS ONE 2018, 13, e0192616. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.T.; De Cosmo, S.; Viazzi, F.; Pacilli, A.; Ceriello, A.; Genovese, S.; Guida, P.; Giorda, C.; Cucinotta, D.; Pontremoli, R.; et al. Plasma Triglycerides and HDL-C Levels Predict the Development of Diabetic Kidney Disease in Subjects with Type 2 Diabetes: The AMD Annals Initiative. Diabetes Care 2016, 39, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Swerdlow, D.I.; Preiss, D.; Fairhurst-Hunter, Z.; Keating, B.J.; Asselbergs, F.W.; Sattar, N.; Humphries, S.E.; Hingorani, A.D.; Holmes, M.V. Association of Lipid Fractions With Risks for Coronary Artery Disease and Diabetes. JAMA Cardiol. 2016, 1, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Fall, T.; Xie, W.; Poon, W.; Yaghootkar, H.; Mägi, R.; GENESIS Consortium; Knowles, J.W.; Lyssenko, V.; Weedon, M.; Frayling, T.M.; et al. Using Genetic Variants to Assess the Relationship Between Circulating Lipids and Type 2 Diabetes. Diabetes 2015, 64, 2676–2684. [Google Scholar] [CrossRef]
- Liu, H.; Yao, X.; Wang, L.; Liu, J.; Li, X.; Fu, X.; Liu, J.; Dong, S.; Wang, Y. The causal relationship between 5 serum lipid parameters and diabetic nephropathy: A Mendelian randomization study. Front. Endocrinol. 2024, 15, 1358358. [Google Scholar] [CrossRef] [PubMed]
- Hanai, K.; Babazono, T.; Yoshida, N.; Nyumura, I.; Toya, K.; Hayashi, T.; Bouchi, R.; Tanaka, N.; Ishii, A.; Iwamoto, Y. Gender differences in the association between HDL cholesterol and the progression of diabetic kidney disease in type 2 diabetic patients. Nephrol. Dial. Transplant. 2012, 27, 1070–1075. [Google Scholar] [CrossRef] [PubMed]
- Yadegar, A.; Mohammadi, F.; Rabizadeh, S.; Ayati, A.; Seyedi, S.A.; Nabipoorashrafi, S.A.; Esteghamati, A.; Nakhjavani, M. Correlation between different levels and patterns of dyslipidemia and glomerular filtration rate in patients with type 2 diabetes: A cross-sectional survey of a regional cohort. J. Clin. Lab. Anal. 2023, 37, e24954. [Google Scholar] [CrossRef] [PubMed]
- Moradi, H.; Streja, E.; Kashyap, M.L.; Vaziri, N.D.; Fonarow, G.C.; Kalantar-Zadeh, K. Elevated high-density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol. Dial. Transplant. 2014, 29, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Lamprea-Montealegre, J.A.; Sharrett, A.R.; Matsushita, K.; Selvin, E.; Szklo, M.; Astor, B.C. Chronic kidney disease, lipids and apolipoproteins, and coronary heart disease: The ARIC study. Atherosclerosis 2014, 234, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Yang, W.; Akkina, S.; Alper, A.; Anderson, A.H.; Appel, L.J.; He, J.; Raj, D.S.; Schelling, J.; Strauss, L.; et al. Relation of serum lipids and lipoproteins with progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 2014, 9, 1190–1198. [Google Scholar] [CrossRef]
- Nakajima, K.; Higuchi, R.; Iwane, T.; Shibata, M.; Takada, K.; Sugiyama, M.; Matsuda, M.; Nakamura, T. High Incidence of Diabetes in People with Extremely High High-Density Lipoprotein Cholesterol: Results of the Kanagawa Investigation of Total Checkup Data from the National Database-1 (KITCHEN-1). J. Clin. Med. 2019, 8, 381. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, C.; Wang, Y.; Shao, B.; Fong, T.L.; Lau, N.C.; Zhang, H.; Li, H.; Wang, J.; Lu, X.; et al. Dose-response association of diabetic kidney disease with routine clinical parameters in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. EClinicalMedicine 2024, 69, 102482. [Google Scholar] [CrossRef] [PubMed]
- Igata, M.; Nakajima, K. U-Shaped Relationship Between Proteinuria and High-Density Lipoprotein Cholesterol: Results of Cross-Sectional and Six Years Cohort Studies (KITCHEN-10). J. Clin. Med. Res. 2022, 14, 300–308. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Lin, M.; Hu, Y.; Ma, Y. High levels of high-density lipoprotein cholesterol may increase the risk of diabetic kidney disease in patients with type 2 diabetes. Sci. Rep. 2024, 14, 15362. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.E.; Williams, D.H.; Oganov, R.G.; Tao, S.C.; Rywik, S.L.; Stein, Y.; Little, J.A. Sex difference in high density lipoprotein cholesterol in six countries. Am. J. Epidemiol. 1996, 143, 1100–1106. [Google Scholar] [CrossRef]
- Abbas, A.; Fadel, P.J.; Wang, Z.; Arbique, D.; Jialal, I.; Vongpatanasin, W. Contrasting effects of oral versus transdermal estrogen on serum amyloid A (SAA) and high-density lipoprotein-SAA in postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e164–e167. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, F.Y.; Shih, C.M.; Au, H.K.; Chang, Y.J.; Nakagami, H.; Morishita, R.; Chang, N.C.; Shyu, K.G.; Chen, J.W. Moderate to high concentrations of high-density lipoprotein from healthy subjects paradoxically impair human endothelial progenitor cells and related angiogenesis by activating Rho-associated kinase pathways. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, J.P.; Ryan, D.; Rainwater, D.L.; Moss, A.J.; Zareba, W.; Sparks, C.E. Cholesteryl ester transfer protein polymorphism (TaqIB) associates with risk in postinfarction patients with high C-reactive protein and high-density lipoprotein cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Wang, C.; Ning, G.; Wang, W.; Chen, G.; Wan, Q.; Qin, G.; Yan, L.; Wang, G.; Qin, Y.; et al. High concentrations of triglycerides are associated with diabetic kidney disease in new-onset type 2 diabetes in China: Findings from the China Cardiometabolic Disease and Cancer Cohort (4C) Study. Diabetes Obes. Metab. 2021, 23, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Peng, D. The emerging role of apolipoprotein C-III: Beyond effects on triglyceride metabolism. Lipids Health Dis. 2016, 15, 184. [Google Scholar] [CrossRef] [PubMed]
- Ben-Aicha, S.; Badimon, L.; Vilahur, G. Advances in HDL: Much More than Lipid Transporters. Int. J. Mol. Sci. 2020, 21, 732. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, B.; Rysz, J.; Ławiński, J.; Rysz-Górzyńska, M.; Gluba-Brzózka, A. Is a High HDL-Cholesterol Level Always Beneficial? Biomedicines 2021, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Cardner, M.; Yalcinkaya, M.; Goetze, S.; Luca, E.; Balaz, M.; Hunjadi, M.; Hartung, J.; Shemet, A.; Kränkel, N.; Radosavljevic, S.; et al. Structure-function relationships of HDL in diabetes and coronary heart disease. JCI Insight 2020, 5, e131491. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Kaneko, H.; Matsuoka, S.; Suzuki, Y.; Ueno, K.; Ohno, R.; Okada, A.; Fujiu, K.; Michihata, N.; Jo, T. HDL cholesterol and clinical outcomes in diabetes mellitus. Eur. J. Prev. Cardiol. 2023, 30, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Leiter, L.A. The prevention of diabetic microvascular complications of diabetes: Is there a role for lipid lowering? Diabetes Res. Clin. Pract. 2005, 68 (Suppl. 2), S3–S14. [Google Scholar] [CrossRef]
- Zoppini, G.; Negri, C.; Stoico, V.; Casati, S.; Pichiri, I.; Bonora, E. Triglyceride-high-density lipoprotein cholesterol is associated with microvascular complications in type 2 diabetes mellitus. Metabolism 2012, 61, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.J.; Mark, P.B.; Kanbay, M.; Sarafidis, P.; Heine, G.H.; Rossignol, P.; Massy, Z.A.; Mallamaci, F.; Valdivielso, J.M.; Malyszko, J.; et al. Lipid management in patients with chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 727–749. [Google Scholar] [CrossRef]
- Tsuruya, K.; Yoshida, H.; Nagata, M.; Kitazono, T.; Iseki, K.; Iseki, C.; Fujimoto, S.; Konta, T.; Moriyama, T.; Yamagata, K.; et al. Impact of the Triglycerides to High-Density Lipoprotein Cholesterol Ratio on the Incidence and Progression of CKD: A Longitudinal Study in a Large Japanese Population. Am. J. Kidney Dis. 2015, 66, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Nakanishi, S.; Yoneda, M.; Awaya, T.; Yamane, K.; Hirano, T.; Kohno, N. Associations between small dense LDL, HDL subfractions (HDL2, HDL3) and risk of atherosclerosis in Japanese-Americans. J. Atheroscler. Thromb. 2012, 19, 444–452. [Google Scholar] [CrossRef]
- Fukui, T.; Hirano, T. High-density lipoprotein subspecies between patients with type 1 diabetes and type 2 diabetes without/with intensive insulin therapy. Endocr. J. 2012, 59, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Bulum, T.; Duvnjak, L.; Prkacin, I. Lower levels of HDL2 cholesterol are associated with microalbuminuria in patients with type 1 diabetes. Acta Med. Croat. 2011, 65, 243–250. [Google Scholar]
- Sheng, G.; Liu, D.; Kuang, M.; Zhong, Y.; Zhang, S.; Zou, Y. Utility of Non-High-Density Lipoprotein Cholesterol to High-Density Lipoprotein Cholesterol Ratio in Evaluating Incident Diabetes Risk. Diabetes Metab. Syndr. Obes. 2022, 15, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Gao, J.; Pu, C.; Feng, G.; Wang, L.; Huang, L.; Zhang, Y. ApoM/HDL-C and apoM/apoA-I ratios are indicators of diabetic nephropathy in healthy controls and type 2 diabetes mellitus. Clin. Chim. Acta 2017, 466, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bardini, G.; Innocenti, M.; Rotella, C.M.; Giannini, S.; Mannucci, E. Variability of triglyceride levels and incidence of microalbuminuria in type 2 diabetes. J. Clin. Lipidol. 2016, 10, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.S.; Park, Y.M.; Kwon, H.S.; Yoon, K.H.; Han, K.; Kim, M.K. HDL-Cholesterol, Its Variability, and the Risk of Diabetes: A Nationwide Population-Based Study. J. Clin. Endocrinol. Metab. 2019, 104, 5633–5641. [Google Scholar] [CrossRef]
- Wang, M.C.; Li, C.I.; Liu, C.S.; Lin, C.H.; Yang, S.Y.; Li, T.C.; Lin, C.C. Effect of blood lipid variability on mortality in patients with type 2 diabetes: A large single-center cohort study. Cardiovasc. Diabetol. 2021, 20, 228. [Google Scholar] [CrossRef] [PubMed]
- Hammad, S.M.; arth, J.L.; Knaak, C.; Argraves, W.S. Megalin acts in concert with cubilin to mediate endocytosis of high density lipoproteins. J. Biol. Chem. 2000, 275, 12003–12008. [Google Scholar] [CrossRef] [PubMed]
- Mack, S.; Coassin, S.; Vaucher, J.; Kronenberg, F.; Lamina, C.; ApoA-IV-GWAS Consortium. Evaluating the Causal Relation of ApoA-IV with Disease-Related Traits—A Bidirectional Two-sample Mendelian Randomization Study. Sci. Rep. 2017, 7, 8734. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.T.; Giandalia, A.; Romeo, E.L.; Muscianisi, M.; Ruffo, M.C.; Alibrandi, A.; Bitto, A.; Forte, F.; Grillone, A.; Asztalos, B.; et al. HDL subclasses and the common CETP TaqIB variant predict the incidence of microangiopatic complications in type 2 diabetic women: A 9years follow-up study. Diabetes Res. Clin. Pract. 2017, 132, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.G.; Farnier, M.; Krempf, M.; Bergeron, J.; Luc, G.; Averna, M.; Stroes, E.S.; Langslet, G.; Raal, F.J.; El Shahawy, M.; et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1489–1499. [Google Scholar] [CrossRef]
- Viljoen, A.J.S.; Wierzbicki, A.S. Diabetic Dyslipidemia and Risk of Cardiovascular Disease. In Textbook of Diabetes; Holt, R.I.G., Cockram, C.S., Flyvbjerg, A., Goldstein, B.J., Eds.; Blackwell Pub: Oxford, UK, 2017. [Google Scholar]
- Zhou, S.; Su, L.; Xu, R.; Li, Y.; Chen, R.; Cao, Y.; Gao, P.; Zhang, X.; Luo, F.; Gao, Q.; et al. Statin initiation and risk of incident kidney disease in patients with diabetes. CMAJ 2023, 195, E729–E738. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Nault, P.; Giugliano, R.P.; Keech, A.C.; Pineda, A.L.; Kanevsky, E.; Kuder, J.; Murphy, S.A.; Jukema, J.W.; Lewis, B.S.; et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 2015, 372, 1500–1509. [Google Scholar] [CrossRef]
- Keech, A.; Simes, R.J.; Barter, P.; Best, J.; Scott, R.; Taskinen, M.R.; Forder, P.; Pillai, A.; Davis, T.; Glasziou, P.; et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R., 3rd; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; Probstfield, J.; et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar] [PubMed]
- Ansquer, J.C.; Foucher, C.; Rattier, S.; Taskinen, M.R.; Steiner, G.; DAIS Investigators. Fenofibrate reduces progression to microalbuminuria over 3 years in a placebo-controlled study in type 2 diabetes: Results from the Diabetes Atherosclerosis Intervention Study (DAIS). Am. J. Kidney Dis. 2005, 45, 485–493. [Google Scholar] [CrossRef]
- Tsunoda, F.; Asztalos, I.B.; Horvath, K.V.; Steiner, G.; Schaefer, E.J.; Asztalos, B.F. Fenofibrate, HDL, and cardiovascular disease in Type-2 diabetes: The DAIS trial. Atherosclerosis 2016, 247, 35–39. [Google Scholar] [CrossRef]
- Agrawal, S.; Zaritsky, J.J.; Fornoni, A.; Smoyer, W.E. Dyslipidaemia in nephrotic syndrome: Mechanisms and treatment. Nat. Rev. Nephrol. 2018, 14, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Mika, D.; Guruvayoorappan, C. Myeloperoxidase: The yin and yang in tumour progression. J. Exp. Ther. Oncol. 2011, 9, 93–100. [Google Scholar]
- Shao, B.; Oda, M.N.; Oram, J.F.; Heinecke, J.W. Myeloperoxidase: An oxidative pathway for generating dysfunctional high-density lipoprotein. Chem. Res. Toxicol. 2010, 23, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J. Linking oxidative stress and inflammation in kidney disease: Which is the chicken and which is the egg? Semin. Dial. 2004, 17, 449–454. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Rocha, M.; Falcon, R.; de Pablo, C.; Alvarez, A.; Jover, A.; Hernandez-Mijares, A.; Victor, V.M. Is myeloperoxidase a key component in the ROS-induced vascular damage related to nephropathy in type 2 diabetes? Antioxid. Redox Signal 2013, 19, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Streja, E.; Kovesdy, C.P.; Streja, D.A.; Moradi, H.; Kalantar-Zadeh, K.; Kashyap, M.L. Niacin and progression of CKD. Am. J. Kidney Dis. 2015, 65, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S.H.; Kamanna, V.S.; Kashyap, M.L. Niacin decreases leukocyte myeloperoxidase: Mechanistic role of redox agents and Src/p38MAP kinase. Atherosclerosis 2014, 235, 554–561. [Google Scholar] [CrossRef]
- Warnholtz, A.; Wild, P.; Ostad, M.A.; Elsner, V.; Stieber, F.; Schinzel, R.; Walter, U.; Peetz, D.; Lackner, K.; Blankenberg, S.; et al. Effects of oral niacin on endothelial dysfunction in patients with coronary artery disease: Results of the randomized, double-blind, placebo-controlled INEF study. Atherosclerosis 2009, 204, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Kim, H.J.; Kamanna, V.S.; Vaziri, N.D. Niacin improves renal lipid metabolism and slows progression in chronic kidney disease. Biochim. Biophys. Acta 2010, 1800, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Yee, M.S.; Pavitt, D.V.; Richmond, W.; Cook, H.T.; McLean, A.G.; Valabhji, J.; Elkeles, R.S. Changes in lipoprotein profile and urinary albumin excretion in familial LCAT deficiency with lipid lowering therapy. Atherosclerosis 2009, 205, 528–532. [Google Scholar] [CrossRef]
- Owada, A.; Suda, S.; Hata, T. Antiproteinuric effect of niceritrol, a nicotinic acid derivative, in chronic renal disease with hyperlipidemia: A randomized trial. Am. J. Med. 2003, 114, 347–353. [Google Scholar] [CrossRef]
- Krause, B.R.; Remaley, A.T. Reconstituted HDL for the acute treatment of acute coronary syndrome. Curr. Opin. Lipidol. 2013, 24, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Stenkula, K.G.; Lindahl, M.; Petrlova, J.; Dalla-Riva, J.; Göransson, O.; Cushman, S.W.; Krupinska, E.; Jones, H.A.; Lagerstedt, J.O. Single injections of apoA-I acutely improve in vivo glucose tolerance in insulin-resistant mice. Diabetologia 2014, 57, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.J.; Drummond, G.; Kim, D.H.; Li, M.; Kruger, A.L.; Ikehara, S.; Abraham, N.G. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J. Lipid Res. 2008, 49, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- McGrath, K.C.; Li, X.; Twigg, S.M.; Heather, A.K. Apolipoprotein-AI mimetic peptides D-4F and L-5F decrease hepatic inflammation and increase insulin sensitivity in C57BL/6 mice. PLoS ONE 2020, 15, e0226931. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, S.J.; Liébana-García, R.; Nilsson, O.; Domingo-Espín, J.; Grönberg, C.; Stenkula, K.G.; Lagerstedt, J.O. ApoAI-derived peptide increases glucose tolerance and prevents formation of atherosclerosis in mice. Diabetologia 2019, 62, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.S.; Irigoyen, M.; Sanches, T.R.; Volpini, R.A.; Camara, N.O.; Malheiros, D.M.; Shimizu, M.H.; Seguro, A.C.; Andrade, L. Apolipoprotein A-I mimetic peptide 4F attenuates kidney injury, heart injury, and endothelial dysfunction in sepsis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R514–R524. [Google Scholar] [CrossRef]
- Tao, Y.; Lacko, A.G.; Sabnis, N.A.; Das-Earl, P.; Ibrahim, D.; Crowe, N.; Zhou, Z.; Cunningham, M.; Castillo, A.; Ma, R. Reconstituted HDL ameliorated renal injury of diabetic kidney disease in mice. Physiol. Rep. 2024, 12, e16179. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, Y.; Zhong, J.; Otsuka, T.; Dikalova, A.; Pastan, I.; Anantharamaiah, G.M.; Linton, M.F.; Yancey, P.G.; Ikizler, T.A.; Fogo, A.B.; et al. Lipoprotein modulation of proteinuric renal injury. Lab. Investig. 2019, 99, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D.; Kim, H.J.; Moradi, H.; Farmand, F.; Navab, K.; Navab, M.; Hama, S.; Fogelman, A.M.; Quiroz, Y.; Rodriguez-Iturbe, B. Amelioration of nephropathy with apoA-1 mimetic peptide in apoE-deficient mice. Nephrol. Dial. Transplant. 2010, 25, 3525–3534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Franceschini, G.; Calabresi, L.; Chiesa, G.; Parolini, C.; Sirtori, C.R.; Canavesi, M.; Bernini, F. Increased cholesterol efflux potential of sera from ApoA-IMilano carriers and transgenic mice. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Tsunoda, T.; Tuzcu, E.M.; Schoenhagen, P.; Cooper, C.J.; Yasin, M.; Eaton, G.M.; Lauer, M.A.; Sheldon, W.S.; Grines, C.L.; et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: A randomized controlled trial. JAMA 2003, 290, 2292–2300. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Puri, R.; Ballantyne, C.M.; Jukema, J.W.; Kastelein, J.J.P.; Koenig, W.; Wright, R.S.; Kallend, D.; Wijngaard, P.; Borgman, M.; et al. Effect of Infusion of High-Density Lipoprotein Mimetic Containing Recombinant Apolipoprotein A-I Milano on Coronary Disease in Patients With an Acute Coronary Syndrome in the MILANO-PILOT Trial: A Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Poulouin, Y.; Bhatt, D.L.; Bittner, V.A.; Chua, T.; Diaz, R.; Goodman, S.G.; Huo, Y.; Jukema, J.W.; et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 2012, 367, 2089–2099. [Google Scholar] [CrossRef]
- Stalenhoef, A.F.; Davidson, M.H.; Robinson, J.G.; Burgess, T.; Duttlinger-Maddux, R.; Kallend, D.; Goldberg, A.C.; Bays, H. Efficacy and safety of dalcetrapib in type 2 diabetes mellitus and/or metabolic syndrome patients, at high cardiovascular disease risk. Diabetes Obes. Metab. 2012, 14, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Bowman, L.; Hopewell, J.C.; Chen, F.; Wallendszus, K.; Stevens, W.; Collins, R.; Wiviott, S.D.; Cannon, C.P.; Braunwald, E.; Sammons, E.; et al. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N. Engl. J. Med. 2017, 377, 1217–1227. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Leiter, L.A.; Ballantyne, C.M.; Barter, P.J.; Black, D.M.; Kallend, D.; Laghrissi-Thode, F.; Leitersdorf, E.; McMurray, J.J.V.; Nicholls, S.J.; et al. Dalcetrapib Reduces Risk of New-Onset Diabetes in Patients With Coronary Heart Disease. Diabetes Care 2020, 43, 1077–1084. [Google Scholar] [CrossRef]
- Barter, P.J.; Rye, K.A.; Tardif, J.C.; Waters, D.D.; Boekholdt, S.M.; Breazna, A.; Kastelein, J.J. Effect of torcetrapib on glucose, insulin, and hemoglobin A1c in subjects in the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Circulation 2011, 124, 555–562. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Tao, H.; Davies, S.S. HDL Function and Atherosclerosis: Reactive Dicarbonyls as Promising Targets of Therapy. Circ. Res. 2023, 132, 1521–1545. [Google Scholar] [CrossRef] [PubMed]
- Dangas, K.; Navar, A.M.; Kastelein, J. The effect of CETP inhibitors on new-onset diabetes: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, T.; Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 2018, 8, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Lacko, A.G.; Nair, M.; Prokai, L.; McConathy, W.J. Prospects and challenges of the development of lipoprotein-based formulations for anti-cancer drugs. Expert. Opin. Drug Deliv. 2007, 4, 665–675. [Google Scholar] [CrossRef]
- Fox, C.A.; Moschetti, A.; Ryan, R.O. Reconstituted HDL as a therapeutic delivery device. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159025. [Google Scholar] [CrossRef]
- Lacko, A.G.; abnis, N.A.; Nagarajan, B.; McConathy, W.J. HDL as a drug and nucleic acid delivery vehicle. Front. Pharmacol. 2015, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Kaneko, S.; Yanai, K.; Aomatsu, A.; Hirai, K.; Ookawara, S.; Morishita, Y. MicroRNA Expression Profiling in Diabetic Kidney Disease. Transl. Res. 2021, 237, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, A.; Simental-Mendía, M.; Millán-Alanís, J.M.; Simental-Mendía, L.E. Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: A systematic review and meta-analysis of 48 randomized controlled trials. Pharmacol. Res. 2020, 160, 105068. [Google Scholar] [CrossRef] [PubMed]
- Filippas-Ntekouan, S.; Tsimihodimos, V.; Filippatos, T.; Dimitriou, T.; Elisaf, M. SGLT-2 inhibitors: Pharmacokinetics characteristics and effects on lipids. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 1113–1121. [Google Scholar] [CrossRef]
- Fadini, G.P.; Bonora, B.M.; Zatti, G.; Vitturi, N.; Iori, E.; Marescotti, M.C.; Albiero, M.; Avogaro, A. Effects of the SGLT2 inhibitor dapagliflozin on HDL cholesterol, particle size, and cholesterol efflux capacity in patients with type 2 diabetes: A randomized placebo-controlled trial. Cardiovasc. Diabetol. 2017, 16, 42. [Google Scholar] [CrossRef]
- Hayashi, T.; Fukui, T.; Nakanishi, N.; Yamamoto, S.; Tomoyasu, M.; Osamura, A.; Ohara, M.; Yamamoto, T.; Ito, Y.; Hirano, T. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: Comparison with sitagliptin. Cardiovasc. Diabetol. 2017, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Breder, I.; Cunha Breder, J.; Bonilha, I.; Munhoz, D.B.; Medorima, S.T.K.; Oliveira, D.C.; do Carmo, H.R.; Moreira, C.; Kontush, A.; Zimetti, F.; et al. Rationale and design of the expanded combination of evolocumab plus empagliflozin in diabetes: EXCEED-BHS3 trial. Ther. Adv. Chronic Dis. 2020, 11, 2040622320959248. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Tan, M.H.; Prince, M.J.; Erickson, P.P. The effects of oral anti-hyperglycaemic medications on serum lipid profiles in patients with type 2 diabetes. Diabetes Obes. Metab. 2004, 6, 133–156. [Google Scholar] [CrossRef] [PubMed]
- Nowrouzi-Sohrabi, P.; Soroush, N.; Tabrizi, R.; Shabani-Borujeni, M.; Rezaei, S.; Jafari, F.; Hosseini-Bensenjan, M.; Stricker, B.H.; van Hoek, M.; Ahmadizar, F. Effect of Liraglutide on Cardiometabolic Risk Profile in People with Coronary Artery Disease with or without Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2021, 12, 618208. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wu, S.; Wang, J.; Guo, S.; Chai, S.; Yang, Z.; Li, L.; Zhang, Y.; Ji, L.; Zhan, S. Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: A systematic review and network meta-analysis. Clin. Ther. 2015, 37, 225–241.e8. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Katagiri, T.; Suzuki, H.; Matsunaga, S.; H Yamada, M.; Ikarashi, T.; Yamamoto, M.; Furukawa, K.; Iwanaga, M.; Hatta, M.; et al. A 52-week randomized controlled trial of ipragliflozin or sitagliptin in type 2 diabetes combined with metformin: The N-ISM study. Diabetes Obes. Metab. 2021, 23, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Monami, M.; Vitale, V.; Ambrosio, M.L.; Bartoli, N.; Toffanello, G.; Ragghianti, B.; Monami, F.; Marchionni, N.; Mannucci, E. Effects on lipid profile of dipeptidyl peptidase 4 inhibitors, pioglitazone, acarbose, and sulfonylureas: Meta-analysis of placebo-controlled trials. Adv. Ther. 2012, 29, 736–746. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Cooper, M.E.; Chai, Z.; Liu, F. High-Density Lipoprotein in Patients with Diabetic Kidney Disease: Friend or Foe? Int. J. Mol. Sci. 2025, 26, 1683. https://doi.org/10.3390/ijms26041683
Liu K, Cooper ME, Chai Z, Liu F. High-Density Lipoprotein in Patients with Diabetic Kidney Disease: Friend or Foe? International Journal of Molecular Sciences. 2025; 26(4):1683. https://doi.org/10.3390/ijms26041683
Chicago/Turabian StyleLiu, Ke, Mark E. Cooper, Zhonglin Chai, and Fang Liu. 2025. "High-Density Lipoprotein in Patients with Diabetic Kidney Disease: Friend or Foe?" International Journal of Molecular Sciences 26, no. 4: 1683. https://doi.org/10.3390/ijms26041683
APA StyleLiu, K., Cooper, M. E., Chai, Z., & Liu, F. (2025). High-Density Lipoprotein in Patients with Diabetic Kidney Disease: Friend or Foe? International Journal of Molecular Sciences, 26(4), 1683. https://doi.org/10.3390/ijms26041683




