An Osteoblast-Specific Enhancer and Subenhancer Cooperatively Regulate Runx2 Expression in Chondrocytes
Abstract
1. Introduction
2. Results
2.1. Deletion of the Osteoblast-Specific 1.3 kb Enhancer Showed No Phenotypes
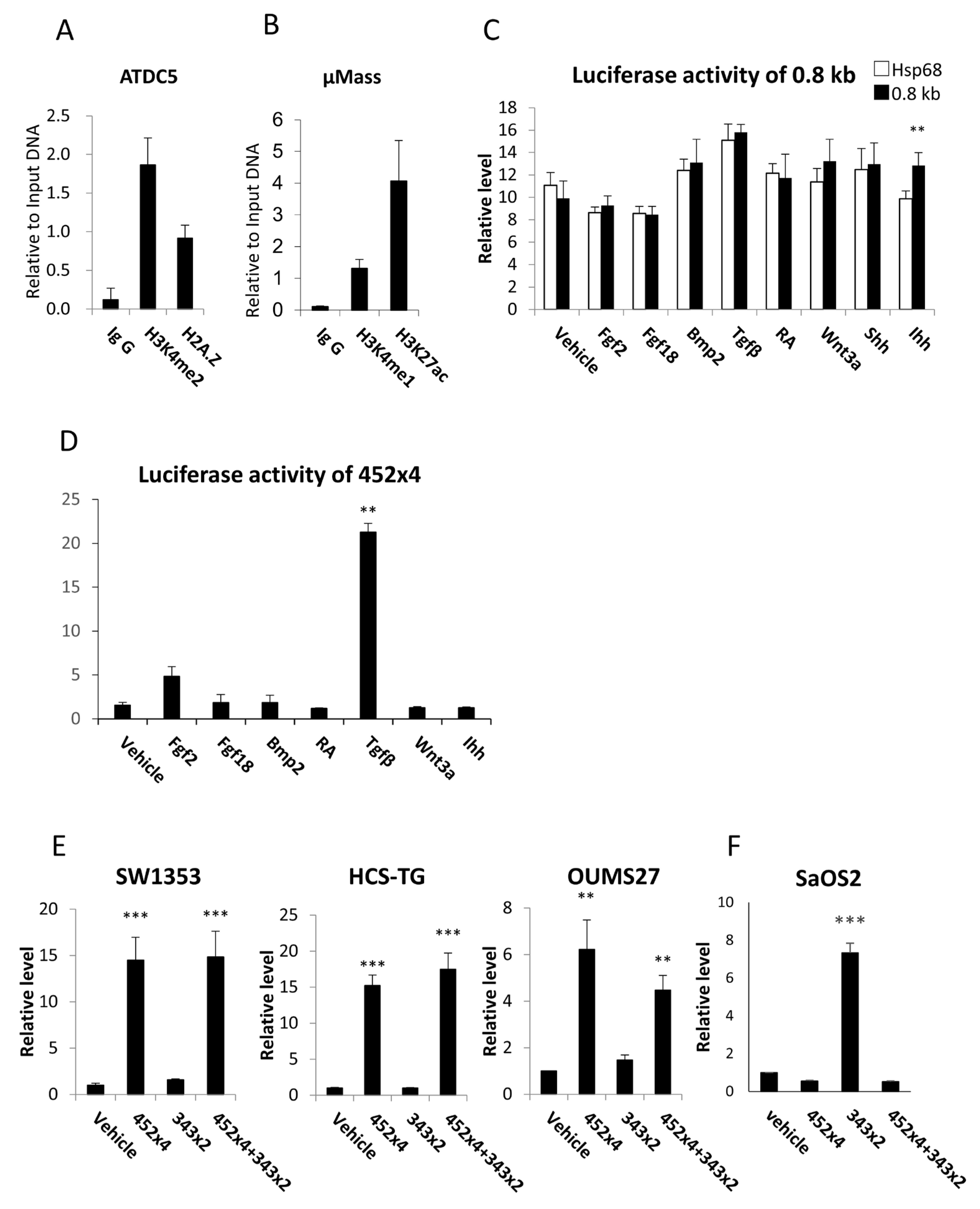
2.2. The 0.8 kb Region Showed Enhancer-like Histone Modifications, and Tandem Repeats of the Core Region Induced Luciferase Activity in Chondrocyte Cell Lines
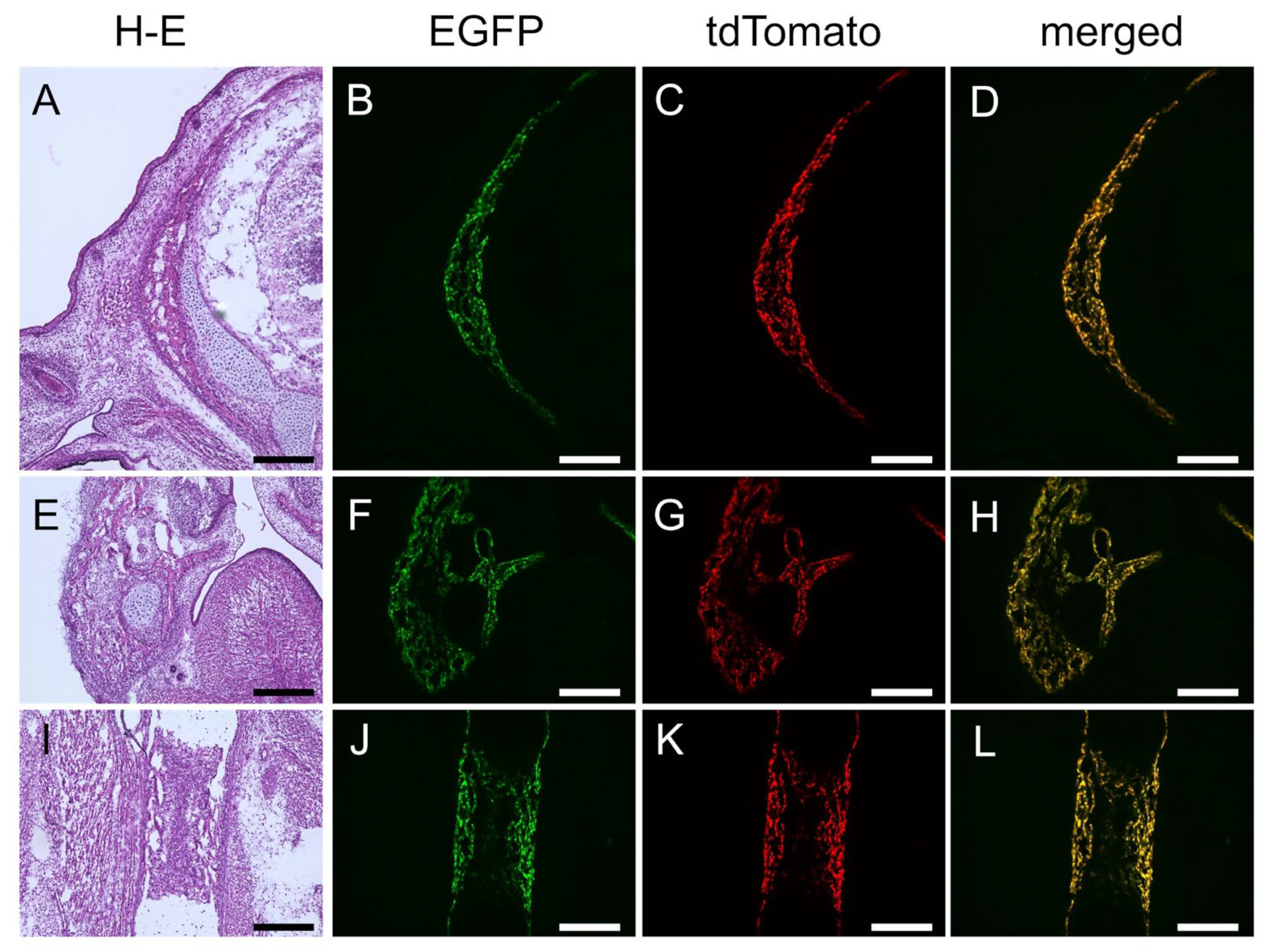
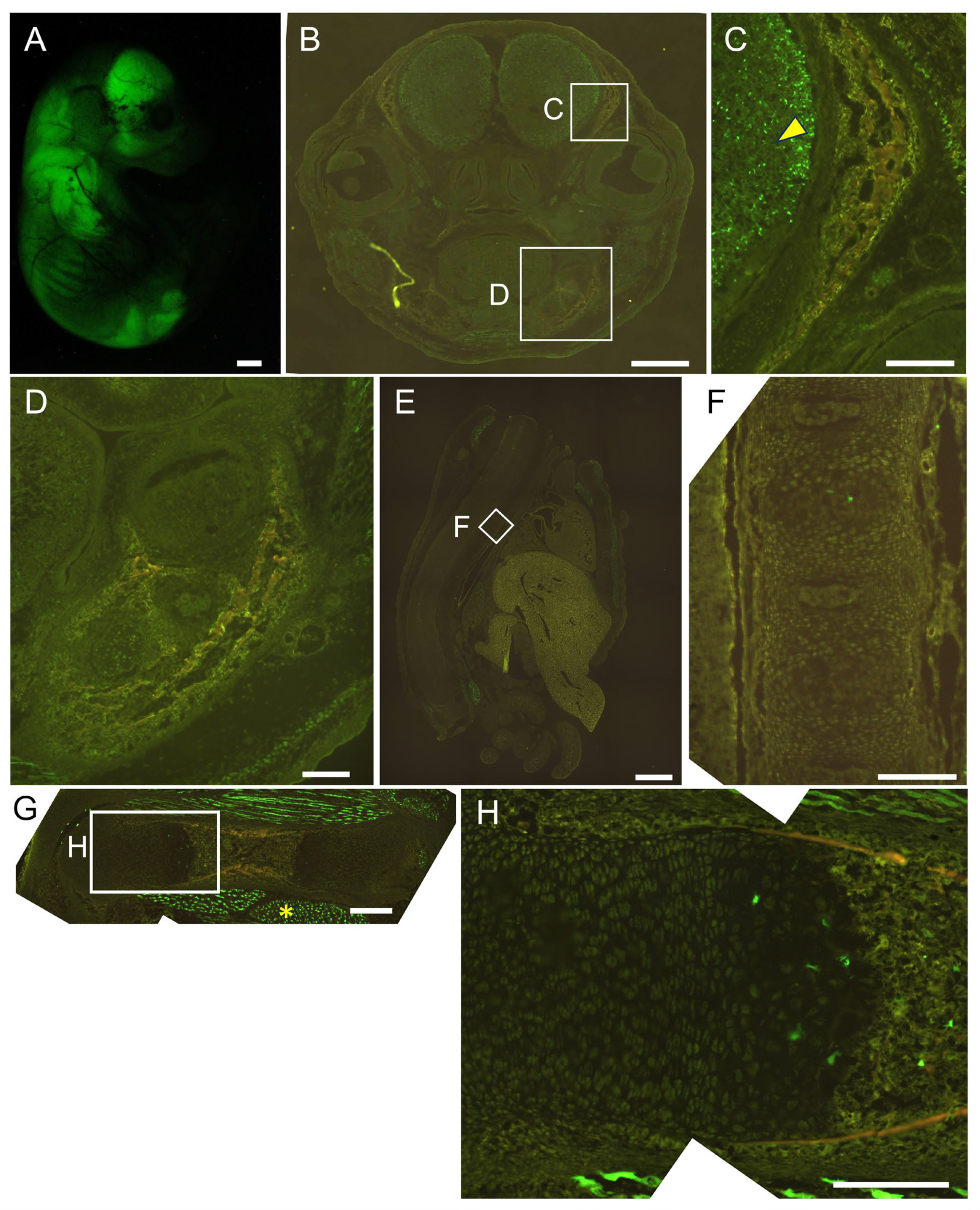
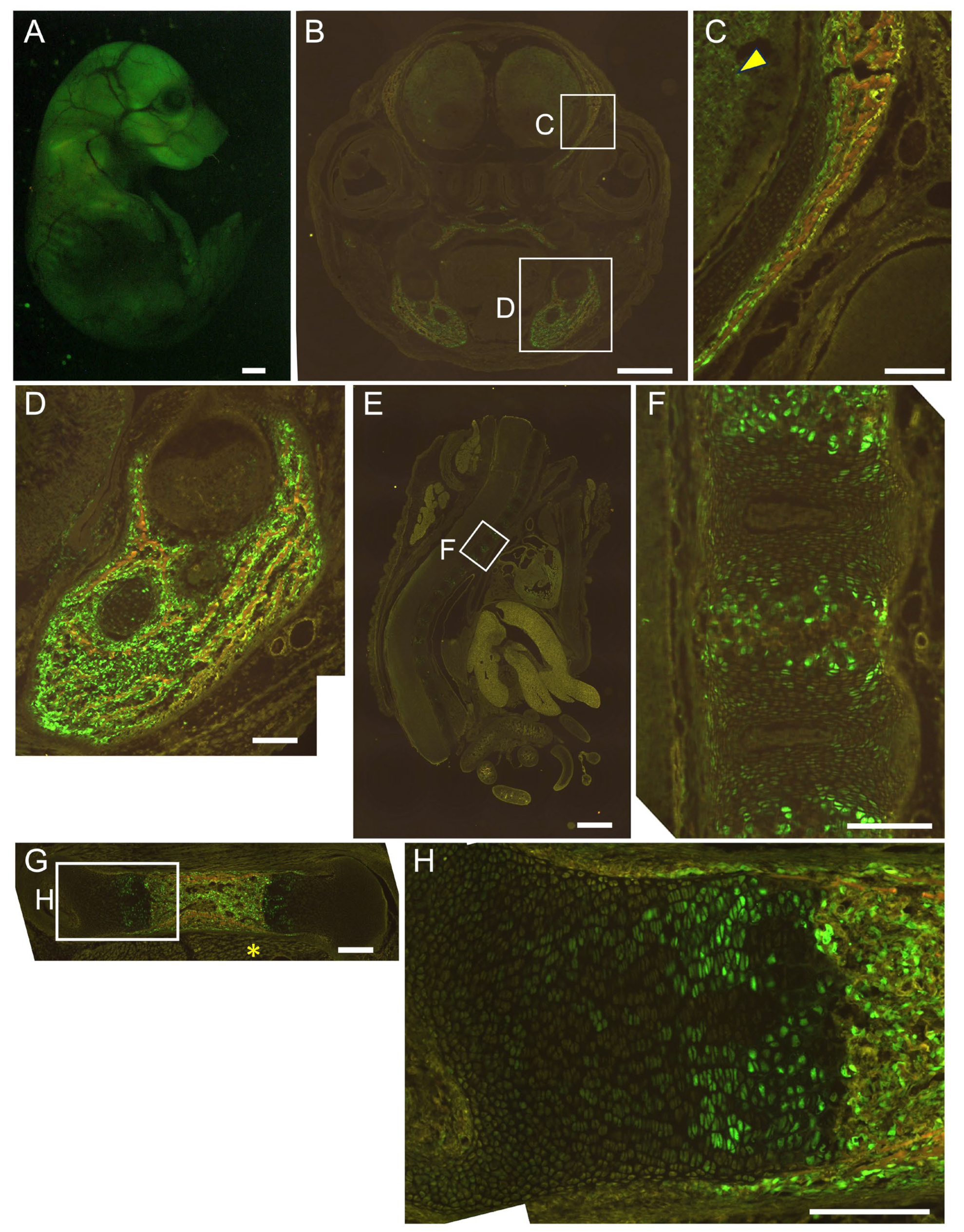
2.3. The 0.8 kb DNA Region and 1.3 kb Enhancer Cooperatively Directed Reporter EGFP Expression to Chondrocytes
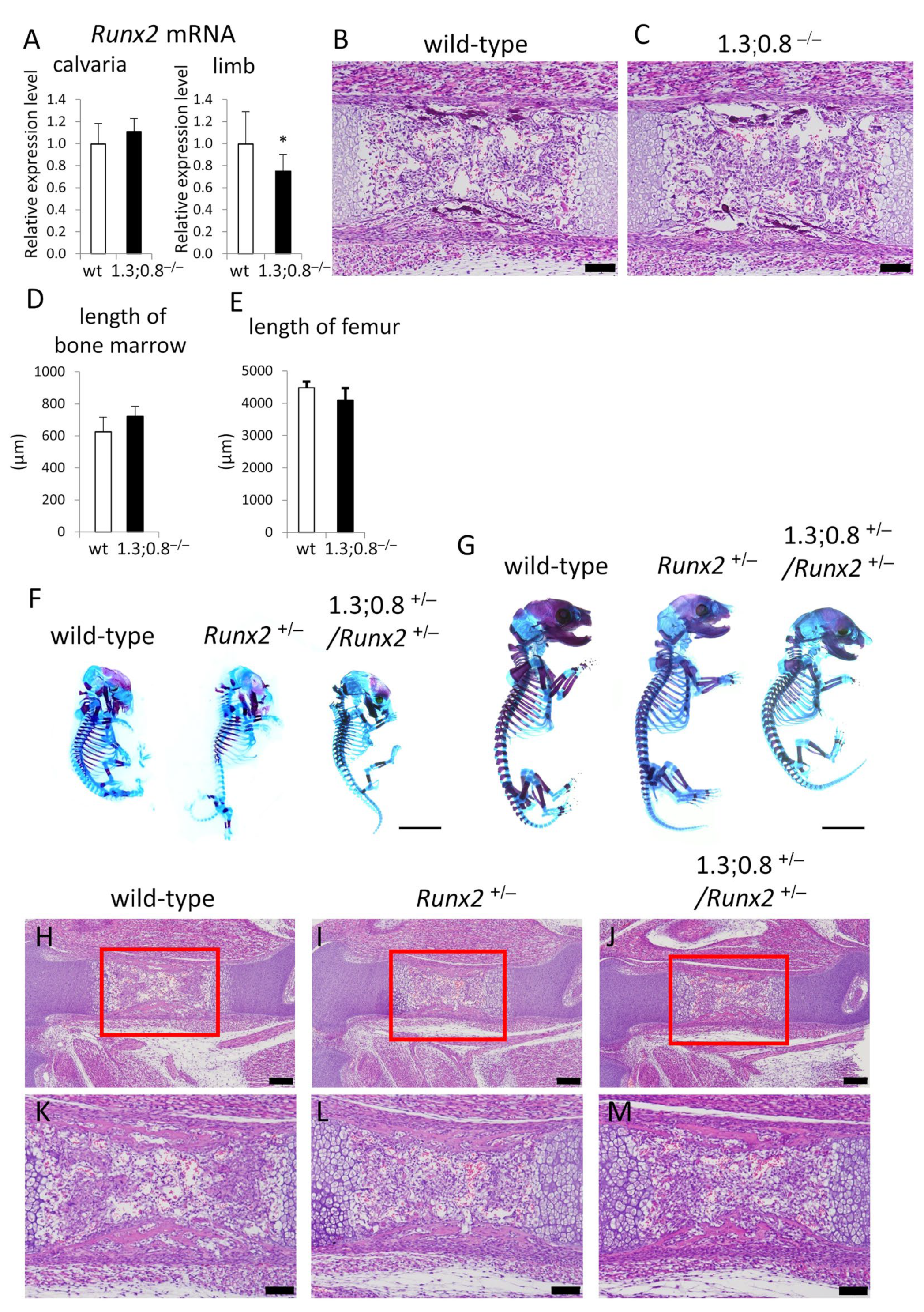
2.4. The Deletion of 1.3 kb and 0.8 kb Regions Showed No Apparent Phenotypes
3. Discussion

4. Materials and Methods
4.1. Generation of Transgenic and Runx2 Enhancer-Deletion Mice
4.2. Skeletal Preparation and Micro-CT Analysis
4.3. Cell Lines
4.4. Cell Culture and ChIP Analysis
4.5. Histological Analyses
4.6. Reporter Assay
4.7. Real-Time RT-qPCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, S.; Wysocka, J. Deciphering the multi-scale, quantitative cis-regulatory code. Mol. Cell 2023, 83, 373–392. [Google Scholar] [CrossRef]
- Ong, C.T.; Corces, V.G. Enhancers: Emerging roles in cell fate specification. EMBO Rep. 2012, 13, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Osterwalder, M.; Barozzi, I.; Tissières, V.; Fukuda-Yuzawa, Y.; Mannion, B.J.; Afzal, S.Y.; Lee, E.A.; Zhu, Y.; Plajzer-Frick, I.; Pickle, C.S.; et al. Enhancer redundancy provides phenotypic robustness in mammalian development. Nature 2018, 554, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Hendrix, D.A.; Levine, M.S. Shadow enhancers as a source of evolutionary novelty. Science 2008, 321, 1314. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.; Wunderlich, Z.; Enciso, G. Shadow enhancers mediate trade-offs between transcriptional noise and fidelity. PLoS Comput. Biol. 2023, 19, e1011071. [Google Scholar] [CrossRef]
- Frankel, N.; Davis, G.K.; Vargas, D.; Wang, S.; Payre, F.; Stern, D.L. Phenotypic robustness conferred by apparently redundant transcriptional enhancers. Nature 2010, 466, 490–493. [Google Scholar] [CrossRef]
- Waymack, R.; Fletcher, A.; Enciso, G.; Wunderlich, Z. Shadow enhancers can suppress input transcription factor noise through distinct regulatory logic. Elife 2020, 9, e59351. [Google Scholar] [CrossRef]
- Perry, M.W.; Boettiger, A.N.; Levine, M. Multiple enhancers ensure precision of gap gene-expression patterns in the Drosophila embryo. Proc. Natl. Acad. Sci. USA 2011, 108, 13570–13575. [Google Scholar] [CrossRef]
- Cannavò, E.; Khoueiry, P.; Garfield, D.A.; Geeleher, P.; Zichner, T.; Gustafson, E.H.; Ciglar, L.; Korbel, J.O.; Furlong, E.E. Shadow Enhancers Are Pervasive Features of Developmental Regulatory Networks. Curr. Biol. 2016, 26, 38–51. [Google Scholar] [CrossRef]
- Will, A.J.; Cova, G.; Osterwalder, M.; Chan, W.L.; Wittler, L.; Brieske, N.; Heinrich, V.; de Villartay, J.P.; Vingron, M.; Klopocki, E.; et al. Composition and dosage of a multipartite enhancer cluster control developmental expression of Ihh (Indian hedgehog). Nat. Genet. 2017, 49, 1539–1545. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Skeletal Development and Maintenance by Runx2 and Sp7. Int. J. Mol. Sci. 2024, 25, 10102. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Otto, F.; Zabel, B.; Mundlos, S. Regulation of chondrocyte differentiation by Cbfa1. Mech. Dev. 1999, 80, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Inada, M.; Yasui, T.; Nomura, S.; Miyake, S.; Deguchi, K.; Himeno, M.; Sato, M.; Yamagiwa, H.; Kimura, T.; Yasui, N.; et al. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn. 1999, 214, 279–290. [Google Scholar] [CrossRef]
- Yoshida, C.A.; Yamamoto, H.; Fujita, T.; Furuichi, T.; Ito, K.; Inoue, K.; Yamana, K.; Zanma, A.; Takada, K.; Ito, Y.; et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes. Dev. 2004, 18, 952–963. [Google Scholar] [CrossRef]
- Shu, B.; Zhang, M.; Xie, R.; Wang, M.; Jin, H.; Hou, W.; Tang, D.; Harris, S.E.; Mishina, Y.; O’Keefe, R.J.; et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J. Cell Sci. 2011, 124, 3428–3440. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Hu, J.; Zhang, L.; Wei, C.; Sultana, N.; Cai, X.; Zhang, W.; Cai, C.L. Smad4 deficiency impairs chondrocyte hypertrophy via the Runx2 transcription factor in mouse skeletal development. J. Biol. Chem. 2018, 293, 9162–9175. [Google Scholar] [CrossRef]
- Dong, Y.F.; Soung, D.Y.; Schwarz, E.M.; O’Keefe, R.J.; Drissi, H. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J. Cell Physiol. 2006, 208, 77–86. [Google Scholar] [CrossRef]
- Lengner, C.J.; Hassan, M.Q.; Serra, R.W.; Lepper, C.; van Wijnen, A.J.; Stein, J.L.; Lian, J.B.; Stein, G.S. Nkx3.2-mediated repression of Runx2 promotes chondrogenic differentiation. J. Biol. Chem. 2005, 280, 15872–15879. [Google Scholar] [CrossRef]
- Yamashita, S.; Andoh, M.; Ueno-Kudoh, H.; Sato, T.; Miyaki, S.; Asahara, H. Sox9 directly promotes Bapx1 gene expression to repress Runx2 in chondrocytes. Exp. Cell Res. 2009, 315, 2231–2240. [Google Scholar] [CrossRef]
- Fujiwara, M.; Tagashira, S.; Harada, H.; Ogawa, S.; Katsumata, T.; Nakatsuka, M.; Komori, T.; Takada, H. Isolation and characterization of the distal promoter region of mouse Cbfa1. Biochim. Biophys. Acta 1999, 1446, 265–272. [Google Scholar] [CrossRef]
- Xiao, Z.S.; Hjelmeland, A.B.; Quarles, L.D. Selective deficiency of the “bone-related” Runx2-II unexpectedly preserves osteoblast-mediated skeletogenesis. J. Biol. Chem. 2004, 279, 20307–20313. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Lengner, C.J.; Gaur, T.; Lou, Y.; Hussain, S.; Jones, M.D.; Borodic, B.; Colby, J.L.; Steinman, H.A.; van Wijnen, A.J.; et al. Runx2 protein expression utilizes the Runx2 P1 promoter to establish osteoprogenitor cell number for normal bone formation. J. Biol. Chem. 2011, 286, 30057–30070. [Google Scholar] [CrossRef] [PubMed]
- Kawane, T.; Komori, H.; Liu, W.; Moriishi, T.; Miyazaki, T.; Mori, M.; Matsuo, Y.; Takada, Y.; Izumi, S.; Jiang, Q.; et al. Dlx5 and mef2 regulate a novel runx2 enhancer for osteoblast-specific expression. J. Bone Miner. Res. 2014, 29, 1960–1969. [Google Scholar] [CrossRef]
- Okura, H.; Sato, S.; Kishikawa, S.; Kaneto, S.; Nakashima, T.; Yoshida, N.; Takayanagi, H.; Kiyono, H. Runx2-I isoform contributes to fetal bone formation even in the absence of specific N-terminal amino acids. PLoS ONE 2014, 9, e108294. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Awad, H.A.; Liu, S.; Mahlios, J.; Zhang, S.; Guilak, F.; Mayo, M.S.; Quarles, L.D. Selective Runx2-II deficiency leads to low-turnover osteopenia in adult mice. Dev. Biol. 2005, 283, 345–356. [Google Scholar] [CrossRef]
- Zambotti, A.; Makhluf, H.; Shen, J.; Ducy, P. Characterization of an osteoblast-specific enhancer element in the CBFA1 gene. J. Biol. Chem. 2002, 277, 41497–41506. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, Y.J.; Yoon, W.J.; Kim, J.I.; Kim, B.G.; Hwang, Y.S.; Wozney, J.M.; Chi, X.Z.; Bae, S.C.; Choi, K.Y.; et al. Dlx5 specifically regulates Runx2 type II expression by binding to homeodomain-response elements in the Runx2 distal promoter. J. Biol. Chem. 2005, 280, 35579–35587. [Google Scholar] [CrossRef]
- Hassan, M.Q.; Tare, R.; Lee, S.H.; Mandeville, M.; Weiner, B.; Montecino, M.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. HOXA10 controls osteoblastogenesis by directly activating bone regulatory and phenotypic genes. Mol. Cell Biol. 2007, 27, 3337–3352. [Google Scholar] [CrossRef]
- Zhang, Y.; Hassan, M.Q.; Xie, R.L.; Hawse, J.R.; Spelsberg, T.C.; Montecino, M.; Stein, J.L.; Lian, J.B.; van Wijnen, A.J.; Stein, G.S. Co-stimulation of the bone-related Runx2 P1 promoter in mesenchymal cells by SP1 and ETS transcription factors at polymorphic purine-rich DNA sequences (Y-repeats). J. Biol. Chem. 2009, 284, 3125–3135. [Google Scholar] [CrossRef]
- Gaur, T.; Lengner, C.J.; Hovhannisyan, H.; Bhat, R.A.; Bodine, P.V.; Komm, B.S.; Javed, A.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 2005, 280, 33132–33140. [Google Scholar] [CrossRef]
- Lengner, C.J.; Drissi, H.; Choi, J.Y.; van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Lian, J.B. Activation of the bone-related Runx2/Cbfa1 promoter in mesenchymal condensations and developing chondrocytes of the axial skeleton. Mech. Dev. 2002, 114, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Lee, S.W.; Park, M.H.; Bae, Y.C.; Shin, H.I.; Nam, S.; Kim, Y.J.; Kim, H.J.; Ryoo, H.M. Spatio-temporal expression patterns of Runx2 isoforms in early skeletogenesis. Exp. Mol. Med. 2002, 34, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Enomoto-Iwamoto, M.; Iwamoto, M.; Nomura, S.; Himeno, M.; Kitamura, Y.; Kishimoto, T.; Komori, T. Cbfa1 is a positive regulatory factor in chondrocyte maturation. J. Biol. Chem. 2000, 275, 8695–8702. [Google Scholar]
- Tasic, B.; Hippenmeyer, S.; Wang, C.; Gamboa, M.; Zong, H.; Chen-Tsai, Y.; Luo, L. Site-specific integrase-mediated transgenesis in mice via pronuclear injection. Proc. Natl. Acad. Sci. USA 2011, 108, 7902–7907. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.; Alves, M.R.; Crocker, J. Multi-enhancer transcriptional hubs confer phenotypic robustness. Elife 2019, 8, e45325. [Google Scholar] [CrossRef]
- Arnold, M.A.; Kim, Y.; Czubryt, M.P.; Phan, D.; McAnally, J.; Qi, X.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev. Cell 2007, 12, 377–389. [Google Scholar] [CrossRef]
- Galouzis, C.C.; Furlong, E.E.M. Regulating specificity in enhancer-promoter communication. Curr. Opin. Cell Biol. 2022, 75, 102065. [Google Scholar] [CrossRef]
- Otani, S.; Date, Y.; Ueno, T.; Ito, T.; Kajikawa, S.; Omori, K.; Taniuchi, I.; Umeda, M.; Komori, T.; Toguchida, J.; et al. Runx3 is required for oncogenic Myc upregulation in p53-deficient osteosarcoma. Oncogene 2022, 41, 683–691. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, Q.; Nagano, K.; Moriishi, T.; Miyazaki, T.; Komori, H.; Ito, K.; Mark, K.V.; Sakane, C.; Kaneko, H.; et al. Runx2 is essential for the transdifferentiation of chondrocytes into osteoblasts. PLoS Genet. 2020, 16, e1009169. [Google Scholar] [CrossRef]
- Kiyonari, H.; Kaneko, M.; Abe, S.; Aizawa, S. Three inhibitors of FGF receptor, ERK, and GSK3 establishes germline-competent embryonic stem cells of C57BL/6N mouse strain with high efficiency and stability. Genesis 2010, 48, 317–327. [Google Scholar] [CrossRef]
- Kudawara, I.; Araki, N.; Myoui, A.; Kato, Y.; Uchida, A.; Yoshikawa, H. New cell lines with chondrocytic phenotypes from human chondrosarcoma. Virchows Arch. 2004, 444, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Kunisada, T.; Miyazaki, M.; Mihara, K.; Gao, C.; Kawai, A.; Inoue, H.; Namba, M. A new human chondrosarcoma cell line (OUMS-27) that maintains chondrocytic differentiation. Int. J. Cancer 1998, 77, 854–859. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, Y.; Qin, X.; Moriishi, T.; Kawata-Matsuura, V.K.S.; Komori, H.; Sakane, C.; Yabuta, S.; Jiang, Q.; Kaneko, H.; Ito, K.; et al. An Osteoblast-Specific Enhancer and Subenhancer Cooperatively Regulate Runx2 Expression in Chondrocytes. Int. J. Mol. Sci. 2025, 26, 1653. https://doi.org/10.3390/ijms26041653
Matsuo Y, Qin X, Moriishi T, Kawata-Matsuura VKS, Komori H, Sakane C, Yabuta S, Jiang Q, Kaneko H, Ito K, et al. An Osteoblast-Specific Enhancer and Subenhancer Cooperatively Regulate Runx2 Expression in Chondrocytes. International Journal of Molecular Sciences. 2025; 26(4):1653. https://doi.org/10.3390/ijms26041653
Chicago/Turabian StyleMatsuo, Yuki, Xin Qin, Takeshi Moriishi, Viviane K. S. Kawata-Matsuura, Hisato Komori, Chiharu Sakane, Suemi Yabuta, Qing Jiang, Hitomi Kaneko, Kosei Ito, and et al. 2025. "An Osteoblast-Specific Enhancer and Subenhancer Cooperatively Regulate Runx2 Expression in Chondrocytes" International Journal of Molecular Sciences 26, no. 4: 1653. https://doi.org/10.3390/ijms26041653
APA StyleMatsuo, Y., Qin, X., Moriishi, T., Kawata-Matsuura, V. K. S., Komori, H., Sakane, C., Yabuta, S., Jiang, Q., Kaneko, H., Ito, K., Shigeta, M., Abe, T., & Komori, T. (2025). An Osteoblast-Specific Enhancer and Subenhancer Cooperatively Regulate Runx2 Expression in Chondrocytes. International Journal of Molecular Sciences, 26(4), 1653. https://doi.org/10.3390/ijms26041653





