Anthelmintic Potential and In Silico Studies of Ricinoleic Acid from the Seed Oil of Ricinus communis L.
Abstract
1. Introduction
2. Results
2.1. Extraction Yield
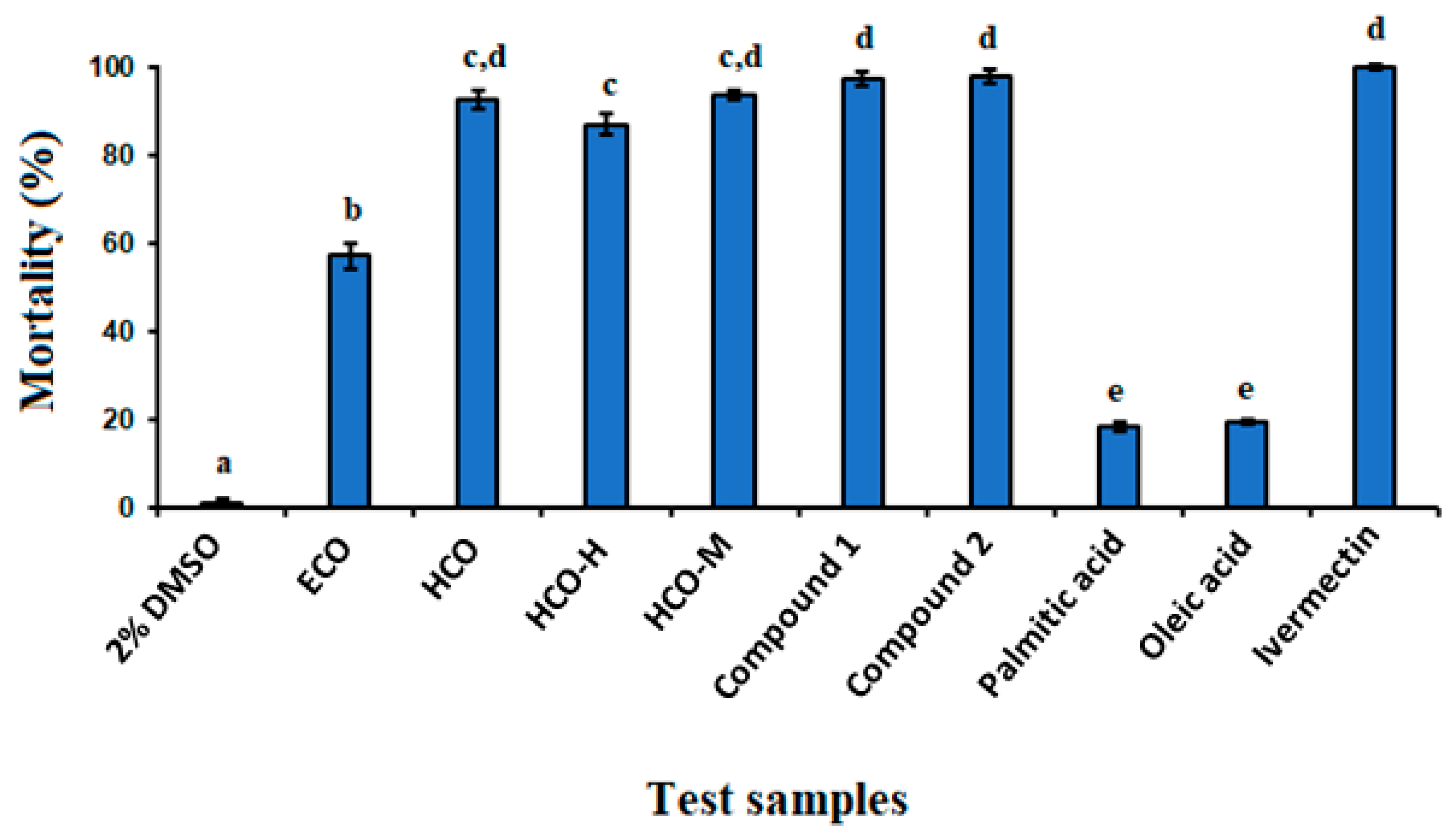
2.2. In Vitro Anthelmintic Activity
2.3. Structural Elucidation of Compounds 1 and 2
2.4. Anthelmintic Activity of Ricinoleic Acid and Methyl Ricinoleate
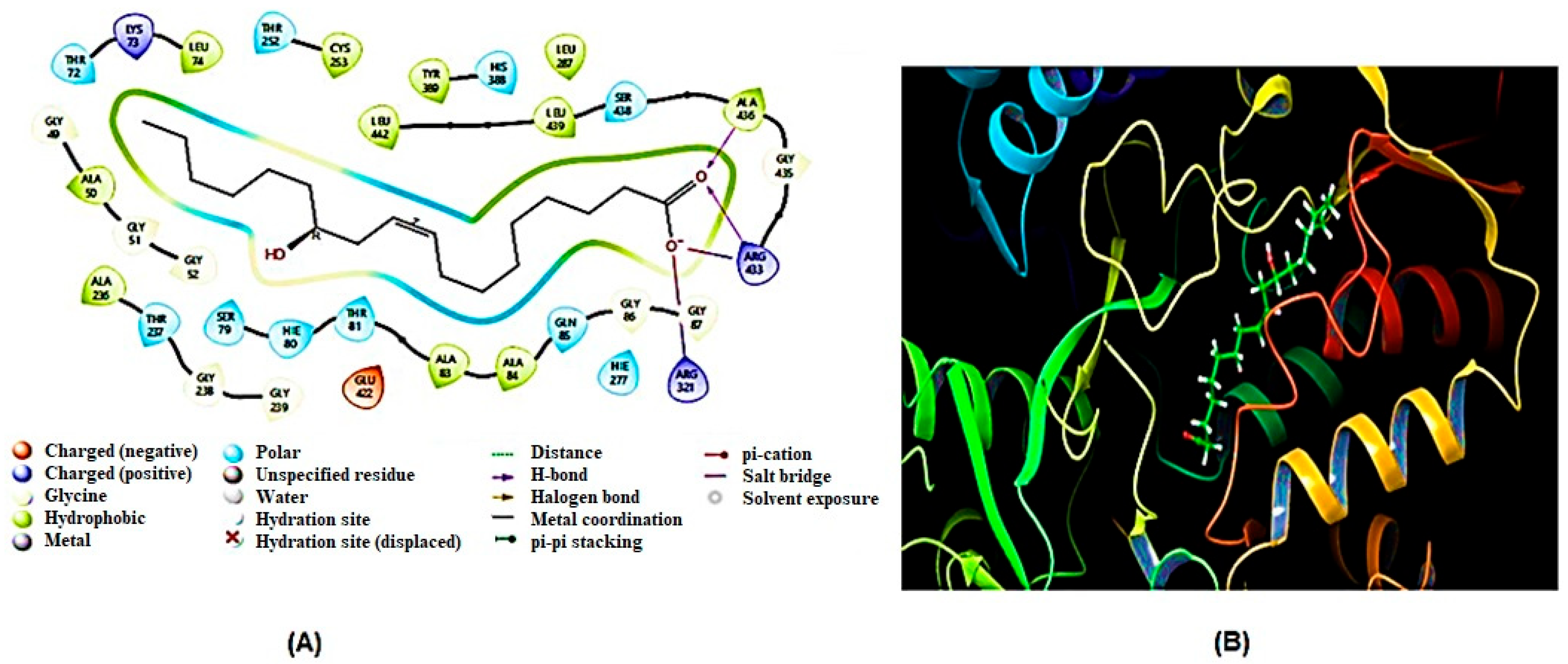
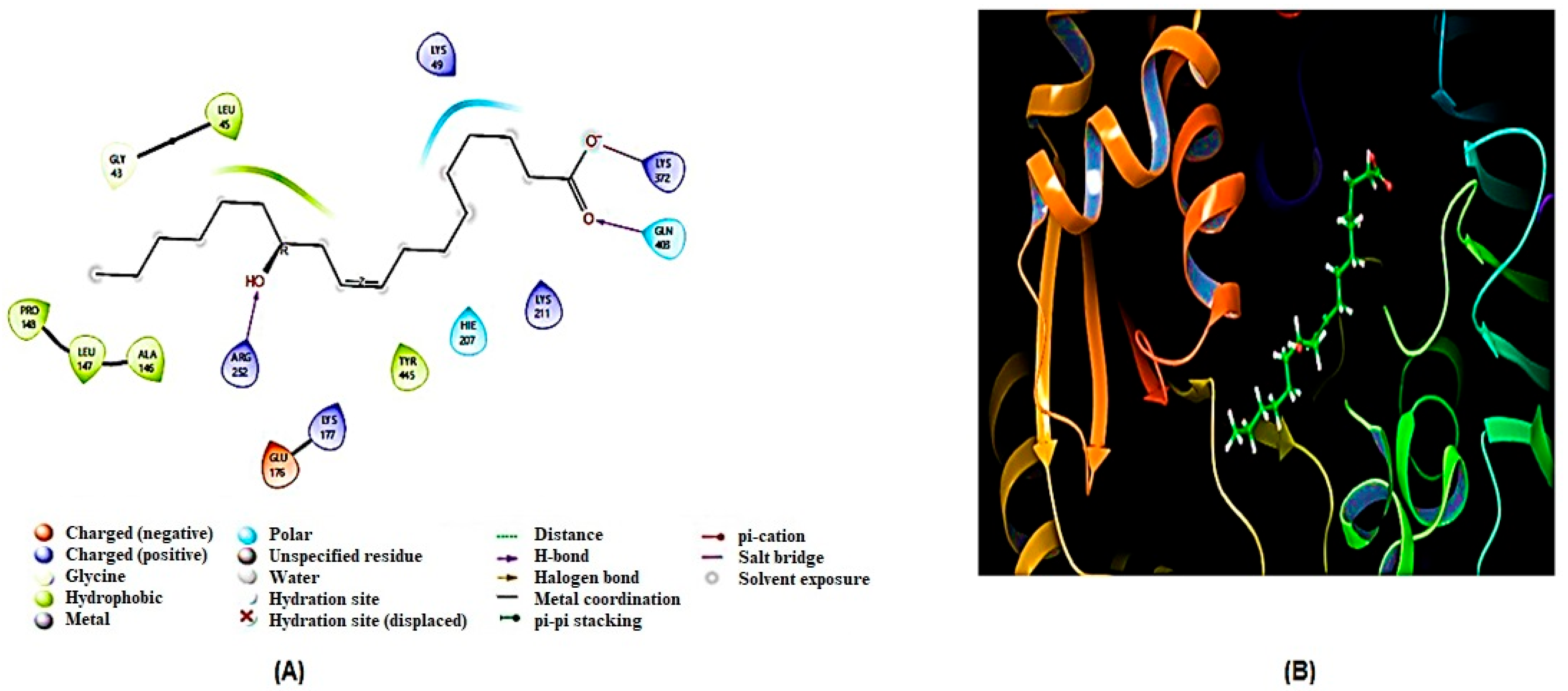
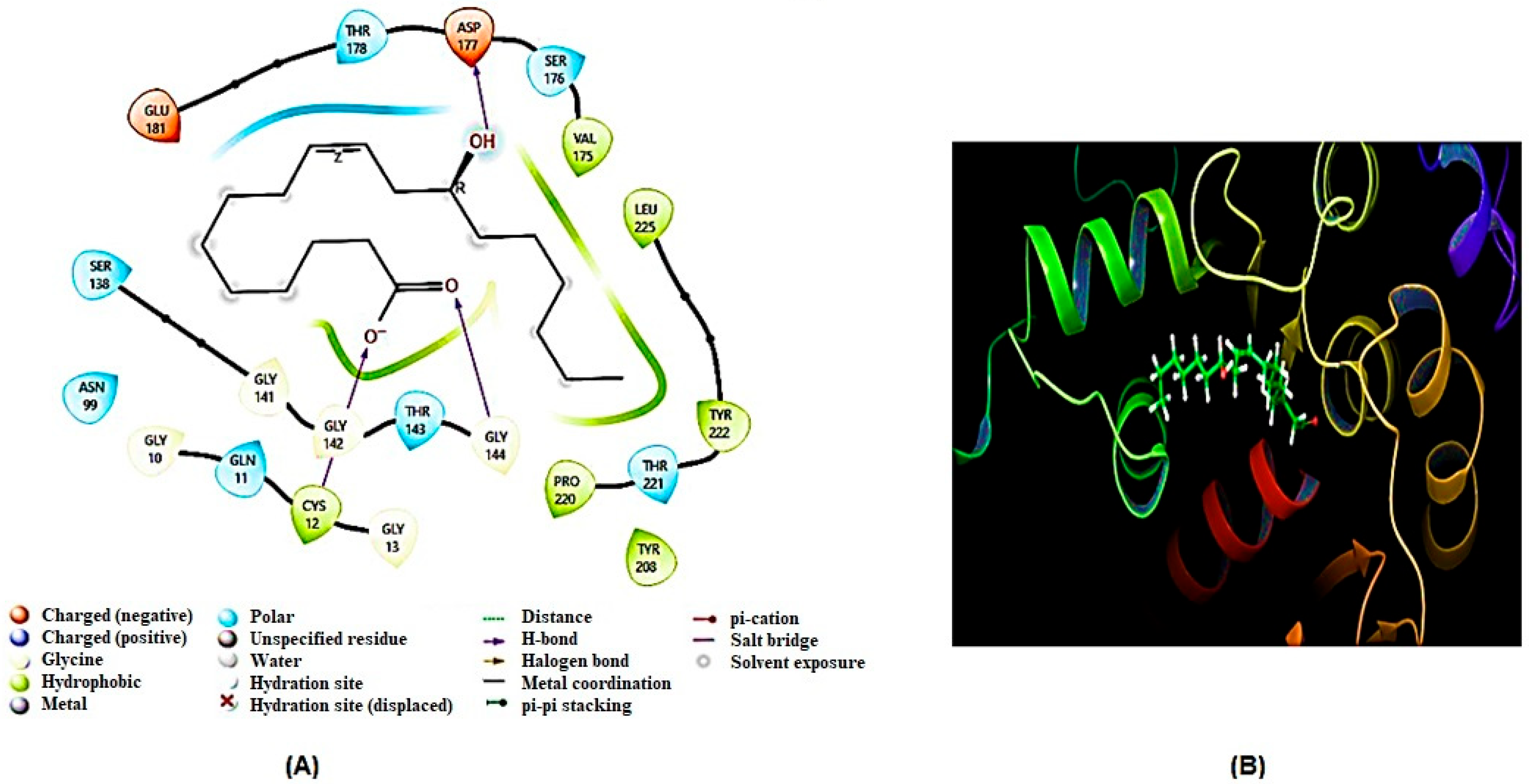
2.5. Molecular Docking Analysis
2.5.1. Homology Modeling and Structure Validations of Modeled Proteins
2.5.2. Docking Results
2.5.3. ADME (Absorption, Distribution, Metabolism, and Excretion) Property Prediction
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Reagents
4.3. Test Organisms
4.4. Extraction
4.5. Hydrolysis of the Oil
4.6. Isolation of Compound
4.7. Esterification of Compound 1
4.8. Caenorhabditis Elegans Assay
4.9. Molecular Docking Studies
4.9.1. Structure Validation of Homology Modeled Protein
4.9.2. Preparation of the Target Proteins
4.9.3. Ligand Preparation
4.9.4. Prediction for Active Protein Site
4.9.5. Ligand Docking
4.9.6. ADME Descriptors Analysis
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Brooker, S.; Bethony, J.M.; Bottazzi, M.E.; Loukas, A.; Xiao, S. Hookworm infection. N. Engl. J. Med. 2004, 351, 799–807. [Google Scholar] [CrossRef]
- Farrell, S.H.; Coffeng, L.E.; Truscott, J.E.; Werkman, M.; Toor, J.; de Vlas, S.J.; Anderson, R.M. Investigating the effectiveness of current and modified World Health Organization guidelines for the control of soil-transmitted helminth Infections. Clin. Infect. Dis. 2018, 66, S253–S259. [Google Scholar] [CrossRef]
- Pabalan, N.; Singian, E.; Tabangay, L.; Jarjanazi, H.; Boivin, M.J.; Ezeamama, A.E. Soil-transmitted helminth infection, loss of education and cognitive impairment in school-aged children: A systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2018, 12, e0005523. [Google Scholar] [CrossRef]
- Baker, S.M.; Ensink, J.H. Helminth transmission in simple pit latrines. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 709–710. [Google Scholar] [CrossRef]
- Asaolu, S.; Ofoezie, I. The role of health education and sanitation in the control of helminth infections. Acta Trop. 2003, 86, 283–294. [Google Scholar] [CrossRef]
- Mutombo, P.N.; Man, N.W.; Nejsum, P.; Ricketson, R.; Gordon, C.A.; Robertson, G.; Clements, A.C.; Chacón-Fonseca, N.; Nissapatorn, V.; Webster, J.P.; et al. Diagnosis and drug resistance of human soil-transmitted helminth infections: A public health perspective. Adv. Parasitol. 2019, 104, 247–326. [Google Scholar]
- Coles, G.C. Developments in the chemotherapy of parasitic flatworms. In Parasitic Flatworms: Molecular Biology Biochemistry, Unology and Physiology; Maule, A.G., Marks, N.J., Eds.; CABI: Wallingford, UK, 2006; Chapter 12; pp. 243–255. [Google Scholar]
- Cheraghipour, K.; Moridnia, A.; Sharifi, M.; Mohaghegh, M.A.; Khanizadeh, S.; Nourmohammadi, M.; Kalani, H. The effect of medicinal plant extracts on helminthes: A systematic review. J. Isfahan Med. Sch. 2019, 37, 462–474. [Google Scholar]
- David, B.; Wolfender, J.-L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Hrčková, G.; Velebný, S. Parasitic Helminths of Humans and Animals: Health Impact and Control. In Pharmacological Potential of Selected Natural Compounds in the Control of Parasitic Diseases; Hrckova, G., Velebny, S., Eds.; Springer: Vienna, Austria, 2013; pp. 29–99. [Google Scholar]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef]
- World Health Organization. National Policy on Traditional Medicine and Regulation of Herbal Medicines: Report of a WHO Global Survey; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. Indian Traditional Ayurvedic System of Medicine and Nutritional Supplementation. Evidence-Based Complement. Altern. Med. 2013, 2013, 376327. [Google Scholar] [CrossRef]
- Das, S.; Choudhury, M.D. Ethnomedicinal uses of some traditional medicinal plants found in Tripura, India. J. Med. Plants Res. 2012, 6, 908–914. [Google Scholar] [CrossRef]
- Scantlebury, C.E.; Peachey, L.; Hodgkinson, J.; Matthews, J.B.; Trawford, A.; Mulugeta, G.; Tefera, G.; Pinchbeck, G.L. Participatory study of medicinal plants used in the control of gastrointestinal parasites in donkeys in Eastern Shewa and Arsi zones of Oromia region, Ethiopia. BMC Vet. Res. 2013, 9, 179. [Google Scholar] [CrossRef]
- Mesfin, F.; Demissew, S.; Teklehaymanot, T. An ethnobotanical study of medicinal plants in Wonago Woreda, SNNPR, Ethiopia. J. Ethnobiol. Ethnomed. 2009, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Giday, M.; Asfaw, Z.; Woldu, Z. Medicinal plants of the Meinit ethnic group of Ethiopia: An ethnobotanical study. J. Ethnopharmacol. 2009, 124, 513–521. [Google Scholar] [CrossRef]
- Singh, A.; Gowri, K.; Chitra, V. A review on phytochemical constituents and pharmacological activities of the plant: Aerva lanata. Res. J. Pharm. Technol. 2020, 13, 1580–1586. [Google Scholar] [CrossRef]
- Odunsi, T.A.A.; Olabode, O.S.; Ojediran, T.K. Physical and nutrient characterisation of raw and processed castor (Ricinus communis L.) seeds in Nigeria. World J. Agric. Sci. 2012, 8, 89–95. [Google Scholar]
- Franke, H.; Scholl, R.; Aigner, A. Ricin and Ricinus communis in pharmacology and toxicology-from ancient use and “Papyrus Ebers” to modern perspectives and “poisonous plant of the year 2018. Naunyn Schmiedebergs Arch Pharmacol. 2019, 392, 1181–1208. [Google Scholar] [CrossRef] [PubMed]
- McKeon, T.A. Emerging Industrial Oil Crops; AOCS Press, 2016; pp. 75–112. [Google Scholar]
- Mahadev, N.D.; Thorat, A.T.; Vitthal, B.P. An evaluation of anthelmintic activity of Ricinus communis Linn. leaves by using different type of solvent. J. Pharmacogn. Phytochem. 2017, 6, 1845–1847. [Google Scholar]
- Rana, M.; Kumar, H.; Parashar, B. In vitro anthelmintic activity of bark of Ricinus communis Linn. J. Chem. Pharm. Res. 2013, 5, 40–42. [Google Scholar]
- Manthri, S.; Sravanthi, K.C.; Sidagonde, S. Anthelmintic activity of castor oil and mustard oil. J. Phytol. 2011, 3, 12–14. [Google Scholar]
- Palikaras, K.; Tavernarakis, N. Caenorhabditis elegans (Nematode). In Brenner’s Encyclopedia of Genetics; Academic Press: Cambridge, MA, USA, 2013; Volume 1, pp. 186–188. [Google Scholar]
- Jolayemi, O.S.; Alagbe, C.I. Influence of seed variety and extraction technique on fatty acid distribution and quality parameters of tropical castor (Ricinus communis L.) Oils. J. Oleo Sci. 2022, 71, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Dotto, J.M.; Chacha, J.S. The potential of pumpkin seeds as a functional food ingredient: A review: Biofunctional ingredients of pumpkin seeds. Sci. Afr. 2020, 10, e00575. [Google Scholar]
- Costa, J.d.L.; da Silva, A.L.L.; Bier, M.C.J.; Brondani, G.E.; Gollo, A.L.; Letti, L.A.J.; Erasmo, E.A.L.; Soccol, C.R. Callus growth kinetics of physic nut (Jatropha curcas L.) and content of fatty acids from crude oil obtained in vitro. Appl. Biochem. Biotechnol. 2015, 176, 892–902. [Google Scholar] [CrossRef]
- Katase, M.; Kubo, C.; Ushio, S.; Ootsuka, E.; Takeuchi, T.; Mizukubo, T. Nematicidal activity of volatile fatty acids generated from wheat bran in reductive soil disinfestation. Nematol. Res. (Jpn. J. Nematol.) 2009, 39, 53–62. [Google Scholar] [CrossRef]
- Bataglion, G.A.; da Silva, F.M.; Santos, J.M.; dos Santos, F.N.; Barcia, M.T.; de Lourenço, C.C.; Salvador, M.J.; Godoy, H.T.; Eberlin, M.N.; Koolen, H.H. Comprehensive characterization of lipids from Amazonian vegetable oils by mass spectrometry techniques. Food Res. Int. 2014, 64, 472–481. [Google Scholar] [CrossRef]
- Wafa, G.; Amadou, D.; Larbi, K.M.; Héla, E.F.O. Larvicidal activity, phytochemical composition, and antioxidant properties of different parts of five populations of Ricinus communis L. Ind. Crop. Prod. 2014, 56, 43–51. [Google Scholar] [CrossRef]
- Ahmad, M.U.; Husain, S.K.; Osman, S.M. Ricinoleic acid in Phyllanthus niruri seed oil. J. Am. Oil Chem. Soc. 1981, 58, 673–674. [Google Scholar] [CrossRef]
- Kołodziej, P.; Wujec, M.; Doligalska, M.; Makuch-Kocka, A.; Khylyuk, D.; Bogucki, J.; Demkowska-Kutrzepa, M.; Roczeń-Karczmarz, M.; Studzińska, M.; Tomczuk, K.; et al. Synthesis and anthelmintic activity of novel thiosemicarbazide and 1,2,4-triazole derivatives: In vitro, in vivo, and in silico study. J. Adv. Res. 2023, 60, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.P.; van Vliet, A.H.M.; LaCourse, E.J.; Betson, M. In silico docking of nematode β-tubulins with benzimidazoles points to gene expression and orthologue variation as factors in anthelmintic resistance. Front. Trop. Dis. 2022, 3. [Google Scholar] [CrossRef]
- QikProp, version 4.4; Schrödinger, LLC: New York, NY, USA, 2015.
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Pink, R.; Hudson, A.; Mouriès, M.-A.; Bendig, M. Opportunities and challenges in antiparasitic drug discovery. Nat. Rev. Drug Discov. 2005, 4, 727–740. [Google Scholar] [CrossRef]
- Buckingham, S.D.; Partridge, F.A.; Sattelle, D.B. Automated, high-throughput, motility analysis in Caenorhabditis elegans and parasitic nematodes: Applications in the search for new anthelmintics. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 226–232. [Google Scholar] [CrossRef]
- Aboobaker, A.A.; Blaxter, M.L. Medical significance of Caenorhabditis elegans. Ann. Med. 2000, 32, 23–30. [Google Scholar] [CrossRef] [PubMed]
- McCarter, J.P. Genomic filtering: An approach to discovering novel antiparasitics. Trends Parasitol. 2004, 20, 462–468. [Google Scholar] [CrossRef]
- Stadler, M.; Mayer, A.; Anke, H.; Sterner, O. Fatty acids and other compounds with nematicidal activity from cultures of Basidiomycetes. Planta Med. 1994, 60, 128–132. [Google Scholar] [CrossRef]
- Hirazawa, N.; Oshima, S.I.; Mitsuboshi, T.; Hata, K. The anthelmintic effect of medium-chain fatty acids against the monogenean Heterobothrium okamotoi in the tiger puffer Takifugu rubripes: Evaluation of doses of caprylic acid at different water temperatures. Aquaculture 2001, 195, 211–223. [Google Scholar] [CrossRef]
- Bonde, C.S.; Bornancin, L.; Lu, Y.; Simonsen, H.T.; Martínez-Valladares, M.; Peña-Espinoza, M.; Mejer, H.; Williams, A.R.; Thamsborg, S.M. Bio-guided fractionation and molecular networking reveal fatty acids to be principal anti-parasitic compounds in Nordic seaweeds. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Davis, E.L.; Meyers, D.M.; Dullum, C.J.; Feitelson, J.S. Nematicidal activity of fatty acid esters on soybean cyst and root-knot. Nematodes 1997, 29, 677–684. [Google Scholar]
- Pineda-Alegría, J.; Sánchez, J.; González-Cortazar, M.; Fernex, E.v.S.-D.; González-Garduño, R.; Gives, P.M.-D.; Zamilpa, A.; Aguilar-Marcelino, L. In vitro nematocidal activity of commercial fatty acids and β-sitosterol against Haemonchus contortus. J. Helminthol. 2020, 94, e135. [Google Scholar] [CrossRef]
- Carvalho, D.C.C.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Kumarasingha, R.; Young, N.D.; Yeo, T.-C.; Lim, D.S.L.; Tu, C.-L.; Palombo, E.A.; Shaw, J.M.; Gasser, R.B.; Boag, P.R. Transcriptional alterations in Caenorhabditis elegans following exposure to an anthelmintic fraction of the plant Picria fel-terrae Lour. Parasites Vectors 2019, 12, 181. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.R.; Luciani, G.M.; Musso, G.; Bagg, R.; Yeo, M.; Zhang, Y.; Rajendran, L.; Glavin, J.; Hunter, R.; Redman, E.; et al. Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat. Commun. 2015, 6, 7485. [Google Scholar] [CrossRef]
- Lalthanpuii, P.B.; Lalchhandama, K. Phytochemical analysis and in vitro anthelmintic activity of Imperata cylindrica underground parts. BMC Complement. Med. Ther. 2020, 20, 332. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Das, R.; Mai, A.H.; De Borggraeve, W.M.; Luyten, W. Nematicidal activity of Holigarna caustica (Dennst.) oken fruit is due to linoleic acid. Biomolecules 2020, 10, 1043. [Google Scholar] [CrossRef]
- Stadler, M.; Anke, H.; Sterner, O. Linoleic acid ˗ The nematicidal principle of several nematophagous fungi and its production in trap-forming submerged cultures. Arch. Microbiol. 1993, 160, 401–405. [Google Scholar] [CrossRef]
- Grzybek, M.; Kukula-Koch, W.; Strachecka, A.; Jaworska, A.; Phiri, A.M.; Paleolog, J.; Tomczuk, K. Evaluation of anthelmintic activity and composition of pumpkin (Cucurbita pepo L.) seed extracts-in vitro and in vivo studies. Int. J. Mol. Sci. 2016, 17, 1456. [Google Scholar] [CrossRef]
- King, A.; Selak, M.A.; Gottlieb, E. Succinate dehydrogenase and fumarate hydratase: Linking mitochondrial dysfunction and cancer. Oncogene 2006, 25, 4675–4682. [Google Scholar] [CrossRef]
- Vaisman, B.; Shikanov, A.; Domb, A.J. The isolation of ricinoleic acid from castor oil by salt-solubility-based fractionation for the biopharmaceutical applications. J. Am. Oil Chem. Soc. 2007, 85, 169–184. [Google Scholar] [CrossRef]
- Aldai, N.; Murray, B.E.; Nájera, A.I.; Troy, D.J.; Osoro, K. Derivatization of fatty acids and its application for conjugated linoleic acid studies in ruminant meat lipids. J. Sci. Food Agric. 2005, 85, 1073–1083. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 643684, Ricinoleic acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ricinoleic-acid (accessed on 2 February 2023).
- Thomsen, H.; Reider, K.; Franke, K.; Wessjohann, L.A.; Keiser, J.; Dagne, E.; Arnold, N. Characterization of constituents and anthelmintic properties of Hagenia abyssinica. Sci. Pharm. 2012, 80, 433–446. [Google Scholar] [CrossRef]
- Schrödinger Suite Release 2023-2, Maestro v.13.5; Schrödinger, LLC.: New York, NY, USA, 2022.
- Abongwa, M.; Martin, R.J.; Robertson, A.P. A brief review on the mode of action of antinematodal drugs. Acta Vet. 2017, 67, 137–152. [Google Scholar] [CrossRef]
- UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [PubMed]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Abdullahi, M.; Adeniji, S.E.; Arthur, D.E.; Haruna, A. Homology modeling and molecular docking simulation of some novel imidazo [1,2-a]pyridine-3-carboxamide (IPA) series as inhibitors of Mycobacterium tuberculosis. J. Genet. Eng. Biotechnol. 2021, 19, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, D.; De Groot, B.L. Conformational transitions upon ligand binding: Holo-structure prediction from apo conformations. Biophys. J. 2010, 98, 428a. [Google Scholar] [CrossRef]

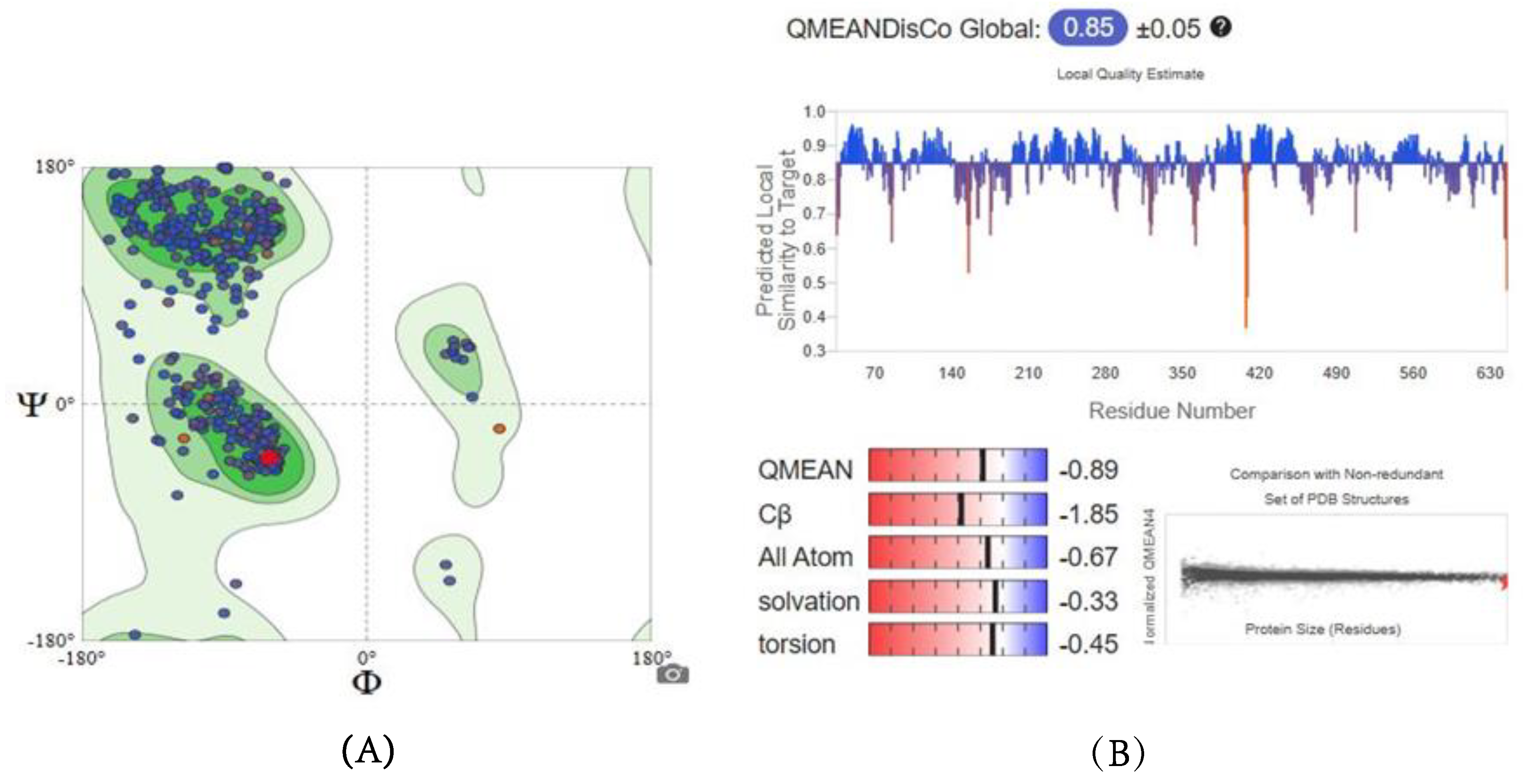
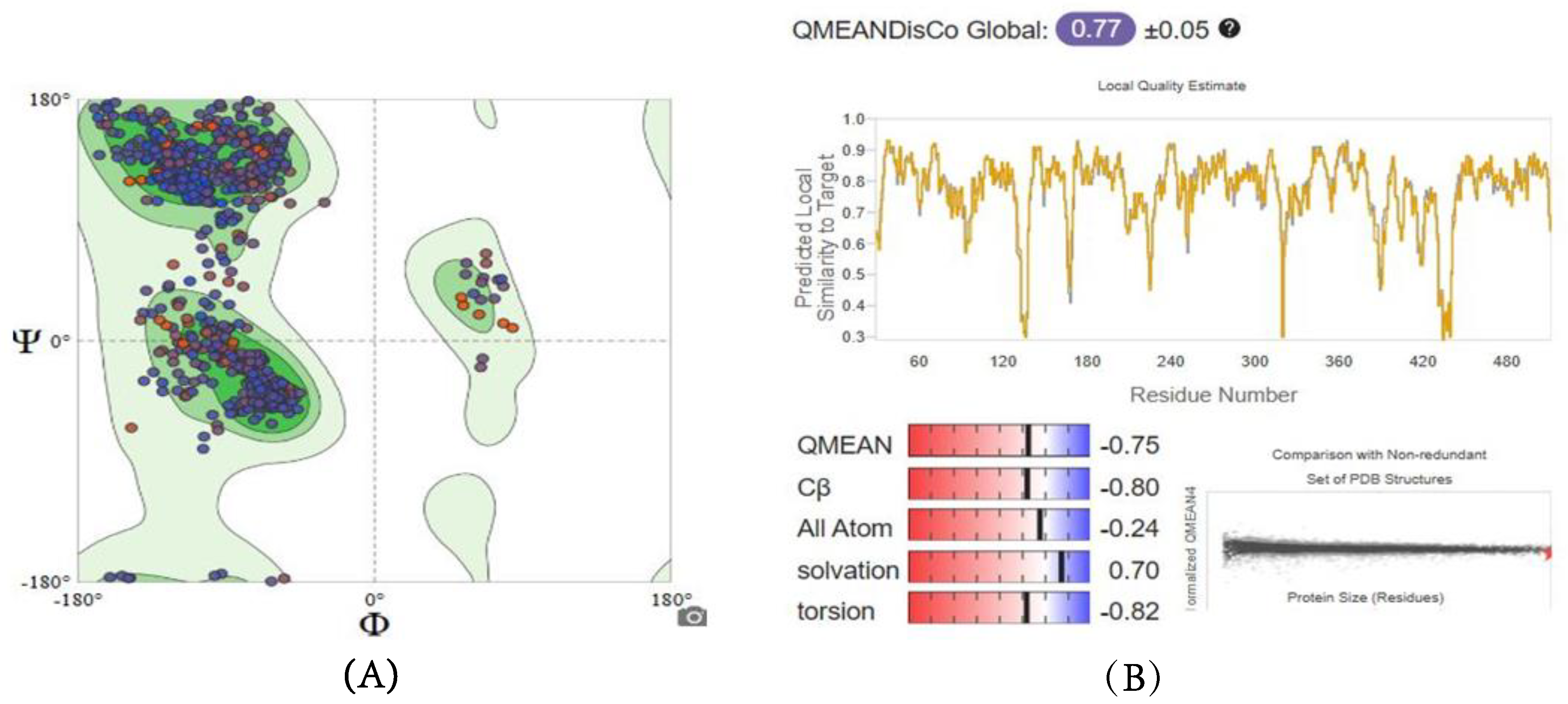
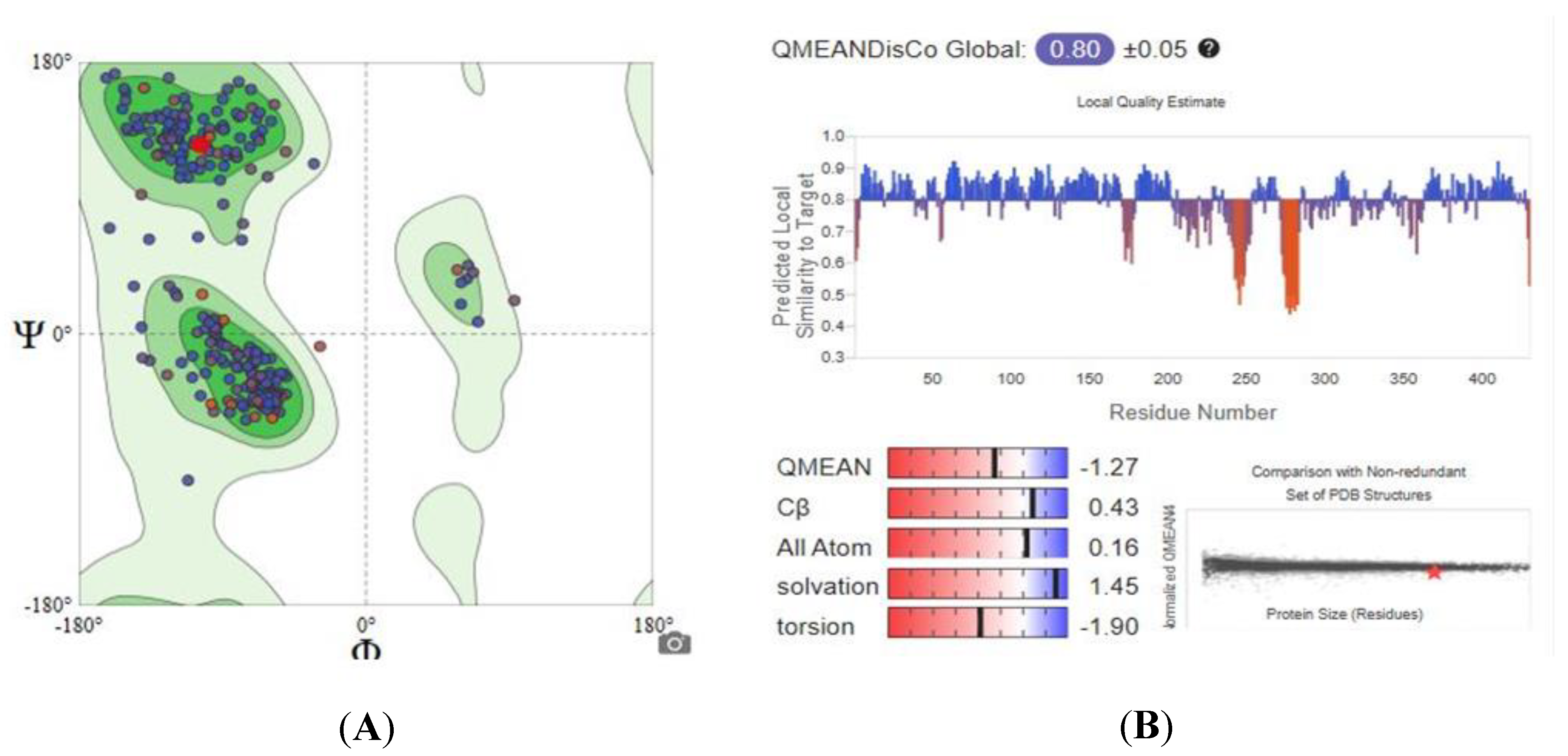
| Enzymes | GMQE Score | QMEAN Score | Sequence Identity Percentage | Ramachandran Plot |
|---|---|---|---|---|
| SDH | 0.86 | −0.89 | 73.62% | 96.39% favored |
| G6PD | 0.86 | −0.75 | 60.53% | 94.61% favored |
| TBB2C | 0.82 | −1.27 | 89.16% | 96.50% favored |
| No | Enzyme | Docking Score (kcal/mol) | Glide Score (kcal/mol) | Glide Model (kcal/mol) | |||
|---|---|---|---|---|---|---|---|
| Ricinoleic Acid | Mebendazole | Ricinoleic Acid | Mebendazole | Ricinoleic Acid | Mebendazole | ||
| 1 | SDH | −5.408 | −6.243 | −5.413 | −6.558 | −71.326 | −66.032 |
| 2 | G6PD | −3.758 | −3.814 | −3.763 | −4.130 | −31.923 | −41.351 |
| 3 | TBB2C | −1.444 | −4.756 | −1.449 | −5.072 | −35.155 | −50.874 |
| No | Enzyme | H–Bonds AA | Non–H–Bonds AA |
|---|---|---|---|
| 1 | SDH | Arg433, Ala436, Ala236 | Thr72, Leu442, Ala50, Gly49, Ala53, Thr237, Lys73, Leu74, Thr81, Ser438, Leu439, Glu422, Ala436, Gly85, Hie80 |
| 2 | G6PD | Gly403, Arg252 | Lys49, Lys211, 297, Lys177, Glu176, Ala146, Leu147, Pro148, Leu45, Tyr445 |
| 3 | TBB2C | Cys12, Asp177 | Leu225, Ala228, Trp231, Met213, Val214, Gln215, Asn216, Leu217, Met218, Val219, Arg221, Thr221, Phe222, Lys281 |
| Descriptor | Predicted Values | Recommended Values [36] |
|---|---|---|
| Molecular weight (mol MW) | 298.465 g/mol | 130.0–725.0 |
| Hydrogen bond donor (donorHB) | 2 | 0.0–6.0 |
| Hydrogen bond acceptor (acceptHB) | 3.7 | 2.0–20.0 |
| High lipophilicity (QPlogPo/W) | 4.801 | −2.0–6.5 |
| Molar refractivity (QPpolrz) | 32.945 | 13–70.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berhanu, T.; Tewelde, E.; Yeshak, M.Y.; Bisrat, D.; Asres, K. Anthelmintic Potential and In Silico Studies of Ricinoleic Acid from the Seed Oil of Ricinus communis L. Int. J. Mol. Sci. 2025, 26, 1636. https://doi.org/10.3390/ijms26041636
Berhanu T, Tewelde E, Yeshak MY, Bisrat D, Asres K. Anthelmintic Potential and In Silico Studies of Ricinoleic Acid from the Seed Oil of Ricinus communis L. International Journal of Molecular Sciences. 2025; 26(4):1636. https://doi.org/10.3390/ijms26041636
Chicago/Turabian StyleBerhanu, Temesgen, Eyael Tewelde, Mariamawit Y. Yeshak, Daniel Bisrat, and Kaleab Asres. 2025. "Anthelmintic Potential and In Silico Studies of Ricinoleic Acid from the Seed Oil of Ricinus communis L." International Journal of Molecular Sciences 26, no. 4: 1636. https://doi.org/10.3390/ijms26041636
APA StyleBerhanu, T., Tewelde, E., Yeshak, M. Y., Bisrat, D., & Asres, K. (2025). Anthelmintic Potential and In Silico Studies of Ricinoleic Acid from the Seed Oil of Ricinus communis L. International Journal of Molecular Sciences, 26(4), 1636. https://doi.org/10.3390/ijms26041636






