Regulation of NO-Generating System Activity in Cucumber Root Response to Cold
Abstract
1. Introduction
2. Results
2.1. Growth of Cucumber Roots Under LT Conditions
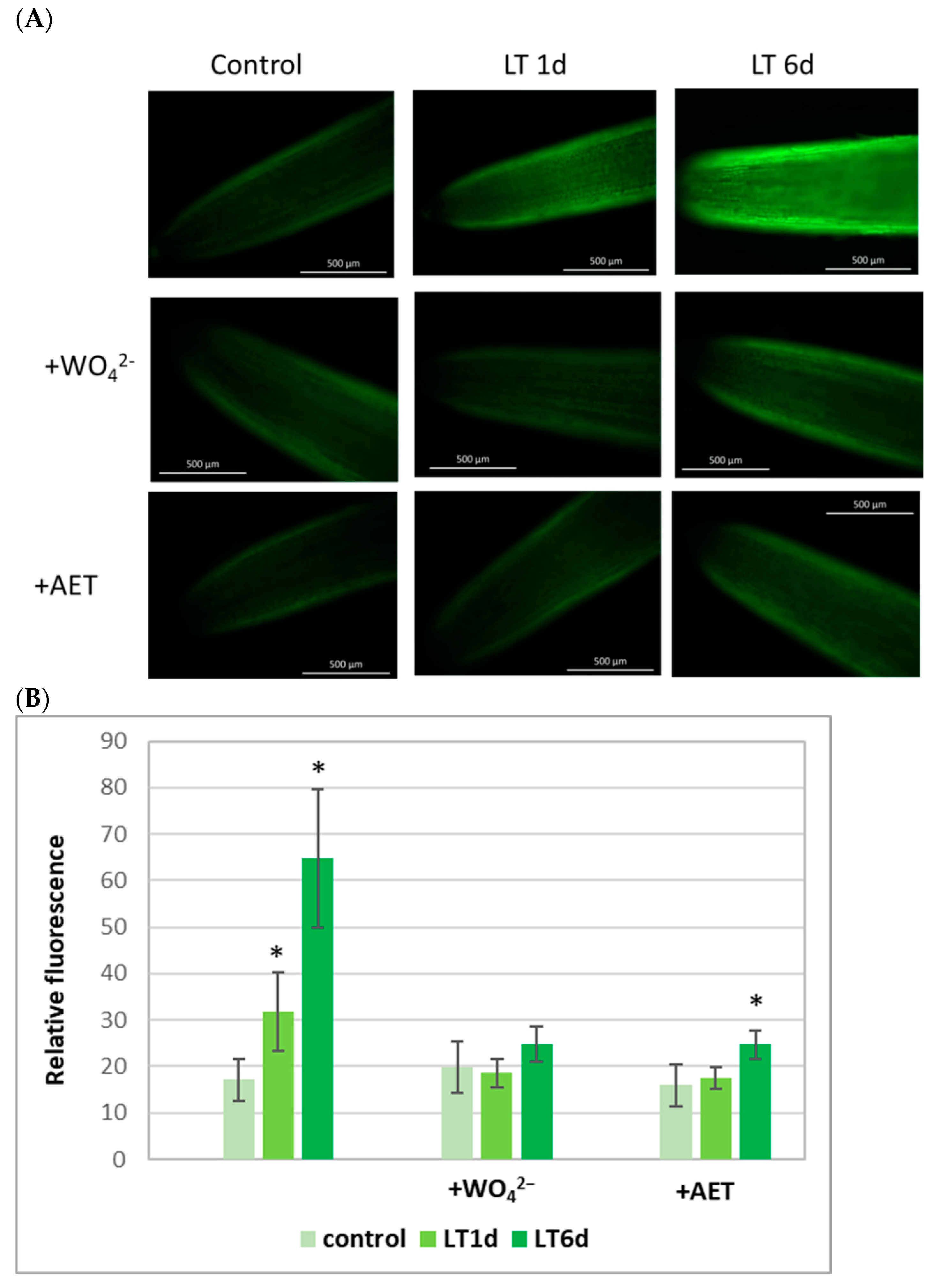
2.2. Endogenous NO Level in Cucumber Roots
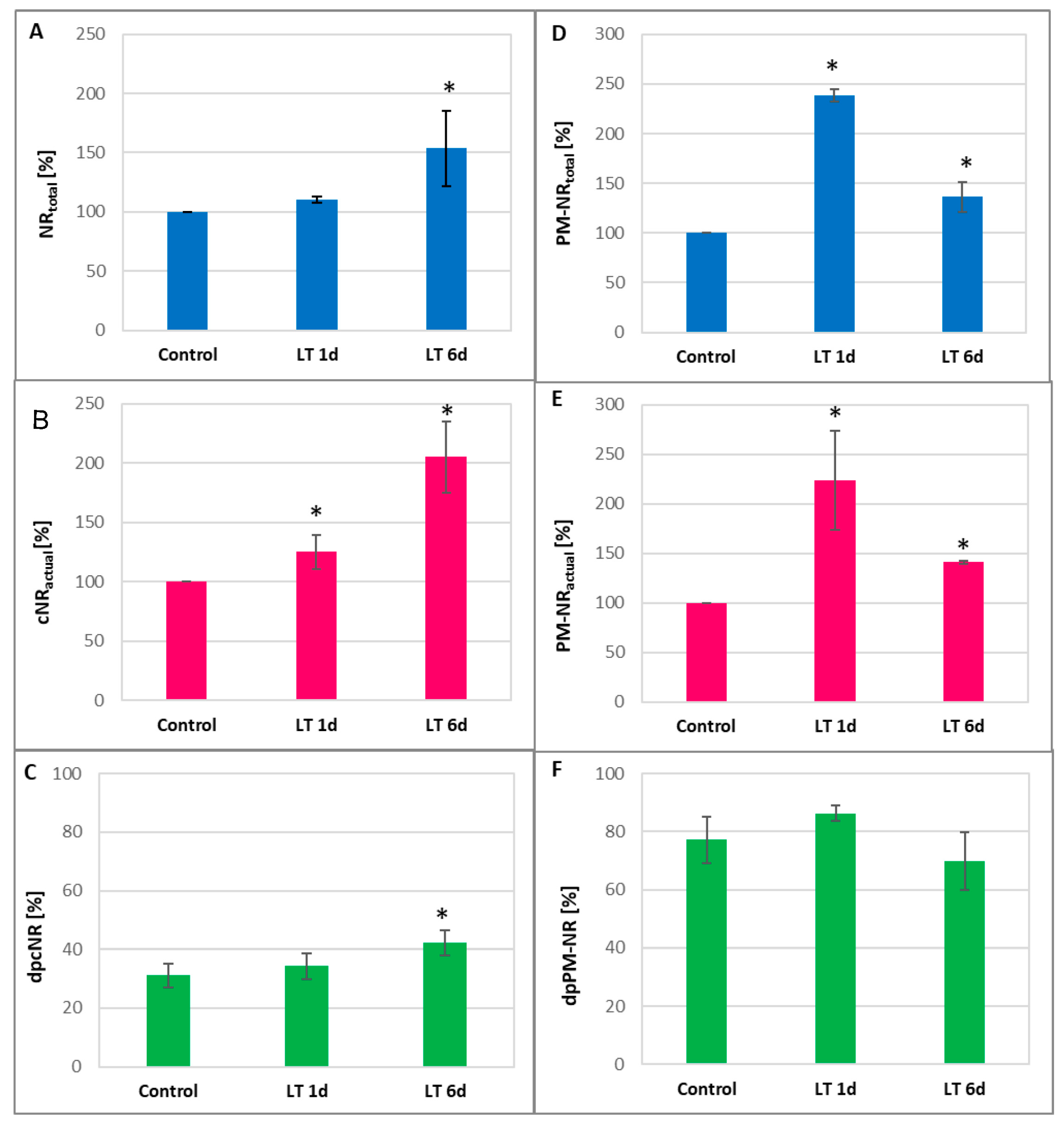
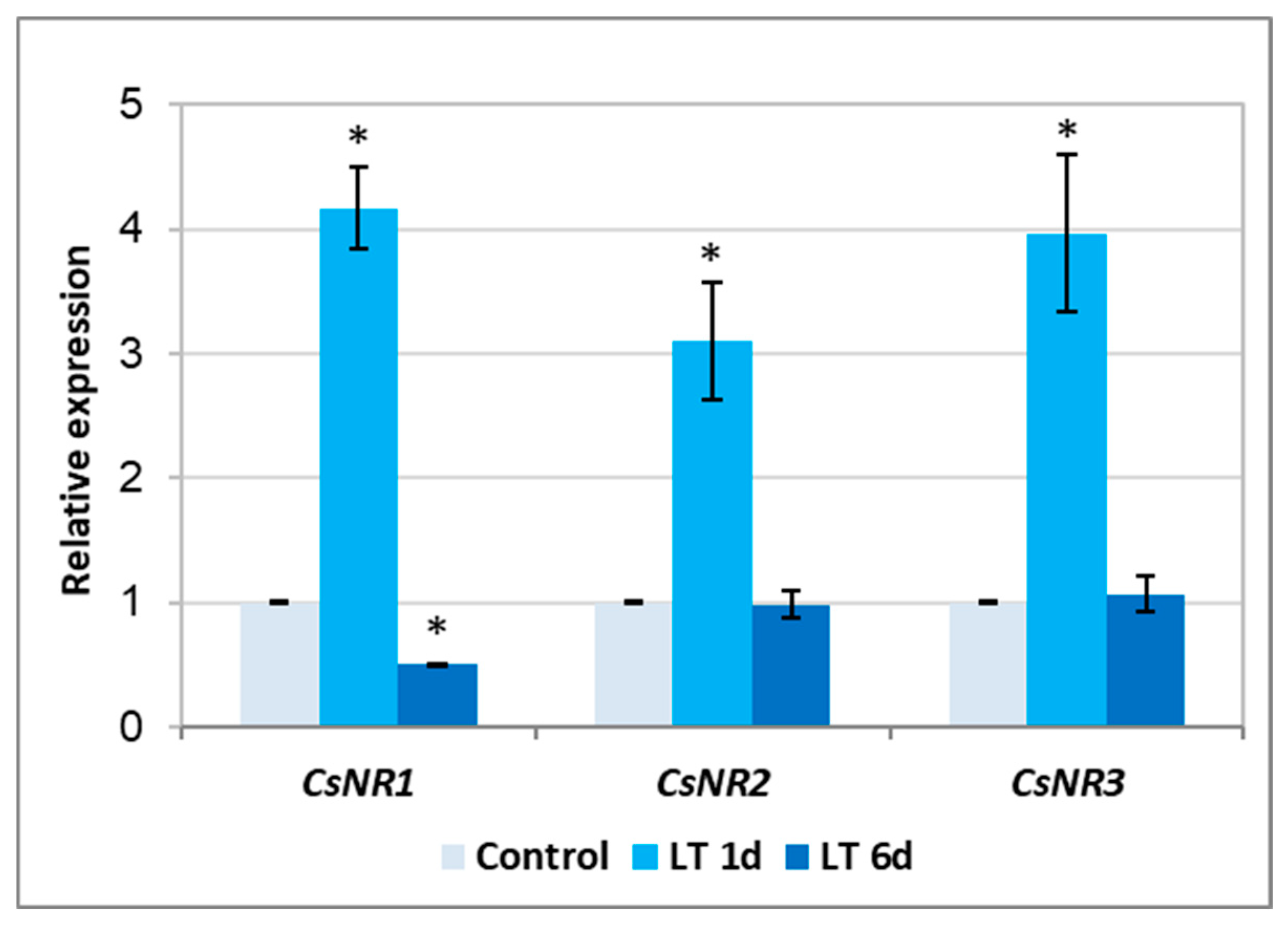
2.3. Activity of NR and Expression of CsNR Genes
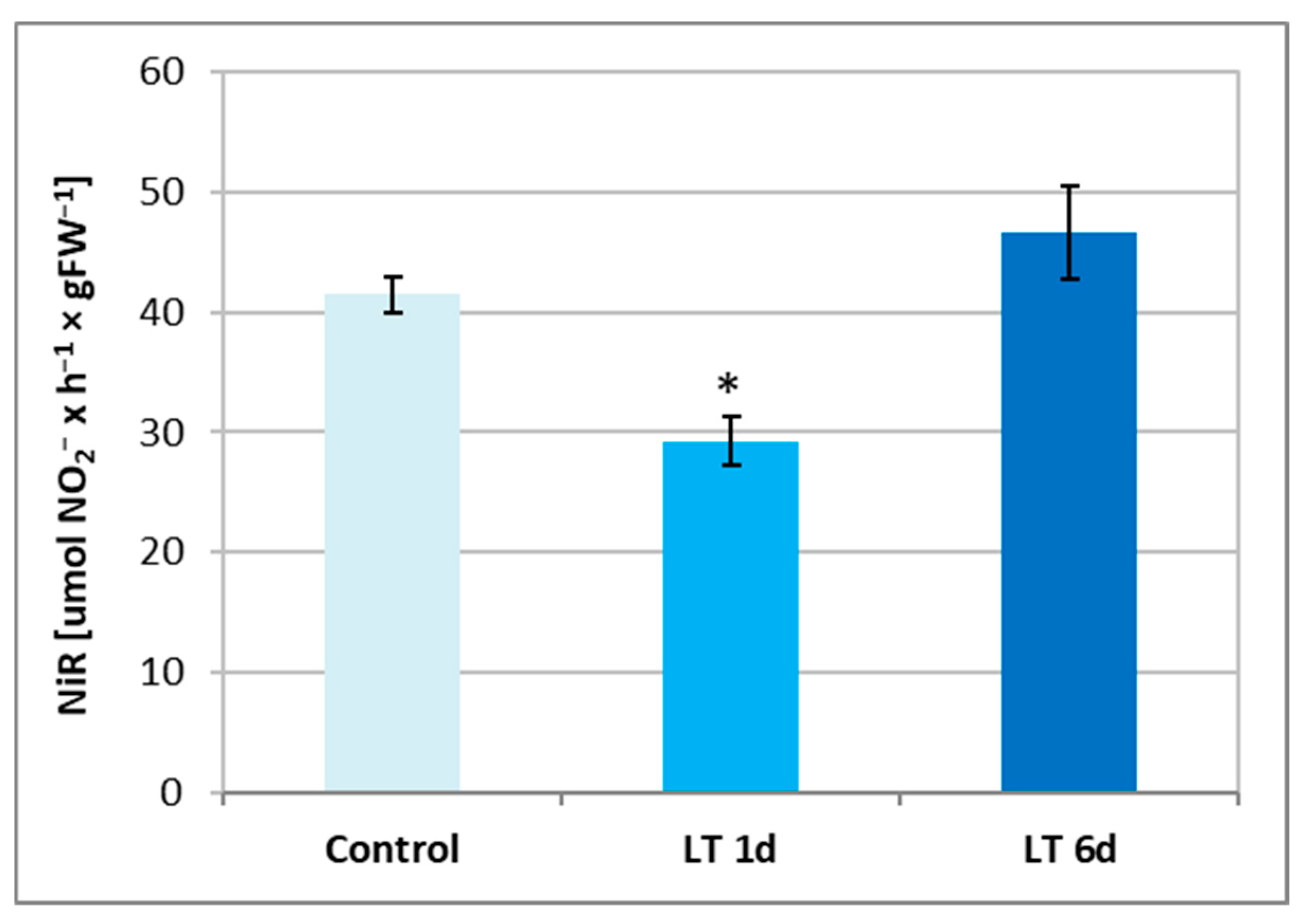
2.4. Activity of Nitrite Reductase and Nitrate/Nitrite Content
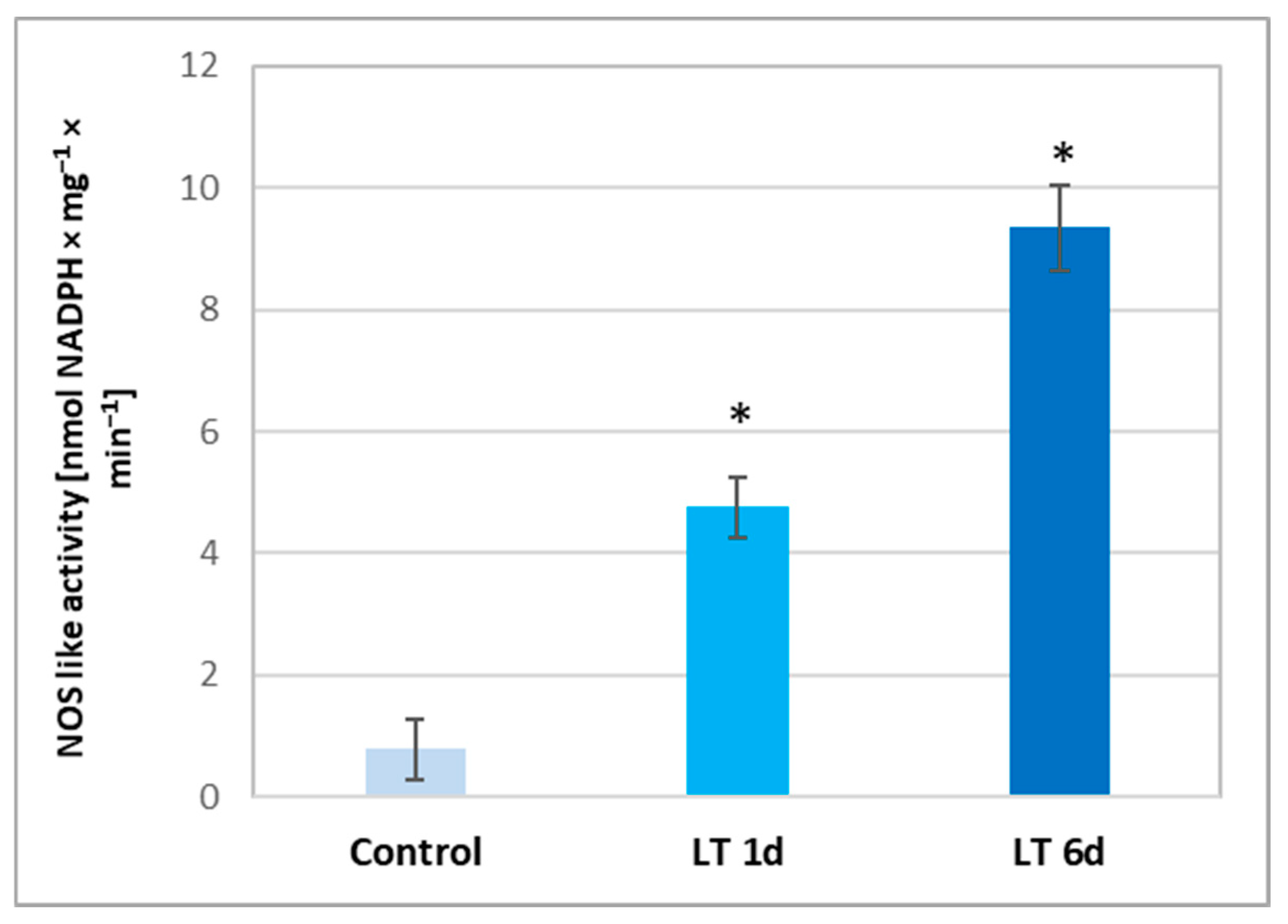
2.5. NOS-like Activity
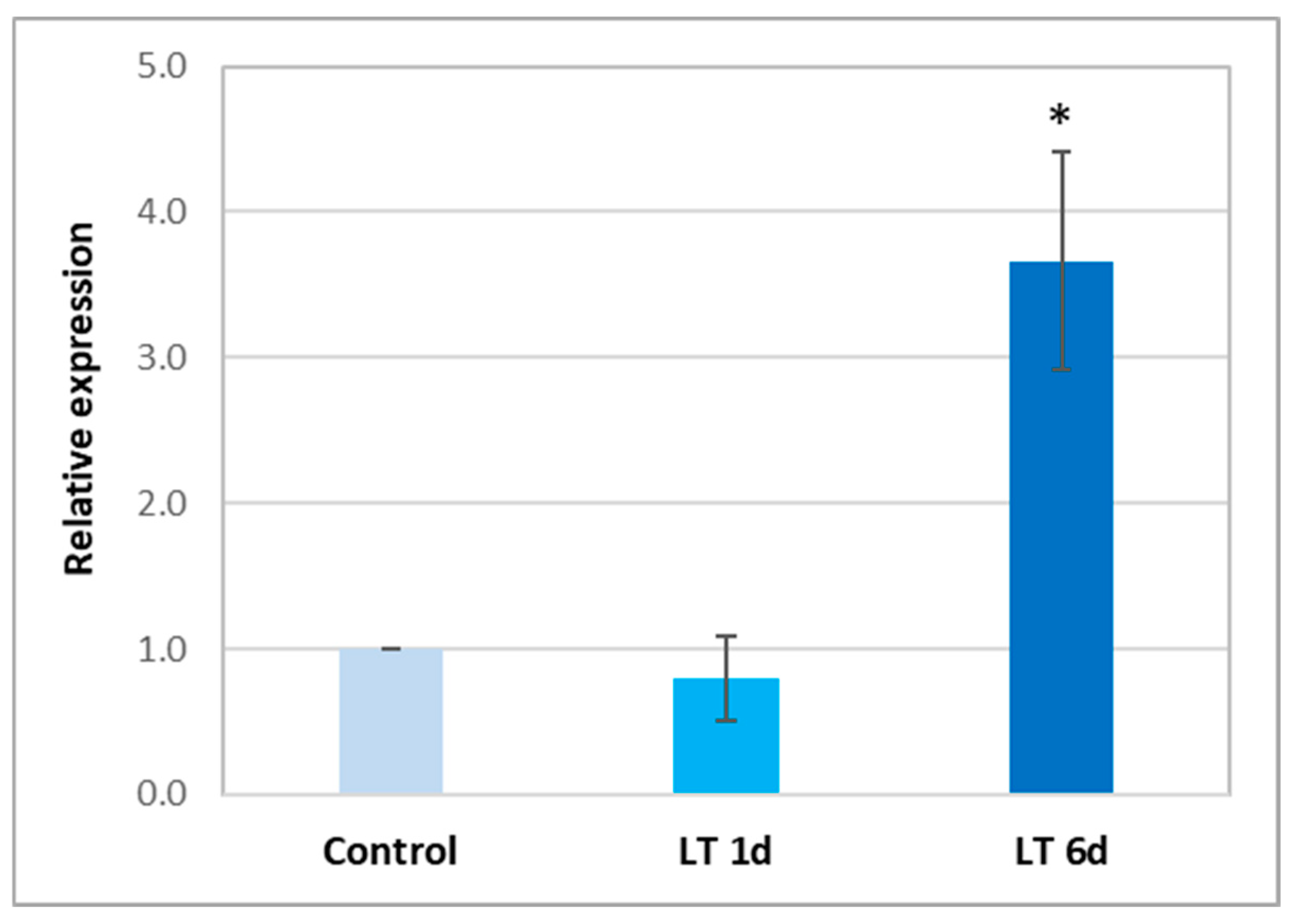
2.6. Expression of ARC Gene
3. Discussion
4. Materials and Methods
4.1. Plant Material, Growth Conditions, and Measurements of Growth Parameters
4.2. Detection of Endogenous NO
4.3. Preparation of Cytosol and Isolation of Plasma Membrane Fraction
4.4. Determination of Nitrate Reductase and Nitrite Reductase Activities
4.5. NOS-like Activity Assay
4.6. RNA Isolation, cDNA Synthesis, and Real-Time PCR Analysis
4.7. Endogenous Nitrate and Nitrite Level Measurement
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Vicente, I.; Lorenzo, O. Nitric Oxide Regulation of Temperature Acclimation: A Molecular Genetic Perspective. J. Exp. Bot. 2021, 72, 5789–5794. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Advances and Challenges in Uncovering Cold Tolerance Regulatory Mechanisms in Plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.A.; Cheng, Y.; Wang, B.; Qin, Y. Plant Low-Temperature Stress: Signaling and Response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Guy, C.L. Cold Acclimation and Freezing Stress Tolerance: Role of Protein Metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J. Abiotic Stress Signal Transduction in Plants: Molecular and Genetic Perspectives. Physiol. Plant 2001, 112, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Chinnusamy, V.; Zhu, J.; Zhu, J. Gene Regulation during Cold Acclimation in Plants. Physiol. Plant 2006, 126, 52–61. [Google Scholar] [CrossRef]
- Muzi, C.; Camoni, L.; Visconti, S.; Aducci, P. Cold Stress Affects H+-ATPase and Phospholipase D Activity in Arabidopsis. Plant Physiol. Biochem. 2016, 108, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, R.; Ashraf, M.A.; Ali, S.; Iqbal, M.; Zafar, S.; Akbar, A.; Banik, A. Role of NO in Plants. In Nitric Oxide in Plant Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 139–168. [Google Scholar]
- Napieraj, N.; Janicka, M.; Augustyniak, B.; Reda, M. Exogenous Putrescine Modulates Nitrate Reductase-Dependent NO Production in Cucumber Seedlings Subjected to Salt Stress. Metabolites 2023, 13, 1030. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ohri, P. Say “NO” to Plant Stresses: Unravelling the Role of Nitric Oxide under Abiotic and Biotic Stress. Nitric Oxide 2023, 130, 36–57. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, Z.; Barroso, J.B.; Brouquisse, R.; Corpas, F.J.; Gupta, K.J.; Lindermayr, C.; Loake, G.J.; Palma, J.M.; Petřivalský, M.; Wendehenne, D.; et al. A Forty Year Journey: The Generation and Roles of NO in Plants. Nitric Oxide 2019, 93, 53–70. [Google Scholar] [CrossRef]
- Tejada-Jimenez, M.; Llamas, A.; Galván, A.; Fernández, E. Role of Nitrate Reductase in NO Production in Photosynthetic Eukaryotes. Plants 2019, 8, 56. [Google Scholar] [CrossRef]
- Gupta, K.J.; Kaladhar, V.C.; Fitzpatrick, T.B.; Fernie, A.R.; Møller, I.M.; Loake, G.J. Nitric Oxide Regulation of Plant Metabolism. Mol. Plant 2022, 15, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Reda, M.; Janicka, M.; Kabała, K. Nitrate Reductase Dependent Synthesis of NO in Plants. In Nitric Oxide in Plant Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 95–110. [Google Scholar]
- Allagulova, C.R.; Lubyanova, A.R.; Avalbaev, A.M. Multiple Ways of Nitric Oxide Production in Plants and Its Functional Activity under Abiotic Stress Conditions. Int. J. Mol. Sci. 2023, 24, 11637. [Google Scholar] [CrossRef]
- Campbell, W.H. Nitrate Reductase Structure, Function and Regulation: Bridging the Gap between Biochemistry and Physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 277–303. [Google Scholar] [CrossRef] [PubMed]
- Rockel, P.; Strube, F.; Rockel, A.; Wildt, J.; Kaiser, W.M. Regulation of Nitric Oxide (NO) Production by Plant Nitrate Reductase in Vivo and in Vitro. J. Exp. Bot. 2002, 53, 103–110. [Google Scholar] [CrossRef]
- Wilson, I.D.; Neill, S.J.; Hancock, J.T. Nitric Oxide Synthesis and Signalling in Plants. Plant Cell Environ. 2008, 31, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, C.; Strube, F.; Marx, G.; Ullrich, W.R.; Rockel, P. A Plasma Membrane-Bound Enzyme of Tobacco Roots Catalyses the Formation of Nitric Oxide from Nitrite. Planta 2001, 212, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, C.; Stremlau, S. Formation and Possible Roles of Nitric Oxide in Plant Roots. J. Exp. Bot. 2006, 57, 463–470. [Google Scholar] [CrossRef]
- Reda, M.; Golicka, A.; Kabała, K.; Janicka, M. Involvement of NR and PM-NR in NO Biosynthesis in Cucumber Plants Subjected to Salt Stress. Plant Sci. 2018, 267, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, Á.; Ocaña-Calahorro, F.; Mariscal, V.; Carreras, A.; Barroso, J.B.; Galván, A.; Fernández, E. A Dual System Formed by the ARC and NR Molybdoenzymes Mediates Nitrite-dependent NO Production in Chlamydomonas. Plant Cell Environ. 2016, 39, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate Reductase Regulates Plant Nitric Oxide Homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Gross, I.; Durner, J. Nitric Oxide Production in Plants: An Update. J. Exp. Bot. 2018, 69, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Maiber, L.; Koprivova, A.; Bender, D.; Kopriva, S.; Fischer-Schrader, K. Characterization of the Amidoxime Reducing Components ARC1 and ARC2 from Arabidopsis thaliana. FEBS J. 2022, 289, 5656–5669. [Google Scholar] [CrossRef]
- Tang, X.; Peng, Y.; Li, Z.; Guo, H.; Xia, X.; Li, B.; Yin, W. The Regulation of Nitrate Reductases in Response to Abiotic Stress in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 1202. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Fan, X.; Miller, A.J.; Xu, G. Plant Nitrogen Uptake and Assimilation: Regulation of Cellular pH Homeostasis. J. Exp. Bot. 2020, 71, 4380–4392. [Google Scholar] [CrossRef] [PubMed]
- Tischner, R. Nitrate Uptake and Reduction in Higher and Lower Plants. Plant Cell Environ. 2000, 23, 1005–1024. [Google Scholar] [CrossRef]
- Tossi, V.; Lamattina, L.; Cassia, R. Pharmacological and Genetical Evidence Supporting Nitric Oxide Requirement for 2,4-Epibrassinolide Regulation of Root Architecture in Arabidopsis thaliana. Plant Signal Behav. 2013, 8, e24712. [Google Scholar] [CrossRef] [PubMed]
- Jeandroz, S.; Wipf, D.; Stuehr, D.J.; Lamattina, L.; Melkonian, M.; Tian, Z.; Zhu, Y.; Carpenter, E.J.; Wong, G.K.-S.; Wendehenne, D. Occurrence, Structure, and Evolution of Nitric Oxide Synthase–like Proteins in the Plant Kingdom. Sci. Signal 2016, 9, re2. [Google Scholar] [CrossRef]
- Phillips, K.; Majola, A.; Gokul, A.; Keyster, M.; Ludidi, N.; Egbichi, I. Inhibition of NOS-like Activity in Maize Alters the Expression of Genes Involved in H2O2 Scavenging and Glycine Betaine Biosynthesis. Sci. Rep. 2018, 8, 12628. [Google Scholar] [CrossRef]
- Reda, M.; Migocka, M.; Kłobus, G. Effect of Short-Term Salinity on the Nitrate Reductase Activity in Cucumber Roots. Plant Sci. 2011, 180, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-G.; Chen, L.; Zhang, L.-L.; Zhang, W.-H. Nitric Reductase-Dependent Nitric Oxide Production Is Involved in Cold Acclimation and Freezing Tolerance in Arabidopsis. Plant Physiol. 2009, 151, 755–767. [Google Scholar] [CrossRef]
- Janicka, M.; Reda, M.; Czyżewska, K.; Kabała, K. Involvement of Signalling Molecules NO, H2O2 and H2S in Modification of Plasma Membrane Proton Pump in Cucumber Roots Subjected to Salt or Low Temperature Stress. Funct. Plant Biol. 2018, 45, 428. [Google Scholar] [CrossRef]
- Carvalho, C.P.; Cardoso-Gustavson, P.; Rodrigues, E.; Braga, M.R.; Mercier, H.; Nievola, C.C. Low Temperature Acclimation and De-Acclimation of the Subtropical Bromeliad Nidularium Minutum: Implications of Changes in the NO, Sugar Content and NR Activity. Environ. Exp. Bot. 2019, 159, 34–43. [Google Scholar] [CrossRef]
- Zhang, P.; Li, S.; Zhao, P.; Guo, Z.; Lu, S. Comparative Physiological Analysis Reveals the Role of NR-Derived Nitric Oxide in the Cold Tolerance of Forage Legumes. Int. J. Mol. Sci. 2019, 20, 1368. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; Foresi, N.; Lamattina, L. Structure Diversity of Nitric Oxide Synthases (NOS): The Emergence of New Forms in Photosynthetic Organisms. Front. Plant Sci. 2013, 4, 232. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, B.; Xue, S.; Cai, Y.; Qi, W.; Jian, C.; Xu, S.; Wang, T.; Ren, H. Cucumber (Cucumis sativus L.) Nitric Oxide Synthase Associated Gene1 (CsNOA1) Plays a Role in Chilling Stress. Front. Plant Sci. 2016, 7, 1652. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. NO Source in Higher Plants: Present and Future of an Unresolved Question. Trends Plant Sci. 2022, 27, 116–119. [Google Scholar] [CrossRef]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in Plants: From Nutrition to the Modulation of Abiotic Stress Adaptation. Stress Biol. 2022, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.; Wdowikowska, A.; Janicka, M. Plant Plasma Membrane Proton Pump: One Protein with Multiple Functions. Cells 2022, 11, 4052. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.M.; Glass, A.D.M. Molecular and Physiological Aspects of Nitrate Uptake in Plants. Trends Plant Sci. 1998, 3, 389–395. [Google Scholar] [CrossRef]
- Ullrich, W. Nitrate and Ammonium Uptake in Green Algae and Higher Plants: Mechanism and Relationship with Nitrate Metabolism. In Inorganic Nitrogen Metabolism; Ullrich, W.R., Aparicio, P.J., Syrett, P.J., Castillo, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 32–38. [Google Scholar]
- Tischner, R. Nitrate Uptake and Reduction in Plants. J. Crop Improv. 2006, 15, 53–95. [Google Scholar] [CrossRef]
- Kaiser, W.M.; Weiner, H.; Kandlbinder, A.; Tsai, C.; Rockel, P.; Sonoda, M.; Planchet, E. Modulation of Nitrate Reductase: Some New Insights, an Unusual Case and a Potentially Important Side Reaction. J. Exp. Bot. 2002, 53, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Lillo, C.; Meyer, C.; Lea, U.S.; Provan, F.; Oltedal, S. Mechanism and Importance of Post-Translational Regulation of Nitrate Reductase. J. Exp. Bot. 2004, 55, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Mohn, M.; Thaqi, B.; Fischer-Schrader, K. Isoform-Specific NO Synthesis by Arabidopsis Thaliana Nitrate Reductase. Plants 2019, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Fu, X.; Han, L.; Xu, C.; Liu, C.; Bi, H.; Ai, X. Nitric Oxide Functions as a Downstream Signal for Melatonin-Induced Cold Tolerance in Cucumber Seedlings. Front. Plant Sci. 2021, 12, 686545. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, L.; Sun, M.; Wang, J.; Li, S.; Miao, L.; Yan, Y.; He, C.; Yu, X.; Li, Y. Adaptation of Cucumber Seedlings to Low Temperature Stress by Reducing Nitrate to Ammonium during It’s Transportation. BMC Plant Biol. 2021, 21, 189. [Google Scholar] [CrossRef] [PubMed]
- Goel, P.; Singh, A.K. Abiotic Stresses Downregulate Key Genes Involved in Nitrogen Uptake and Assimilation in Brassica juncea L. PLoS ONE 2015, 10, e0143645. [Google Scholar] [CrossRef]
- Yaneva, I.A.; Hoffmann, G.W.; Tischner, R. Nitrate Reductase from Winter Wheat Leaves Is Activated at Low Temperature via Protein Dephosphorylation. Physiol. Plant 2002, 114, 65–72. [Google Scholar] [CrossRef]
- Floryszak-Wieczorek, J.; Arasimowicz-Jelonek, M.; Izbiańska, K. The Combined Nitrate Reductase and Nitrite-Dependent Route of NO Synthesis in Potato Immunity to Phytophthora Infestans. Plant Physiol. Biochem. 2016, 108, 468–477. [Google Scholar] [CrossRef]
- Costa-Broseta, Á.; Castillo, M.; León, J. Nitrite Reductase 1 Is a Target of Nitric Oxide-Mediated Post-Translational Modifications and Controls Nitrogen Flux and Growth in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 7270. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Janicka, M.; Wdowikowska, A.; Kłobus, G. Assay of Plasma Membrane H+-ATPase in Plant Tissues under Abiotic Stresses. In Plant Membrane Proteomics. Methods in Molecular Biology; Mock, H.P., Matros, A., Witzel, K., Eds.; Springer Protocols: Berlin/Heidelberg, Germany, 2018; Volume 1696, pp. 205–215. [Google Scholar]
- Chung, H.-J.; Ferl, R.J. Arabidopsis Alcohol Dehydrogenase Expression in Both Shoots and Roots Is Conditioned by Root Growth Environment. Plant Physiol. 1999, 121, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Spalding, M.H.; Edwards, G.E. Photosynthesis in Enzymatically Isolated Leaf Cells from the CAM Plant Sedum telephium L. Planta 1978, 141, 59–63. [Google Scholar] [CrossRef]
- Kaiser, W.M.; Huber, S.C. Correlation between Apparent Activation State of Nitrate Reductase (NR), NR Hysteresis and Degradation of NR Protein. J. Exp. Bot. 1997, 48, 1367–1374. [Google Scholar] [CrossRef]
- Orea, A.; Pajuelo, P.; Pajuelo, E.; Márquez, A.J.; Romero, J.M. Characterisation and Expression Studies of a Root cDNA Encoding for Ferredoxin-nitrite Reductase from Lotus japonicus. Physiol. Plant 2001, 113, 193–202. [Google Scholar] [CrossRef]
- Sun, C.; Lu, L.; Liu, L.; Liu, W.; Yu, Y.; Liu, X.; Hu, Y.; Jin, C.; Lin, X. Nitrate Reductase-mediated Early Nitric Oxide Burst Alleviates Oxidative Damage Induced by Aluminum through Enhancement of Antioxidant Defenses in Roots of Wheat (Triticum aestivum). New Phytol. 2014, 201, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Migocka, M.; Papierniak, A. Identification of Suitable Reference Genes for Studying Gene Expression in Cucumber Plants Subjected to Abiotic Stress and Growth Regulators. Mol. Breed. 2011, 28, 343–357. [Google Scholar] [CrossRef]
- Reda, M. Regulation of Nitrate Reduction in Arabidopsis WT and Hxk1 Mutant under C and N Metabolites. Physiol. Plant 2013, 149, 260–272. [Google Scholar] [CrossRef]
| Control | LT 1 d | LT 6 d | |
|---|---|---|---|
| FW [g] | 0.141 ± 0.011 | 0.140 ± 0.007 | 0.030 ± 0.006 * |
| DW [g] | 0.0063 ± 0.0006 | 0.0064 ± 0.0006 | 0.0016 ± 0.0002 * |
| WC [%] | 95.64 ± 0.14 | 95.45 ± 0.27 | 94.85 ± 0.19 |
| Total soluble protein [mg × g FW−1] | 1.53 ± 0.13 | 1.42 ± 0.09 | 1.87 ± 0.07 * |
| Control | LT 1 d | LT 6 d | |
|---|---|---|---|
| NO2− | 7.56 ± 0.74 | 8.33 ± 0.71 | 10.86 ± 1.24 * |
| NO3− | 32.47 ± 1.97 | 17.89 ± 3.69 * | 33.86 ± 3.20 |
| NO2−/NO3− | 0.23 ± 0.02 | 0.48 ± 0.14 * | 0.31 ± 0.01 * |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Temp of Anniling | Ref. |
|---|---|---|---|---|
| CsNR1 CsNR2 CsNR3 | GGACGGTAGAGTAAAGAAGGC CGACTCCTCCTCCAACTCC TCCAATGGCGACTGCTG | TATCCCTTTTACTCCATTCA CCACTTCCATGTTGTCCAA CATCATCATCAATAAGGAGCGG | 56 °C | [32] |
| CsARC | TTCTTGTTGATGGCTGCGA | AGTTTCATTCAGCTCAGGTC | 60 °C | |
| CsCACS CsTIP41 | TGGGAAGATTCTTATGAAGTGC CAACAGGTGATATTGGATTATGATTATAC | CTCGTCAAATTTACACATTGGT GCCAGCTCATCCTCATATAAG | 60 °C 56 °C | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reda, M.; Kabała, K.; Stanisławski, J.; Szczepski, K.; Janicka, M. Regulation of NO-Generating System Activity in Cucumber Root Response to Cold. Int. J. Mol. Sci. 2025, 26, 1599. https://doi.org/10.3390/ijms26041599
Reda M, Kabała K, Stanisławski J, Szczepski K, Janicka M. Regulation of NO-Generating System Activity in Cucumber Root Response to Cold. International Journal of Molecular Sciences. 2025; 26(4):1599. https://doi.org/10.3390/ijms26041599
Chicago/Turabian StyleReda, Małgorzata, Katarzyna Kabała, Jan Stanisławski, Kacper Szczepski, and Małgorzata Janicka. 2025. "Regulation of NO-Generating System Activity in Cucumber Root Response to Cold" International Journal of Molecular Sciences 26, no. 4: 1599. https://doi.org/10.3390/ijms26041599
APA StyleReda, M., Kabała, K., Stanisławski, J., Szczepski, K., & Janicka, M. (2025). Regulation of NO-Generating System Activity in Cucumber Root Response to Cold. International Journal of Molecular Sciences, 26(4), 1599. https://doi.org/10.3390/ijms26041599






